Abstract
EMBO J (2012) 31 19, 3809–3820 doi:; DOI: 10.1038/emboj.2012.233; published online August 24 2012
In this issue of The EMBO Journal, Gaucher et al (2012) demonstrate that Brdt, a testes-specific member of the BET family of double bromodomain proteins, is intricately involved in the transcriptional regulation and genome reorganization required for the production of viable, fertile sperm. These findings are complimented by a recent publication in Cell (Matzuk et al, 2012) where JQ1, a small molecule BET inhibitor, is effective as a male contraceptive in mice. Both studies establish Brdt as an integral factor in sperm development.
Gametogenesis is a complex, conserved process that has emerged as a preeminent model system for epigenetic study due to the vast changes in transcription and chromatin structure involved (Govin and Berger, 2009). Spermatogenesis occurs in several stages, each controlled by the highly regulated expression of specific genes. First, diploid undifferentiated germ cells go through meiosis to generate four genetically unique haploid cells. These cells then undergo a striking differentiation process: most of the cytoplasm is shed and the nuclear volume is reduced 10-fold. Nuclear reduction is accomplished by dramatic chromatin compaction during which most histones are replaced with sperm-specific, highly basic protamines (Govin et al, 2004). This chromatin reorganization protects the genetic information, and the location and modification state of the remaining histones prepare the genome for reactivation after fertilization (Hammoud et al, 2009).
Although the gross chromatin changes in spermatogenesis have been defined, many key players in these processes remain elusive. Functional studies are challenging due to lack of a reliable culture system and common deleterious effects of knocking out chromatin modifying enzymes and chromatin binding proteins in mice. Fortunately, recent studies have identified Brdt as an epigenetic target that is expressed exclusively in the testes. Brdt is in the BET protein family, which also includes Brd2, Brd3, and Brd4. All BET members have two tandem N-terminal bromodomains that bind to acetylated histone tails. Brd2 and Brd4 have an interesting attribute as epigenetic memory factors that ‘bookmark’ active genes through mitosis, a time when cells are largely transcriptionally silent (Muller et al, 2011). Bookmarking BET proteins are believed to restore gene expression quickly after mitosis is complete by association with members of the transcriptional machinery. While Brd2 and Brd4 knockout mice are embryonic lethal, mutant mice expressing a truncated Brdt show a strikingly specific phenotype: male sterility (Houzelstein et al, 2002; Shang et al, 2007, 2009). Histones are hyperacetylated after meiosis, prior to their vast removal, and Brdt may function to facilitate post-meiotic chromatin compaction by binding to acetylated histone tails (Pivot-Pajot et al, 2003; Morinière et al, 2009).
In the featured study, Gaucher et al (2012) use the first Brdt knockout mouse to demonstrate that Brdt is a master regulator of the spermatogenic gene expression program and is required for progression through meiosis. In brdt−/− mice, over 1000 testes-specific meiotic and post-meiotic genes fail to be activated. ChIP sequencing revealed that Brdt and acetylated histones are bound at the promoters of many of these genes, suggesting a direct regulation. In fact, Brdt binds through meiosis to the promoters of numerous post-meiotic genes that are downregulated in its absence. Brdt also binds to pTEFb, the transcriptional elongation factor, similarly to Brd4. This interaction suggests that Brdt, like other BET proteins, may act as a bookmarking factor and ‘pre-mark’ post-meiotic gene expression during meiosis. In contrast to the knockouts, mice expressing Brdt lacking the first bromodomain (BD1) are able to complete meiosis, but show defects in spermatid elongation and histone replacement. These fascinating results suggest that Brdt directly functions in multiple spermatogenesis pathways in a domain-specific manner.
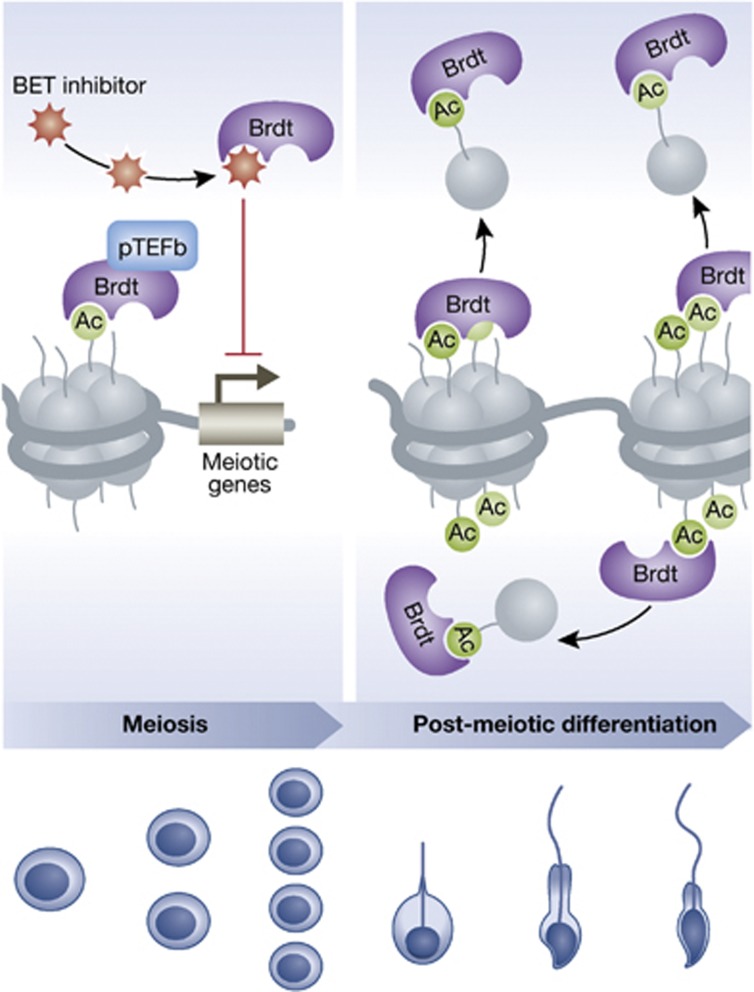
In this breakthrough study, Gaucher et al, 2012 confirm that Brdt is a complex transcriptional regulator of the gene expression program during spermatogenesis. Brdt appears to activate meiotic and post-meiotic genes by binding to acetylated histones at their promoters. Then, paradoxically, Brdt facilitates the vast transcriptional silencing caused by the replacement of hyperacetylated histones with protamines. Because the function of Brdt is testes specific and critical for spermatogenesis, it is an ideal target for contraceptive drug development (see Figure 1).
