Abstract
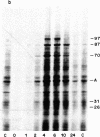
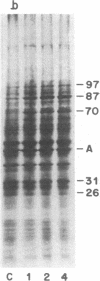
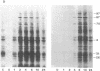
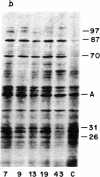
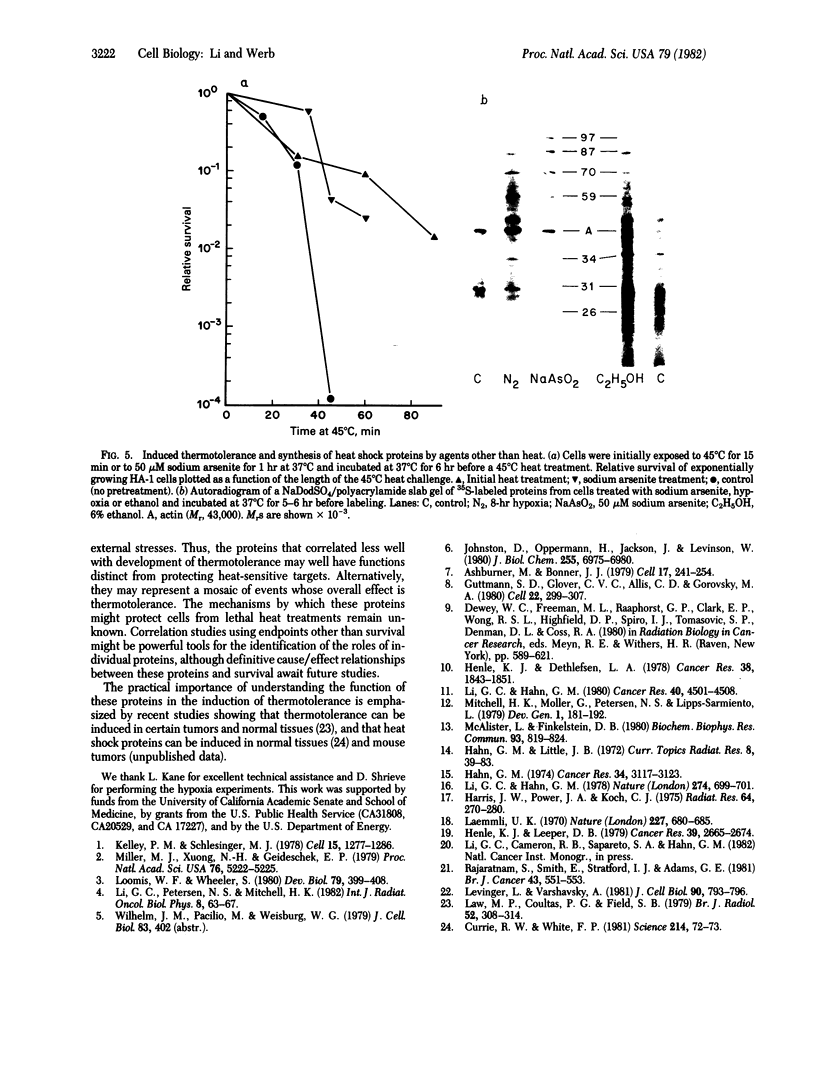
Synthesis of a family of proteins called "heat shock" proteins is induced or enhanced in cells in response to various environmental stresses, suggesting that these proteins may perform functions essential to cell survival. Because a brief, nonlethal heat treatment can dramatically induce a transient resistance to a subsequent lethal heat treatment (thermotolerance), we examined the effect of heat treatment (41-46 degrees C) on protein synthesis and cell survival in plateau-phase Chinese hamster fibroblast (HA-1) cells. After heat treatments that either drastically inhibited total protein synthesis (46 degrees C) or did not suppress it (41 degrees C), the synthesis of heat shock proteins was greatly enhanced over that in unheated cells, and cell survival was increased 10(2)- to 10(6)-fold when cells were challenged by a subsequent lethal heat treatment. The synthesis of heat shock proteins correlated well with the development of thermotolerance, and the stability of these proteins correlated well with the persistence of thermotolerance up to 36 hr. Sodium arsenite, hypoxia, and ethanol also induced both the synthesis of heat shock proteins and transient thermotolerance. A qualitative analysis of individual proteins suggests that the synthesis and persistence of polypeptides of Mr 70,000 or 87,000 most closely conformed to the kinetics of thermotolerance.
