Abstract
The blood–spinal cord barrier (BSCB) regulates molecular exchange between blood and spinal cord. Pericytes are presumed to be important cellular constituents of the BSCB. However, the regional abundance and vascular functions of spinal cord pericytes have yet to be determined. Utilizing wild-type mice, we show that spinal cord pericyte capillary coverage and number compared with the brain regions are reduced most prominently in the anterior horn. Regional pericyte variations are highly correlated with: (1) increased capillary permeability to 350 Da, 40,000 Da, and 150,000 Da, but not 2,000,000 Da fluorescent vascular tracers in cervical, thoracic, and lumbar regions and (2) diminished endothelial zonula occludens-1 (ZO-1) and occludin tight junction protein expression. Pericyte-deficient mutations (PdgfrβF7/F7 mice) resulted in additional pericyte reductions in spinal cord capillaries leading to overt BSCB disruption to serum proteins, accumulation in motor neurons of cyotoxic thrombin and fibrin and motor neuron loss. Barrier disruption in perciyte-deficient mice coincided with further reductions in ZO-1 and occludin. These data suggest that pericytes contribute to proper function of the BSCB at the capillary level. Regional reductions in spinal cord pericytes may provide a cellular basis for heightened spinal cord barrier capillary permeability and motor neuron loss.
Keywords: blood–brain barrier, capillaries, endothelium, pericytes, vascular biology
Introduction
The blood–brain barrier (BBB) and blood–spinal cord barrier (BSCB) tightly regulate the blood–central nervous system (CNS) molecular exchange required for normal neuronal function (Zlokovic, 2008, 2011). In contrast to the highly permeable capillaries in the systemic circulation (Mann et al, 1985), blood–CNS vascular barriers are formed by continuous endothelial cells with tight junction protein complexes and low rates of vesicular transport (Winkler et al, 2011). This largely prohibits transport of large molecules and polar solutes without specific transport systems (Zlokovic and Apuzzo, 1997; Zlokovic, 2011). Although the BBB and BSCB are functionally similar, many differences have been identified (Bartanusz et al, 2011). For example, the rodent BSCB has heightened permeability to mannitol and inulin (Daniel et al, 1985; Prockop et al, 1995), interferons (Pan et al, 1997a), albumin, sucrose, and tumor necrosis factor α (Pan et al, 1997b). The source of these permeability changes is largely unknown, although reduced tight junction protein expression in the spinal cord has been reported (Ge and Pachter, 2006).
Pericytes are multifunctional members of the neurovascular unit (Armulik et al, 2011; Winkler et al, 2011). Pericytes ensheathe microvascular endothelial cells covering much of the vascular wall (Bell et al, 2012). Central nervous system pericytes have been demonstrated to promote development and maintenance of the BBB (Armulik et al, 2010; Bell et al, 2010; Daneman et al, 2010; Li et al, 2011), regulate capillary structure (Armulik et al, 2010; Bell et al, 2010; Enge et al, 2002; Hellstrom et al, 2001) and diameter (Armulik et al, 2010; Li et al, 2011; Lindahl et al, 1997; Peppiatt et al, 2006), controversially, modulate capillary blood flow (Bell et al, 2010; Fernandez-Klett et al, 2010; Peppiatt et al, 2006), and participate in spinal cord scar formation (Goritz et al, 2011).
Although, pericytes are important cellular constituents of the BSCB (Bartanusz et al, 2011), their role in BSCB formation, maintenance and/or function is not known. Whether significant variations between brain and spinal cord pericyte populations exist and the functional consequences therein have been largely unexplored. In the present study, we analyzed pericyte populations and changes in barrier properties in multiple brain and spinal cord regions in mice under physiologic conditions and in pericyte-deficient mice.
Materials and methods
Animals
All experimental protocols and animal handling procedures were performed in accordance with the National Institutes of Health (NIH, USA) guidelines for the use of experimental animals and the experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Southern California. Adult 2-month-old male B6SJLF1/J mice devoid of CNS neuropathology were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Adult male 4- to 6-month-old pericyte-deficient PdgfrβF7/F7 mice on a 129S1/SvlmJ background were generated and maintained as described (Tallquist et al, 2003). Adult 4- to 6-month-old nontransgenic 129S1/SvlmJ littermates were used as controls for PdgfrβF7/F7 mice. Adult 13-month-old PdgfrβF7/F7 mice and 13-month-old nontransgenic 129S1/SvlmJ littermates were utilized for neuronal analysis.
Tissue Preparation
Animals were anesthetized with intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine and transcardially perfused with phosphate-buffered saline (PBS) containing 5 mM EDTA (Sigma-Aldrich, St Louis, MO, USA). In a separate set of studies, mice were intravenously injected with 0.1 mL of Alexa Fluor 546-conjugated cadaverine diluted 0.5 μg/μL. Cadaverine was allowed to circulate for 2 hours and then mice were transcardially perfused. The brains were excised from the skull and prepared for subsequent analysis. For spinal cord isolation, mice were decapitated and the vertebral column was dissected. An 18-gauge needle attached to a 3-mL syringe was placed in the sacral opening of the vertebral column and the intact spinal cord was discharged with cold PBS. Once isolated, the brain and spinal cords were either prepared for microvessel isolation (see below), immersion fixed in 4% PFA (paraformaldehyde) (Sigma-Aldrich) overnight at 4°C, or embedded in optimal cutting temperature compound (Tissue-Tek, Torrance, CA, USA). Fixed tissues were sectioned at a thickness of 40 μm with a Leica VT1000S vibrating blade microtome (Leica Instruments, Nussloch, Germany) in preparation for immunofluorescent analysis. Tissues embedded in optimal cutting temperature were cryosectioned at a thickness of 14 μm and subsequently fixed in ice-cold acetone in preparation for immunofluorescent analysis.
