Abstract
Inflammatory cytokines may mediate hypoxic-ischemic (HI) injury and offer insights into the severity of injury and the timing of recovery. In our randomized, multicenter trial of hypothermia, we analyzed the temporal relationship of serum cytokine levels in neonates with hypoxic-ischemic encephalopathy (HIE) with neurodevelopmental outcome at 12 months. Serum cytokines were measured every 12 hours for 4 days in 28 hypothermic (H) and 22 normothermic (N) neonates with HIE. Monocyte chemotactic protein-1 (MCP-1) and interleukins (IL)-6, IL-8, and IL-10 were significantly higher in the H group. Elevated IL-6 and MCP-1 within 9 hours after birth and low macrophage inflammatory protein 1a (MIP-1a) at 60 to 70 hours of age were associated with death or severely abnormal neurodevelopment at 12 months of age. However, IL-6, IL-8, and MCP-1 showed a biphasic pattern in the H group, with early and delayed peaks. In H neonates with better outcomes, uniform down modulation of IL-6, IL-8, and IL-10 from their peak levels at 24 hours to their nadir at 36 hours was observed. Modulation of serum cytokines after HI injury may be another mechanism of improved outcomes in neonates treated with induced hypothermia.
Keywords: chemokines, cytokines, hypoxic-ischemic brain injury, induced hypothermia
Introduction
Hypoxic-ischemic (HI) injury induces release of cytokines and chemokines, which amplify inflammatory cascades and recruit neutrophils and monocytes to sites of injury. Serum inflammatory proteins are readily measurable and may be useful biomarkers of phases of injury. Combinations of cytokines are beginning to be used as early, discriminating predictors of severe traumatic brain injury and multiorgan system failure in adults and children (Bogner et al, 2009; Berger et al, 2008; Kirchoff et al, 2008; Jastrow et al, 2009). In neonates with hypoxic-ischemic encephalopathy (HIE), elevated interleukin (IL)-6 and IL-8 in serum and cerebrospinal fluid have been associated with high lactate/choline ratios by magnetic resonance spectroscopy and more severe neurologic outcomes (Bartha et al, 2004; Savman et al, 1998; Nelson et al, 1998; Martin-Ancel et al, 1997).
Although IL-6, macrophage inflammatory protein 1a (MIP-1a), and other inflammatory cytokines are traditionally viewed as augmenting injury, a growing body of evidence from animal and human studies describes a dual role for these mediators in injury and repair after stroke (Matsuda et al, 1996; Loddick et al, 1998; Suzuki et al, 2009). This duality may add to their potential usefulness as biomarkers, if different peaks are indicative of different phases of recovery. However, the natural time course and hypothermia's effect on serum cytokines after HI injury need to be elucidated before such markers can be developed for neonatal HIE.
Hypothermia is reported to have variable effects on inflammatory cytokines, depending on site, timing of measurement, and the presence of infection (Yanagawa et al, 2002; Diestel et al, 2008; Xiong et al, 2009). In a lipopolysaccharide pediatric mouse model, hypothermia increased IL-6 and IL-10 within 6 hours (Stewart et al, 2009). Additionally, IL-6 and IL-8 in cerebrospinal fluid increased within 18 to 24 hours in hypothermic pediatric traumatic brain injury and adult cardiac arrest patients compared with normothermia patients (Buttram et al, 2007; Fries et al, 2009).
In our multicenter trial of induced systemic hypothermia in neonatal HIE, we investigated the effects of time and hypothermia on serum cytokine levels as a secondary outcome. We postulated a priori that proinflammatory IL-6 and antiinflammatory IL-10 at enrollment would predict outcome. This is the first report of a time course of circulating inflammatory mediators in neonates with HIE and with and without systemic hypothermia.
Materials and methods
This analysis was performed as a secondary outcome of a multicenter randomized, controlled phase II trial of systemic hypothermia of 33°C for 48 hours in neonates with evidence of HIE (Eicher et al, 2005a, 2005b). Thirty-two patients were enrolled and randomized to hypothermia (H) and thirty-three to normothermia (N) groups. Newborns qualified for the study if they were ⩾35 weeks gestation, >2,000 g birth weight, and ⩽6 hours after birth or HI insult. Consent was obtained before study enrollment, using a consent form approved by the local Institutional Review Board at each site. Medical University of South Carolina's Institutional Review Board and the local institutional review boards at each of the six sites ensured human subjects' protection and performed regulatory oversight of the study. Our entry criteria have been published along with safety and clinical outcomes. Exclusion criteria included maternal chorioamnionitis, sepsis at birth and either birth weight or head circumference <10th percentile for gestational age. An adverse neurodevelopmental outcome was defined as death or severe motor disability at 12 months of age (Bayley II psychomotor developmental indices ⩽70). One milliliter of blood for serum cytokines was collected via umbilical catheter within 3 hours of enrollment, then every 12 hours for 72 hours. Time of blood draw after enrollment and time after birth, in parentheses, were 0 hours (0 to 8 hours), 12 hours (11 to 21 hours), 24 hours (22 to 33 hours), 36 hours (34 to 45 hours), 48 hours (46 to 56 hours), 60 hours (57 to 69 hours), and 72 hours (70 to 81 hours). Blood drawn 0 to 48 hours after enrollment was obtained during hypothermia, and all subjects were normothermic at the 60- to 72-hour draws. Serum was separated, transported on dry ice, and frozen at −80°C until analysis. If thawed on arrival, then samples were not used for analysis.
For multiplex cytokine assays, 25 μL of serum was prepared according to manufacturer's protocol for multiplexed fluorescent bead immunoassays (Beadlyte; Upstate Biotechnology, Waltham, MA, USA). Samples were analyzed in duplicate on BioPlex platform (BioRad, Luminex Technology, Hercules, CA, USA). Values were reported as median fluorescent activity. Concentrations were then extrapolated from an 8-point standard curve for each cytokine using either a 4 or 5 parameter logistic fit using the Bioplex software, and reported as picograms per milliliter (pg/mL). Lower limits of assays were between 0.5 and 6 pg/mL, depending on the cytokine. Cross-reactivities of both reporter and antibody standards using this kit have been quantified for these Upstate multiplex kits and are minimal for all cytokines analyzed. Levels of IL-1b, 2, 6, 8, 10, 12, 13, tumor necrosis factor tumor necrosis factor α, MIP-1a, monocyte chemotactic protein-1 (MCP-1), interferon γ, and interferon inducible protein 10 were measured in a 1:2 dilution.
