Abstract
Both the transforming growth factor β (TGF-β) and integrin signalling pathways have well-established roles in angiogenesis. However, how these pathways integrate to regulate angiogenesis is unknown. Here, we show that the extracellular matrix component, fibronectin, and its cellular receptor, α5β1 integrin, specifically increase TGF-β1- and BMP-9-induced Smad1/5/8 phosphorylation via the TGF-β superfamily receptors endoglin and activin-like kinase-1 (ALK1). Fibronectin and α5β1 integrin increase Smad1/5/8 signalling by promoting endoglin/ALK1 cell surface complex formation. In a reciprocal manner, TGF-β1 activates α5β1 integrin and downstream signalling to focal adhesion kinase (FAK) in an endoglin-dependent manner. α5β1 integrin and endoglin form a complex on the cell surface and co-internalize, with their internalization regulating α5β1 integrin activation and signalling. Functionally, endoglin-mediated fibronectin/α5β1 integrin and TGF-β pathway crosstalk alter the responses of endothelial cells to TGF-β1, switching TGF-β1 from a promoter to a suppressor of migration, inhibiting TGF-β1-mediated apoptosis to promote capillary stability, and partially mediating developmental angiogenesis in vivo. These studies provide a novel mechanism for the regulation of TGF-β superfamily signalling and endothelial function through crosstalk with integrin signalling pathways.
Keywords: angiogenesis, crosstalk, endoglin, integrin, TGF-β
Introduction
Transforming growth factor β (TGF-β) superfamily signalling pathways have important roles in regulating endothelial cell biology and angiogenesis. Deletion of components of this pathway results in abnormalities in the formation of the primitive vascular plexus, decreased vessel wall integrity and embryonic lethality in murine models due to defects in angiogenesis (Goumans et al, 2009; Pardali and ten Dijke, 2009). The canonical TGF-β superfamily signalling pathway is triggered when TGF-β superfamily ligands bind to cell surface receptors, including co-receptors, type II, and type I receptors. Upon ligand binding these receptors form complexes, which facilitate the transphosphorylation and activation of the type I receptor by the type II receptor; the type I receptor then phosphorylates receptor-regulated Smads (R-Smads), which bind the co-Smad, Smad4, and accumulate in the nucleus where they act in concert with co-activators and co-repressors to regulate target gene expression (Massague, 1992, 1996). Endothelial cells express two type I TGF-β superfamily receptors, activin-like kinase-1 (ALK1), which is expressed preferentially in the endothelium, and ALK5 (also referred to as the type I TGF-β receptor, TβRI), which is expressed ubiquitously (Massague, 1998). In endothelial cells, TGF-β can activate two Smad signalling pathways, the Smad1/5/8 pathway (via ALK1) and the Smad2/3 pathway (via ALK5). Recent studies have shown that another TGF-β superfamily ligand, BMP-9, also binds with high affinity to ALK1 and endoglin in endothelial cells, induces phosphorylation of Smad1 (David et al, 2007), and plays a physiological role in the control of adult blood vessel quiescence (David et al, 2008). While the balance of signalling between ALK1/Smad1/5/8 and ALK5/Smad2/3 is thought to be a major determinant of TGF-β superfamily responsiveness in endothelial cell biology (Letamendia et al, 1998), how the balance between these two TGF-β signalling pathways is regulated during angiogenesis is largely unknown.
Endoglin is a TGF-β superfamily co-receptor also preferentially expressed in endothelial cells. Like many other TGF-β superfamily receptors, endoglin is essential for angiogenesis and vascular development, as endoglin-null (endoglin−/−) mice experience embryonic lethality at day 10.5 due to defects in vascular development (Li et al, 1999; Arthur et al, 2000). Moreover, mutations in endoglin and ALK1 cause hereditary haemorrhagic telangiectasia (HHT), an autosomal dominant vascular disease characterized by dilated vessels and arteriovenous malformations that lead to recurrent haemorrhage and shunting in the lung, brain, and the gastrointestinal tract (Bourdeau et al, 1999). In addition, endoglin is overexpressed in neoangiogenic vessels, during inflammation (Torsney et al, 2002; Mazibrada et al, 2008), and in solid tumours (Fonsatti et al, 2003; Bernabeu et al, 2009). Although, our previous work has demonstrated that endoglin can regulate both canonical and non-canonical TGF-β signalling and endothelial function through interaction with GIPC (Lee et al, 2008) and β-arrestin2 (Lee and Blobe, 2007), the mechanisms by which endoglin mediates these effects remain largely unknown.
During angiogenesis, growth factors and their receptors coordinate with the extracellular matrix (ECM) and ECM receptors, including integrins, to regulate angiogenesis (Eliceiri, 2001). Upon integrin engagement, the ECM triggers activation of numerous intracellular signalling pathways essential for endothelial cell survival, proliferation, migration, and angiogenesis (Ingber, 2002; Comoglio et al, 2003; Ross, 2004; Mahabeleshwar et al, 2007). Although certain ECM components, including laminin, emerged early in evolution, other components, notably fibronectin, are present only in vertebrates with an endothelial cell-lined circulatory system (Whittaker et al, 2006), suggesting a potential role for fibronectin in regulating angiogenesis. In addition, genetic studies in mice and fish support a fundamental role for fibronectin and its primary receptor, integrin α5β1, in early blood vessel development and vascular physiology (George et al, 1993; Francis et al, 2002; Hynes, 2007; Astrof and Hynes, 2009). We noted that, fibronectin, together with the two TGF-β superfamily receptors that are preferentially expressed on endothelial cells, ALK1 and endoglin, are all expressed predominantly in developing vessels, with diminished expression in mature vessels, where laminin and collagen predominate the ECM (Tonnesen et al, 1985; Nicosia and Madri, 1987; Risau and Lemmon, 1988; Wikstrom et al, 2002; Seki et al, 2003; Mahmoud et al, 2009). Further, both fibronectin-null and endoglin-null mice die at embryonic day 9.5–10.5 due to defects in vascular development (George et al, 1993; Li et al, 1999; Arthur et al, 2000; Francis et al, 2002). Based on these observations, we hypothesized that the ECM might interact with TGF-β superfamily signalling pathway to regulate signalling and endothelial cell biology. Here, we investigate the crosstalk between TGF-β and fibronectin/integrin α5β1 pathways and the role of this crosstalk in regulating endothelial cell biology and angiogenesis.
Results
Endoglin specifically increases TGF-β1- and BMP-9-induced Smad1/5/8 activation in endothelial cells
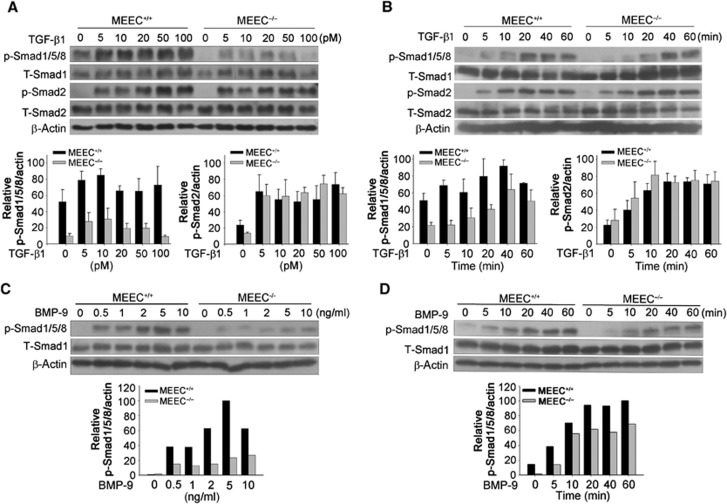
To investigate the role of endoglin in TGF-β superfamily signalling in endothelial cells, we stimulated murine embryonic endothelial cells (MEEC) from endoglin wild-type (MEEC+/+) and knockout (MEEC−/−) mice (Supplementary Figure S1) with two of the main physiological ligands for endoglin, TGF-β1 and BMP-9. Treatment of MEEC+/+ with TGF-β1 induced both Smad1/5/8 and Smad2 phosphorylation in a dose- (Figure 1A) and time- (Figure 1B) dependent manner. In contrast, treatment of MEEC−/− with TGF-β1 resulted in decreased and delayed Smad1/5/8 phosphorylation relative to MEEC+/+ (Figure 1A and B), while Smad2 phosphorylation was not effected (Figure 1A and B). Importantly, restoring endoglin expression in MEEC−/− restored both basal and TGF-β1-induced Smad1/5/8 phosphorylation (Supplementary Figure S5). Treatment of MEEC+/+ with BMP-9 also induced Smad1/5/8 phosphorylation in a dose- (Figure 1C) and time- (Figure 1D) dependent manner, while having no effect on Smad2 phosphorylation (Figure 2E), consistent with a previous report (David et al, 2008). In contrast, treatment of MEEC−/− with BMP-9 resulted in decreased and delayed Smad1/5/8 phosphorylation relative to MEEC+/+ (Figure 1C and D). These results indicate that endoglin specifically facilitates TGF-β1- and BMP-9-induced Smad1/5/8 activation in endothelial cells.
Figure 1.
Endoglin specifically increases TGF-β1- and BMP-9-induced Smad1/5/8 activation in endothelial cells. Murine embryonic endothelial cells (MEEC) from endoglin wild-type (MEEC+/+) and knockout (MEEC−/−) mice were serum starved for 6 h, treated with indicated concentrations of TGF-β1 (A) or BMP-9 (C) for 30 min, or treated with 50 pM TGF-β1 (B) or 2 ng/ml BMP-9 (D) for the indicated times, and the cell lysates analysed with the indicated antibodies. Quantitated data normalized to β-actin from three independent experiments ±s.d. are presented below. Figure source data can be found with the Supplementary data.
