Abstract
OBJECTIVES:
In patients with celiac disease, enteropathy is caused by the entry of gluten peptides into the lamina propria of the intestine, in which their immunogenicity is potentiated by tissue transglutaminase (tTG) and T-helper type 1–mediated immune responses are triggered. Tight junction disassembly and paracellular permeability are believed to have an important role in the transport of gluten peptides to the lamina propria. Larazotide acetate is a tight-junction regulator peptide that, in vitro, prevents the opening of intestinal epithelial tight junctions. The aim of this study was to evaluate the efficacy and tolerability of larazotide acetate in protecting against gluten-induced intestinal permeability and gastrointestinal symptom severity in patients with celiac disease.
METHODS:
In this dose-ranging, placebo-controlled study, 86 patients with celiac disease controlled through diet were randomly assigned to larazotide acetate (0.25, 1, 4, or 8 mg) or placebo three times per day with or without gluten challenge (2.4 g/day) for 14 days. The primary efficacy outcome was the urinary lactulose/mannitol (LAMA) fractional excretion ratio. Secondary endpoints included gastrointestinal symptom severity, quality-of-life measures, and antibodies to tTG.
RESULTS:
LAMA measurements were highly variable in the outpatient setting. The increase in LAMA ratio associated with the gluten challenge was not statistically significantly greater than the increase in the gluten-free control. Among patients receiving the gluten challenge, the difference in the LAMA ratios for the larazotide acetate and placebo groups was not statistically significant. However, larazotide acetate appeared to limit gluten-induced worsening of gastrointestinal symptom severity as measured by the Gastrointestinal Symptom Rating Scale at some lower doses but not at the higher dose. Symptoms worsened significantly in the gluten challenge–placebo arm compared with the placebo–placebo arm, suggesting that 2.4 g of gluten per day is sufficient to induce reproducible gluten toxicity. Larazotide acetate was generally well tolerated. No serious adverse events were observed. The most common adverse events were headache and urinary tract infection.
CONCLUSIONS:
LAMA variability in the outpatient setting precluded accurate assessment of the effect of larazotide acetate on intestinal permeability. However, some lower doses of larazotide acetate appeared to prevent the increase in gastrointestinal symptom severity induced by gluten challenge.
INTRODUCTION
Celiac disease is one of the most common autoimmune disorders, affecting 1% of individuals in many regions (1,2,3,4). Subjects with celiac disease frequently present with intestinal symptoms such as diarrhea, abdominal pain and bloating, and may also experience extraintestinal signs (5,6,7,8,9). Severe complications of celiac disease include gastrointestinal carcinoma and T-cell lymphoma, which may develop owing to chronic inflammation and sustained activation of intestinal lymphocytes and T cells (10).
Disease activity is triggered and sustained by the entry of gluten peptides into the lamina propria of the intestine after crossing the epithelial barrier. Gluten, an amorphous mixture of proteins found in the endosperm of cereals like wheat, rye, and barley, is a major component of the human diet. In the lamina propria, tissue transglutaminase (tTG) modifies gluten peptides and potentiates their immunogenicity. These events subsequently trigger both T-helper type 1–mediated immune responses (11).
Currently, the only management option for patients with celiac disease is strict adherence to a gluten-free diet. Adherence to this highly restrictive diet is difficult due to the pervasiveness of gluten in foods. Patients maintaining a gluten-free diet may still be inadvertently exposed to up to 2 g per day of gluten (12,13,14,15). Exposure even to small amounts (i.e., 50 mg per day) can trigger signs and symptoms of celiac disease (4,14,16). Consequently, even after long-term maintenance of a gluten-free diet, many patients still have symptoms and/or mucosal damage (2,17,18,19). Therefore, a gluten-free diet alone may be insufficient to fully control the disease in some patients, and safe and effective pharmacological therapy are needed (20,21).
Immune responses in patients with celiac disease are initiated when immunogenic, incompletely digested gluten peptides gain entry into the lamina propria of the small intestine by transcellular transport (22,23,24,25) and/or through the paracellular space between epithelial cells (23,24). In transcellular transport, partially degraded gliadin moves through epithelial cells in an immunoglobulin-mediated process, making them available for antigen presentation (24). Paracellular transport of gliadin peptides occurs in the setting of increased paracellular permeability in patients with celiac disease due to gliadin-induced innate and adaptive immune responses (11,26,27,28) and subsequent tight junction disassembly (28,29,30). In addition, genetic defects have been identified in the cytoskeletal proteins involved in tight junction functioning (31,32,33).
Larazotide acetate (formerly referred to as AT-1001) is a first-in-class, tight-junction regulator peptide that in vitro prevents the opening of intestinal epithelial tight junctions induced by multiple stimuli, including cytokines, bacterial antigens, and gluten peptides (34,35). Its immunological activity is limited to the luminal surface of the small intestine (36,37). In an inpatient, randomized, double-blind, placebo-controlled, single-dose study 37), patients who received larazotide acetate had a significant reduction in gastrointestinal symptoms, particularly diarrhea, after a 2.5-g gluten challenge compared with those who received placebo. In addition, patients in the placebo group had a 70% increase in the urinary lactulose-to-mannitol (LAMA) ratio, a measure of intestinal permeability, whereas those receiving larazotide acetate had no change. Larazotide acetate was not detected in the plasma after supra-therapeutic doses, and no significant systemic toxicities were observed.