Data Analysis
Mean concentration of replicate experiments was calculated for each sample, and the database was constructed with double data entry verification for over 3,000 cytokine values. Three patients placed on extracorporeal membrane oxygenation after enrollment 3 (N), and 2 (N) patients with positive initial blood cultures were not included in the analysis, as both conditions will induce their own cytokine storm. Samples were also missing due to improper transport, nursing error, or early death. Serum cytokine values were analyzed for 21 to 28 subjects (H) and 15 to 22 subjects (N) at each time point.
We used median values for nonparametric data, and logarithmic transformations were performed on cytokine values before statistical analysis. The independent relationship of hypothermia and time was assessed using multivariate, mixed effects models based on log-transformed values. Parametric tests (Analysis of Variance) were performed in N and H groups over time. Kruskal–Wallis test determined time points that there were significantly different between groups. For prediction of good or adverse outcome, a Mann–Whitney test was performed on median log-transformed cytokine values. We performed Spearman's rank correlation on non-log-transformed cytokine values and actual patient temperatures at enrollment to determine the relationship between temperature and cytokine value. Chi-squared was performed for discriminant analysis of temperature at enrollment predicting outcome. We did not correct for multiple comparisons in these exploratory analyses. We note specific P values when P<0.1 (SAS version 9.2, SAS Institute, Inc., Cary, NC, USA).
Results
Cytokines at Enrollment
Demographics of the 50 patients (28 H, 22 N) and outcomes for the patients were similar for baseline and clinically prognostic variables (Table 1). Death with multiorgan failure was also similar between treatment groups (4 H and 3 N) and includes those who were withdrawn from support with multiorgan failure.
Table 1. Demographics.
| Hypothermia | Normothermia | Total | |
|---|---|---|---|
| n=28 | n=22 | n=50 | |
| Gender | |||
| Male | 16 | 11 | 27 (54%) |
| Female | 12 | 11 | 23 (46%) |
| Ethnic group | |||
| Caucasian | 15 | 12 | 27 (54%) |
| African-American | 11 | 8 | 19 (38%) |
| Other | 2 | 2 | 4 (8%) |
| Entry strata | |||
| Inborn @ level III | 6 | 7 | 13 (26%) |
| Outborn (transported) | 22 | 15 | 37 (74%) |
| Gestational age | 38.7±1.9 weeks | 39.2±1.3 weeks | P=0.3 |
| Birth weight | 3,222±757 g | 3,571±768 g | P=0.1 |
| Clinical status at enrollment | |||
| Chest compressions | 19 | 14 | 33 (66%) |
| Mean cord pH | 6.95±0.19 | 6.97±0.23 | P=0.8 |
| Mean cord base deficit | –18±8.3 | –16±8.4 | P=0.7 |
| Median Apgar @ 1 minute | 1 | 1 | P=0.3 |
| Median Apgar @ 5 minutes | 2 | 2 | P=0.7 |
| Median Apgar @ 10 minutes | 4 | 3 | P=0.6 |
| Sarnat stage III | 24 | 16 | 40 (82%) |
| Sarnat stage II | 4 | 4 | 8 (16%) |
| Outcome status at 1 year of age | |||
| Expired | 9 | 8 | 17 (34%) |
| With multiorgan failure | 4 | 3 | |
| Withdrawal of support | 8 | 6 | |
| Severe impairment | 5 | 5 | 10 (20%) |
| Mild/moderate impairment | 13 | 4 | 17 (34%) |
| Survived but lost to follow-up | 1 | 5 | 6 (12%) |
Hypothermia treatment in our trial was initiated immediately and on transport from outlying hospitals. Most hypothermia patients were already cooled for several hours by the time of the first blood draw at the tertiary care center, and enrollment cytokine levels could potentially show a treatment effect. We found that median serum cytokine values and interquartile ranges (IQR) at enrollment were similar between groups, with the exception of IL-8, which was significantly higher in the H group (Table 2). To clarify whether the differences in initial cytokine values were directly related to the rectal temperature very soon after hypothermia was initiated, we performed Spearman's rank correlation between rectal temperatures at the time of the initial sample, and non-transformed enrollment cytokine levels. For IL-1b, IL-6, IL-10, IL-12, MCP-1, MIP-1a, and interferon inducible protein 10 there were no significant correlations with enrollment temperature. However, IL-8 showed a trend to association with P=0.075. Taken with the significant difference between treatment groups for IL-8 at enrollment, these data could indicate an early effect of treatment on IL-8 levels.
Table 2. Median and IQR (pg/mL) for serum cytokine concentrations at enrollment.
| Hypothermia | Normothermia | P value | |
|---|---|---|---|
| IL-1b | 0.6 (0.4 to 1.3) | 0.6 (0.4 to 2.3) | NS |
| IL-6 | 122.0 (40.5 to 987.5) | 44.6 (13.6 to 471.3) | NS |
| IL-8 | 65.3 (17.5 to 158.8) | 25.9 (6.4 to 69.1) | 0.028 |
| IL-10 | 180.9 (38.5 to 401.4) | 68.4 (10.9 to 158.7) | NS |
| IL-12 | 1.1 (0.5 to 4.9) | 1.3 (0.5 to 4.9) | NS |
| MCP-1 | 169.8 (14.8 to 549.6) | 20.4 (2.4 to 311.7) | 0.067 |
| IP-10 | 1,678 (281 to 3,232) | 378 (227 to 3,434) | NS |
| MIP-1a | 13.4 (11.7 to 38.9) | 12.7 (12.7 to 35.9) | NS |
IL, interleukin; IP-10, interferon inducible protein 10; IQR, interquartile range; MCP-1, monocyte chemotactic protein-1; MIP-1a, macrophage inflammatory protein 1a.