Microvessel Isolation
Following tissue removal, microvessels were isolated as we previously described for the brain (Bell et al, 2010; Zlokovic et al, 1993, 2000) and spinal cord (Zhong et al, 2008, 2009). Meninges and large pial vessels were carefully removed and regions of interest including cortex, caudate, hippocampus, cervical, thoracic, and lumbar spinal cord were isolated under a dissecting microscope. White matter was excised and discarded from each region. The spinal cord was bisected and only anterior portions were used for isolation. Identical regions from five individual mice were pooled to maximize yield from each preparation. Tissue was then cut into small pieces with a scalpel (∼1 to 2 mm in size) and homogenized in a Dounce homogenizer containing PBS with 2% FBS (fetal bovine serum) (Invitrogen, Carlsbad, CA, USA). Homogenate was centrifuged at 6000 g for 20 minutes in 18% dextran solution (MW: ∼70,000 Da; Sigma-Aldrich) in PBS containing 2% FBS. Following centrifugation, floating glial and neuronal elements were gently aspirated and the vascular pellet was resuspended in PBS containing 2% FBS. Isolated microvessels were then passed through a 100-μm cell strainer to remove larger vessels and debris. Flow through was collected and then passed through a 40-μm cell strainer to remove red blood cells. Microvessels were trapped on top of cell strainer, which was subsequently inverted and rinsed to collect microvessels in 50 mL conical tube. A small aliquot was visualized at × 10 magnification with a standard light microscope to confirm purity and yield. Microvessel preparations from each region were adhered to glass histology slides using a Cytospin III Cytocentrifuge (Shandon, Pittsburgh, PA, USA) and fixed in 4% PFA for 20 minutes for immunofluorescent analysis. Three independent preparations were utilized for all studies (15 mice in total).
Immunofluorescent and Fluorescent Analysis
Free-floating 40 μm thick PFA-fixed coronal brain and spinal cord sections were initially blocked and permeabilized with 10% swine serum (Vector Laboratories, Burlingame, CA, USA) containing 0.1% Triton X-100 (Sigma-Aldrich). Sections were then incubated in the following primary antibodies: goat α-mouse CD13 (10 μg/mL; R&D Systems, Minneapolis, MN, USA), goat α-mouse platelet-derived growth factor receptor β (PDGFRβ) (10 μg/mL; R&D Systems), mouse α-mouse neuronal-specific antigen A60 (NeuN) (1:100, EMD Millipore, Billerica, MA, USA) or rabbit α-mouse collagen IV (1:200, EMD Millipore). To visualize pericytes, sections were then incubated in bovine anti-goat Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:100 to detect CD13 or PDGFRβ. To visualize endothelial cells, sections were incubated in either donkey anti-rabbit Alexa Fluor 488 (Jackson ImmunoResearch) diluted 1:100 to detect collagen IV or biotin-labeled Lycoperiscon esculentum (tomato) lectin (Vector Labs) diluted 1:100. Sections treated with lectin were then incubated in Dylight 649-conjugated streptavidin (Vector Labs) to detect endothelial-specific fluorescent signal. Nuclei were detected with Hoechst 33342 (Invitrogen) diluted 1:10,000.
In a separate set of studies, 14 μm thick acetone fixed coronal spinal cord sections were blocked with 10% swine serum. Sections were then incubated in the following primary antibodies: goat α-mouse CD13 (10 μg/mL, R&D Systems), mouse α-mouse desmin (1:100, Dako, Carpinteria, CA, USA), rabbit α-mouse collagen IV (1:200, EMD Millipore), rabbit α-human fibrin which cross reacts with mouse fibrin (1:500, Dako), goat α-mouse thrombin (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse α-mouse zonula occludens-1 (ZO-1) (1:50, Invitrogen), mouse α-mouse occludin (1:50, Invitrogen), mouse α-mouse neuronal-specific antigen A60 (NeuN) (1:100, EMD Millipore) and goat anti-mouse ChAT (choline acetyl transferase) (1:50, EMD Millipore). To visualize pericytes, sections were incubated in bovine anti-goat Cy3 or donkey anti-mouse Cy3 (Jackson ImmunoResearch Laboratories) diluted 1:100 to detect CD13 or desmin, respectively. To visualize plasma proteins, sections were incubated with donkey anti-rabbit Cy3 or bovine anti-goat Alexa Fluor 488 (Jackson ImmunoResearch) diluted 1:100 to visualize fibrin and thrombin, respectively. To visualize tight junction proteins, sections were incubated in donkey anti-mouse Alexa Fluor 488 (Jackson ImmunoResearch) diluted 1:100 to visualize occludin and ZO-1 and for collagen IV-positive endothelial cells in donkey anti-rabbit Cy3, Alexa Fluor 488 or DyLight 649. To visualize motor neurons, sections were incubated with donkey anti-mouse Alexa Fluor 488 (Jackson ImmunoResearch) and bovine anti-goat Cy3 (Jackson ImmunoResearch) diluted 1:100 to detect NeuN and ChAT, respectively.
In a separate set of studies, PFA-fixed microvessel preparations were blocked and permeabilized with 10% swine serum containing 0.05% Triton X-100. Microvessels were then incubated in the following primary antibodies: mouse α-mouse ZO-1 (1:50, Invitrogen), rabbit α-mouse occludin (1:100, Invitrogen) or rabbit α-mouse claudin-5 (1:200, Millipore). To visualize tight junctions, microvessels were incubated in donkey anti-mouse Alexa Fluor 488 (Jackson ImmunoResearch) or donkey anti-rabbit Alexa Fluor 488 (Jackson ImmunoResearch) to detect ZO-1 or occludin and claudin-5, respectively. To visualize endothelial cells, sections were incubated in DyLight 594-conjugated L. esculentum (tomato) lectin (Vector Labs) diluted 1:100.
Tissue sections and microvessel preparations were mounted with fluorescent mounting media (Dako) and coverslipped. All slides were scanned with a custom built Zeiss 510 meta confocal laser scanning microscope with a Zeiss Apochromat × 25/0.8 NA water immersion objective (Car Zeiss Microimaging, Thornwood, NY, USA). A 488-nm argon laser was used to excite Alexa Fluor 488 and the emission was collected through a 500 to 550 nm band pass (bp) filter. A 543-nm HeNe laser was used to excite Cy3, Alexa Fluor 546 and DyLight 549 and the emission was collected through a 560 to 615 nm bp filter. A 633-nm HeNe laser was used to excite DyLight 649 and the emission was collected through a 650 to 700 nm bp filter. A 800-nm tuned Ti:sapphire laser (Specra Physics, Irvine, CA, USA) was used to excite Hoechst 33342 and the emission was collected through a 435 to 485 bp filter.