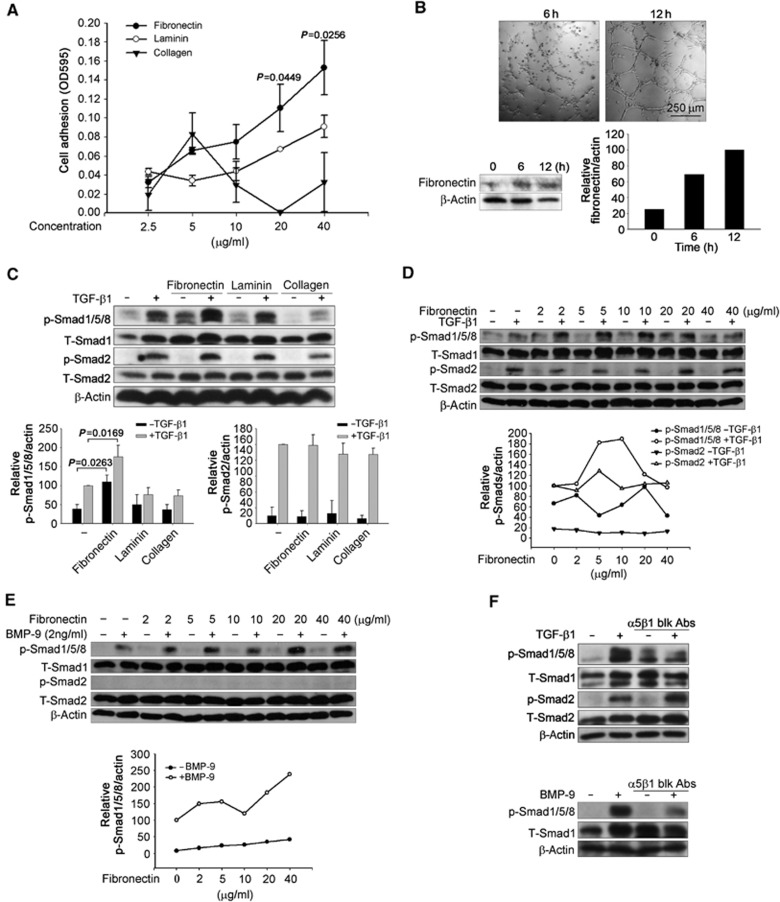
Figure 2.
Fibronectin and its receptor, integrin α5β1, specifically increase TGF-β1- and BMP-9-induced Smad1/5/8 phosphorylation. (A) Human microvascular endothelial-1 cells (HMEC-1) were incubated on dishes coated with the indicated concentrations of fibronectin, collagen, and laminin for 15 min and the level of adhesion assessed. The cell adhesion ±s.d. of three independent experiments is presented. (B) HMEC-1 were cultured on Matrigel for indicated times to form tubules. Fibronectin expression levels were assessed, and quantitated with β-actin as a loading control. (C) HMEC-1 were cultured in the dishes coated with 10 μg/ml fibronectin, collagen, or laminin, serum starved for 6 h, treated with 50 pM TGF-β1 for 30 min, the cell lysates analysed with the indicated antibodies. Quantitated data normalized to β-actin from three independent experiments ±s.d. are presented below. (D, E) HMEC-1 were cultured in the dishes coated with indicated doses of fibronectin, serum starved for 6 h, treated with 50 pM TGF-β1 (D) or 2 ng/ml BMP-9 (E) for 30 min, the cell lysates analysed with the indicated antibodies, and the data quantitated below. (F) HMEC-1 were cultured in the dishes coated with 10 μg/ml fibronectin, serum starved for 5 h, pretreated with 10 μg/ml integrin α5β1 function blocking antibody (blk Abs) for 1 h, followed by treatment with 50 pM TGF-β1 or 2 ng/ml BMP-9 for 30 min, and the cell lysates analysed with the indicated antibodies. Figure source data can be found with the Supplementary data.
Fibronectin and its receptor, integrin α5β1, increase TGF-β1- and BMP-9-induced Smad1/5/8 phosphorylation
Angiogenesis occurs in the context of a stroma composed of ECM components and stromal cells, including fibroblasts and immune cells. To explore the potential roles of distinct ECM components in regulating TGF-β superfamily signalling in endothelial cells, we assessed the adhesion of human microvascular endothelial cells (HMEC-1) to different ECM components that have prominent roles in regulating angiogenesis, including fibronectin (Astrof and Hynes, 2009), collagen (Twardowski et al, 2007), and laminin (Ljubimova et al, 2006). While HMEC-1 adhered to all three of these ECM components (Figure 2A), adhesion to fibronectin was most robust, followed by adhesion to laminin and collagen (Figure 2A). The expression of fibronectin also increased during angiogenesis on Matrigel in vitro (Figure 2B), with HMEC-1 forming fibronectin fibres (Supplementary Figure S2), suggesting a potential role for fibronectin in regulating endothelial cell signalling.
To examine the effect of these ECM components on TGF-β superfamily signalling in endothelial cells, HMEC-1 were plated on non-ECM coated plastic, or plastic coated with fibronectin, laminin or collagen and then stimulated with TGF-β1 or BMP-9. While laminin had no effect and collagen slightly decreased Smad1/5/8 signalling (Figure 2C), fibronectin modestly increased basal Smad1/5/8 phosphorylation (Figure 2C–E), and potently increased TGF-β1- (Figure 2C and D) and BMP-9- (Figure 2E) induced Smad1/5/8 phosphorylation. Fibronectin more effectively promoted TGF-β1-induced Smad1/5/8 phosphorylation, with an optimal concentration of 10 μg/ml, relative to the 40 μg/ml required for optimal stimulation of BMP-9-induced Smad1/5/8 phosphorylation (Figure 2D and E). In addition, fibronectin, laminin, or collagen had no effect on basal or TGF-β1-induced Smad2 phosphorylation (Figure 2C–E). These data suggest that fibronectin specifically promotes TGF-β1- and BMP-9-induced Smad1/5/8 activation in endothelial cells.
As integrin α5β1 is the predominant cellular receptor for fibronectin, we investigated whether integrin α5β1 regulates TGF-β1- or BMP-9-induced Smad1/5/8 activation. An integrin α5β1 function-blocking antibody effectively suppressed fibronectin and TGF-β1- or BMP-9-induced Smad1/5/8 phosphorylation in the presence (Figure 2F) or absence (Supplementary Figure S3) of exogenous fibronectin, while having no effect on Smad2 phosphorylation (Figure 2F). Taken together, these data support a role for fibronectin and its cellular receptor, integrin α5β1, in specifically regulating TGF-β1- and BMP-9-induced Smad1/5/8 activation in endothelial cells.
Regulation of TGF-β signalling by fibronectin/integrin α5β1 in endothelial cells depends on endoglin and ALK1
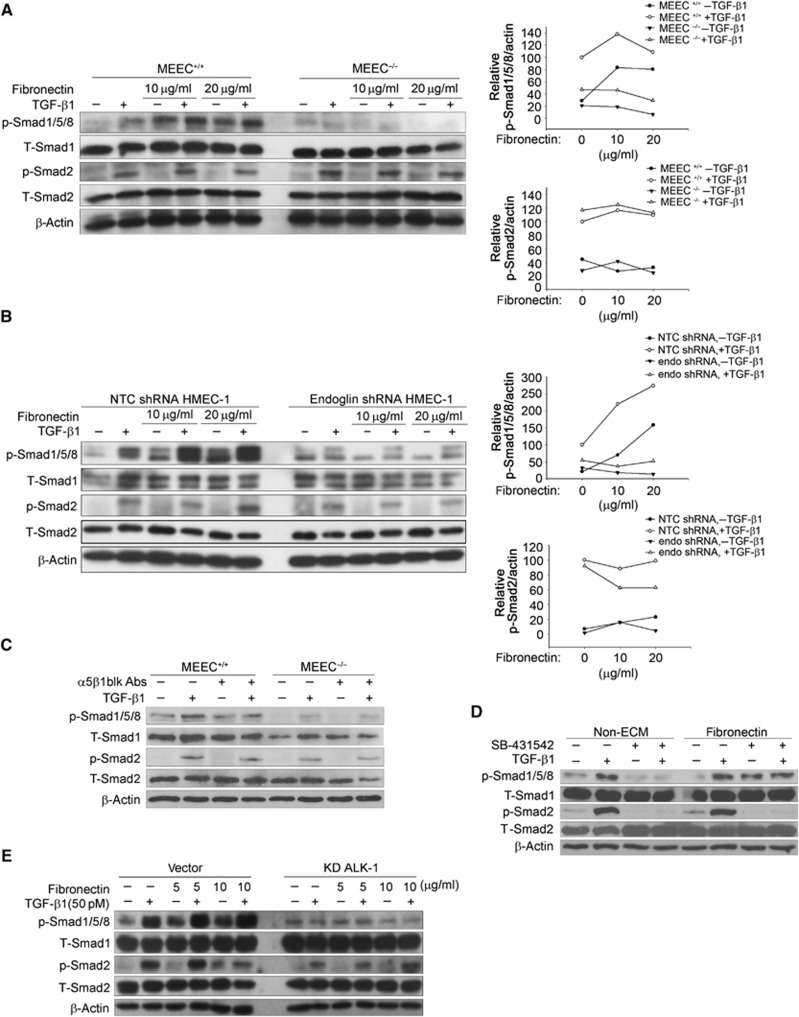
As endoglin specifically regulates Smad1/5/8 signalling in endothelial cells (Figure 1), we asked whether regulation of Smad1/5/8 signalling by fibronectin/integrin α5β1 occurs in an endoglin-dependent manner. We assessed the effects of fibronectin on TGF-β signalling between MEEC+/+ and MEEC−/− or control and endoglin knockdown HMEC-1 (Supplementary Figure S4). Fibronectin increased the TGF-β1-induced Smad1/5/8 phosphorylation in a dose-dependent manner in MEEC+/+ or control HMEC-1, but not in MEEC−/− or HMEC-1 with shRNA-mediated silencing of endoglin expression (Figure 3A and B). Consistent with our prior results, fibronectin had no effect on TGF-β1-induced Smad2 phosphorylation in either MEEC or HMEC-1 (Figure 3A and B). The difference between MEEC+/+ and MEEC−/− was endoglin specific, as expression of human endoglin in MEEC−/− rescued fibronectin/TGF-β1-induced Smad1/5/8 signalling (Supplementary Figure S5). The integrin α5β1 function-blocking antibody also specifically suppressed fibronectin and TGF-β1-induced Smad1/5/8 phosphorylation in MEEC+/+, but not in MEEC−/−, and had no effects on TGF-β1-induced Smad2 phosphorylation in either cell line (Figure 3C). Taken together, these studies strongly support a role for endoglin in mediating the effects of fibronectin and integrin α5β1 on TGF-β1-induced Smad1/5/8 signalling.