Hypothermia and Time had Significant Effects on Cytokine Levels, But Gender Did Not
Levels of IL-6, IL-8, IL-10, and MCP-1 showed significant independent effects of time and H treatment (IL-6 P<0.0001, IL-8 P<0.007, IL-10 P<0.0001, MCP-1 P<0.05 for both H and time). MIP-1a showed no significant effects of H treatment, but MIP-1a levels tended to be different over time (P=0.06). IL-1b, IL-12, and interferon inducible protein 10 values showed no significant effect of time or hypothermia treatment on serum levels. Gender did not have a significant independent effect on any cytokine in our study.
Induced Hypothermia Increases Serum Cytokines in Neonatal Hypoxic-Ischemic Encephalopathy Compared with Normothermic Subjects
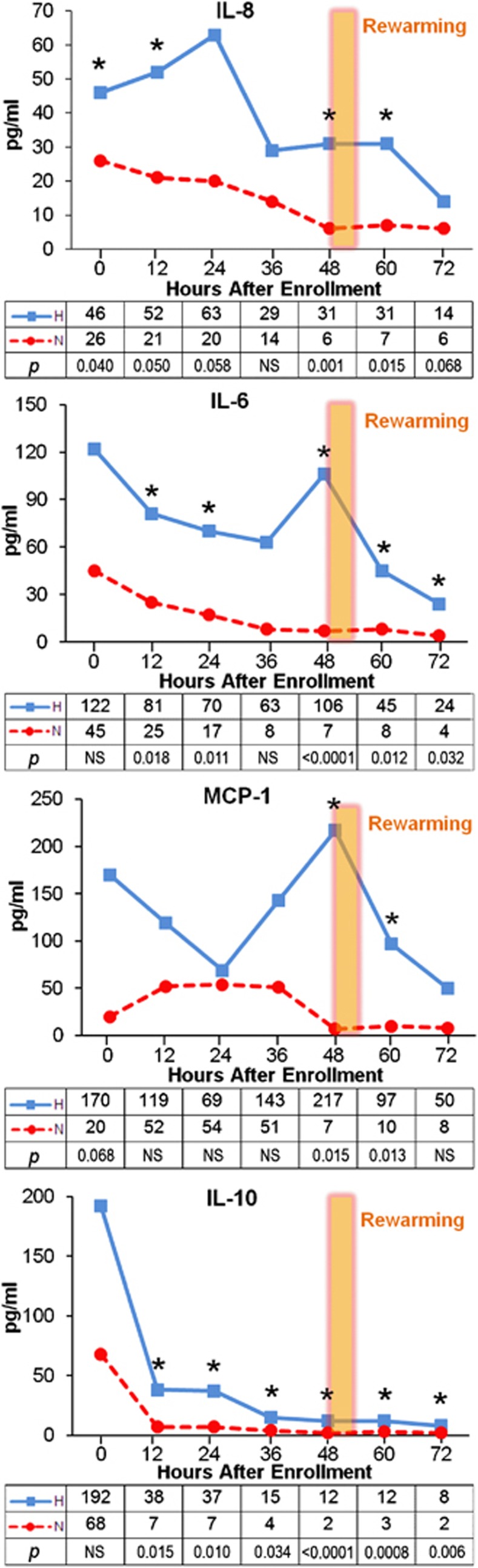
Median levels of IL-8, IL-6, IL-10, and MCP-1 are significantly higher in the H group than in the N group at most time points over the 80 hours after birth, and the pattern of serum levels over time is notably different between H and N groups (Figure 1). Interleukin-8, IL-6, and MCP-1 have early peaks (22 to 33 hours) and either a second peak or an elevated plateau (46 to 56 hours after birth) only in the H group. Since these samples were obtained before rewarming, this second peak cannot be due to the rewarming process itself. Both pro-inflammatory and anti-inflammatory serum cytokines are elevated with systemic H treatment, and IL-6, IL-8, and MCP-1 have unique, biphasic expression patterns in the H neonates.
Figure 1.
Median serum interleukin (IL)-8, IL-6, monocyte chemotactic protein-1 (MCP-1), IL-10 concentrations over time by treatment group, with median (pg/mL) and P values for each time point noted. *indicates P values <0.05.
High Interleukin-6 and Monocyte Chemotactic Protein-1 at Enrollment Predict Poor Outcome
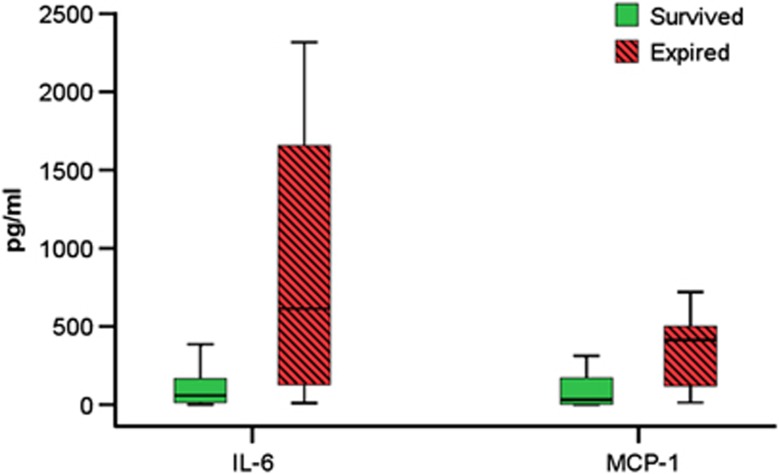
Levels of IL-6 and MCP-1 at enrollment were significantly elevated in subjects who later succumbed regardless of treatment (n=29 survivors and 15 non-survivors; Figure 2). Median IL-6 for surviving subjects was 58 pg/mL (IQR 13 to 217 pg/mL) versus 613 pg/mL in non-survivors (IQR 115 to 1,662 pg/mL, P=0.0023). Median MCP-1 was 31 pg/mL (IQR 1.6 to 172 pg/mL) in survivors versus 414 pg/mL (IQR 39 to 506 pg/mL) in non-survivors (P=0.0063).
Figure 2.