Image Analysis
All image analysis was conducted utilizing NIH ImageJ software. All image analyses were performed by a blinded investigator. For pericyte coverage analysis, CD13-, PDGFRβ-, or desmin-positive surface area was determined by the ImageJ Area measurement tool and divided by the lectin- or collagen IV-positive capillary surface area, respectively, as we previously described (Bell et al, 2010). For pericyte number analysis, PDGFRβ- or CD13-positive perivascular cell bodies located on the abluminal endothelial cell wall that co-localized with Hoechst-positive nuclei were counted using the ImageJ Cell Counter plug-in and expressed per mm2 lectin- or collagen IV-positive capillary surface area, respectively, as previously reported (Bell et al, 2010; Pfister et al, 2008). For tight junction protein analysis, ZO-1-, occludin- or claudin-5-positive immunofluorescent length was determined by the ImageJ Length measurement tool divided by the lectin-positive capillary surface area as we previously described (Bell et al, 2010). Values were expressed as mm tight junction length per mm2 capillary surface area. For motor neuron analysis, NeuN- and ChAT-positive cells were manually counted in the anterior horn of the lumbar spinal cord in six nonadjacent sections (∼100 μm apart) and expressed as motor neurons per lumbar section as previously described (Jaarsma et al, 2008).
For all analyses, a field size of 420 × 420 μm2 was utilized and 10 to 12 μm maximum projection z-stack images were reconstructed. For all studies examining region variability, three randomly selected fields per section from three nonadjacent sections (∼100 μm apart) were analyzed. Six mice were analyzed per region. For studies utilizing Pdgfrβ+/+ and PdgfrβF7/F7 mice, three randomly selected fields per section from three nonadjacent sections (∼100 μm apart) were analyzed. Three to four mice were analyzed per region. For studies utilizing isolated microvessels, a minimum of five randomly selected 420 × 420 μm2 fields were selected per preparation (five mice per preparation). Three to four independent preparations were utilized per parameter.
Noninvasive Fluorometric Permeability Studies
Regional BBB and BSCB permeability to fluorescent tracers of different molecular weights including tetramethylrhodamine (TMR)-dextran (40,000 Da; Invitrogen), TMR-dextran (150,000 Da; Invitrogen) and Fluorescein-mega-dextan (MW: 2,000,000) were determined as we previously described (Bell et al, 2010). Dextrans of multiple molecular weights were individually diluted to a final concentration of 10 mg/mL and injected via the femoral vein at a volume of 0.1 mL. Orbital sinus blood samples were collected before injection and 2, 10, 15, 20, and 30 minutes postinjection. Following 30 minutes of fluorescent tracer circulation, animals were transcardially perfused with PBS containing 5 mM EDTA to remove residual tracer confined within the brain and spinal cord vasculature. Cortex, caudate, hippocampus, cervical, thoracic, and lumbar spinal cord regions were separately dissected. Tissues from each CNS region were individually weighed, homogenized in 10 volumes of 1% Triton X-100 (Sigma-Aldrich), and centrifuged to obtain a supernatant for analysis. Plasma samples were diluted (1:25 to 1:100) and 100 μL of plasma samples and brain homogenates (undiluted) were then analyzed using a Victor fluorometric plate reader (Perkin-Elmer, Waltham, MA, USA). A wavelength of 543 nm and 488 nm was used for excitation and emission was collected at 590 nm and 535 nm for TMR and Fluorescein, respectively.
Calculation of Permeability-Surface Area (PS) Product from Fluorometric Permeability Studies
The PS product (mL per gram per minute) was determined for each tracer in each CNS region by dividing the brain fluorescent intensity values by the integrated plasma fluorescent intensity value over 30 minutes of tracer circulation. The PS product was calculated using equation (1) as we previously described (Mackic et al, 1998).
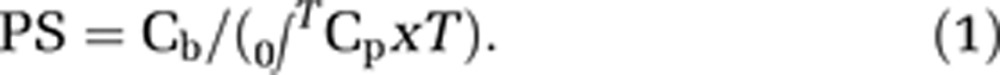 |
Where Cb and 0∫TCp are defined as the fluorescent tracer concentration in the brain (per gram tissue) and integrated plasma concentration, respectively, and T represents experimental time.
Statistical Analysis
All data were analyzed using multifactorial analysis of variance (ANOVA) followed by Tukey posthoc tests. Correlations were determined using Pearson's correlation analysis. A P value <0.05 was considered statistically significant in all studies.