This dose-ranging, exploratory study was designed to evaluate the effect of multiple doses of larazotide acetate in patients with celiac disease who were given a 2-week gluten challenge in an outpatient setting. In addition, we sought to evaluate the tolerability of multiple doses of larazotide acetate, to explore elements of the design of studies involving controlled gluten challenges, and to gain experience with experimental outcome measures for drug development in celiac disease.
METHODS
The study protocol was approved by the institutional review boards of the participating institutions and all patients provided written informed consent (ClinicalTrials. gov Identifier: NCT00362856). The study was conducted at 10 clinical sites in the United States between August 2006 and March 2007. Patients were recruited by the investigators directly and through peer-to-peer communication.
Patients
Patients aged 18 to 65 years were eligible for the study if they had a body mass index between 18.5 and 38 kg/m2 and a diagnosis of celiac disease confirmed via biopsys ≥6 months before study entry. Patients must have been following a gluten-free diet for ≥6 months before study entry and have been in remission, as measured by antibodies to tTG (≤10 ELISA (enzyme-linked immunosorbent assay) Units (EU) for IgA and IgG, with 10 EU as the cutoff level for a positive test result). Women must have been post-menopausal or surgically sterile or must have had a negative result on the serum β-human chorionic gonadotropin pregnancy test and agreed to use acceptable methods of contraception.
Patients were excluded if they had any food intolerances or allergies other than to gluten that would have interfered with the conduct of the study, had any chronic active gastrointestinal disease other than celiac disease, had diabetes mellitus, were receiving any medications that could have interfered with LAMA testing or other study measures, used nicotine-containing products for 6 months before study entry, had clinically significant abnormal laboratory test results at the time of screening, had significant comorbidities (including positive HIV, hepatitis B surface antigen, or hepatitis C test results), were pregnant or breast-feeding mothers, had donated blood within 56 days of randomization or plasma within 7 days, had abused alcohol or drugs within 2 years of randomization, had a positive urine drug test result at screening, or had participated in any clinical trial with an active drug within 30 days of randomization. Alcohol consumption of ≥3 fluid ounces within 48 h of producing urine samples for LAMA ratio testing was not permitted.
Study design and dosing
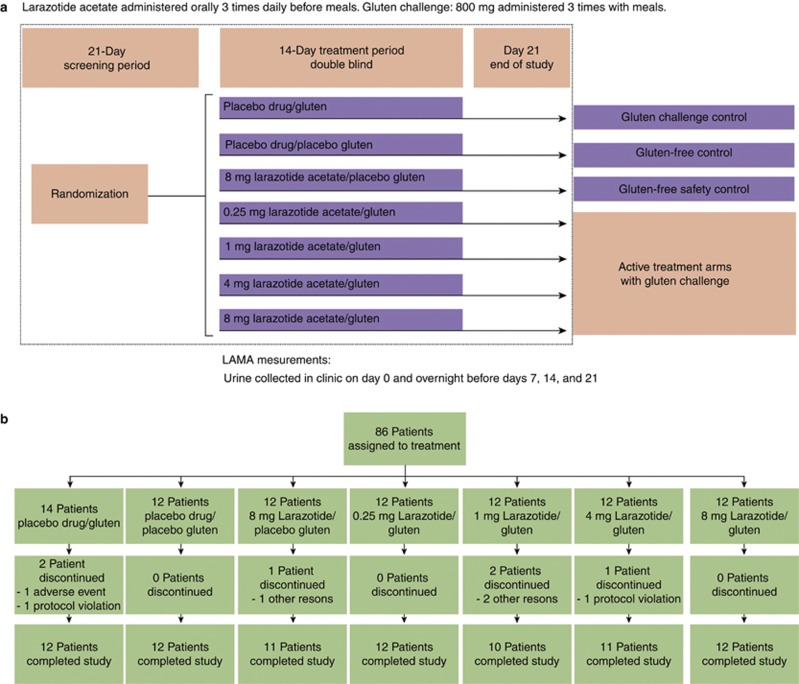
In this prospective, multicenter, double-blind trial, 86 patients were randomly assigned to one of seven treatment groups (Figure 1a). Patients in four groups received a gluten challenge along with doses of 0.25, 1, 4, or 8 mg larazotide acetate three times daily. The other three groups were a safety control arm with the highest dose of larazotide acetate (8 mg) and no gluten challenge, a gluten-free control arm of patients in remission (placebo drug/placebo gluten), and a gluten challenge control arm (placebo drug/gluten challenge).
Figure 1.
Schema of overall study design and participant allocation. (a) Study design and (b) disposition of patients. LAMA, lactulose/mannitol.
The study treatment regimen started with breakfast on day 1 and continued through day 14. Patients ingested study medication capsules three times daily 15 min before meals. Larazotide acetate capsules contained enteric-coated multi-particulate beads. Placebo drug was provided in similarly colored capsules, with beads that were composed like those of the active drug except for the absence of larazotide acetate. Neither larazotide acetate nor placebo drug capsules contained gluten.
The gluten challenge consisted of two capsules of gluten 400 mg (Amgluten 160 powder, Tate and Lyle of Decatur, Illinois, consisting of 45% gliadin, 45% glutenins, and 10% globulins). Matching placebo gluten capsules consisted of 100% cornstarch. Gluten challenge or placebo was ingested three times daily during meals. Thus, the gluten challenge consisted of a total of 2.4 g per day. Patients were asked to eat three meals per day and remain on a gluten-free diet during the study.
Study conduct and assessments
This study was performed according to good clinical practice guidelines established by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. There were five clinic visits: screening and days 0, 7, 14, and 21.