Box plot of medians and interquartile ranges of serum interleukin (IL)-6, and monocyte chemotactic protein (MCP)-1 concentrations at enrollment by survival regardless of treatment group (IL-6, P=0.0023, MCP-1 P=0.0063).
Receiver operating characteristic curve analyses generated independent cutoff values of 103 pg/mL for IL-6 and 187 pg/mL for MCP-1 within 0 to 9 hours after birth, with higher values predicting death with 81% sensitivity, 62% specificity for IL-6, and 73% sensitivity, 79% specificity for MCP-1. In a logistic regression model, if either MCP-1>187 pg/mL or IL-6>103 pg/mL, then death was correctly predicted in 14 out of 15 non-surviving subjects (94%, P<0.01). If either IL-6 or MCP-1 was lower than their cutoff values, then survival was correctly predicted in 25 out of 29 of those that survived (86%, P<0.003).
As noted by other investigations in normothermic HI injury, serum IL-6 levels that are extremely elevated very early after HI indicate patients with more severe injury (Martin-Ancel et al, 1997; Fassbender et al, 1994). In our trial, hypothermia subjects' serum IL-6 levels at enrollment tended to predict severely abnormal motor scores at 12 months or death, with odds ratio=1.582, and 95% confidence interval=0.95 to 2.636 (P=0.05; n=12 good outcome, 13 adverse outcome). The median serum IL-6 concentration at enrollment in H patients with adverse outcome was 205 pg/mL (110 to 1,669 pg/mL IQR), compared with 94 pg/mL (32 to 250 pg/mL IQR) in the H subjects with a better neurologic outcome.
Cytokine Profiles by Outcome
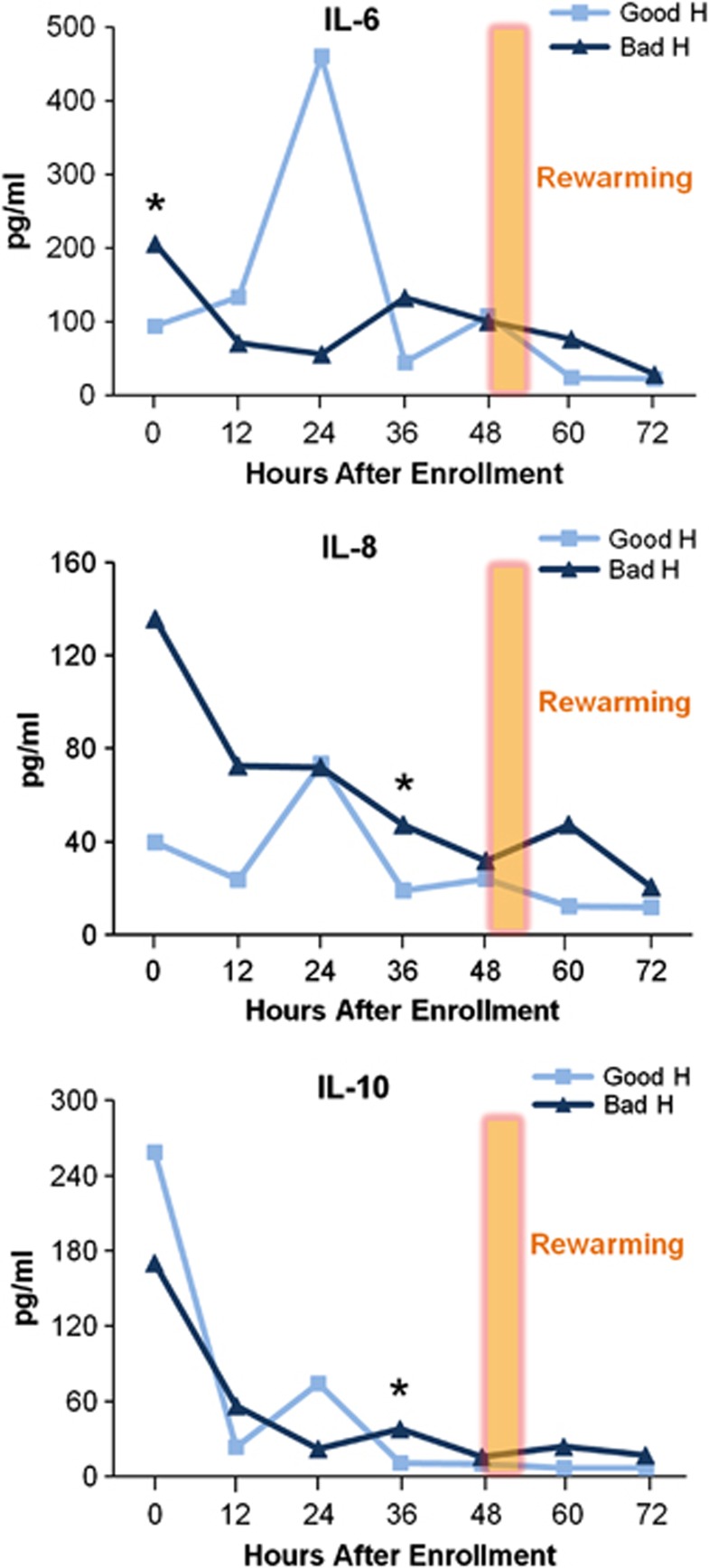
We postulated that within the hypothermia group, subjects with different neurodevelopmental outcomes would have different cytokine profiles. Although elevated IL-6 levels shortly after birth in neonates with encephalopathy were associated with non-survival, H patients with a better neurodevelopmental outcome have second peaks of IL-6, IL-8, and IL-10 at 24 hours after enrollment (Figure 3). From 24 to 36 hours, median IL-6, IL-8, and IL-10 level values in the H good outcome group decreased significantly (P=0.02 IL-6, P=0.015 IL-8, P=0.008 IL-10). Thus, the 36-hour time period is notable for a trend reversal among cytokines in the H group with a better outcome, indicating an active modulation of cytokine values in this group, not simply a result of cytokine variability.
Figure 3.
Median serum interleukin (IL)-6, IL-8, and IL-10 concentrations over time in the hypothermia (H) treatment group by death or severe disability at 12 months (*IL-6 and IL-8, P=0.05, *IL-10 P=0.056).