Results
Blood–Spinal Cord Barrier Pericyte Coverage and Number Are Reduced in Comparison to Brain
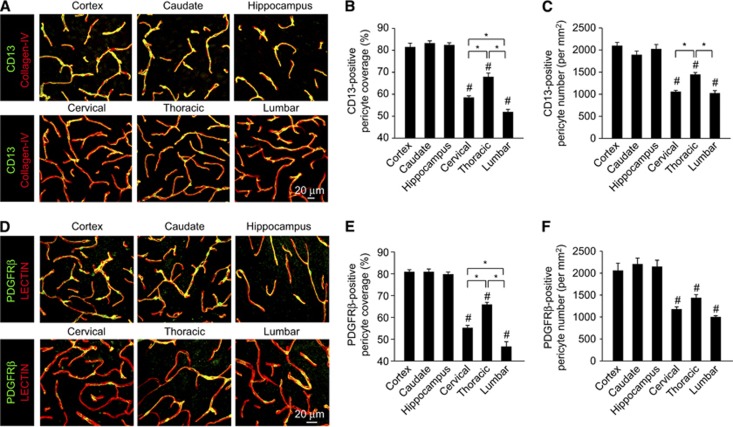
Pericytes represent a heterogenous cell population not covered by a single cell marker that is specific or inclusive (Armulik et al, 2011; Winkler et al, 2011). Therefore, both, Aminopeptidase-N (CD13) (Armulik et al, 2010) and PDGFRβ (Bell et al, 2010; Daneman et al, 2010; Lindahl et al, 1997; Winkler et al, 2010) previously established to be abundantly expressed in CNS pericytes were independently analyzed to determine CNS pericyte populations. Consistent with previous reports, quantification of the percentage of the capillary wall covered by pericyte cell processes positive for CD13 (Figures 1A and 1B) or PDGFRβ (Figures 1D and E) in the brain regions was ∼80% (Armulik et al, 2010; Bell et al, 2010; Daneman et al, 2010; Li et al, 2011) and did not vary in cortex, caudate, and/or hippocampus (Bell et al, 2010). In contrast, CD13-positive and PDGFRβ-positive pericyte coverages were significantly reduced in cervical, thoracic, and lumbar spinal cord anterior horn capillaries covering only 59% to 55%, 68% to 66%, and 52% to 48% of the endothelial cell wall, respectively (Figures 1A, 1B, 1D, and 1E). Next, using previously published methods (Armulik et al, 2010; Bell et al, 2010; Pfister et al, 2008), we evaluated whether there were similar reductions in pericyte number that may contribute to the observed reductions in capillary coverage. Counting CD13-positive (Figure 1C) or PDGFRβ-positive (Figure 1F) pericytes per mm2 capillary area revealed ∼47% to 45%, 28% to 33%, and 49% to 53% reductions in cervical, thoracic, and lumbar anterior horn pericyte number, respectively, when compared with the brain regions.
Figure 1.
Pericyte number and coverage is reduced along blood–spinal cord barrier. (A) Confocal microscopy analysis of CD13-positive pericytes (green) and collagen IV-positive capillary profiles (red) in 2-month-old wild-type mouse cortex, caudate, and hippocampal brain regions and cervical, thoracic, and lumbar spinal cord regions (anterior horns). (B) Quantification of regional CD13-positive pericyte coverage of collagen IV-positive brain and spinal cord capillaries. Mean±s.e.m., n=5 to 7 animals per group. #P<0.05 when compared with the brain regions; *P<0.05. (C) Quantification of regional CD13-positive pericyte cell number normalized to collagen IV-positive capillary surface area in the brain and spinal cord. Mean±s.e.m., n=5 to 7 animals per group. #P<0.05 when compared with the brain regions; *P<0.05. (D) Confocal microscopy analysis of platelet-derived growth factor receptor β (PDGFRβ)-positive pericytes (green) and lectin capillary profiles (red) in 2-month-old wild-type mouse cortex, caudate, and hippocampal brain regions and cervical, thoracic, and lumbar spinal cord regions. (E) Quantification of regional PDGFRβ-positive pericyte coverage of lectin-positive brain and spinal cord capillaries. Mean±s.e.m., n=7 animals per group. #P<0.05 when compared with the brain regions; *P<0.05. (F) Quantification of regional PDGFRβ-positive pericyte cell number normalized to lectin-positive capillary surface area in the brain and spinal cord. Mean±s.e.m., n=7 animals per group. #P<0.05 when compared with the brain regions; *P<0.05.
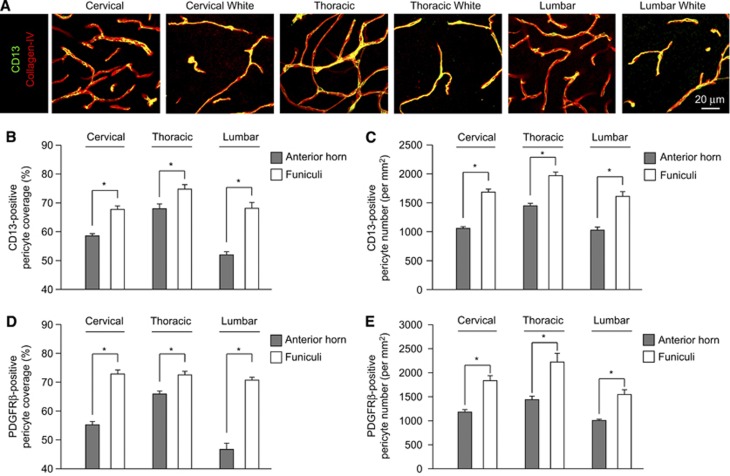
Pericyte coverage and/or populations were not uniformly reduced within the spinal cord regions. For example, CD13-positive pericyte capillary coverage was much higher in cervical, thoracic, and lumbar white matter funiculi as evidenced by capillary coverage values of 68%, 75%, and 68%, respectively (Figures 2A and 2B). Platelet-derived growth factor receptor β-positive pericyte processes showed similarly higher white matter capillary coverage values of 73%, 73%, and 68% in cervical, thoracic, and lumbar funiculi (Figure 2D). CD13- (Figure 2C) and PDGFRβ-positive (Figure 2E) pericyte numbers per mm2 capillary area were significantly higher in white matter regions when compared with gray matter. These data suggest that BSCB pericyte reductions are most pronounced in the spinal cord regions enriched with neuronal cell bodies.
Figure 2.
Subregional variation in spinal cord pericyte number and coverage between anterior horn gray matter and lateral and dorsal funiculi. (A) Confocal microscopy analysis of CD13-positive pericytes (green) and collagen IV-positive capillary profiles (red) in 2-month-old wild-type mouse anterior horn gray matter or dorsal columns for cervical thoracic and lumbar spinal cord. (B) Quantification of CD13-positive pericyte coverage of collagen IV-positive capillaries in anterior horn gray matter (gray) or dorsal and lateral funiculi (white) from cervical, thoracic, and lumbar spinal cord. Mean±s.e.m., n=5 to 7 animals per group; *P<0.05. (C) Quantification of CD13-positive pericyte cell number normalized to lectin-positive capillary surface area in anterior horn gray matter (gray) or dorsal and lateral funiculi (white) from cervical, thoracic, and lumbar spinal cord. Mean±s.e.m., n=5 to 7 animals per group; *P<0.05. (D) Quantification of platelet-derived growth factor receptor β (PDGFRβ)-positive pericyte coverage of lectin-positive capillaries in anterior horn gray matter (gray) or dorsal and lateral funiculi (white) from cervical, thoracic, and lumbar spinal cord. Mean±s.e.m., n=7 animals per group; *P<0.05. (E) Quantification of PDGFRβ-positive pericyte cell number normalized to lectin-positive capillary surface area in anterior horn gray matter (gray) or dorsal and lateral funiculi (white) from cervical, thoracic, and lumbar spinal cord. Mean±s.e.m., n=7 animals per group; *P<0.05.