Figure 1.
Brdt seems to play two stage-specific roles during mammalian spermatogenesis. This figure represents a possible model for the dual function of Brdt. First, Brdt binds via its two bromodomains to acetylated histones at the promoters of specific meiotic and post-meiotic genes and facilitates their activation at the appropriate time. In the post-meiotic phase of spermatogenesis, Brdt binds to hyperacetylated histones and aids in their general removal from DNA. Small molecule BET inhibitors like JQ1 bind to the bromodomains of Brdt, preventing it from binding to acetylated histones and performing its dual role during spermatogenesis.
A recent study in Cell used the small molecule BET inhibitor JQ1 to disrupt spermatogenesis in mice (Matzuk et al, 2012). JQ1 has been shown to bind to the bromodomain of Brd4 and was used to attenuate cancerous properties of Brd4-dependent carcinoma cells (Filippakopoulos et al, 2010). This current study demonstrated that JQ1 can cross the blood-testis barrier, resulting in decreased spermatozoa number and motility. Importantly for any potential human male contraceptive, the effects of JQ1 on sperm production were reversible and did not affect hormone levels. Although JQ1 can affect other BET family members, the similarities between the brdt−/− mice and JQ1-treated mice are striking. Thus, both Gaucher et al, 2012 and Matzuk et al have independently established Brdt, a testes-specific histone acetyl-binding protein, as a central and versatile epigenetic player in mammalian spermatogenesis. Further manipulation of Brdt and other BET proteins will continue to aid in characterizing the still enigmatic and ever-changing chromatin landscape in sperm and beyond.
Acknowledgments
Support to JMB from the T32 Genetics Training Grant at the University of Pennsylvania (GM008216). Support to SLB from NIH grants GM055360 and U54-HD068157.
Footnotes
The authors declare that they have no conflict of interest.
References
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N et al. (2010) Selective inhibition of BET bromodomains. Nature 468: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher J, Boussouar F, Montellier E, Curtet S, Buchou T, Bertrand S, Hery P, Jounier S, Depaux A, Vitte A-L, Guardiola P, Pernet K, Debernardi A, Lopez F, Holota H, Imbert J, Wolgemuth DJ, Gérard M, Rousseaux S, Khochbin S (2012) Bromodomain-dependent stage-specific male genome programming by Brdt. EMBO J 31: 3809–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govin J, Berger SL (2009) Genome reprogramming during sporulation. Int J Dev Biol 53: 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S (2004) The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur J Biochem 271: 3459–3469 [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR (2009) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460: 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzelstein D, Bullock SL, Lynch DE, Grigorieva EF, Wilson VA, Beddington RSP (2002) Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol Cell Biol 22: 3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno Julio E, Lemieux Madeleine E, Picaud S, Yu Richard N, Qi J, Knapp S, Bradner James E (2012) Small-molecule inhibition of BRDT for male contraception. Cell 150: 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinière J, Rousseaux S, Steuerwald U, Soler-López M, Curtet S, Vitte A-L, Govin J, Gaucher J, Sadoul K, Hart DJ, Krijgsveld J, Khochbin S, Müller CW, Petosa C (2009) Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature 461: 664–668 [DOI] [PubMed] [Google Scholar]
- Muller S, Filippakopoulos P, Knapp S (2011) Bromodomains as theraputic targets. Expert Rev Mol Med 13: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivot-Pajot C, Caron C, Govin J, Vion A, Rousseaux S, Khochbin S (2003) Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol Cell Biol 23: 5354–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ (2007) The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development 134: 3507–3515 [DOI] [PubMed] [Google Scholar]
- Shang E, Wang X, Wen D, Greenberg DA, Wolgemuth DJ (2009) Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev Dyn 238: 908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]