Median IL-6, IL-8, and IL-10 levels are higher in those H patients with adverse outcomes at 36 hours and remain so over the next 2 days (Table 3). This difference was marginally significant for IL-8 and IL-10 with our sample size (n=10 good outcome and n=12 adverse outcome). Other investigators have also found detrimental aspects of persistent elevation of IL-10 (Kirchoff et al, 2008).
Table 3. Median cytokine values pg/mL (IQR) at 36 hours in hypothermia group between good and bad outcomes.
| Hypothermia | Good outcome | Bad outcome | |
|---|---|---|---|
| IL-6 | 44 (7 to 110) | 132 (21 to 810) | P=0.28 |
| IL-8 | 19 (3 to 95) | 47 (28 to 267) | P=0.049 |
| IL-10 | 10 (2 to 45) | 38 (14 to 109) | P=0.056 |
| MCP-1 | 438 (71 to 1,667) | 20 (6 to 569) | P=0.106 |
IL, interleukin; IQR, interquartile range; MCP-1, monocyte chemotactic protein-1.
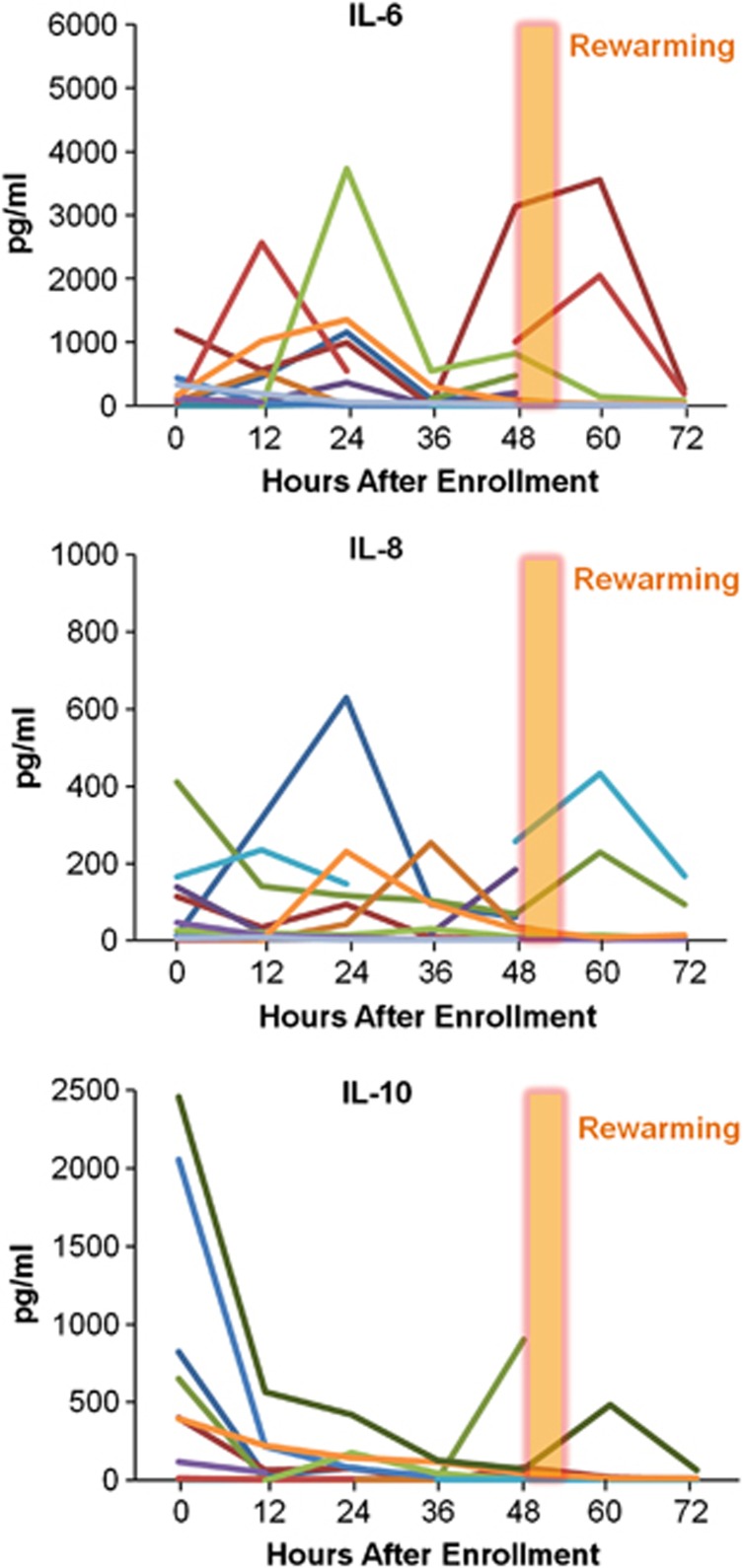
The individual time courses of serum inflammatory cytokines in the H group with good outcomes show a distinctive pattern (Figure 4). A pattern of peak levels during the initial 24 hours followed by a significant decrease from 24 to 36 hours in the better outcome H group is consistent across several cytokines. All 10 H patients with good outcomes had peak IL-6 levels from 0 to 24 hours after enrollment and much lower levels at 36 hours. In contrast, the H adverse outcome group had no cohesive pattern, with 7 out of 12 having late IL-6 peaks ⩾36 hours, and 6 patients with persistently high levels of IL-6 (>800 pg/mL) >60 hours after injury. Five patients had elevation of IL-6 after rewarming (2 good and 3 adverse outcomes).
Figure 4.
Individual patient serum concentrations of IL-6, IL-8, and IL-10 over time in the hypothermia treatment group with a favorable outcome.