Full text
PDF
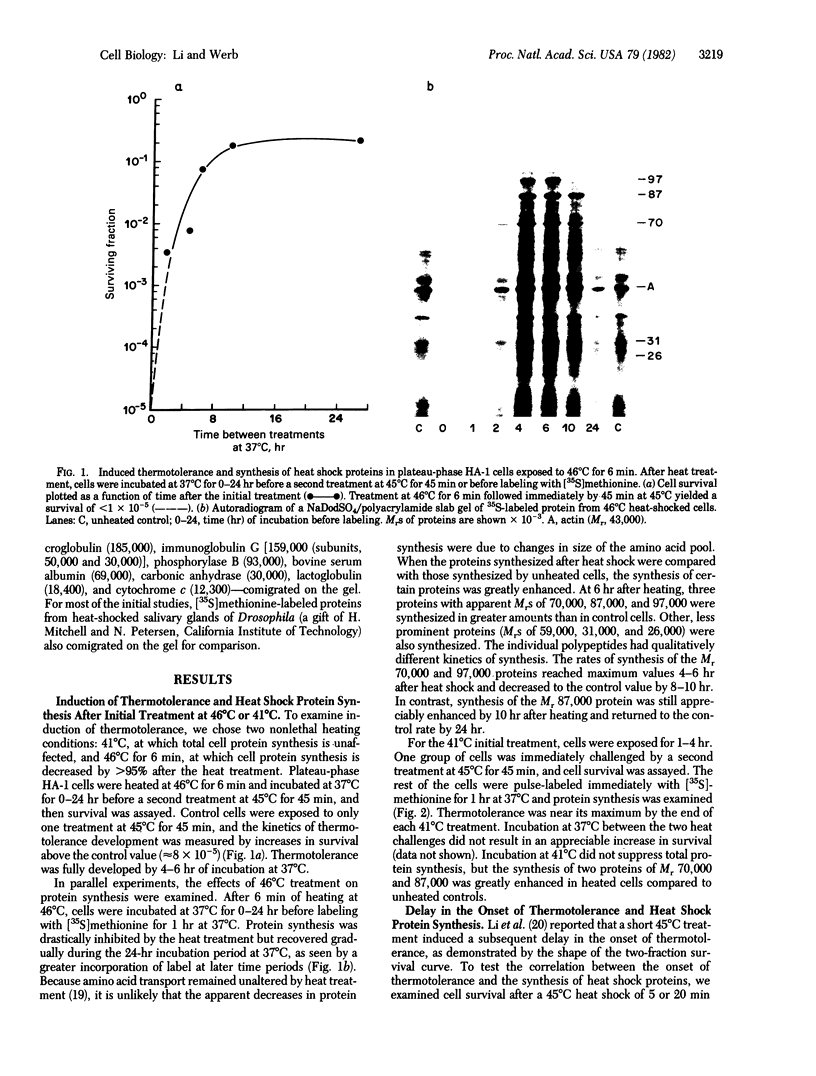
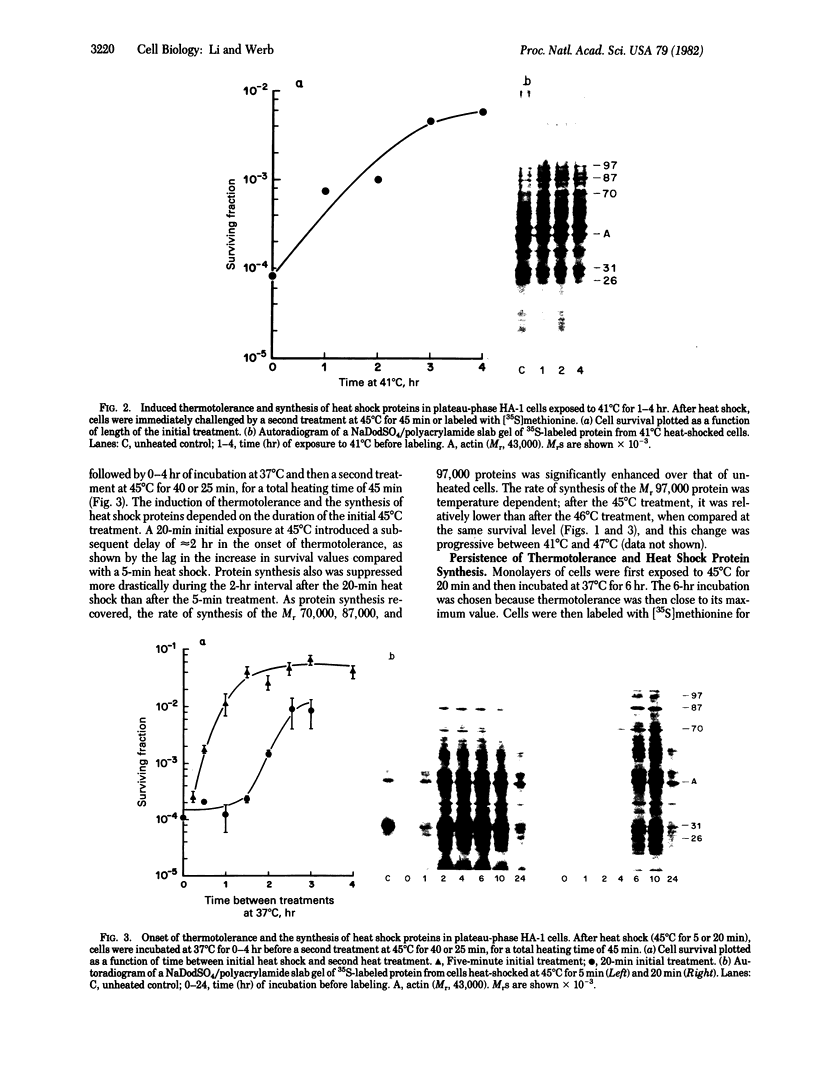
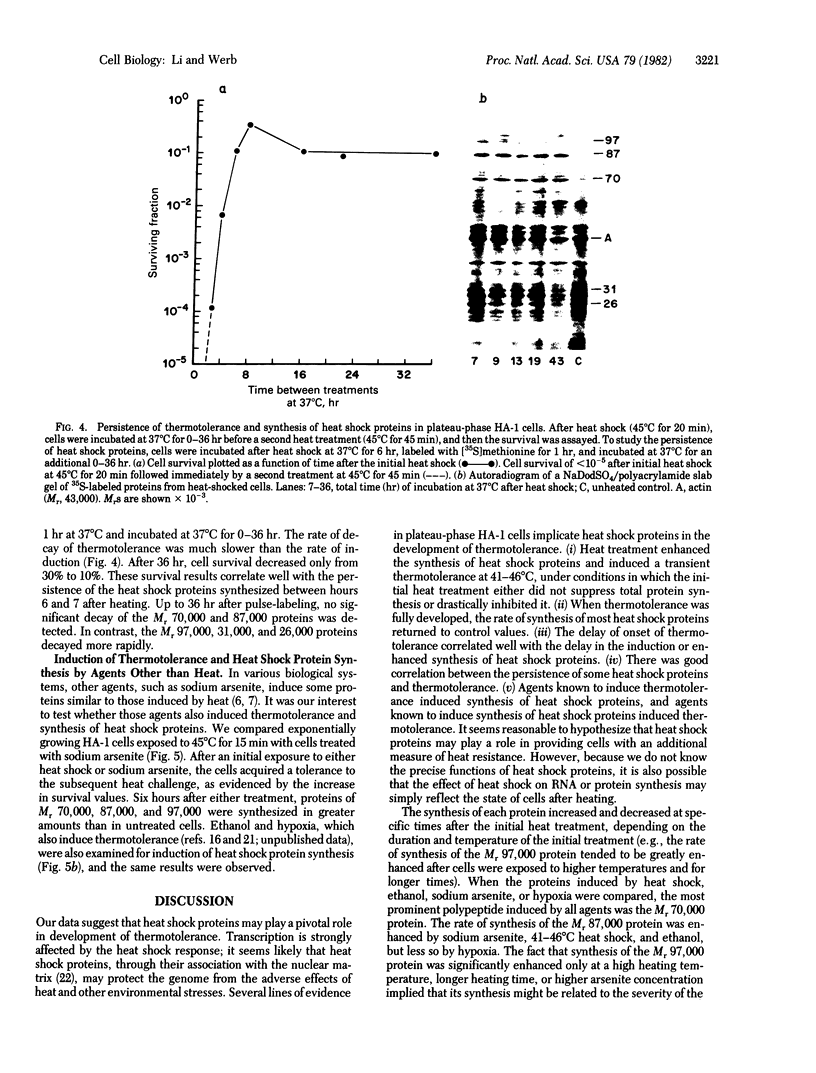
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Currie R. W., White F. P. Trauma-induced protein in rat tissues: a physiological role for a "heat shock" protein? Science. 1981 Oct 2;214(4516):72–73. doi: 10.1126/science.7280681. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., Little J. B. Plateau-phase cultures of mammalian cells: an in vitro model for human cancer. Curr Top Radiat Res Q. 1972 Jul;8(1):39–43. [PubMed] [Google Scholar]
- Hahn G. M. Metabolic aspects of the role of hyperthermia im mammalian cell inactivation and their possible relevance to cancer treatment. Cancer Res. 1974 Nov;34(11):3117–3123. [PubMed] [Google Scholar]
- Harris J. W., Power J. A., Koch C. J. Radiosensitization of hypoxic mammalian cells by diamide. I. Effects of experimental conditions on survival. Radiat Res. 1975 Nov;64(2):270–280. [PubMed] [Google Scholar]