Figure 3.
Regulation of TGF-β signalling by fibronectin/integrin α5β1 in endothelial cells depends on endoglin and ALK1. (A, B) MEEC+/+ and MEEC−/− (A), or HMEC-1 adenovirus transfected with shRNA of non-target control (NTC) or endoglin (B) were cultured in the dishes coated with indicated doses of fibronectin, serum starved for 6 h, treated with 50 pM TGF-β1 for 30 min, the cell lysates analysed with the indicated antibodies, and the data quantitated. (C) MEEC+/+ and MEEC−/− were cultured in the dished coated with 10 μg/ml fibronectin, serum starved for 5 h, pretreated with 10 μg/ml integrin α5β1 function blocking antibody for 1 h, treated with 50 pM TGF-β1 for 30 min, and the cell lysates analysed with the indicated antibodies. (D) HMEC-1 were cultured in the non-coated or 10 μg/ml fibronectin-coated dishes, serum starved for 5 h, pretreated with 10 μM ALK5 inhibitor, SB-431542 for 1 h, then treated with or without 50 pM TGF-β1, and the cell lysates were analysed with the indicated antibodies. (E) HMEC-1 were transfected with vector or ALK1 kinase dead (KD ALK1) plasmids using adenovirus for 48 h, and cultured in the dishes coated with indicated doses of fibronectin, treated with 50 pM TGF-β1 for 30 min, and the cell lysates were analysed with the indicated antibodies. Figure source data can be found with the Supplementary data.
To determine whether ALK5 and ALK1 are involved in fibronectin-mediated TGF-β signalling, we either treated HMEC-1 with SB-431542, an ALK5 inhibitor that does not inhibit ALK1, or expressed a dominant-negative kinase dead ALK1 mutant (ALK1 KD) in HMEC-1. SB-431542 pretreatment effectively inhibited TGF-β1-induced Smad1/5/8 and Smad2 phosphorylation in the absence of fibronectin (Figure 3D), or in the presence of laminin or collagen (Supplementary Figure S6). However, in HMEC-1 cultured in fibronectin, SB-431542 only inhibited TGF-β1-induced Smad2 phosphorylation, with no effect on fibronectin/TGF-β1-induced Smad1/5/8 phosphorylation (Figure 3D; Supplementary Figure S10). These data suggested that ALK5 is not required for fibronectin-mediated regulation of Smad1/5/8 signalling in endothelial cells. In contrast, dominant-negative ALK1 (ALK1 KD) abolished TGF-β1-induced Smad1/5/8 phosphorylation as well as fibronectin augmented Smad1/5/8 phosphorylation (Figure 3E), suggesting that the regulation of TGF-β1-induced Smad1/5/8 signalling by fibronectin occurs in an ALK1-dependent manner.
TGF-β activates integrin α5β1 signalling in an endoglin-dependent manner
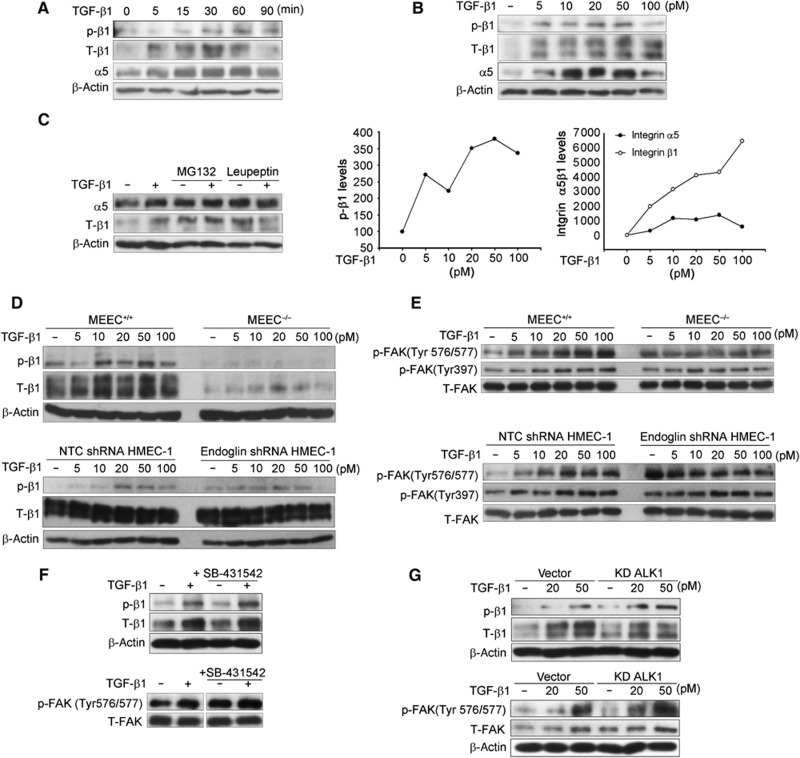
As TGF-β has been reported to regulate integrin α5β1 expression in non-endothelial cells (Collo and Pepper, 1999; Nesti et al, 2002), we investigated whether TGF-β1 might regulate integrin α5β1 expression in endothelial cells. TGF-β1 increased integrin α5β1 expression levels in a time- (Figure 4A) and dose- (Figure 4B) dependent manner in endothelial cells. TGF-β treatment had no effect on integrin α5 and β1 levels at the mRNA level (Supplementary Figure S7), and induced integrin α5β1 levels quickly, beginning at 15 min (Figure 4A), suggesting an effect at the protein level. In addition, while pretreatment with the lysosome inhibitor, leupeptin, increased α5 and β1 basal levels, pretreatment inhibited TGF-β1-induced increase in integrin α5β1 levels (Figure 4C). However, the proteasome inhibitor, MG132, failed to inhibit TGF-β1-induced-α5 and β1 levels (Figure 4C). These results suggest that TGF-β1 increases integrin α5β1 expression by preventing lysosome-mediated integrin α5β1 degradation.
Figure 4.
TGF-β activates integrin α5β1 signalling in an endoglin-dependent manner. (A) HMEC-1 were treated with 50 pM TGF-β1 for the indicated times, the cell lysates were then analysed with the indicated antibodies. p-β1: phosphorylated integrin β1 at Tyrosine 788/789; T-β1: total integrin β1. (B) HMEC-1 were serum starved for 6 h prior to treatment with the indicated doses of TGF-β1 for 30 min, the cell lysates were then analysed with the indicated antibodies, and the data quantitated below. (C) HMEC-1 were pretreated with 10 μM MG132 or 50 μM leupeptin for 1 h, followed by treatment with 50 pM TGF-β1 for 30 min, and integrin α5 and β1 subunits expression levels were assessed by western blot analysis. (D, E) MEEC+/+ and MEEC−/− or HMEC-1 adenovirally infected with shRNA to non-targeting control (NTC) or endoglin were serum starved for 6 h prior to treatment with the indicated doses of TGF-β1 for 30 min, and the cell lysates analysed with the indicated antibodies. (F) HMEC-1 were serum starved for 5 h, pretreated with 10 μM SB-431542 for 1 h prior to treatment with 50 pM TGF-β1 for 30 min, and the cell lysates analysed with the indicated antibodies. (G) HMEC-1 were adenovirally infected with vector or KD ALK1 for 48 h, serum starved for 6 h prior to treatment with indicated doses of TGF-β1 for 30 min, and the cell lysates analysed with the indicated antibodies. Figure source data can be found with the Supplementary data.
Phosphorylation of integrin β1 on threonines 788/789 is indicative of integrin α5β1 activation (Wennerberg et al, 1998). In addition to increasing integrin α5β1 expression, TGF-β induced phosphorylation of integrin β1 on threonines 788/789 in HMEC-1 and MEEC (Figure 4A, B). However, TGF-β1 did not stimulate phosphorylation of integrin β1 to the same extent in the MEEC−/− or HMEC-1 with silenced endoglin expression (Figure 4D). Focal adhesion kinase (FAK) is phosphorylated after integrin activation and is an important downstream mediator of integrin signalling (Lipfert et al, 1992; Guan, 1997). Consistent with the effects on TGF-β1-mediated integrin α5β1 activation, TGF-β1 treatment significantly increased FAK phosphorylation at Tyr576/577 and modestly increased FAK phosphorylation at Tyr397 in MEEC+/+ (Figure 4E, upper panel) and HMEC-1 (Figure 4E, bottom panel), while TGF-β1 had no effect on FAK phosphorylation in MEEC−/− (Figure 4E, upper panel) or HMEC-1 with silenced endoglin expression (Figure 4E, bottom panel). Further, as integrin phosphorylation of FAK at Tyr 576/577 requires Src recruitment, TGF-β1 increased Src phosphorylation at Tyr416 in MEEC+/+, while having no effect in MEEC−/− (Supplementary Figure S8). In contrast to the effects of TGF-β1, BMP-9 did not induce integrin α5β1 expression and only transiently induced integrin β1 phosphorylation (Supplementary Figure S9). Taken together, these data indicate that endoglin is required for TGF-β1-mediated integrin α5β1 activation and downstream signalling in endothelial cells.
To evaluate the role of ALK5 and ALK1 in TGF-β1 activation of integrin signalling, we pretreated HMEC-1 with SB-431542 or expressed dominant-negative ALK1 (ALK1 KD) to block ALK5 (Figure 3D; Supplementary Figure S10) and ALK1 activity (Figure 3E; Supplementary Figure S11), respectively. Neither SB-431542 nor overexpression of ALK1 KD inhibited TGF-β-induced integrin β1 subunit phosphorylation (Figure 4F and G) or FAK phosphorylation at Tyr 576/577 (Figure 4F and G), suggesting that neither ALK1 nor ALK5 was involved in TGF-β1-induced and endoglin-dependent integrin α5β1 activation.