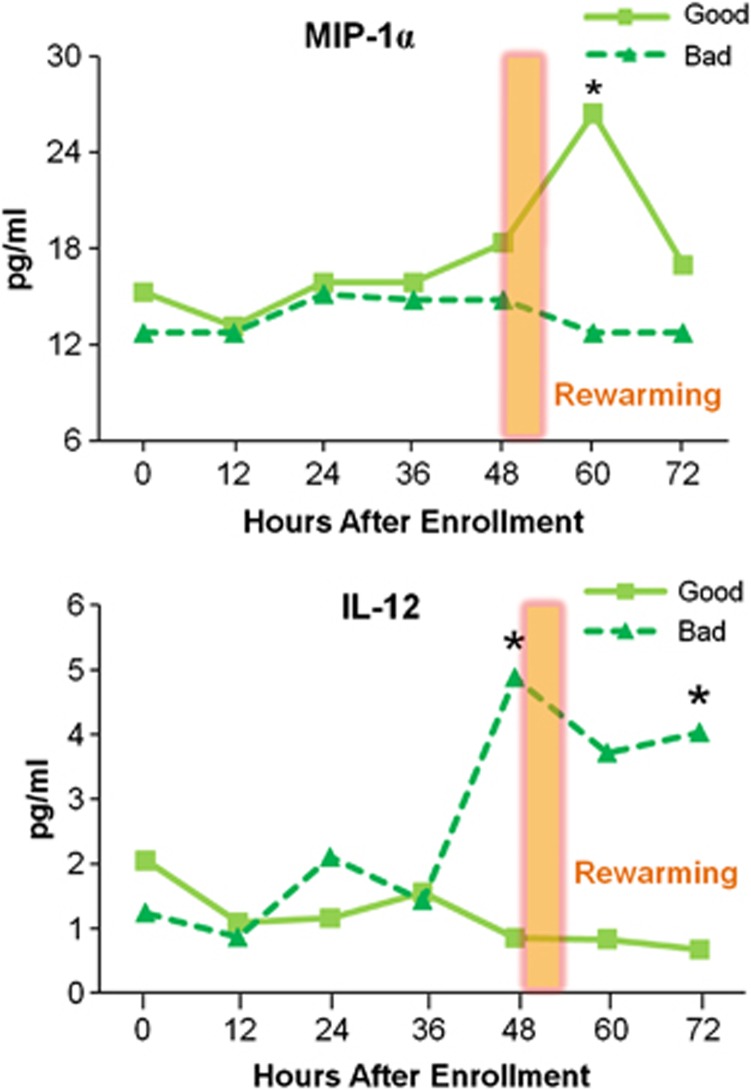
For All Hypoxic-Ischemic Encephalopathy Neonates, High Interleukin-12 and Low Macrophage Inflammatory Protein 1a Are Associated with Adverse Outcomes
Levels of IL-12 and MIP-1a over the entire study period were not significantly different by treatment, and were analyzed by outcome regardless of treatment group (Figure 5). Interleukin-12 was significantly different between outcome groups (P=0.051 at 48 hours and P=0.028 at 72 hours), with a peak in those subjects with worse outcomes. In contrast, in HIE patients with favorable outcomes, median serum MIP-1a levels are higher at 60 to 70 hours after birth. A MIP-1a level <15.9 pg/mL at 60 to 70 hours of age trended toward association with an adverse outcome (P=0.052).
Figure 5.
Median serum macrophage inflammatory protein 1a (MIP-1a) and interleukin (IL)-12 concentrations over time by outcome, regardless of treatment. Median MIP-1a at 60 hours in all patients with better outcomes was 26.4 pg/mL (interquartile range (IQR) 10.7 to 56.3 pg/mL, n=20), compared with 12.7 pg/mL in those with worse outcomes (IQR 7.5 to 17.3 pg/mL, n=12) (*P=0.0507). Median IL-12 concentration at 48 hours for those patients with better outcomes was 0.85 pg/mL (IQR 0.57 to 3.18 pg/mL, n=16) compared with those with adverse outcomes, 3.72 pg/mL (IQR 0.55 to 6.06, n=20, *P=0.0507). At 72 hours, median IL-12 was 0.67 pg/mL (IQR 0.51 to 0.91, n=12) for those patients with better outcomes versus 4.03 pg/mL (IQR 0.83 to 7.79, n=17, *P=0.027) for those with adverse outcomes.
Discussion
Circulating inflammatory cytokines and chemokines are readily measurable and attractive bedside biomarkers of phases of injury (Tekgul et al, 2004; Buttram et al, 2007). Substantial evidence links elevated serum cytokines with inflammatory disease processes that involve vascular endothelial activation, a hallmark of significant HI injury (Yan et al, 1995). Cytokines, such as IL-6, IL-8, MIP-1a, and MCP-1, are secreted by activated endothelial cells, microglia, neurons, and astrocytes, and are chemotactic for blood-borne neutrophils and monocytes/macrophages, potentially increasing leukocyte recruitment as well as directly amplifying inflammatory cascades in the injured central nervous system. Supporting this pro-inflammatory mechanism of secondary injury, investigations in neonates suggest that elevated cytokine levels may serve as valuable predictors of outcome after HIE. Serum cytokines, including IL-6, IL-8, MIP-1a, and MCP-1, from term newborn blood spots were elevated in infants who later developed cerebral palsy (Nelson et al, 1998). High serum and cerebrospinal fluid levels of IL-6 and IL-8 in normothermic HIE newborns have been shown to be significantly associated with death, more severe injury, and severe motor disability at 12 to 30 months (Bartha et al, 2004; Savman et al, 1998; Aly et al, 2006; Tekgul et al, 2004; Fotopoulos et al, 2005). In our study, elevated serum levels of IL-6 and MCP-1 from 0 to 9 hours after HI birth are associated with non-survival of HIE neonates, regardless of treatment group. We did include infants in extremis in our trial, and the rapid, extreme elevation of IL-6 and MCP-1 in the most severely affected HI neonates does not appear to be amenable to induced hypothermia. Therefore, the time interval immediately after HI birth may be a critical period for additional therapeutics. Interleukin-6 and MCP-1 could be very early biomarkers and valuable predictors of patients who need other adjunctive therapy or who will likely not survive.