- Henle K. J., Dethlefsen L. A. Heat fractionation and thermotolerance: a review. Cancer Res. 1978 Jul;38(7):1843–1851. [PubMed] [Google Scholar]
- Henle K. J., Leeper D. B. Effects of hyperthermia (45 degrees) on macromolecular synthesis in Chinese hamster ovary cells. Cancer Res. 1979 Jul;39(7 Pt 1):2665–2674. [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law M. P., Coultas P. G., Field S. B. Induced thermal resistance in the mouse ear. Br J Radiol. 1979 Apr;52(616):308–314. doi: 10.1259/0007-1285-52-616-308. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Heat-shock proteins of Drosophila are associated with nuclease-resistant, high-salt-resistant nuclear structures. J Cell Biol. 1981 Sep;90(3):793–796. doi: 10.1083/jcb.90.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Hahn G. M. A proposed operational model of thermotolerance based on effects of nutrients and the initial treatment temperature. Cancer Res. 1980 Dec;40(12):4501–4508. [PubMed] [Google Scholar]
- Li G. C., Hahn G. M. Ethanol-induced tolerance to heat and to adriamycin. Nature. 1978 Aug 17;274(5672):699–701. doi: 10.1038/274699a0. [DOI] [PubMed] [Google Scholar]
- Li G. C., Petersen N. S., Mitchell H. K. Induced thermal tolerance and heat shock protein synthesis in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):63–67. doi: 10.1016/0360-3016(82)90386-8. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. A response of protein synthesis to temperature shift in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5222–5225. doi: 10.1073/pnas.76.10.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam S., Smith E., Stratford I. J., Adams G. E. Thermotolerance in chinese hamster cells under oxic conditions after chronic culture under hypoxia. Br J Cancer. 1981 Apr;43(4):551–553. doi: 10.1038/bjc.1981.80. [DOI] [PMC free article] [PubMed] [Google Scholar]