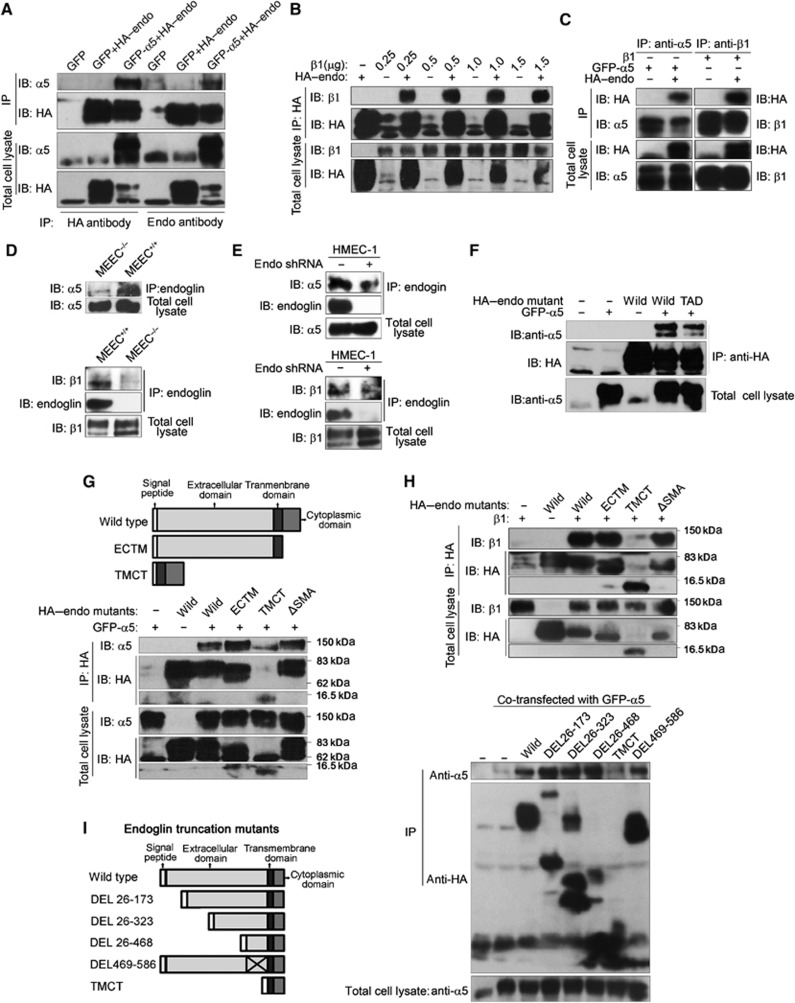
Endoglin interacts with integrin α5β1 via its extracellular domain
As we demonstrated that integrin α5β1 regulates TGF-β signalling in an endoglin-dependent manner, and that TGF-β signalling regulates integrin α5β1 in an endoglin-dependent manner, we next addressed whether endoglin interacted with integrin α5β1. First, we overexpressed GFP-tagged integrin α5 (GFP–α5) or β1 and HA-tagged endoglin (HA–endoglin) in COS7 cells and performed co-immunoprecipitation studies. Immunoprecipitation of endoglin was able to specifically co-immunoprecipitate integrin α5 (Figure 5A; Supplementary Figure S12) and β1 (Figure 5B). In a reciprocal manner, immunoprecipitation of integrin α5 or β1 specifically co-immunoprecipitated exogenous HA–endoglin (Figure 5C). As endoglin is expressed preferentially in endothelial cells, we asked whether endogenous endoglin and endogenous integrin α5β1 interacted in endothelial cells. Immunoprecipitation of endogenous endoglin specifically co-immunoprecipitated endogenous integrin α5 and β1 subunits in MEEC and HMEC-1 (Figure 5D and E). The interaction between endoglin and integrin α5β1 was specific, as endoglin could not co-immunoprecipitate integrin β4 (Supplementary Figure S13), a subunit of the laminin receptor, integrin α6β4, integrin αv or integrin β3, subunits of another fibronectin receptor, integrin αvβ3 (Supplementary Figure S14). Taken together, these data demonstrate that endoglin interacts specifically with integrin α5β1 in endothelial cells.
Figure 5.
Endoglin interacted with integrin α5β1 via its extracellular domain. (A) Anti-HA or anti-endoglin immunoprecipitates (IP) were prepared from COS7 cells expressing HA–endoglin with GFP or GFP–α5. HA–endoglin and α5 were detected in IP and total cell lysates by western blot analysis. (B) Anti-HA immunoprecipitates were prepared from COS7 cells expressing HA–endoglin with indicated amounts of β1 integrin. HA–endoglin and β1 were detected in IP and total cell lysates by western blot analysis. (C) Anti-α5 or β1 integrin immunoprecipitates were prepared from COS7 cells expressing HA–endoglin and GFP–α5 or β1, respectively. HA–endoglin and α5 or β1 were detected in IP and total cell lysates by western blot analysis. (D) Immunoprecipitates were prepared from MEEC+/+ and MEEC−/−, α5 or β1 was detected in IP and cell lysates by western blot analysis. (E) Immunoprecipitates were prepared from wild HMEC-1 or HMEC-1 adenovirally infected with shRNA to endoglin, α5 or β1 was detected in IP and cell lysates by western blot analysis. (F) Anti-HA immunoprecipitates were prepared from COS7 cells expressing GFP–α5 with HA–endoglin or RGD to TAD mutant of HA–endoglin, α5 and HA–endoglin were detected in IP and cell lysates by western blot analysis. (G, H) Anti-HA immunoprecipitates were prepared from COS7 cells expressing GFP–α5 (G) or β1 (H) with HA–endoglin or HA–endoglin lacking cytoplasmic domain (ECTM), extracellular domain (TMCT) or the last three amino acids in c-terminal (ΔSMA), α5 and HA–endoglin were detected in IP and cell lysates by western blot analysis. (I) Anti-HA immunoprecipitates were prepared from COS7 cells expressing GFP–α5 with HA–endoglin or indicated HA–endoglin truncation mutants, α5 and HA–endoglin were detected in IP and cell lysates by western blot analysis. Figure source data can be found with the Supplementary data.
As human endoglin contains an RGD domain, which has the potential to mediate binding to integrin α5β1, we mutated RGD in human endoglin to TAD and tested its ability to interact with the integrin α5 subunit. The endoglin-TAD mutant only slightly decreased endoglin’s interaction with integrin α5 (Figure 5F), suggesting that RGD is not the only domain mediating endoglin and integrin α5β1 interaction. Further, while mutation of endoglin cytoplasmic domain phosphorylation sites (Supplementary Figure S15), deletion of the entire cytoplasmic domain (Figure 5G and H) or deletion of the Class I PDZ binding motif mediating binding to GIPC (ΔSMA, Lee et al, 2008; Figure 5G and H), had no effect on endoglin interaction, deleting the entire extracellular domain (TMCT) abolished the interaction of endoglin with both integrin α5 (Figure 5G) and integrin β1 (Figure 5H). To determine which sequence in the extracellular domain of endoglin was responsible for interaction with integrin α5β1, we made a series of truncation mutants of the endoglin extracellular domain, all of which could localize on the cell surface (Supplementary Figure S16), and we assessed their ability to interact with integrin α5. None of the truncation mutants, DEL 26–173, DEL 26–323, or DEL 26–486, could abolish interaction with integrin α5 (Figure 5I). As TMCT mutant could completely abolish the interaction (Figure 5G and H), we deleted amino acids 486–586, as these represent the difference between DEL 26–486 and TMCT. However, DEL 486–586 also interacted with integrin α5 (Figure 5I). Taken together, these results suggest that endoglin interacts with integrin α5β1 through multiple regions in its extracellular domain.
Fibronectin and integrin α5β1 enhance endoglin/ALK1complex formation
Endoglin potentiates TGF-β1/ALK1/Smad1/5/8 signalling (Figure 1) by interacting with ALK1 via its extracellular domain (Lebrin et al, 2004; Blanco et al, 2005). Given that fibronectin/integrin α5β1 also increase ALK1/Smad1/5/8 signalling and that integrin α5β1 can interact with the extracellular domain of endoglin, we next asked whether fibronectin-induced clustering of integrin α5β1, as demonstrated here (Supplementary Figure S17), could increase Smad1/5/8 phosphorylation by enhancing endoglin complex formation with ALK1. We first tested whether ALK1 or ALK5 interacted with integrin α5. ALK1, and to a lesser extent ALK5, interacted with integrin α5 in an endoglin-independent manner (Figure 6A). We then asked whether fibronectin-induced clustering of integrin α5β1 enhanced endoglin complex formation with ALK1 using a Duolink assay. While this assay was not sensitive enough to detect endogenous complexes in endothelial cells, in COS7 cells expressing endoglin and ALK1, fibronectin, but not collagen, increased complex formation between endoglin and ALK1 (Figure 6B; Supplementary Figure S18). Importantly, integrin α5β1 function-blocking antibody was able to inhibit the effect of fibronectin on endoglin/ALK1 complex formation (Supplementary Figure S18). These data support a model in which fibronectin-induced clustering of integrin α5β1, via integrin α5β1’s interaction with endoglin and ALK1, brings these receptors into proximity, in turn enhancing ligand binding and downstream signalling (Figure 9D).
Figure 6.
Fibronectin enhances endoglin complex formation with ALK1. (A) Anti-HA immunoprecipitates were prepared from COS7 cells expressing HA–ALK1 or HA–ALK5 with GFP–α5 in presence or absence of endoglin. HA–ALK1, HA–ALK5, and α5 were detected in IP and total cell lysates by western blot analysis. (B) COS7 cells expressing HA–endoglin and myc-ALK1 were cultured in dishes coated without or with 10 μg/ml fibronectin. Interaction between HA–endoglin and myc-ALK1 was assessed by Duolink assay. Nuclei were stained using DAPI. The number of endoglin/ALK-1 complexes per cell ±s.d. (N=42 cells) from one representative experiment of three independent experiments is presented. Figure source data can be found with the Supplementary data.