In contrast to most cytokine levels shortly after treatment initiation, after 12 hours hypothermia was associated with significantly increased median serum levels of IL-6, IL-8, IL-10, and MCP-1 compared with N treatment. We found independent effects of hypothermia treatment and time on cytokine flux, such that the H subjects also had a different pattern of serum expression, with IL-6, IL-8, and MCP-1 showing secondary peaks at 24 to 56 hours of age only in the H group. The time course of the elevation we observe seems to contradict the known neuroprotective properties of induced hypothermia, but is consistent with the theory of cytokine-mediated repair at later time points, in which the role of these mediators days after HI may be different than their role immediately after injury (Catalano et al, 2008; Maeda et al, 1994; Suzuki et al, 2009). However, as biomarkers of injury, the second peak of cytokines may indicate worsening of inflammation before eventual recovery. In addition, after 36 hours there is a trend reversal of cytokines values among those in the H group with a better outcome, suggesting an active down modulation of cytokine values in this group, whereas among those with adverse outcomes in the H group, IL-6 patterns showed notable persistent elevation.
The hypothesis of dual roles of well-known inflammatory mediators is difficult to test in human neonates, but has been shown in animal studies. The concept of dual roles was elegantly illustrated in Nijboer's work on inhibition of Nuclear Factor-kb (NF-kb) in HI injury (Nijboer et al, 2008). In their postnatal day 7 rat model, NF-kb mRNA levels peaked shortly after HI injury, normalized by 12 hours, then peaked again at 24 hours. Inhibition of the early peak of NF-kb expression was neuroprotective, but inhibition of the second peak at 24 hours aggravated injury. Similarly, in adults at a mean of 11 hours after stroke onset, elevated serum levels of IL-6 correlate with less neurologic impairment and decreased infarct size (Sotgiu et al, 2006). Potential beneficial effects of IL-6 are decreased glutamate toxicity, decreased tumor necrosis factor a and IL-1, increased JAK-STAT3 signaling, and induction of anti-apoptotic Bcl proteins in peri-infarct areas (Yamashita et al, 2005; Matsuda et al, 1996). In addition, IL-8 may stimulate angiogenesis and modulate glutamate AMPA receptor activity (Catalano et al, 2008). For such dual role mediators (IL-6, matrix metalloprotease 9, nitric oxide, reactive oxygen species, NF-kb, and others), later peaks in expression seem to be important for repair in animal models (Loddick et al, 1998; Herrmann et al, 2003; Pannu and Singh, 2006).
In contrast, investigations of MCP-1 and IL-12 in stroke models point to these specific cytokines solely as pro-inflammatory contributors to HI injury. Overexpression of MCP-1 in the central nervous system has been shown to increase monocytic infiltration into the central nervous system by 48 hours in HI animal models (Chen et al, 2003). Inhibition of MCP-1 also results in neuroprotection after stroke (Hughes et al, 2002; Rankine et al, 2006). Interleukin-12 enhances Th1 responses activating cytotoxic T cells, promoting natural killer cells invasion and autoimmunity (Gee et al, 2009). Consistent with these reports, we observed that high levels of serum MCP-1 shortly after birth correlated with death, and IL-12 increased late in all patients with worse outcomes. Thus, our data may give mechanistic clues as to inflammatory cytokine modulation, or lack thereof, in those HIE patients whose injuries are too severe for recovery.
Significant increases in median serum levels of cytokines induced by hypothermia compared with those in normothermic HIE patients raise the question of whether hypothermia also changes the downstream effects of these cytokines via receptor binding and signal transduction. Receptors for IL-6, IL-8, MCP-1, and MIP-1a are normally expressed but also are induced by ischemia in proliferating and mature neurons, oligodendroglia, astrocytes, and microglial cells (Ransohoff, 2009; Zhou et al, 2010). The induction of inflammatory cytokine receptors over time after HI injury in animal models is not likely to be a protective effect, unless these chemotactic cytokines also trigger some other beneficial second messengers, promoting proliferation and migration of neuronal and oligodendroglial cells, as has been shown to occur during development (Trettel et al, 2003; Vlahakis et al, 2002).
The usefulness of cytokines as biomarkers that predict outcome is hampered by well-known variability among intensive care patients with multiple interventions and organ systems injuries leading to cytokine storm (Ferrara et al, 1993). Also in neonatal HIE, variability may be affected by the fact that HI may occur at varying intervals before delivery, leading to different cytokine time courses after birth. We only included HIE subjects, without extracorporeal membrane oxygenation, sepsis or chorioamnionitis, and found similar patterns of expression in the H group with better outcomes, perhaps indicating serum cytokines were less confounded by these other insults or that the injury was more acute or less severe.
Summary
Our data are consistent with accumulating evidence of different roles that cytokines may have after injury. Inflammatory cytokines have been traditionally viewed solely as mediators of central nervous system injury, but investigations into the actions of IL-6, reactive oxygen species, and NF-kb have provided sound evidence of reparative actions after HI injury. Cytokine and chemokine actions may, therefore, be specific to the phase of injury and recovery and switch roles within a relatively short time after injury. In our investigations of the time course of serum cytokines after birth in HIE neonates enrolled in a hypothermia trial, there was a second peak in serum levels of inflammatory mediators only in H patients. We found that early serum IL-6 and MCP-1 are indicators of death; however, within 24 to 56 hours, IL-6 and MIP-1a may be markers of patients who have activated important mechanisms of repair and consequently will have better outcomes than those who do not have a secondary peak. We speculate that hypothermia may not simply prolong the latent phase or delay secondary injury, but may shorten the time to initiate repair in neonates recovering from HIE.
Further investigation of the temporal relationships of biomarkers in injury and recovery, and the variability of those phases during hypothermia is required for optimal neuroprotection. With a better understanding of the modulation of serum cytokines and chemokines, these biomarkers may serve as an indicator of an individual patient's place in the injury-repair continuum, and which therapies might be helpful or harmful.
No author has received funding for this work or manuscript outside of NINDS funding.
Footnotes
This work was funded by National Institutes of Neurologic Disorders and Stroke, R01 NS38602.