An experimental biomarker, the urinary LAMA fractional excretion ratio, was used to quantify changes in intestinal permeability. In patients with celiac disease, mucosal injury leads to a simultaneous reduction in the transmembrane absorption of monosaccharides (e.g., mannitol) and an increase in the paracellular absorption of disaccharides (e.g., lactulose), resulting in an increase in the LAMA ratio. A probe solution containing 7.5 g lactulose and 2 g mannitol in approximately 100 ml of water was administered orally in the clinic on day 0 and in the evening before the study visits on days 7, 14, and 21. On the day-0 collection in the clinic, urine was collected for 6 h. Patients fasted for at least 4 h before drinking the sugar solution and did not eat or drink (except water) until the end of the 6-h collection. For the overnight collections before days 7, 14, and 21, patients were asked to have a normal dinner around 1800hrs, not to eat or drink afterward (except water until 2200 hrs), void completely at 2200 hrs, drink the sugar solution, fast overnight (drinking water was permitted), and collect their overnight and morning urine. Urine samples were frozen at the clinic and stored for analysis of lactulose and mannitol using standardized methods (Dionex MA-1 ion exchange column with pulsed amperometric detection on a Dionex Ion Chromatograph 3000, Thermo Scientific, Sunnyvale, CA) (38).
Serum was analyzed at a central laboratory for antibodies to tTG at screening and day 21 (ACM Medical Laboratory, Rochester, New York).
Patients completed the Gastrointestinal Symptom Rating Scale (GSRS) (39,40,41) and the Psychological General Well-Being Index (42,43) on days 0, 7, 14, and 21. The GSRS is a widely used questionnaire completed by the patient to assess gastrointestinal symptom severity. The instrument consists of 15 questions that are grouped into five domains (diarrhea, abdominal pain, indigestion, constipation, and reflux). In a post hoc analysis, the three domains that are most relevant to celiac disease (diarrhea, abdominal pain, and indigestion) were evaluated separately, hereafter referred to as the Celiac Disease GSRS (CeD-GSRS). Both the GSRS (42,44,45) and the Psychological General Well-Being Index (42,46,47) have been previously used to evaluate patients with celiac disease. However, the CeD-GSRS has not been previously used.
Tolerability was evaluated at each visit by adverse event surveillance, measurement of vital signs, and clinical laboratory analysis for blood chemistry, hematology, and urinalysis. Twelve-lead electrocardiograms were performed at screening and on days 0 and 21. Plasma levels of larazotide acetate and metabolites were measured on days 0, 7, and 14 using validated high-performance liquid chromatography with tandem mass spectrometry, with a lower limit of quantification of 0.5 ng/ml.
In order to differentiate adverse events that were related to the gluten challenge from those that were related to study medication, the following events were identified a priori as the signs and symptoms of gluten toxicity: abdominal discomfort, dyspepsia, nausea, diarrhea, vomiting, flatulence, constipation, and rash/dermatitis herpetiformis. These events were evaluated as part of the efficacy assessment and were not included in the assessment of safety.
Outcome measures and statistical analysis
The primary outcome measure was the change in LAMA ratios from day 0 to day 14, which was calculated by dividing the LAMA ratio at day 14 by the LAMA ratio at day 0. This calculation results in a fold-ratio that indicates the intestinal permeability, with a ratio of 1 indicating no change in permeability, a ratio >1 indicating an increase in permeability, and a ratio <1 indicating a reduction. LAMA values were log-transformed for the analysis and sample-size calculations that made it possible to use standard sample-size calculation methods for determining the differences between the treatment groups. Because of the log transformation, LAMA values were summarized using the geometric mean.
All analyses presented in this report were conducted using the intent-to-treat population. All analyses were pre-specified unless otherwise indicated. Safety analyses were descriptive in nature.
In the sample-size calculation, it was estimated that eight evaluable patients per group would have provided 80% power to demonstrate a statistically significant difference (α=0.05) between the gluten-challenge control group and the larazotide acetate groups that received the gluten challenge. An additional 3–5 patients per group was projected to allow for screen failures and patients who withdrew during the study (27–45%). The effect size for this outcome was set a priori at a threefold reduction in LAMA ratios between any test group and placebo. The sample size calculation was based on a t test for differences between two groups. The standard deviation for log-transformed fold-ratios was assumed to be 0.3, based on the results of a previous study (37).
RESULTS
Demography and patient disposition
Eighty-six patients signed the informed consent, were randomly assigned to treatment, and were included in the intent-to-treat analysis (Figure 1b). Fifty-three percent were women, and the mean age was 46.3 years. All patients except one were Caucasian. Eighty patients (93.0%) completed the study. Six patients discontinued the study prematurely: 1 in the larazotide acetate 8 mg/placebo gluten arm (because of abdominal discomfort), 2 in the placebo drug/gluten-challenge arm (1 positive drug screen and 1 allergic reaction), 2 in the larazotide acetate 1 mg/gluten-challenge arm (headache, nausea, flatulence, and diarrhea), and 1 in the larazotide acetate 4 mg/gluten-challenge arm (non-compliance with intestinal permeability tests and questionnaires).