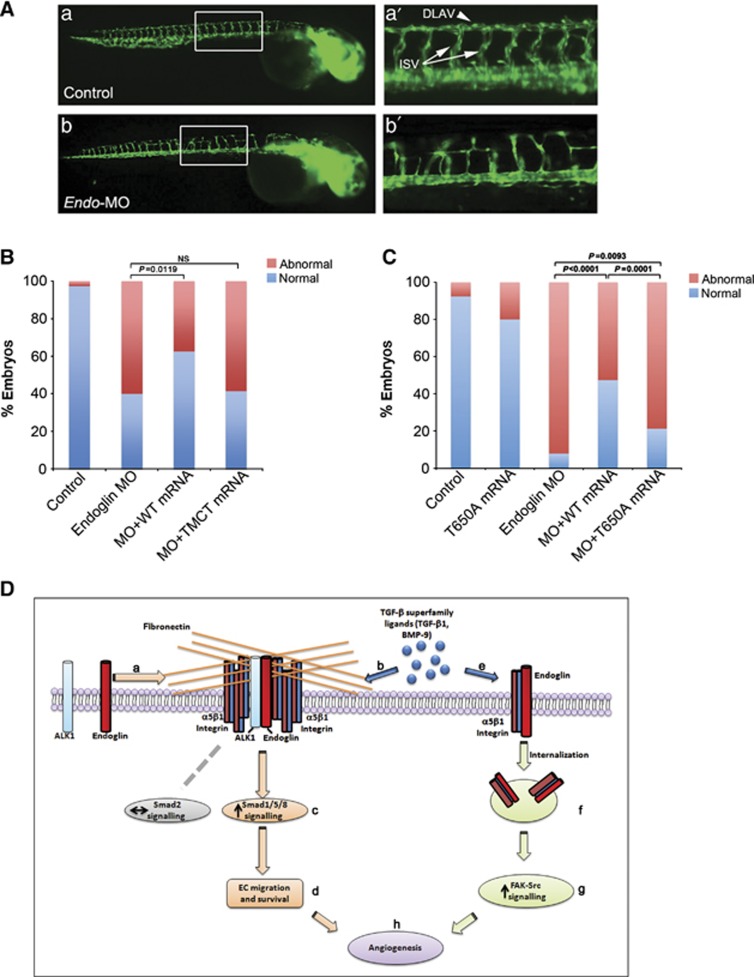
Figure 9.
Endoglin and endoglin/integrin α5β1 complex internalization are required for developmental angiogenesis in vivo. (A) Representative photographs of Fli1-EGFP control embryos (a) and embryos injected with Endo-MO (b) at 48 h post fertilization (h.p.f.), as visualized by fluorescence microscopy. (a′, b′) higher magnifications of the trunk. Intersegmental vessels (ISV; white arrows), dorsal longitudinal anastomotic vessel (DLAV; white arrowhead). (B, C) Bar graph represents the percentage of normal embryos and embryos with ISV sprouting defects defined as mutant. (D) Model for endoglin regulated crosstalk between the fibronectin/α5β1 integrin and TGF-β signalling pathways. Fibronectin induces α5β1 integrin clustering, which through interaction of α5β1 integrin with endoglin and ALK1 promotes endoglin/ALK1 cell surface complex formation (a), subsequently selectively increasing Smad1/5/8 signalling (c) induced by TGF-β superfamily ligands (b), without altering Smad2 signalling. The selective increase in Smad1/5/8 signalling regulates endothelial migration, survival (d) and angiogenesis (h). Endoglin also promotes integrin α5β1 internalization (f) to increase TGF-β1 (e) induced α5β1 integrin signalling to FAK-Src (g), promoting developmental angiogenesis (h).
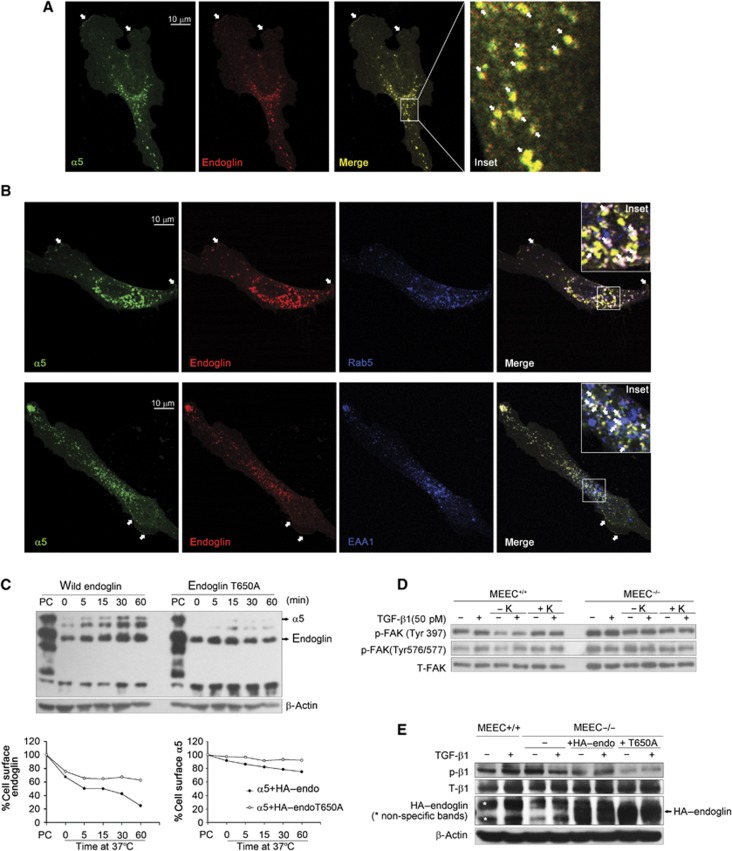
The internalization of endoglin/integrin α5β1 complexes regulates integrin signalling
As endoglin and integrin α5β1 interact physically, we investigated the cellular localization of endoglin-integrin α5β1 complexes using confocal laser scanning microscopy. Endoglin and integrin α5 co-localized at the cell membrane (white arrows in Figure 7A and B) and in intracellular vesicles (white arrow in insets of Figure 7A and B). EEA1 and the GTPase, Rab5, regulate the passage of cargo from the cell surface/plasma membrane into the early endosome (Gorvel et al, 1991; Simonsen et al, 1998). Endoglin/integrin α5β1 co-localized into Rab5- and EAA1-positive vesicles (Figure 7B, white arrows in insets), suggesting that endoglin/integrin α5β1 complexes internalize. To assess directly the fate of these complexes, we co-transfected COS7 cells with HA–endoglin and integrin α5 and performed a time course of endoglin/α5 internalization using a trypsin-biotinylation internalization assay, which assesses internalized receptors from an initially labelled pool of biotinylated cell surface receptors. Both endoglin and integrin α5 internalized in a time-dependent manner (Figure 7C). Interestingly, internalized biotinylated integrin α5 could be co-immunoprecipitated with internalized biotinylated endoglin (Figure 7C), supporting complex formation at the cell surface, followed by co-internalization. However, co-expression of integrin α5 and HA–endoglin T650A mutant, which cannot bind β-arrestin2 (Supplementary Figure S19) or internalize (Lee and Blobe, 2007), suppressed endoglin and integrin α5 internalization, suggesting the internalization of endoglin/α5 complex was triggered by endoglin’s interaction with β-arrestin2.
Figure 7.
The endoglin/integrin α5β1 complex undergoes internalization. (A) MEEC−/− expressing HA–endoglin with GFP–α5 integrin were stained using P3D1 to endoglin and anti-GFP antibodies. Images were obtained using confocal laser scanning microscopy. (B) MEEC−/− expressing HA–endoglin with GFP–α5 integrin were stained using P3D1, anti-GFP and anti-Rab5 or anti-EAA1 antibodies. Images were obtained using confocal laser scanning microscopy. (C) COS7 cells were transfected with HA–endoglin or HA–endoglin T650A mutant and GFP–α5 for 24 h, labelled the cell surface proteins with Sulfo-NHS-LC-Biotin, the cell surface protein cleaved with trypsin for the indicated time course at 37°C, immunoprecipitated with anti-HA antibody, resolved by SDS–PAGE, and western blots were performed using streptavidin-horseradish peroxidase. The surface receptors without trypsin digestion were used as a positive control (PC), whose values were set as 100%. (D) MEEC+/+ and MEEC−/− were serum starved for 5 h. Clathrin-dependent endocytosis was inhibited by potassium (K) depletion for 1 h. Cells were treated with 50 pM TGF-β1 for 30 min, phosphorylation of FAK was then detected. (E) MEEC+/+, MEEC−/−, and MEEC−/− transfected with HA–endoglin or HA–endoglin T650A for 24 h, were serum starved for 6 h, treated without or with 50 pM TGF-β1 for 30 min, and phosphorylation of integrin β1 was detected. Figure source data can be found with the Supplementary data.
Receptor endocytosis has important regulatory roles in signal transduction (Ceresa and Schmid, 2000; Cavalli et al, 2001). To investigate whether the co-internalization of integrin α5β1 and endoglin had effects on either ALK1/Smad1/5/8 or integrin α5β1 signalling, we assayed the effects of potassium depletion and nystatin, which inhibit clathrin-dependent or -independent endocytosis, respectively. Neither potassium depletion nor nystatin significantly affected TGF-β1-induced Smad1/5/8 or Smad2 phosphorylation in either MEEC+/+ or MEEC−/− (Supplementary Figures S20 and S21), suggesting that endoglin/integrin α5β1 internalization did not mediate the effects of fibronectin/integrin α5β1 on Smad 1/5/8 signalling.
While nystatin had no effect on TGF-β1-induced FAK phosphorylation (Supplementary Figure S22), potassium depletion inhibited both the basal and TGF-β1-induced FAK phosphorylation at Tyr397 and Tyr 576/577; these effects could be rescued by restoring potassium (Figure 7D). Notably, potassium depletion had no effect on TGF-β1-induced FAK phosphorylation in MEEC−/− (Figure 7D), suggesting that endoglin is required for integrin α5 endocytosis and endocytosis-regulated integrin signalling. Consistent with this hypothesis, endoglin expression rescued TGF-β1-induced integrin β1 phosphorylation in MEEC−/− (Figure 7E), while expression of endoglin-T650A mutant, which is unable to support integrin α5 endocytosis (Figure 7C), was unable to rescue TGF-β1-induced integrin β1 phosphorylation (Figure 7E). These data suggest that the endocytosis of endoglin and integrin α5β1 are mediated by a clathrin-dependent pathway, with this endocytosis regulating integrin α5β1 activation and signalling, while having no effect on TGF-β1-induced Smad1/5/8 signalling.