Reduced Pericyte Coverage is Correlated with Increased Blood–Spinal Cord Barrier Permeability
To determine regional BBB and BSCB permeability in wild-type mice, we first injected the fluorescently conjugated vascular tracer cadaverine (MW: 350 Da). In vessels with tight barrier properties, such as the BBB, no residual tissue and/or intravascular fluorescent signal was observed following perfusion (Figure 3A). In contrast, increased parenchymal fluorescence greatest in anterior horn regions were detected in cervical, thoracic, and lumbar spinal cord following perfusion, suggesting heightened barrier tracer flux (Figure 3A). Importantly, no significant differences in tissue autofluorescence were detected between brain and spinal cord as evidenced by the noninjected control (Figure 3A). Spinal cord accumulation of 40,000 Da and 150,000 Da dextans was not detected by microscopy (data not shown).
Figure 3.
Heightened blood–spinal cord barrier (BSCB) permeability is highly correlated with regional reductions in pericyte coverage. (A) Regional fluorescent detection of intravascularly administered cadaverine (green) in 2-month-old wild-type spinal cord (longitudinal section, but not brain coronal section). Broken line represents outline of tissue section. Left, noninjection control; Right, injected mouse. (B, C) Blood–brain barrier (BBB) and BSCB permeability PS products for tetramethylrhodamine (TMR)-dextran (40,000 Da) (B) and TMR-dextran (150,000 Da) and fluorescein conjugated-mega-dextran (2,000,000 Da) (C) in 2-month-old mouse cortex, caudate, hippocampus, cervical, thoracic, and lumbar spinal cord. Mean±s.e.m., n=6 mice per group for TMR-dextran, n=3 mice per group for mega-dextran. #P<0.05 when compared with the brain regions; *P<0.05. (D, E) Negative correlation between the PS products for TMR-dextran (40,000 Da) (D) and TMR-dextran (150,000 Da) (E) and regional pericyte capillary coverage in 2-month-old wild-type mice. r, Pearson's coefficient.
To quantify the magnitude of vascular leakage and determine the molecular cutoff and size specificity of regional BSCB permeability changes, we next injected individual fluorescently labeled dextrans of different molecular weights and utilized a more sensitive assay to measure residual tissue fluorescence following perfusion as previously established (Bell et al, 2010). Analysis revealed ∼3.8-, 3.1-, and 4.3-fold increases in barrier permeability to 40,000 Da dextran in cervical, thoracic, and lumbar spinal cords, respectively, when compared with the brain regions (Figure 3B). Blood–spinal cord barrier permeability to 150,000 Da was also increased, albeit to a lesser degree, as evidenced by 2.3-, 1.5-, and 2.6-fold increases in PS product in cervical, thoracic, and lumbar spinal cord (Figure 3C). In contrast, there was no detectable leakage of mega-dextran (2,000,000 Da), indicating barely detectable values in all spinal cord and/or brain regions (Figure 3C). Importantly, heightened BSCB permeability to both 40,000 Da (Figure 3D) and 150,000 Da (Figure 3E) showed a significant negative correlation with regional pericyte capillary coverage, suggesting that regional pericyte coverage reductions in the spinal cord are associated with permeability changes.
Increased Blood–Spinal Cord Barrier Barrier Permeability is Associated with Reduced Tight Junction Protein Expression
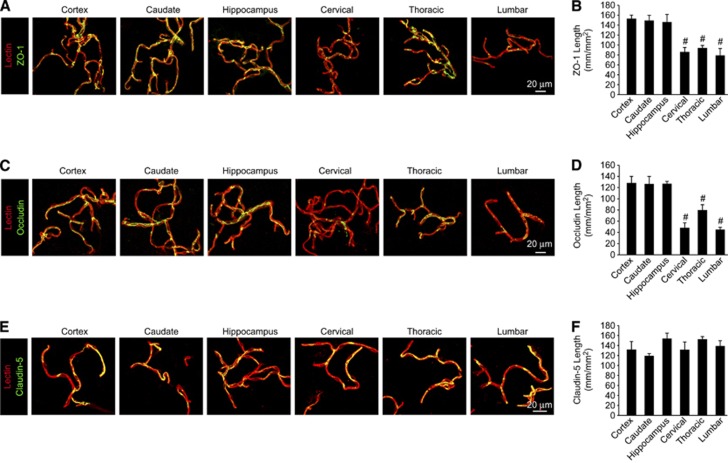
Pericyte reductions have been previously associated with reduced tight junction protein expression and misalignment (Bell et al, 2010; Daneman et al, 2010). To evaluate tight junctional proteins, we next measured endothelial ZO-1, occludin, and claudin-5 tight junctional length in the brain and spinal cord capillaries. Tight junctional length measurements for ZO-1, an important adaptor protein linking transmembrane junctional complexes to the underyling actin cytoskeleton (Zlokovic, 2008, 2011), revealed ∼42%, 37%, and 47% reductions in cervical, thoracic, and lumbar spinal cord capillaries, respectively, when compared with the brain regions (Figures 4A and 4B). Similar analysis revealed even greater 62%, 37%, and 65% reductions in the transmembrane tight junctional protein occludin complexes in cervical, thoracic, and lumbar capillaries, respectively (Figures 4C and 4D). Consistent with a previous report (Ge and Pachter, 2006), no significant changes in the transmembrane tight junctional protein claudin-5 were detected in the spinal cord and brain capillaries (Figures 4E and 4F). Together, these data suggest that BSCB permeability increases might arise from increased paracellular flow through diminished tight junctional protein complexes in BSCB regions with reduced pericyte coverage.
Figure 4.