Primary efficacy outcome: LAMA ratio
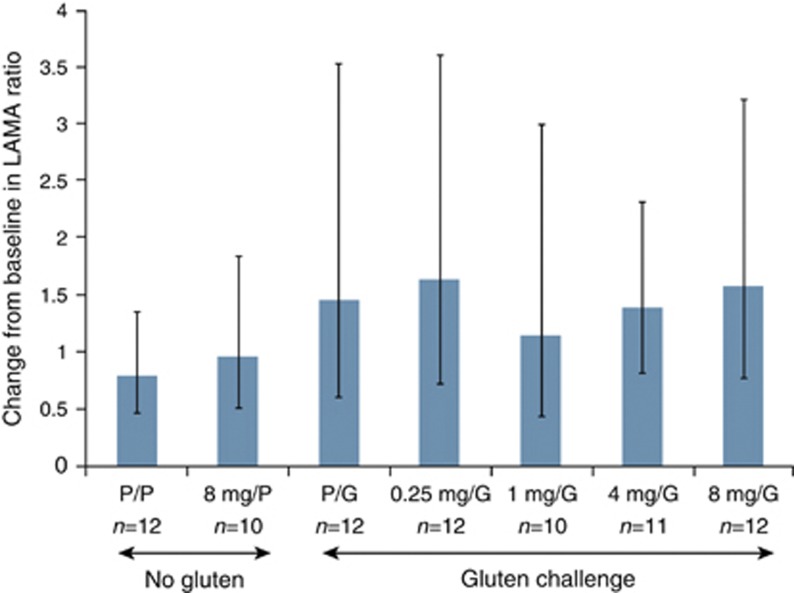
The geometric mean change in the LAMA ratio in the gluten-challenge control group was greater than that of patients in the gluten-free control group, although the difference between the groups was not statistically significant (Figure 2). Among the patients who received the gluten challenge, no statistically significant differences in the LAMA ratios were observed between the larazotide acetate groups and the placebo group (Figure 2). The LAMA ratios varied widely. Geometric mean LAMA fold-ratios numerically decreased between baseline and day 7 in the gluten-free control groups and the gluten-challenge control group (see Supplementary Figure S1 online).
Figure 2.
Change from baseline LAMA levels in the individual treatment groups. Mean change from baseline in the urinary lactulose-to-mannitol (LAMA) ratio. Values are the geometric mean fold-ratio on day 14 over baseline (day 0). Vertical bars represent 95% confidence intervals. Dashed horizontal line indicates level for gluten-free control. G, gluten; P, placebo.
Secondary efficacy outcomes
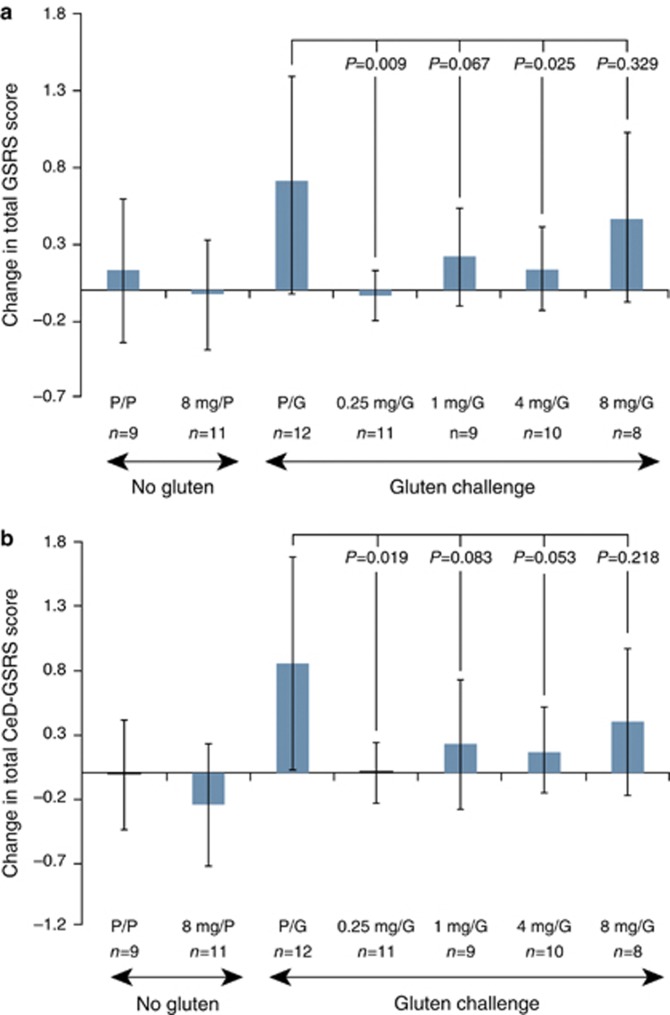
The changes in total GSRS and the CeD-GSRS for the each treatment group from baseline to day 14 are shown in Figure 3. Gastrointestinal symptoms were stable in the two treatment groups that received placebo gluten, but symptoms grew more severe in the gluten-challenge control group.
Figure 3.
Change from baseline in gastrointestinal symptoms in the individual treatment groups as measured by the GSRS and CeD-GSRS. Mean (95% confidence interval) changes from baseline to day 14 in the total (a) Gastrointestinal Symptom Rating Scale (GSRS) scores and (b) Celiac Disease GSRS (CeD-GSRS) scores. n=9–13 per group. P values comparing larazotide acetate/gluten-challenge (G) groups to the placebo (P) drug/gluten-challenge group were calculated using an analysis of covariance model, with treatment as a fixed effect and the corresponding baseline value as a covariate.