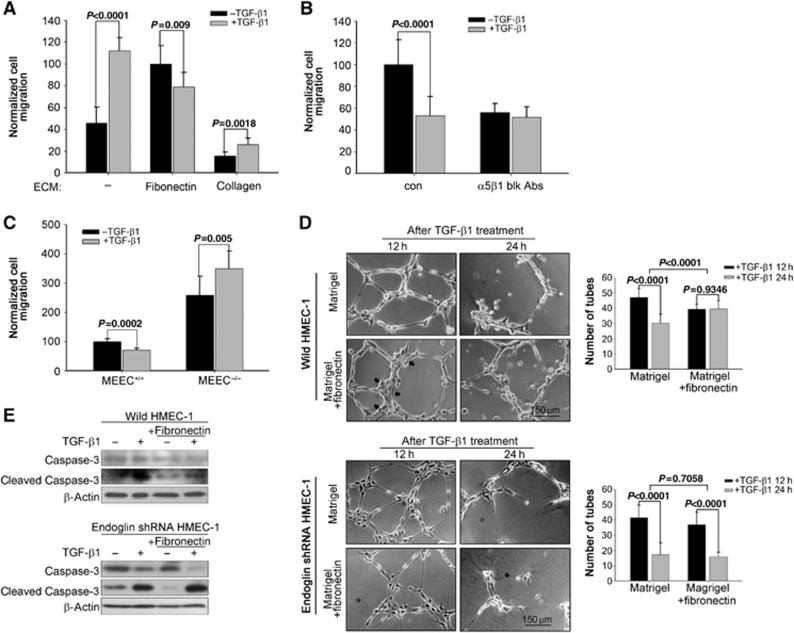
Fibronectin/integrin α5β1 switch TGF-β from a promoter to a suppressor of migration and stabilized newly formed tubules
As fibronectin/integrin α5β1 and TGF-β signalling pathways crosstalk, we investigated the role of this crosstalk on endothelial cell biology. Although TGF-β1 increased HMEC-1 migration through non-ECM and collagen-coated transwells (Figure 8A), TGF-β1 suppressed endothelial cell migration through fibronectin-coated transwells (Figure 8A), suggesting that fibronectin, through selectively enhancing Smad1/5/8 signalling, can alter endothelial cell responses to TGF-β1. The integrin α5β1 function-blocking antibody also blocked the ability of TGF-β1 to suppress endothelial cell migration through fibronectin-coated transwells (Figure 8B). Further, consistent with the role for endoglin in both fibronectin/integrin α5β1-mediated increases in Smad1/5/8 signalling and TGF-β-induced integrin α5β1 activation, TGF-β1 suppressed endothelial cell migration on fibronectin in MEEC+/+, while TGF-β increased migration on fibronectin in MEEC−/− (Figure 8C). These effects were specific to TGF-β1, as BMP-9 decreased endothelial cell migration in the presence and absence of fibronectin (Supplementary Figure S23). Taken together, these data suggest that endoglin, fibronectin, and its major receptor, integrin α5β1, switch TGF-β1 from a promoter to a suppressor of endothelial cell migration through TGF-β and integrin α5β1 crosstalk.
Figure 8.
Fibronectin/integrin α5β1 switch TGF-β from a migration promoter to migration suppressor. (A) HMEC-1 were plated in the transwells coated with non-ECM, 20 μg/ml fibronectin or collagen, pretreated with or without 100 pM TGF-β1 and assessed for migration after 8 h. Quantitated data from three independent experiments ±s.d. are presented. (B) MEEC+/+ pretreated with or without 10 μg/ml α5β1 function-blocking antibody were plated in transwells coated with 20 μg/ml fibronectin, treated with or without 100 pM TGF-β1 and assessed for migration after 8 h. Quantitated data from two independent experiments ±s.d. are presented. (C) MEEC+/+ or MEEC−/− were plated in transwells coated with 20 μg/ml fibronectin, pretreated with or without 100 pM TGF-β1 and assessed for migration after 8 h. Quantitated data from three independent experiments ±s.d. are presented. (D) Wild HMEC-1 or HMEC-1 adenovirally infected with shRNA to endoglin were cultured on the Matrigel mixed with or without 50 μg/ml fibronectin with 100 pM TGF-β1 treatment for indicated times. Images were taken at 4 × microscopy, and tubules were counted using Image J. Quantitated data ±s.d. of one representative experiment out of two independent experiments are presented. (E) HMEC-1 or HMEC-1 adenovirally infected with shRNA to endoglin were cultured in Matrigel for 12 h, lysed and pro- and cleaved caspase-3 were detected using anti caspase-3 antibody. Figure source data can be found with the Supplementary data.
As Matrigel does not contain fibronectin, we assessed the effects of fibronectin on angiogenesis on Matrigel in vitro in the presence or absence of fibronectin. After 12 h on Matrigel, HMEC-1 spontaneously organized into tubule-like structures (Figure 8D), with the structures deteriorating after 24 h due to apoptosis (Figure 8E). TGF-β1 treatment increased cell apoptosis as detected by pro-caspase-3 cleavage (Figure 8E) and tubule degradation (Figure 8D). In the presence of fibronectin, TGF-β1 induced less tubule formation (Figure 8D), with many of the endothelial cells aggregating together (Figure 8D, 12 h panel, black arrow), consistent with the role of fibronectin in mediating TGF-β1-induced inhibition of endothelial cell migration in this context (Figure 8A). However, both TGF-β-induced apoptosis as assessed using pro-caspase-3 cleavage (Figure 8E) and tubule degradation (Figure 8D) were significantly decreased in the presence of fibronectin (Figure 8D and E). Again, the effect of fibronectin was endoglin dependent, as fibronectin had no effect on TGF-β-induced pro-caspase-3 cleavage and tubule degradation in HMEC-1 with endoglin expression silenced (Figure 8D and E). Further, similarly to the effects on migration, fibronectin has no significant effect on BMP-9-mediated inhibition of tubule formation (Supplementary Figure 24). Collectively, these data suggest that fibronectin cooperates with the TGF-β signalling pathway to decrease apoptosis and maintain the stability of newly formed tubule-like structures.
Endoglin and endoglin/integrin α5β1 internalization are required for developmental angiogenesis in vivo
Our in vitro data highlight an important role for endoglin in mediating the crosstalk between TGF-β and fibronectin/integrin α5β1 pathways. To explore the physiological relevance of our findings, we assessed the role of this endoglin function during capillary formation in vivo using the transgenic Fli1-EGFP zebrafish developmental angiogenesis model. Fli1-driven expression of GFP begins early during embryonic development, with angiogenesis evident within the first 24 h, as monitored via fluorescence microscopy. We generated morpholinos (Endo-MO) to suppress translation of the endogenous endoglin orthologue in Fli1-EGFP embryos, and observed significant defects in the formation of both intersegmental vessels (ISVs) and dorsal longitudinal anastomotic vessel (DLAV) at 48 h post fertilization (h.p.f.) (Figure 9A). The injection of wild-type human endoglin mRNA along with Endo-MO into Fli1-EGFP transgenic embryos effectively rescued the phenotype (Figure 9B and C). However, the endoglin TMCT mutant, which was the only mutant identified that could not interact with integrin α5β1 (Figure 5), failed to rescue the phenotype (Figure 9B). To test whether the endoglin/integrin α5β1 complex endocytosis was critical for promoting angiogenesis in vivo, embryos were injected with Endo-MO and human endoglin mRNA with T650A mutant, which is unable to support internalization of endoglin and integrin α5β1 (Figure 7C). We found that the Endo-T650A mRNA is unable to fully rescue the MO phenotype compared to WT rescue (Figure 9C). Taken together, our Fli1-EGFP zebrafish model supports a pivotal role for endoglin/integrin α5β1 crosstalk and endoglin-mediated integrin α5β1 endocytosis in mediating developmental angiogenesis in vivo.
Discussion
Here, we have shown that the prominent ECM component, fibronectin, and its primary cellular receptor, α5β1 integrin, specifically increase TGF-β1- and BMP9-induced Smad1/5/8 phosphorylation in an endoglin- and ALK1-dependent manner. In a reciprocal fashion, TGF-β1 activates α5β1 integrin and downstream signalling to FAK in an endoglin-dependent manner (Figure 9D).
How might endoglin cooperate with fibronectin and α5β1 integrin to enhance ALK1/Smad1/5/8 signalling? As demonstrated here, endoglin interacts with α5β1 integrin through its extracellular domain. While human endoglin has an RGD motif, which has the potential to bind α5β1 integrin, this motif is not conserved across evolution (Supplementary Figure S25), suggesting that the RGD motif is not the only domain responsible for endoglin–integrin α5β1 interaction. Consistent with that notion, our data show that mouse endoglin, which lacks the RGD domain, and human endoglin with a mutation in the RGD motif can still interact with integrin α5β1. Despite extensive structure/function studies, we were unable to identify a more discrete endoglin domain responsible for this interaction (Figure 5), suggesting that there may be more than one structure in the extracellular domain that mediates this interaction. We also demonstrate that integrin α5β1 interacts with ALK1, but not with ALK5, and is able to enhance endoglin and ALK1 complex formation in a fibronectin- and integrin α5β1-dependent manner. Taken together, these data support a model in which fibronectin induces clustering of integrin α5β1, thereby bringing endoglin and ALK1 into proximity, selectively enhancing ligand binding, and downstream signalling to the Smad1/5/8 pathway (Figure 9D). This model can explain why fibronectin and integrin α5β1 only enhance Smad1/5/8 signalling in the presence of endoglin (Figure 3A and B), while having no effects on the Smad2 signalling pathway downstream of ALK5 (Figure 3A and B), and why the ALK5 inhibitor has no effect on fibronectin/integrin α5β1-mediated Smad1/5/8 signalling (Figure 3D).