Reduced pericyte coverage along blood–spinal cord barrier is associated with reduced tight junction protein expression. (A, C, E) Confocal microscopy analysis of tight junction zonula occludens-1 (ZO-1) (A), occludin (C), or claudin-5 (E) immunodetection (green) in acutely isolated lectin-positive capillaries (red) from 2-month-old mouse cortex, caudate, hippocampus, cervical, thoracic, and lumbar spinal cord. (B, D, F) Quantification of endothelial ZO-1 (B), occludin (D), or claudin-5 (F) tight junctional length in acutely isolated vessel preparations from the brain and spinal cord regions. Mean±s.e.m. n=3 to 4 preparations per group (five mice per preparation); #P<0.05 when compared with the brain regions.
Pericyte Deficiency Exacerbates Spinal Cord Capillary Leakage and Tight Junctional Abnormalities
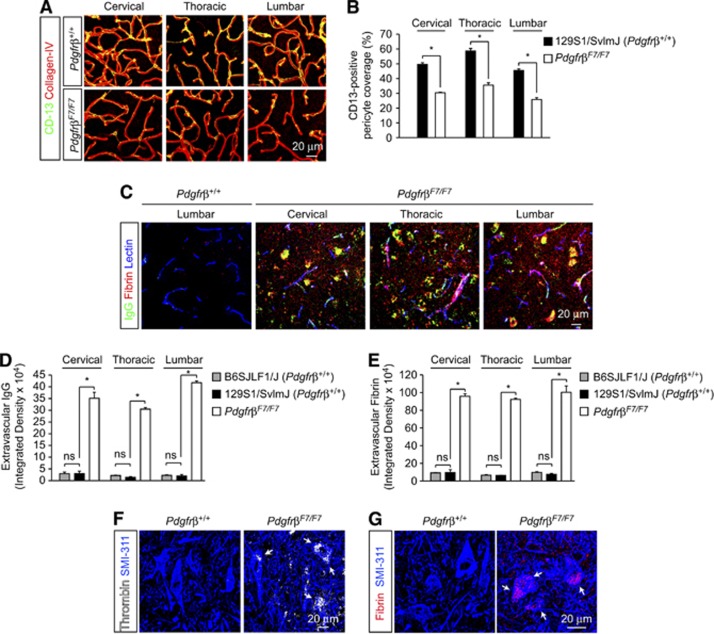
To more directly study the roles of pericytes in BSCB function, we next analyzed permeability and structural changes in well-characterized pericyte-deficient PdgfrβF7/F7 mice (Bell et al, 2010; Tallquist et al, 2003). Quantitative immunofluorescent analysis revealed 39%, 39%, and 43% reductions in CD13-positive pericyte capillary coverage in cervical, thoracic, and lumbar spinal cord capillaries, respectively (Figures 5A and 5B). Similar 40%, 35%, and 43% reductions in desmin-positive pericyte capillary coverage were observed in cervical, thoracic, and lumbar spinal cord (data not shown), thereby confirming that BSCB pericyte populations are further reduced in PdgfrβF7/F7 mutants and may serve as a valuable model to deduce BSCB pericyte function.
Figure 5.
Exacerbation of pericyte deficiency leads to overt blood–spinal cord barrier disruption and leakage of endogenous plasma proteins. (A) Confocal microscopy analysis of CD13-positive pericytes (green) and collagen IV-positive capillary profiles (red) in 6-month-old Pdgfrβ+/+ and PdgfrβF7/F7 mouse cervical, thoracic, and lumbar spinal cord anterior horn. (B) Quantification of regional CD13-positive pericyte coverage of collagen IV-positive anterior horn spinal cord capillaries. Mean±s.e.m., n=3 animals per group; *P<0.05. (C) Representative confocal microscopy analysis of IgG (green), fibrin (red), and lectin-positive capillaries (blue) in 6-month-old Pdgfrβ+/+ mouse lumbar anterior horn and anterior horn from PdgfrβF7/F7 cervical, thoracic, and lumbar spinal cord. (D, E) Quantification of IgG (D) and fibrin (E) extravascular deposits in the spinal cord regions in tissue sections from 2-month-old B6SJL/F1 Pdgfrβ+/+, 6-month-old Pdgfrβ+/+ 129S1/SvlmJ, and 6-month-old PdgfrβF7/F7 mice. Mean±s.e.m., n=3 animals per group; *P<0.05. (F, G) Confocal microscopy analysis of thrombin (white) (F) or fibrin (red) (G) and SMI-311-positive neurons (blue) in 6-month-old Pdgfrβ+/+ and PdgfrβF7/F7 lumbar spinal cord. Arrows indicate neuronal accumulation.
We next evaluated whether BSCB pericyte deficiency directly exacerbates capillary leakage and motor neuron accumulation of the endogenous plasma proteins IgG and Fibrin (Figure 5C). Quantification of extravascular IgG (MW: 150,000 Da) and fibrin (MW: 53,000 to 73,000 Da) demonstrated significant increases in spinal cord accumulation in cervical, thoracic, and lumbar regions of PdgfrβF7/F7 mice (Figures 5D and 5E). No strain-specific differences in deposition of plasma-derived IgG (Figure 5D) or fibrin (Figure 5E) were detected between nontransgenic mouse lines. Increased spinal barrier disruption led to neuronal accumulation of several potentially neurotoxic plasma proteins including thrombin (MW: 36,000 Da) (Figure 5F) and fibrin (Figure 5G) in lumbar spinal cord of PdgfrβF7/F7 mice. Similar results were obtained in cervical and thoracic spinal cord (data not shown). In contrast, plasma proteins were not seen to accumulate in the spinal cord of nontransgenic mice (Figures 5F and 5G). These data suggest that increased BSCB disruption caused by an additional pericyte loss in PdgfrβF7/F7 mice is required to render motor neurons vulnerable to accumulation of larger molecular weight and potentially harmful plasma proteins.