Gastrointestinal symptoms in patients who received the larazotide acetate and the gluten challenge did not become as severe as they did in the gluten-challenge control group. The 0.25 and 4.0 mg doses of larazotide acetate showed statistically significant prevention of gastrointestinal symptom severity worsening. The 4- and 8-mg doses of larazotide acetate did not show statistically significant prevention of gastrointestinal symptom severity worsening (P=0.067 and 0.329, respectively). Results for the CeD-GSRS were generally similar to those of the total GSRS.
Because the individual treatment groups were small (≤13 patients per group) and there were similar trends among the groups receiving larazotide acetate and the gluten challenge, the groups were combined into a single active treatment group (n=48) to make possible further analysis of symptoms and simplify data presentation for subsequent exploratory analyses. Likewise, the two groups receiving placebo gluten along with either placebo drug or 8 mg larazotide acetate were pooled into an aggregate gluten-free control group (n=20). The gluten-challenge control group (n=13) indicates the response to gluten ingestion in the absence of larazotide acetate.
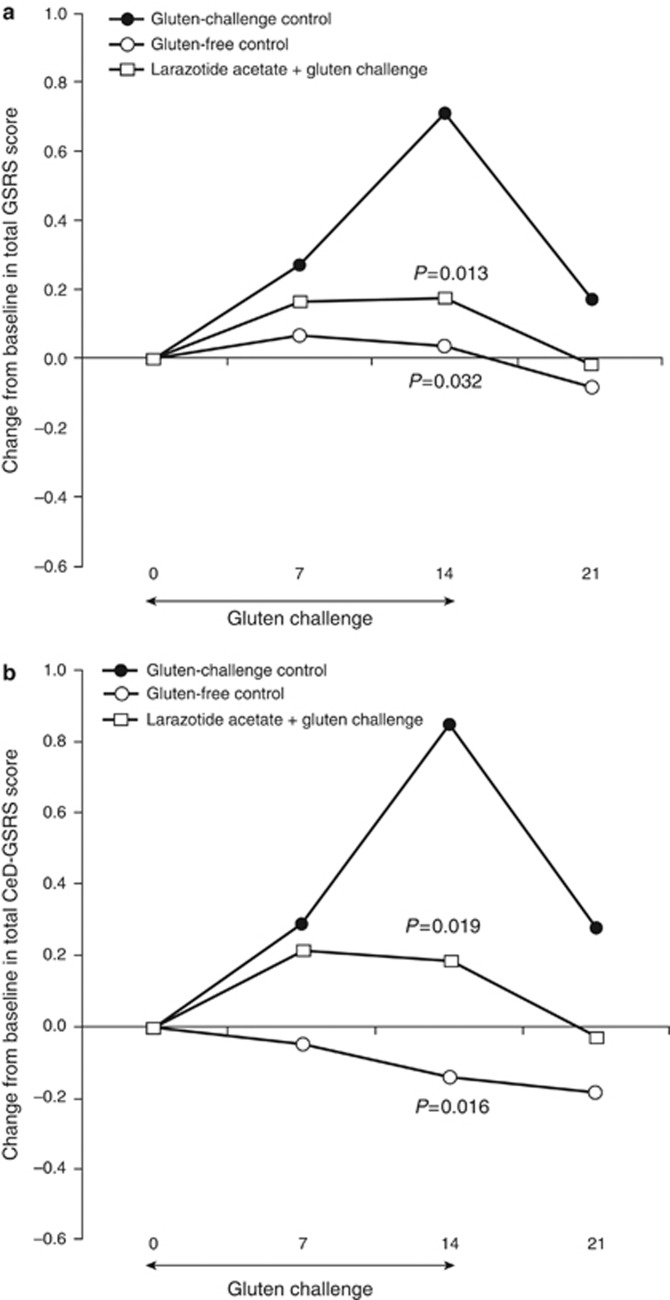
Figure 4 shows the time course of the change in the GSRS scores for these combined treatment groups. In the gluten-challenge control group, the severity of gastrointestinal symptoms increased substantially during the gluten-challenge period (baseline to day 14), as measured by both the total GSRS and the CeD-GSRS. The scores rapidly decreased after the challenge was over (day 14 to day 21). By contrast, there was no increase in severity in the groups that did not receive the gluten challenge (gluten-free control), and only a modest increase in the patients who received larazotide acetate and the gluten challenge. Symptoms were statistically significantly less severe in both the gluten-free control group and the group consisting of patients who received larazotide acetate and the gluten challenge compared with the gluten-challenge control group (P<0.05). Thus, an increase in symptom severity in response to gluten, as well as protection from the exposure to gluten provided by larazotide acetate, was demonstrated using both the GSRS and the CeD-GSRS in this study.
Figure 4.
Change in gastrointestinal symptoms as measured by the GSRS and CeD-GSRS during and after gluten challenge in the gluten challenge/placebo drug cohort compared to the aggregated gluten-free control cohorts and the Larazotide-treated gluten challenge groups. Time course of mean change from baseline in the total (a) Gastrointestinal Symptom Rating Scale (GSRS) score and (b) Celiac Disease (CeD-GSRS) scores. The gluten control group includes patients who received placebo drug and the gluten challenge (n=13). Gluten-free control includes patients who received placebo drug or 8 mg larazotide acetate and gluten placebo (n=20). Patients who received larazotide acetate and the gluten challenge were also combined into one group (n=38). P values were calculated using an analysis of covariance model with treatment as a fixed effect and the corresponding baseline value as a covariate.