In terms of how TGF-β regulates the fibronectin/integrin α5β1 signalling pathway, we show that TGF-β, but not BMP-9, increases both integrin α5β1 expression and activation. While TGF-β has been reported to increase integrin α5β1 transcription in human hepatocellular carcinoma cells (Cai et al, 2000), and integrin α5β1 biosynthesis in human microvascular endothelial cells (Enenstein et al, 1992), the effects here occurred rapidly, suggesting that TGF-β might stabilize integrin α5β1 at the protein level. Consistent with that notion, a lysosomal inhibitor mimicked this effect, suggesting that TGF-β stabilizes integrin α5β1 through inhibition of lysosome degradation. In addition, TGF-β activated integrin α5β1 signalling to FAK in an endoglin-dependent manner. Integrin trafficking has been shown to play important roles in regulating integrin signalling (Liu et al, 2011; Skalski et al, 2011), with a recent study showing that β1 integrin on the plasma membrane is primarily inactive, whereas active β1 integrin receptor is predominantly intracellular (Arjonen et al, 2012). As we have shown here, TGF-β cannot induce integrin α5β1 activation in MEEC−/− and endoglin knockdown HMEC-1. Further, the endoglin T650A mutant, which cannot promote internalization (Lee and Blobe, 2007), suppresses endoglin/integrin α5β1 complex internalization and TGF-β-induced α5β1 integrin activation. These data suggest that endoglin regulates TGF-β-induced integrin signalling activation by complexing and co-internalizing with α5β1 integrin (Figure 9C). The trafficking of endoglin and integrin is also important for endothelial function and angiogenesis (Valdembri et al, 2009; di Blasio et al, 2010), as endoglin deficient in internalizing, endoglin T650A, failed to rescue endoglin-silencing mediated defects in developmental angiogenesis in vivo. These data suggest that TGF-β-mediated regulation of angiogenesis may function, in part, through stabilization and activation of integrin α5β1 signalling.
The crosstalk between the TGF-β and fibronectin/integrin signalling pathways switches TGF-β from a promoter to a suppressor of endothelial cell migration, and promotes endothelial cell survival. How might this crosstalk regulate endothelial cell migration? Our data indicate that fibronectin and integrin α5β1 increase specifically TGF-β1-induced Smad1/5/8 phosphorylation in an endoglin- and ALK1-dependent manner, by increasing complex formation between endoglin and ALK1. At the same time, the level of TGF-β1-induced Smad2 phosphorylation remains unchanged, potentially due to the relative inability of integrin α5β1 to interact with ALK5. Thus, either shifting the balance of Smad1/5/8 and Smad2 signalling towards Smad1/5/8, or selectively increasing Smad1/5/8 signalling, is predicted to result in decreased endothelial cell migration. These results are consistent with our previous findings in which endoglin/GIPC (Lee et al, 2008), constitutively activated ALK1, or expression of the ALK1 activator, CK2β (Lee et al, 2009), increased Smad1/5/8 signalling and inhibited endothelial migration. The mechanisms by which these diverse factors might coordinate to regulate TGF-β superfamily signalling and endothelial cell function are currently being explored.
Interestingly, while the ALK5 inhibitor, SB-431542, inhibited TGF-β-induced Smad2 and Smad1/5/8 phosphorylation in endothelial cells cultured in the absence of fibronectin, as well as TGF-β-induced Smad2 phosphorylation in the presence of fibronectin, SB-431542 was not able to inhibit TGF-β-induced Smad1/5/8 phosphorylation in the presence of fibronectin (Figure 3D; Supplementary Figure S10). As SB-431542 does not inhibit ALK1, the effects of SB-431542 are thought to be mediated through ALK5, which has been shown to be important for ALK1 signalling (Goumans et al, 2003). In this context, the inability of SB-431542 to inhibit TGF-β-induced Smad1/5/8 phosphorylation in the presence of fibronectin suggests that fibronectin bypasses the requirement for ALK5. As we demonstrate that fibronectin increases Smad1/5/8 phosphorylation by increasing complex formation between endoglin and ALK1, ALK5 could be functioning to increase ALK1 signalling in a similar manner. In addition, in the context of maturing blood vessels, where fibronectin is a predominant component (Tonnesen et al, 1985; Nicosia and Madri, 1987; Risau and Lemmon, 1988), ALK1/Smad1/5/8 signalling would dominate, and would not be dependent on ALK5 signalling, consistent with what has been reported in murine models (Park et al, 2008).
In addition to effects on endothelial cell migration, fibronectin increased capillary stability through decreasing TGF-β-induced endothelial cell apoptosis. These results suggest that either increased integrin α5β1 signalling, increased Smad1/5/8 signalling or both result in increased capillary stability. In support of a role for increased Smad1/5/8 signalling, we have recently defined a role for BMP-9, which only increases Smad1/5/8 signalling, in increasing capillary stability (Lee et al, 2012). Thus, fibronectin and TGF-β-induced Smad1/5/8 signalling may serve as a survival signal in newly formed blood vessels, with a specific role in the maturation stage of angiogenesis, regulating TGF-β signalling to inhibit endothelial migration and stabilize the newly formed vessels.
Mutations in endoglin and ALK1 result in hereditary HHT (Johnson et al, 1996; Bourdeau et al, 1999), suggesting that they function in the same signalling pathway. Here, we demonstrate that endoglin is required for fibronectin and α5β1 integrin-mediated stimulation of ALK1/Smad1/5/8 signalling, as well as for TGF-β-mediated activation of α5β1 integrin signalling. While fibronectin and α5β1 integrin signalling are known to be important for regulating angiogenesis and vascular remodelling, and the current studies indicate that these effects may be mediated by crosstalk with the endoglin/ALK1 signalling pathway, the role of fibronectin, α5β1 integrin and their crosstalk with the endoglin/ALK1 signalling pathway in HHT pathogenesis remains to be explored.
Materials and methods
Cell culture and antibodies
HMEC-1 were grown in MCDB-131 medium (Invitrogen), supplemented with 10% fetal bovine serum (FBS), 1 μg/ml hydrocortisone (Sigma), 10 ng/ml EGF (Sigma), and 2 mM L-glutamine (Invitrogen). Endoglin wild-type (MEEC+/+) and endoglin-null (MEEC−/−) MEEC were grown in MCDB-131 supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate (Invitrogen), 100 μg of heparin (Sigma), and 50 μg/ml endothelial cell growth supplement (ECGS; Sigma). Both HMEC-1 and MEEC were cultured in flasks coated with 0.05% gelatin. HEK 293 cells were grown in Dulbecco’s modified Eagle’s medium, supplemented with 10% FBS.
Trypsin internalization assay
COS7 cells were plated in 10 cm dishes to reach 60% confluence, followed by transfection of 6 μg HA–endoglin or HA–endoglin T650 mutant and 6 μg GFP-α5 plasmids. 48 h later, cells were split in 6 cm dishes. After culture overnight, cells were rinsed three times with ice-cold PBS. Then, cells were incubated with 0.5 mg/ml Sulfo-NHS-LC-Biotin (Pierce Biotechnology, Rockford, IL) in Hepes Buffer (150 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2 and 10 mM Hepes) while rocking at 4°C for 2 h. Cells on ice were rinsed twice with ice-cold PBS. In all, 1 ml of room temperature COS7 media was added to select samples followed by a time course at 37°C. Cells were then rinsed once with PBS on ice, incubated at 37°C with trypsin for 1 min, followed by immediate addition of DMEM+10%FBS on ice. Cells were lysed on ice with 1 ml RIPA plus protease inhibitors (leupeptin, pepstatin, PMSF, and DTT), kept on ice for 30 min and centrifuged at 14 000 r.p.m. for 30 min at 4°C. Pellets were removed and the supernatants were used to perform immunoprecipitation using anti-HA antibody. After running SDS–PAGE and transfer, internalized HA–endoglin and α5 were detected with strep-HRP at 1:10 000.
Duolink assay
The Duolink assay (Olink Bioscience) was performed according to manufacturer’s protocol. Briefly, COS7 cells cultured in 10 ml dishes were transfected with 6 μg HA–endoglin and 6 μg myc-ALK1 (HA and myc tags are localized in N terminals of extracellular domains of endoglin and ALK1) for 24 h and plated on coverslides in 6-well plate. Cells were then washed with PBS, fixed with 4% paraformaldehyde, permeabilized in 0.1% Triton X-100/PBS for 5 min and then blocked with 5% bovine serum albumin in PBS for 1 h. Slides were incubated with anti-myc (from rabbit) and anti-HA (from mouse) primary antibodies for 1 h in room temperature and then incubated with PLA probe MINUS and PLUS mixture for 1 h at 37°C. After ligation for 30 min, amplification for 100 min at 37°C, the slides were labelled with DAPI and mounted with Prolong Anti-Fade (Sigma), digitally imaged and counted for number of the red dots per cells manually using ImageJ software.
Transwell migration assay
Transwells (Costar Corning Inc., 8 μM polycarbonate membrane 6.5 mm insert, 24-well plate) were coated with 20 μg/ml fibronectin, laminin or collagen prior to plating. In all, 30 000 cells were plated onto the top of each of the 24-well membrane and allowed to migrate for 8 h prior to cell fixation and staining of the nuclei of the migrated cells on the bottom side of the membrane. The transwell membranes containing stained cells were digitally imaged, then counted using image J software.
Endothelial tubule formation
In all, 24-well plates were coated with 150 μl Matrigel matrix (BD Bioscience, San Jose, CA, USA) mixed with or without 50 μg/ml fibronectin on ice and were incubated at 37°C for 30 min. In all, 5 × 104 cells/500 μl MEECs were plated on 24-well plates coated with Matrigel matrix and were treated as indicated. Tubules formed were digitally imaged after indicated time and counted for number of tubes formed per field.