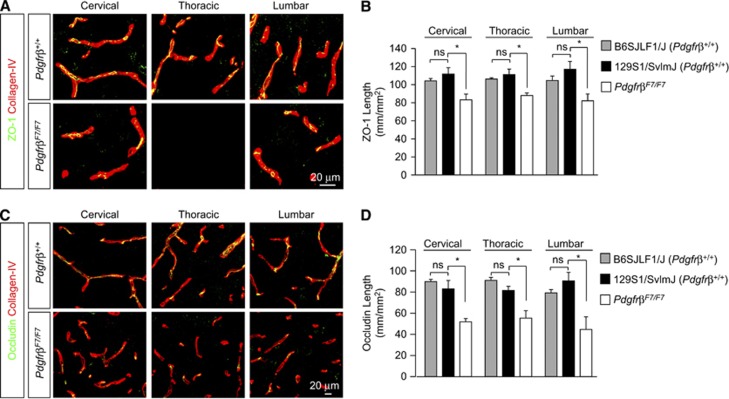
To further deduce whether pericyte-associated BSCB permeability deficits were paracellular in character, we utilized similar immunofluorescent analysis of tight junctional complexes in PdgfrβF7/F7 mice as we did in nontransgenic mice. Analysis of ZO-1 tight junctional length revealed further 26%, 21%, and 30% reductions in junctional length in the anterior horn of cervical, thoracic, and lumbar spinal cord from PdgfrβF7/F7mice when compared with age-matched nontransgenic mice (Figures 6A and 6B). Similar analysis revealed 34%, 39%, and 51% reductions in occludin (Figures 6C and 6D) junctional length in anterior horn cervical, thoracic, and lumbar PdgfrβF7/F7 mouse spinal cord. No strain-specific differences were detected in ZO-1 (Figure 6B) or occludin (Figure 6D) tight junctional length between nontransgenic mouse lines.
Figure 6.
Pericyte deficiency reduces blood–spinal cord barrier (BSCB) tight junction protein expression. (A, C) Confocal microscopy analysis of tight junction zonula occludens-1 (ZO-1) (A) and occludin (C) immunodetection (green) in anterior horn from 6-month-old Pdgfrβ+/+ 129S1/SvlmJ and PdgfrβF7/F7 mouse cervical, thoracic, and lumbar spinal cord. (B, D) Quantification of endothelial ZO-1 (B) or occludin (D) tight junctional length in spinal cord anterior horn regions in tissue sections from 2-month-old B6SJL/F1 Pdgfrβ+/+, 6-month-old Pdgfrβ+/+ 129S1/SvlmJ and 6-month-old PdgfrβF7/F7 mice. Mean±s.e.m. n=3 to 4 preparations per group (five mice per preparation); *P<0.05.
Pericyte Deficiency Contributes to Motor Neuron Loss in the Spinal Cord
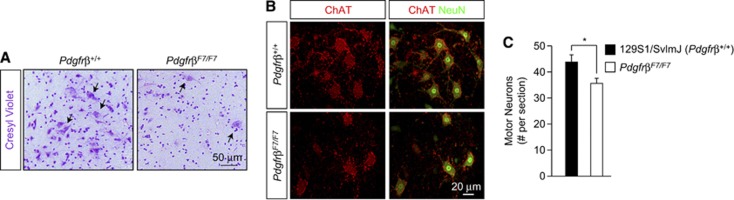
Previously, pericyte reduction and/or dysfunction and plasma protein leakage has been shown to contribute to neurodegenerative changes in the brain (Bell et al, 2010, 2012; Winkler et al, 2011). To deduce whether similar changes may contribute to motor neuron injury in the spinal cord, we first analyzed gross structural changes in the lumbar spinal cord of 13-month-old PdgfrβF7/F7 mice and age-matched nontransgenic littermates. Bright-field microscopy analysis demonstrated reduced cellularity, especially in large cells morphologically consistent with motor neurons, in the lumbar spinal cord anterior horn of PdgfrβF7/F7 mice when compared with nontransgenic littermates (Figure 7A). To confirm motor neuron loss, we utilized an established technique to quantitate motor neurons (Jaarsma et al, 2008). Analysis revealed approximately a 19% reduction in the number of motor neurons in the lumbar spinal cord anterior horn in PdgfrβF7/F7mice when compared with nontransgenic littermates (Figures 7B and 7C).
Figure 7.
Pericyte deficiency leads to spinal cord structural abnormalities and motor neuron loss. (A) Bright-field microscopy analysis of cresyl violet staining (purple) in the lumbar spinal cord anterior horn in a 13-month-old Pdgfrβ+/+ and PdgfrβF7/F7 mouse. Arrows, cells morphologically consistent with motor neurons. (B) Representative confocal microscopy analysis of NeuN-positive (green) and choline acetyl transferase (ChAT)-positive motor neurons (red) in the 13-month-old Pdgfrβ+/+ and PdgfrβF7/F7 lumbar spinal cord anterior horn. (C) Quantification of motor neurons per section in Pdgfrβ+/+ and PdgfrβF7/F7 lumbar spinal cord anterior horn. Mean±s.e.m. n=3 mice per group; *P<0.05.
Discussion
Our findings suggest that as in the brain (Armulik et al, 2010; Bell et al, 2010; Daneman et al, 2010; Li et al, 2011) and retina (Kim et al, 2009), pericytes contribute to vascular barrier properties in the spinal cord. Unlike the relative uniformity of pericyte capillary coverage and cell number in the brain regions (Bell et al, 2010), pericyte coverage and number are reduced in anterior horn spinal cord capillaries in wild-type mice. Consistent with previous reports (Daniel et al, 1985; Pan et al, 1997a, 1997b; Prockop et al, 1995), we found increased spinal cord capillary permeability most pronounced in the cervical and lumbar regions to multiple tracers ≤150,000 Da, which correlated with reductions in pericyte coverage. The present study also shows that deficient PDGFRβ signaling resulting from genetic mutations of cytoplasmic signal transduction domains in PdgfrβF7/F7 mice (Tallquist et al, 2003) leads to gross reductions of spinal cord pericyte populations.