To complement the analysis of the effect of larazotide acetate on symptom severity, each sub-domain of the GSRS (diarrhea, abdominal pain, indigestion, constipation, and reflux) was analyzed individually (see Supplementary Figure S2 online). Based on a comparison of the gluten-challenge control to the groups not receiving gluten, the gluten challenge resulted in an increase in the severity of diarrhea, abdominal pain, and indigestion, with the largest increase in symptom severity in the indigestion domain. Larazotide acetate provided a statistically significant protection against an increase in severity of abdominal pain, indigestion, and reflux, while the protection against an increase in the severity of diarrhea nearly reached statistical significance (P=0.051). Although there was an increase in the severity of both the constipation and reflux domains in the gluten-challenge control group, these increases did not appear to be specifically attributable to gluten, as there were similar increases in the gluten-free control group.
No statistically significant difference between the larazotide acetate groups and the gluten-challenge control group was observed in changes in the total Psychological General Well-Being Index scores (see Supplementary Table 1 online).
Changes in the weekly means of the number of bowel movements, number of episodes of diarrhea, stool consistency rating, and abdominal discomfort scores from daily bowel diaries are provided in Supplementary Figure S3 online. In general, daily bowel diary scores for patients in the larazotide acetate 1-mg and 0.25-mg groups did not increase as much as those in the other groups exposed to gluten.
At two of four tested doses, larazotide acetate appeared to protect patients from the signs and symptoms of gluten toxicity, which were defined a priori: abdominal discomfort, dyspepsia, nausea, diarrhea, vomiting, flatulence, constipation, or rash/dermatitis herpetiformis. Compared with the gluten-challenge control, in which 50.0% of patients experienced symptoms of gluten toxicity, 20.8% of participants in both the aggregated gluten-free control and the larazotide acetate treatment arms exhibited these symptoms (P=0.11 and P=0.046, respectively; Fisher's exact test). After other signs and symptoms that were determined post hoc likely to be related to gluten toxicity were included (namely, elevated liver function tests and aphthous stomatitis), 64.3% of participants in the gluten-challenge control were affected, compared with 16.7% in the aggregated gluten-free control and 23.3% in the aggregated larazotide acetate treatment arms (P=0.021 and P=0.008, respectively; Fisher's exact test).
Mean titers at screening for antibodies to tTG ranged from 1.6 to 3.0 U/ml, and no significant differences between the treatment groups were observed at screening. No significant differences between the treatment groups were observed in the mean changes from screening to day 21 in the titers of antibodies to tTG (−0.1 U/ml in the combined gluten-free control group, 2.9 U/ml in the gluten-challenge control group (P=0.204 vs. gluten-free control group), and 0.9 U/ml in the combined larazotide acetate/gluten-challenge groups (P=0.256 vs. gluten-challenge control group).
Tolerability
The multiple oral doses of larazotide acetate used in this study were well tolerated. Of the 86 patients in the intent-to-treat population, 44 (51.2%) had at least one treatment-emergent adverse event ( Table 1). Fifteen of the 60 patients who received larazotide acetate (25.0%) had adverse events that the investigator considered to be related to study medication, while 7 of the 26 patients who received placebo drug (26.9%) had such events. Headache was the most common adverse event, reported by 17 patients, with no differences among treatment groups. None of the patients had serious adverse events. There were also no clinically significant findings of hepatic, bone, or renal toxicity. Vital signs, electrocardiogram, hematology, and clinical chemistry parameters showed no changes from baseline that were considered clinically significant.
Table 1. Summary of adverse events.
| Category |
Gluten-free control |
Gluten challenge |
|||||
|---|---|---|---|---|---|---|---|
| Placebo (n=12) | 8 mg (n=12) | Placebo (n=14) | 0.25 mg (n=12) | 1 mg (n=12) | 4 mg (n=12) | 8 mg (n=12) | |
| Patients with ≥1 adverse event | 5 (41.7) | 4 (33.3) | 7 (50.0) | 7 (58.3) | 8 (66.7) | 6 (50.0) | 7 (58.3) |
| Patients with ≥1 adverse event related to study medicationa | 2 (16.7) | 1 (8.3) | 5 (35.7) | 3 (25.0) | 4 (33.3) | 3 (25.0) | 4 (33.3) |
| Patients with ≥1 severe adverse event | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) |
| Patients with ≥1 serious adverse events | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Patients who discontinued study medication because of an adverse event | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adverse events that occurred in ≥5% of patients | |||||||
| Headache | 4 (33.3) | 0 (0.0) | 3 (21.4) | 3 (25.0) | 2 (16.7) | 3 (25.0) | 2 (16.7) |
| Urinary tract infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 1 (8.3) | 1 (8.3) | 1 (8.3) |
Events that were considered by the investigator to be possibly or probably related to the study medication.
Five (5.8%) patients had urinary tract infections, all of which occurred in patients who received larazotide acetate and the gluten challenge. There was no evidence of a dosage effect or an association to the duration of dosing at the onset of the event. Microbiological confirmation was attempted in only one of these events, and the result was negative. Only one patient received antibiotic medication. All cases were mild, and none were considered to be related to study drug.
Plasma levels of larazotide acetate were measured at days 0, 7, and 14 and were below the limit of quantification (0.5 ng/ml) in all the groups. Metabolites were measured in the highest dose group (8 mg) and were also below the lower limit of quantification.