Morpholino and embryo manipulations
Zebrafish (Danio rerio) embryos were raised and maintained as described (Westerfield, 1995). Embryos at 24 h.p.f.) were raised in 0.2 mM 1-phenyl-2-thio-urea (Sigma) to prevent pigment formation and allowed to develop until 48 h.p.f. Splice blocker morpholino (MO) against Endoglin (Endo) (TAGTAGAGAACTTACCCGCACAGGC) (Lee et al, 2012) was designed by and obtained from Gene Tools, LLC. For Endo MO efficacy experiments, RT–PCR was performed using gene-specific primers in exons immediately flanking the targeted region on 72 h.p.f. RNA isolated from control and Endo MO-injected embryos. We injected 1 nl of diluted MO (5 ng) and/or RNA (100 pg) into transgenic (Fli1: EGFP) zebrafish embryos at the 1- to 2-cell stage. Injected embryos were scored at 48 h.p.f. and classified into two groups, normal and mutant, on the basis of the relative vasculogenesis defects compared to age-matched controls from the same clutch. For RNA rescue experiments, human ENDOGLIN mRNAs, WT, mutant p.T650A, and mutant TMCT were transcribed in vitro with the SP6 Message Machine kit (Ambion). All experiments were repeated three times; a t-test was performed to determine the significance of phenotypic rescue.
Statistical analysis
Quantitative data are expressed as means±s.d. Statistical significance was determined by the two-tailed Student’s t-test or one-way ANOVA, followed by the LSD-t test for multiple comparisons. A P-value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grant R01-CA135006 (GCB), and Komen for the Cure Grants KG090154(GCB), SAC10000 (GCB) and KG111383 (HT), AHA Fellowship 11POST7160006 (CG), and seed funding from the Center of Human Disease Modeling. NK is a Distinguished Brumley Professor. We thank Dr Nam Y Lee for technical advice.
Author contributions: HYT, KM, CG, NK, and GCB designed the experiments and analysed the data; HYT and CG performed the experiments; HYT and GCB wrote the paper. All authors contributed to discussing the results and editing the final manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arjonen A, Alanko J, Veltel S, Ivaska J (2012) Distinct recycling of active and inactive beta1 integrins. Traffic 13: 610–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG (2000) Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 217: 42–53 [DOI] [PubMed] [Google Scholar]
- Astrof S, Hynes RO (2009) Fibronectins in vascular morphogenesis. Angiogenesis 12: 165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu C, Lopez-Novoa JM, Quintanilla M (2009) The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta 1792: 954–973 [DOI] [PubMed] [Google Scholar]
- Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C (2005) Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol 204: 574–584 [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Dumont DJ, Letarte M (1999) A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 104: 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Lei QY, Wang LY, Zha XL (2000) TGF-beta 1 modulated the expression of alpha 5 beta 1 integrin and integrin-mediated signaling in human hepatocarcinoma cells. Biochem Biophys Res Commun 274: 519–525 [DOI] [PubMed] [Google Scholar]
- Cavalli V, Corti M, Gruenberg J (2001) Endocytosis and signaling cascades: a close encounter. FEBS Lett 498: 190–196 [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Schmid SL (2000) Regulation of signal transduction by endocytosis. Curr Opin Cell Biol 12: 204–210 [DOI] [PubMed] [Google Scholar]
- Collo G, Pepper MS (1999) Endothelial cell integrin alpha5beta1 expression is modulated by cytokines and during migration in vitro. J Cell Sci 112(Pt 4): 569–578 [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L (2003) Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol 15: 565–571 [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S (2008) Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res 102: 914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S (2007) Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109: 1953–1961 [DOI] [PubMed] [Google Scholar]
- di Blasio L, Droetto S, Norman J, Bussolino F, Primo L (2010) Protein kinase D1 regulates VEGF-A-induced alphavbeta3 integrin trafficking and endothelial cell migration. Traffic 11: 1107–1118 [DOI] [PubMed] [Google Scholar]
- Eliceiri BP (2001) Integrin and growth factor receptor crosstalk. Circ Res 89: 1104–1110 [DOI] [PubMed] [Google Scholar]
- Enenstein J, Waleh NS, Kramer RH (1992) Basic FGF and TGF-beta differentially modulate integrin expression of human microvascular endothelial cells. Exp Cell Res 203: 499–503 [DOI] [PubMed] [Google Scholar]
- Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M (2003) Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 22: 6557–6563 [DOI] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO (2002) Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 22: 927–933 [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO (1993) Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119: 1079–1091 [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J (1991) rab5 controls early endosome fusion in vitro. Cell 64: 915–925 [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Liu Z, ten Dijke P (2009) TGF-beta signaling in vascular biology and dysfunction. Cell Res 19: 116–127 [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P (2003) Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell 12: 817–828 [DOI] [PubMed] [Google Scholar]
- Guan JL (1997) Focal adhesion kinase in integrin signaling. Matrix Biol 16: 195–200 [DOI] [PubMed] [Google Scholar]
- Hynes RO (2007) Cell-matrix adhesion in vascular development. J Thromb Haemost 5(Suppl 1): 32–40 [DOI] [PubMed] [Google Scholar]
- Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91: 877–887 [DOI] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13: 189–195 [DOI] [PubMed] [Google Scholar]
- Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P (2004) Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 23: 4018–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Blobe GC (2007) The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J Biol Chem 282: 21507–21517 [DOI] [PubMed] [Google Scholar]
- Lee NY, Golzio C, Gatza CE, Sharma A, Katsanis N, Blobe GC (2012) Endoglin regulates PI3-kinase/Akt trafficking and signaling to alter endothelial capillary stability during angiogenesis. Mol Biol Cell 23: 2412–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Haney JC, Sogani J, Blobe GC (2009) Casein kinase 2beta as a novel enhancer of activin-like receptor-1 signaling. FASEB J 23: 3712–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ray B, How T, Blobe GC (2008) Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem 283: 32527–32533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letamendia A, Lastres P, Botella LM, Raab U, Langa C, Velasco B, Attisano L, Bernabeu C (1998) Role of endoglin in cellular responses to transforming growth factor-beta. A comparative study with betaglycan. J Biol Chem 273: 33011–33019 [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP (1999) Defective angiogenesis in mice lacking endoglin. Science 284: 1534–1537 [DOI] [PubMed] [Google Scholar]
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS (1992) Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol 119: 905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Chen XM, Zheng HX, Shi SL, Li Y (2011) Knockdown of Rab5a expression decreases cancer cell motility and invasion through integrin-mediated signaling pathway. J Biomed Sci 18: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimova JY, Fujita M, Khazenzon NM, Ljubimov AV, Black KL (2006) Changes in laminin isoforms associated with brain tumor invasion and angiogenesis. Front Biosci 11: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV (2007) Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res 101: 570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M, Borthwick GM, Hislop AA, Arthur HM (2009) Endoglin and activin receptor-like-kinase 1 are co-expressed in the distal vessels of the lung: implications for two familial vascular dysplasias, HHT and PAH. Lab Invest 89: 15–25 [DOI] [PubMed] [Google Scholar]
- Massague J (1992) Receptors for the TGF-beta family. Cell 69: 1067–1070 [DOI] [PubMed] [Google Scholar]
- Massague J (1996) TGFbeta signaling: receptors, transducers, and Mad proteins. Cell 85: 947–950 [DOI] [PubMed] [Google Scholar]
- Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67: 753–791 [DOI] [PubMed] [Google Scholar]
- Mazibrada J, Ritta M, Mondini M, De Andrea M, Azzimonti B, Borgogna C, Ciotti M, Orlando A, Surico N, Chiusa L, Landolfo S, Gariglio M (2008) Interaction between inflammation and angiogenesis during different stages of cervical carcinogenesis. Gynecol Oncol 108: 112–120 [DOI] [PubMed] [Google Scholar]
- Nesti LJ, Caterson EJ, Wang M, Chang R, Chapovsky F, Hoek JB, Tuan RS (2002) TGF-beta1-stimulated osteoblasts require intracellular calcium signaling for enhanced alpha5 integrin expression. Ann NY Acad Sci 961: 178–182 [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Madri JA (1987) The microvascular extracellular matrix. Developmental changes during angiogenesis in the aortic ring-plasma clot model. Am J Pathol 128: 78–90 [PMC free article] [PubMed] [Google Scholar]
- Pardali E, ten Dijke P (2009) Transforming growth factor-beta signaling and tumor angiogenesis. Front Biosci 14: 4848–4861 [DOI] [PubMed] [Google Scholar]
- Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP (2008) ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111: 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Lemmon V (1988) Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol 125: 441–450 [DOI] [PubMed] [Google Scholar]
- Ross RS (2004) Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res 63: 381–390 [DOI] [PubMed] [Google Scholar]
- Seki T, Yun J, Oh SP (2003) Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res 93: 682–689 [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394: 494–498 [DOI] [PubMed] [Google Scholar]
- Skalski M, Sharma N, Williams K, Kruspe A, Coppolino MG (2011) SNARE-mediated membrane traffic is required for focal adhesion kinase signaling and Src-regulated focal adhesion turnover. Biochim Biophys Acta 1813: 148–158 [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Jenkins D Jr., Siegal SL, Lee LA, Huff JC, Clark RA (1985) Expression of fibronectin, laminin, and factor VIII-related antigen during development of the human cutaneous microvasculature. J Invest Dermatol 85: 564–568 [DOI] [PubMed] [Google Scholar]
- Torsney E, Charlton R, Parums D, Collis M, Arthur HM (2002) Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res 51: 464–470 [DOI] [PubMed] [Google Scholar]
- Twardowski T, Fertala A, Orgel JP, San Antonio JD (2007) Type I collagen and collagen mimetics as angiogenesis promoting superpolymers. Curr Pharm Des 13: 3608–3621 [DOI] [PubMed] [Google Scholar]
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G (2009) Neuropilin-1/GIPC1 signalling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol 7: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Fassler R, Warmegard B, Johansson S (1998) Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin beta1 A. Requirement for threonines 788–789 in receptor activation. J Cell Sci 111 (Pt 8): 1117–1126 [DOI] [PubMed] [Google Scholar]
- Westerfield M (1995) The Zebrafish Book Eugene: University of Oregon Press, [Google Scholar]
- Whittaker CA, Bergeron KF, Whittle J, Brandhorst BP, Burke RD, Hynes RO (2006) The echinoderm adhesome. Dev Biol 300: 252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom P, Lissbrant IF, Stattin P, Egevad L, Bergh A (2002) Endoglin (CD105) is expressed on immature blood vessels and is a marker for survival in prostate cancer. Prostate 51: 268–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.