References
- Aly H, Khashaba MT, El-Ayouty M, El-Sayed O, Hasanein BM. IL-1b, IL-6 and TNF-a and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28:178–182. doi: 10.1016/j.braindev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich J, Ferriero DM. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56:960–966. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- Berger RP, Ta'Asan S, Rand A, Lokshin A, Kochanek P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr Res. 2008;65:97–102. doi: 10.1203/PDR.0b013e31818c7e27. [DOI] [PubMed] [Google Scholar]
- Bogner V, Keil L, Kanz KG, Kirchoff C, Leidel BA, Mutschler W, Biberthaler P. Very early posttraumatic serum alterations are significantly associated to initial massive RBC substitution, injury severity, multiple organ failure and adverse clinical outcome in multiple injured patients. Eur J Med Res. 2009;14:284–291. doi: 10.1186/2047-783X-14-7-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttram SDW, Wisnewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RSB, Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid flowing severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- Catalano M, Trettel F, Ciprinai R, Laura C, Sovrero F, Eusebi F, Limatola C. Chemokine CXCL8 modulates GluR1 phosphorylation. J Neuroimmun. 2008;198:75–81. doi: 10.1016/j.jneuroim.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NEJ, Vogel SN. Overexpression of MCP-1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Diestel A, Roessler J, Berger F, Schmitt KRL. Hypothermia down regulates inflammation but enhances IL-6 secretion by stimulated endothelial cells. Cryobiology. 2008;57:216–222. doi: 10.1016/j.cryobiol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni L, Hulsey TC, Bass T, Kaufman D, Horgan M, Languani S, Bhatia J, Givelichian L, Sankaran K, Yager J. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol. 2005a;32:18–25. doi: 10.1016/j.pediatrneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni L, Hulsey TC, Bass T, Kaufman D, Horgan M, Languani S, Bhatia J, Givelichian L, Sankaran K, Yager J. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005b;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammatory cytokines in serum of patient with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Ferrara JL, Abhyankar S, Gilliland DG. ″Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1″. Transplant Proc. 1993;2:1216–1217. [PubMed] [Google Scholar]
- Fotopoulos S, Mouchtouri A, Xanthou G, Lipsou N, Petrakou E, Xanthou M. Inflammatory chemokine expression in the peripheral blood of neonates with perinatal asphyxia and perinatal or nosocomial infections. Acta Paediatr. 2005;94:800–806. doi: 10.1111/j.1651-2227.2005.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Fries M, Stoppe C, Brucken D, Rossaint R, Kuhlen R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care. 2009;24:453–457. doi: 10.1016/j.jcrc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Gee K, Guzzo C, CheMat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. MCP-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Jastrow KM, Gonzalez EA, McGuire MF, Suliburk JW, Kozar RA, Iyengar S, Motschall DA, McKinley BA, Moore FA, Mercer DW. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kirchoff C, Buhmann B, bogner V, Stegmaier J, Leidel BA, Braunstein V, Mutschler W. Cerebrospinal IL-10 concentration is elevated in non-survivors after severe traumatic brain injury. Eur J Med Res. 2008;13:464–468. [PubMed] [Google Scholar]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Matsumoto M, Hori O, Kuwabara K, Ogawa S, Yan SD, Ohtsuki T, Kinoshita T, Kamada T, Stern DM. Hypoxia/reoxygenation-mediated induction of astrocyte IL-6: a paracrine mechanism potentially enhancing neuron survival. J Exp Med. 1994;180:2297–2308. doi: 10.1084/jem.180.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ancel A, Garcia-Alix A, Pascual-Salcedo D, Cabanas F, Vlacarce M, Quero J. IL-6 in the cerebrospinal fluid after perinatal asphyxia is related to early and late neurological manifestations. Pediatrics. 1997;100:789–794. doi: 10.1542/peds.100.5.789. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Wen TC, Morita F, Otsuka H, Igase K, Yoshimura H, Sakanaka M. Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci Lett. 1996;204:109–112. doi: 10.1016/0304-3940(96)12340-5. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int. 2006;49:170–182. doi: 10.1016/j.neuint.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Rankine EL, Hughes PM, Botham MS, Perry VH, Felton LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1 deficient mice prior to leukocyte recruitment. Eur J Neurosci. 2006;24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunology and neurobiology. Immunity. 2009;31:1–11. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savman K, Blennow M, Gustafson K, Tarkowshi E, Hagberg H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res. 1998;43:746–751. doi: 10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Sotgiu S, Marchettis ZB, Fois ML, Arru G, Pes GM, Salaris FS, Arru A, Pirisi A, Rosati G. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. 2006;13:505–513. doi: 10.1111/j.1468-1331.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Landseadel JP, Gurka MJ, Fairchild KD. Hypothermia increases IL-6 and IL-10 in juvenile endotoxemic mice. Pediatr Crit Care. 2009;10:1–7. doi: 10.1097/PCC.0b013e3181b01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of IL-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- Tekgul H, Yalaz M, Kutukculer N, Ozbek S, Kose T, Akisu M, Kutursay N, Gokben S. Value of biochemical markers for outcome in term infants with asphyxia. Pediatr Neurol. 2004;31:326–332. doi: 10.1016/j.pediatrneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Trettel F, Di Bartolomeo S, Lauro C, Catalano M, Ciotti MT, Limatola C. Ligand-independent CXCR2 dimerization. J Biol Chem. 2003;278:40980–40988. doi: 10.1074/jbc.M306815200. [DOI] [PubMed] [Google Scholar]
- Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- Xiong M, Yang Y, Chen GQ, Zhou WH. Post-ischemic hypothermia for 24 h in P7 rats rescues hippocampal neuron: association with decreased astrocytes activation and inflammatory cytokine expression. Brain Res Bull. 2009;79:351–357. doi: 10.1016/j.brainresbull.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Sawamoto K, Suzuki S, Suzuki N, Adachi K, Kawase T, Mihara M, Ohsugi Y, Abe K, Okano H. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem. 2005;94:459–468. doi: 10.1111/j.1471-4159.2005.03227.x. [DOI] [PubMed] [Google Scholar]
- Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D. Induction of interleukin-6 (IL-6) by hypoxia in vascular cells. J Bio Chem. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Kawakami M, Okada Y. Moderate hypothermia alters IL-6 and IL-1a reactions in ischemic brain injury. Resuscitation. 2002;53:93–99. doi: 10.1016/s0300-9572(01)00499-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem. 2010;116:406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]