DISCUSSION
The purpose of the study was to evaluate the efficacy and tolerability of multiple doses of larazotide acetate in preventing the exacerbation of symptoms after a gluten challenge in patients with celiac disease well controlled through a gluten-free diet. The primary efficacy outcome was the LAMA ratio, which was used as an experimental biomarker for intestinal permeability. The results indicate that the gluten challenge induced both an increase in symptoms as measured by the GSRS and intestinal permeability in patients who received placebo drug, as indicated by an increase in the LAMA ratio; however, this increase was not statistically significant. LAMA ratios for patients who received larazotide acetate and the gluten challenge were also not statistically significantly different from those who received placebo and the gluten challenge. The significant deterioration in gastrointestinal symptoms in the group receiving gluten and placebo compared with the group receiving no gluten suggests that 2.4 g of gluten per day is sufficient to induce measurable and clinically important symptoms in clinical trial settings.
LAMA ratio values varied markedly among all treatment groups, which may be the result of conducting the measurements in an outpatient setting. In addition, a decrease in LAMA was observed in most treatment groups at day 7, including the gluten-free control group, in which patients received both placebo drug and placebo gluten. These results suggest that there may have been a study effect, by virtue of which patients were more compliant with their gluten-free diets while participating in the study. Future studies should include a run-in period for diet stabilization. In addition, the significant variability in the LAMA ratios in the outpatient setting should be taken into account in future studies utilizing this outcome. Sample size calculations used in the design of this study were based on one preliminary inpatient trial in which the effects of gluten challenge and drug treatment were larger than those observed in this study. Although we cannot exclude the possibility that the primary efficacy outcome in this study was not reached because the treatment did not produce an effect, it is likely that the methods used did not fully detect an effect of both the gluten challenge and the larazotide acetate therapy.
In contrast to the LAMA findings, the results of gastrointestinal symptom severity assessments indicate that larazotide acetate provided protection against the increase in symptoms associated with a gluten challenge in patients with celiac disease. Both the prespecified total GSRS and the exploratory CeD-GSRS scores of patients who received placebo drug increased following the gluten challenge. However, at two of four tested doses (0.25 and 4 mg), patients who received larazotide acetate and the gluten challenge did not show such increases in GSRS and CeD-GSRS scores. Symptom severity was statistically significantly lower than placebo drug in these treatment groups.
As expected, most patients who received the gluten challenge, including those in the gluten-challenge control group, did not develop antibodies to tTG. Thus, the effect of larazotide acetate on antibodies to tTG could not be assessed. Longer gluten challenge studies and/or higher gluten doses will be necessary to assess the effect of the larazotide acetate on the development of antibodies to tTG.
In conclusion, larazotide acetate was well tolerated in this population of patients with celiac disease. Larazotide acetate prevented the increase in gastrointestinal symptom severity induced by gluten challenge at two of the four doses tested. A gluten challenge of 2.4 g per day was sufficient to evaluate this outcome. Additional studies with appropriate run-in periods, larger study populations, additional measures such as histology, and longer duration may be warranted to further evaluate the effect of larazotide acetate in patients with celiac disease.
STUDY HIGHLIGHTS

Acknowledgments
We thank John Jiang, PhD, and Joel Verter for statistical analyses, and Betsy Abraham-Van Parijs, MD, PhD, for comments and suggestions. Scott Newcomer of Cephalon helped prepare the manuscript but did not meet the criteria for authorship. The study coordinators for the investigating co-authors were Carol Van Dyke, Melinda Dennis, BJ Bahl, and Roberta O'Shea. We thank the patients who participated in this trial and the following clinicians and study coordinators: DiMarino A, MD, Moretti D, Miller C, and Wilson K, Thomas Jefferson University, Philadelphia, PA; Kirby D, MD, and Tucker P, VCU Medical Center, Richmond, VA; Medoff J, MD, and Uhl A, Vital re:Search, Greensboro, NC; DeLegge M and Davis CT, Medical University of South Carolina, Charleston, SC; Schuman R, MD, and Dalcomine C, Affiliates in GastroEnterology, Morristown, NJ; Harris L, MD, and Menghini M, Mayo Clinic, Scottsdale, AZ; Pressman MD and Tuohy D, Medical Associates Research Group, San Diego, CA.
Guarantor of the article: J.A. Murray, MD.
Specific author contributions: Study design and execution, patient recruitment, analysis of data, and writing of manuscript: Daniel A. Leffler, C.P. Kelly, and J.A. Murray; patient recruitment and writing of manuscript: H.Z. Abdallah and A.M. Colatrella; study design and execution, analysis of data, and writing of manuscript: L.A. Harris, F. Leon, L.A. Arterburn, and Z.H. Lan; study design and execution, and writing of manuscript: B.M. Paterson.
Financial support: This study was funded by Alba Therapeutics Corporation, Baltimore, Maryland, USA.