Previous work with pericyte-deficient mice identified three potential mechanisms by which pericytes induce endothelial barrier properties: (1) reduction in vesicular transport and bulk flow transcytosis (Armulik et al, 2010; Daneman et al, 2010), (2) proper tight junctional alignment (Daneman et al, 2010), and (3) promotion of endothelial tight junction expression (Bell et al, 2010). Our findings suggest not only that increased BSCB permeability and regional pericyte reductions in the anterior horn of the spinal cord coincide with reductions in tight junction protein expression (i.e., ZO-1 and occludin), consistent with an increased paracellular flow model. However, further experimental elaboration is required utilizing mouse models in which pericyte deficiency may be rescued to definitely establish a causative relationship between pericytes, endothelial permeability, and the underlying molecular coupling.
Overt vascular barrier disruption in the CNS results in parenchymal accumulation of multiple circulating plasma-derived proteins in neurons (Bell et al, 2010; Winkler et al, 2011; Zhong et al, 2008; Zlokovic, 2011). Many plasma-derived products, including thrombin, fibrin, plasmin and hemoglobin, are neurotoxic and/or vasculotoxic at higher concentrations in the CNS (Bell et al, 2010; Chen and Strickland, 1997; Mhatre et al, 2004; Paul et al, 2007; Zhong et al, 2008). In the present report, pericyte reductions in PdgfrβF7/F7 mice correlated with increased BSCB permeability allowing larger toxic plasma proteins such as thrombin (Mhatre et al, 2004) and fibrin (Paul et al, 2007) to accumulate in motor neurons. In contrast, much less pronounced basal increases in BSCB permeability under physiological conditions in the spinal cord of wild-type mice, that is, a PS product for 40,000 Da dextran in the spinal cord under physiological conditions was nearly 20-fold lower than in PdgfrβF7/F7 mice (Bell et al, 2010), did not result in accumulation of larger toxic endogenous macromolecules in motor neurons in either of the nontransgenic mouse strains studied. It is of note, pericytes have been demonstrated to phagocytose multiple endogenous proteins and provide an important clearance pathway for extravasated plasma components (Bell et al, 2010; Diaz-Flores et al, 2009). Therefore, chronic deposition of endogenous plasma-derived proteins in F7 pericyte-deficient mice likely reflect a combination of an increased influx following heightened barrier disruption and reduced pericyte-mediated clearance of these plasma proteins. In contrast, metabolically inert polar exogenous tracers, that is, dextrans, are not taken up by CNS vascular cells in vitro (Weksler et al, 2005; Zhu et al, 2010) or in vivo (Bell et al, 2010, 2012), and their detection in the CNS following acute injection reflects mainly barrier disruption in isolation of cellular clearance. It is of note, the PS products in the brain regions (e.g., cortex) obtained in B6SJLF1/J mice in the present study for both 40,000 and 150,000 Da dextran are virtually identical to values obtained in 129S1/SvlmJ wt mice cortices, as we reported (Bell et al, 2010), further supporting that changes in BSCB permeability are not strain-specific, but could be molecule-specific.
Blood–spinal cord barrier disruption has been implicated early in multiple spinal cord disease processes including amyotrophic lateral sclerosis (Garbuzova-Davis et al, 2011; Zhong et al, 2008, 2009), multiple sclerosis, acute spinal cord injury, and several others (Bartanusz et al, 2011). Whether BSCB disruption contributes to spinal neurodegenerative changes is not known. Consistent with previous reports in the brain (Bell et al, 2010, 2012), our data showing a loss of motor neurons in the lumbar spinal cord suggests that pericyte deficiency and/or breakdown of BSCB may contribute to motor neuron injury. Future studies are needed, however, utilizing inducible models of pericyte deficiency and/or genetic models to rescue pericyte deficiency to better establish the causative relationship between spinal cord microvascular changes and motor neuron injury. At the present time, the mechanisms of motor neuron cell death or whether neuronal dysfunction precedes cellular loss in pericyte-deficient mice are not known and should be addressed in future studies. Proper characterization of spinal cord pericyte populations in human spinal cord diseases may hold considerable promise to better elucidate the interface of vascular dysfunction in spinal cord neurodegeneration.
The authors declare no conflict of interest.
Footnotes
This research was supported by the ALS Association (Grant 1859) and the NIH Grant AG039452 to BVZ.
References
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare A, Singh I, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk B, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PM, Lam DK, Pratt OE. Comparison of the vascular permeability of the brain and the spinal cord to mannitol and inulin in rats. J Neurochem. 1985;45:647–649. doi: 10.1111/j.1471-4159.1985.tb04038.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histo Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR. Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res. 2011;1398:113–125. doi: 10.1016/j.brainres.2011.04.049. [DOI] [PubMed] [Google Scholar]
- Ge S, Pachter JS. Isolation and culture of microvascular endothelial cells from murine spinal cord. J Neuroimmunol. 2006;177:209–214. doi: 10.1016/j.jneuroim.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009;87:653–659. doi: 10.1002/jnr.21884. [DOI] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Mackic JB, Weiss MH, Miao W, Kirkman E, Ghiso J, Calero M, Bading J, Frangione B, Zlokovic BV. Cerebrovascular accumulation and increased blood-brain barrier permeability to circulating Alzheimer's amyloid beta peptide in aged squirrel monkey with cerebral amyloid angiopathy. J Neurochem. 1998;70:210–215. doi: 10.1046/j.1471-4159.1998.70010210.x. [DOI] [PubMed] [Google Scholar]
- Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–248. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997a;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol. 1997b;146:367–373. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57:2495–2502. doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop LD, Naidu KA, Binard JE, Ransohoff J. Selective permeability of [3H]-D-mannitol and [14C]-carboxyl-inulin across the blood-brain barrier and blood-spinal cord barrier in the rabbit. J Spinal Cord Med. 1995;18:221–226. doi: 10.1080/10790268.1995.11719399. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Apuzzo ML. Cellular and molecular neurosurgery: pathways from concept to reality—part I: target disorders and concept approaches to gene therapy of the central nervous system. Neurosurgery. 1997;40:789–803. doi: 10.1097/00006123-199704000-00027. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–1441. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Mackic JB, Wang L, McComb JG, McDonough A. Differential expression of Na,K-ATPase alpha and beta subunit isoforms at the blood-brain barrier and the choroid plexus. J Biol Chem. 1993;268:8019–8025. [PubMed] [Google Scholar]