Potential competing interests: Daniel A. Leffler is a consultant for Alba Therapeutics. C.P. Kelly is a consultant for Alba Therapeutics, Alvine Pharmaceuticals, and ImmunosanT. F. Leon, L.A. Arterburn, and Z.H. Lan were formerly employed by Alba Therapeutics. B.M. Paterson was the former CEO and founder and owns stock in Alba Therapeutics. The other authors declare no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Supplementary Material
References
- Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- Fasano A. European and North American populations should be screened for coeliac disease. Gut. 2003;52:168–169. doi: 10.1136/gut.52.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–166. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–S78. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010;26:116–122. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Green PH. Clinical management of coeliac disease. J Int Med. 2011;269:560–571. doi: 10.1111/j.1365-2796.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- Green PH, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191–195. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–231. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci. 2008;53:1573–1581. doi: 10.1007/s10620-007-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert A, Espadaler M, Angel Canela M, et al. Consumption of gluten-free products: should the threshold value for trace amounts of gluten be at 20, 100 or 200 p.p.m. Eur J Gastroenterol Hepatol. 2006;18:1187–1195. doi: 10.1097/01.meg.0000236884.21343.e4. [DOI] [PubMed] [Google Scholar]
- Mayer M, Greco L, Troncone R, et al. Compliance of adolescents with coeliac disease with a gluten free diet. Gut. 1991;32:881–885. doi: 10.1136/gut.32.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin P, Thorell L, Kaukinen K, et al. The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease. Aliment Pharmacol Ther. 2004;19:1277–1283. doi: 10.1111/j.1365-2036.2004.01961.x. [DOI] [PubMed] [Google Scholar]
- Midhagen G, Hallert C. High rate of gastrointestinal symptoms in celiac patients living on a gluten-free diet: controlled study. Am J Gastroenterol. 2003;98:2023–2026. doi: 10.1111/j.1572-0241.2003.07632.x. [DOI] [PubMed] [Google Scholar]
- Ciacci C, Cirillo M, Cavallaro R, et al. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66:178–185. doi: 10.1159/000066757. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lo W, Memeo L, et al. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest Endosc. 2003;57:187–191. doi: 10.1067/mge.2003.54. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–1420. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- NIH National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28–30, 2004. Gastroenterology. 2005;128:S1–S9. doi: 10.1053/j.gastro.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Zimmer KP, Poremba C, Weber P, et al. Translocation of gliadin into HLA-DR antigen containing lysosomes in coeliac disease enterocytes. Gut. 1995;36:703–709. doi: 10.1136/gut.36.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Candalh C, Dugave C, et al. Alterations of the intestinal transport and processing of gliadin peptides in celiac disease. Gastroenterology. 2003;125:696–707. doi: 10.1016/s0016-5085(03)01049-7. [DOI] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–154. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann M, Richter JF, Wedell I, et al. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut. 2008;57:747–754. doi: 10.1136/gut.2007.136366. [DOI] [PubMed] [Google Scholar]
- Koning F. Celiac disease: caught between a rock and a hard place. Gastroenterology. 2005;129:1294–1301. doi: 10.1053/j.gastro.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjova T, Uibo O, Ojakivi I, et al. Lower expression of tight junction protein 1 gene and increased FOXP3 expression in the small bowel mucosa in coeliac disease and associated type 1 diabetes mellitus. Inter Arch Allergy Immunol. 2011;156:451–461. doi: 10.1159/000324456. [DOI] [PubMed] [Google Scholar]
- Schumann M, Gunzel D, Buergel N, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- van Elburg RM, Uil JJ, Mulder CJ, et al. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993;34:354–357. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- van Heel DA, Franke L, Hunt KA, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet. 2007;39:827–829. doi: 10.1038/ng2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdlickova B, Westra HJ, Franke L, et al. Celiac disease: moving from genetic associations to causal variants. Clin Genet. 2011;80:203–313. doi: 10.1111/j.1399-0004.2011.01707.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Tripathi A, Tamiz AP, et al. Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides. 2012;35:95–101. doi: 10.1016/j.peptides.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Durai M, Kitchens K, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012;35:86–94. doi: 10.1016/j.peptides.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Di Pierro M, Lu R, Uzzau S, et al. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem. 2001;276:19160–19165. doi: 10.1074/jbc.M009674200. [DOI] [PubMed] [Google Scholar]
- Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. doi: 10.1016/s0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]
- Dimenas E, Carlsson G, Glise H, et al. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand J Gastroenterol Suppl. 1996;221:8–13. doi: 10.3109/00365529609095544. [DOI] [PubMed] [Google Scholar]
- Dimenas E, Glise H, Hallerback B, et al. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30:1046–1052. doi: 10.3109/00365529509101605. [DOI] [PubMed] [Google Scholar]
- Revicki DA, Wood M, Wiklund I, et al. Reliability and validity of the Gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75–83. doi: 10.1023/a:1008841022998. [DOI] [PubMed] [Google Scholar]
- Mustalahti K, Lohiniemi S, Collin P, et al. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002;5:105–113. [PubMed] [Google Scholar]
- Dupuy HJ.The psychological general well-being (PGWB) indexIn: Wenger NK, Mattson ME, Furberg CF, et al., (eds). Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies Le Jacq Publishing: New York; 1984170–183. [Google Scholar]
- Lohiniemi S, Maki M, Kaukinen K, et al. Gastrointestinal symptoms rating scale in coeliac disease patients on wheat starch-based gluten-free diets. Scand J Gastroenterol Suppl. 2000;35:947–949. doi: 10.1080/003655200750023002. [DOI] [PubMed] [Google Scholar]
- Hallert C, Granno C, Grant C, et al. Quality of life of adult coeliac patients treated for 10 years. Scand J Gastroenterol Suppl. 1998;33:933–938. doi: 10.1080/003655298750026949. [DOI] [PubMed] [Google Scholar]
- Ukkola A, Maki M, Kurppa K, et al. Diet improves perception of health and well-being in symptomatic, but not asymptomatic, patients with celiac disease. Clin Gastroenterol Hepatol. 2011;9:118–123. doi: 10.1016/j.cgh.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment Pharmacol Ther. 2009;29:811–816. doi: 10.1111/j.1365-2036.2009.03945.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.