Abstract
Tumor necrosis factor (TNF)-α is produced in brain in response to acute cerebral ischemia, and promotes neuronal apoptosis. Biologic TNF inhibitors (TNFIs), such as the etanercept, cannot be developed as new stroke treatments because these large molecule drugs do not cross the blood–brain barrier (BBB). A BBB-penetrating biologic TNFI was engineered by fusion of the type II human TNF receptor (TNFR) to each heavy chain of a genetically engineered chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), designated as cTfRMAb-TNFR fusion protein. The cTfRMAb domain of the fusion protein acts as a molecular Trojan horse to deliver the fused TNFR across the BBB. Etanercept or the cTfRMAb-TNFR fusion protein (1 mg/kg) was administered intravenously in adult mice subjected to 1-hour reversible middle cerebral artery occlusion up to 90 minutes after the occlusion. Neuroprotection was assessed at 24 hours or 7 days after occlusion. The cTfRMAb-TNFR fusion protein treatment caused a significant 45%, 48%, 42%, and 54% reduction in hemispheric, cortical, and subcortical stroke volumes, and neural deficit, respectively. Intravenous etanercept had no therapeutic effect. Biologic TNFIs can be reengineered for BBB penetration, and the IgG-TNFR fusion protein is therapeutic after delayed intravenous administration in experimental stroke.
Keywords: blood–brain barrier; stroke; TNFα inhibitors, monoclonal antibody
Introduction
Tumor necrosis factor (TNF)-α is a proinflammatory cytokine that is synthesized in brain within 1 hour of an acute experimental ischemic stroke (Liu et al, 1994). The intracerebroventricular injection of TNFα exacerbates the extent of the infarction in experimental stroke (Barone et al, 1997). The TNFα knock-out mice are more resistant, and TNFα knock-in rats are more susceptible, to experimental ischemia (Martin-Villalba et al, 2001; Pettigrew et al, 2008). The TNFα has been implicated in the pathogenesis of stroke in humans (Sairanen et al, 2001). Therefore, a potential treatment strategy for acute stroke is the blockade of TNFα action in brain with a TNFα inhibitor (TNFI). Biologic TNFIs reduce the volume of the infarct after transcranial injection. The intracerebroventricular injection of a TNFα neutralizing antibody reduces stroke volume (Barone et al, 1997). The TNF decoy receptors reduce stroke volume after either the intracerebroventricular administration (Barone et al, 1997) or via direct topical application to brain in experimental stroke (Nawashiro et al, 1997). The biologic TNFIs were administered by transcranial delivery to brain, because the biologic TNFIs are large molecule drugs that do not cross the blood–brain barrier (BBB). The BBB is intact in the early hours after stroke (Menzies et al, 1993; Belayev et al, 1996), when neuroprotection is still possible (Zivin, 1998). Biologic TNFIs are potential new intravenous treatments for acute stroke should these molecules be made transportable across the BBB.
The leading decoy receptor-type TNFI is etanercept, which is widely used to suppress TNFα action in inflammation in peripheral organs (Fleischmann et al, 2006). Etanercept is formed by fusion of the extracellular domain (ECD) of the type II human TNF receptor (TNFR) to the amino terminus of the Fc region of human IgG1 (Peppel et al, 1991). Etanercept cannot be developed for the treatment of acute stroke, because etanercept does not cross the BBB (Boado et al, 2010). However, the TNF decoy receptor has been reengineered for BBB transport. The ECD of the type II human TNFR was fused to the carboxyl terminus of the heavy chain of a genetically engineered monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), and this protein is designated as cTfRMAb-TNFR fusion protein (Zhou et al, 2011a). The cTfRMAb-TNFR fusion protein is rapidly transported across the BBB in the mouse via receptor-mediated transport on the endogenous BBB TfR (Zhou et al, 2011a). The purpose of the present investigation was to examine the therapeutic effects of the cTfRMAb-TNFR fusion protein in a mouse model of acute brain ischemia, the reversible middle cerebral artery occlusion (MCAO) model. Mice were treated intravenously with the brain-penetrating TNFI, the cTfRMAb-TNFR fusion protein, or the nonbrain-penetrating TNFI, etanercept. Stroke volume and neural deficit were measured at both 24 hours and 7 days after a 1-hour reversible MCAO.
Materials and methods
Fusion Proteins
The cTfRMAb-TNFR fusion protein was produced in stably transfected Chinese hamster ovary cells and purified by protein G affinity chromatography as described previously (Zhou et al, 2011a). Etanercept (Enbrel) was purchased from the Pharmacy of the UCLA Hospital. The TNFα decoy receptor domain in either the cTfRMAb-TNFR fusion protein or etanercept corresponds to amino acids 23 to 257 of the human type II TNFR ECD (NP_001057; Zhou et al, 2011a). Both the cTfRMAb-TNFR fusion protein and etanercept were formulated in 10 mmol/L sodium acetate/0.15 mol/L NaCl/pH=6.0 (ABS buffer).
Reversible Middle Cerebral Artery Occlusion Model
All experimental animal procedures were conducted according to Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. All of our study protocols were approved by the UCLA Animal Research Committee before the study began, and we were given permission to perform the study. The reversible MCAO mouse model was used, as described by Shimin et al (2009). Adult male C57Bl/6J mice (25 g) supplied by Jackson Labs (Bar Harbor, ME, USA) kept under standardized light/dark (12 h), temperature (25°C) and humidity (70%) conditions were used for the MCAO model. Mice were anesthetized with isoflurane (4% for induction and 2% for maintenance) in 30% O2. Body temperature was continuously monitored and maintained constant at 37°C throughout the surgical procedure using a Harvard thermal blanket with a rectal probe (Harvard Apparatus, Inc., Holliston, MA, USA). Mice were placed in a supine position; a midline incision was made in the neck and the common carotid artery and external carotid artery (ECA) were exposed. The branches of the ECA (superior thyroid and occipital arteries) were electrocoagulated. After occlusion of the common carotid artery with a microclip, the ECA was ligated distally to the cranial thyroid artery and an incision was made in the ECA. A silicon rubber coated 6-0 nylon monofilament (6021; Doccol Corp., Redlands, CA, USA) was inserted into the ECA and gently advanced 9 to 11 mm until resistance was felt for occlusion of the middle cerebral artery. The filament was secured in placed by ligation for duration of 60 minutes after which the nylon filament was carefully withdrawn to allow for reperfusion and the neck incision was sutured. Mice were euthanized either 23 hours or 7 days after 60-minute occlusion. Some mice died prematurely and were not included in the data analysis. No significant difference between the mortality rates among different treatment groups was observed. The average mortality across all the groups was 7% for mice subjected to 60-minute occlusion and 23 hours of reperfusion. The mice that died prematurely were not used to compute stroke volumes or neural deficits. The measurements of infarct volume and neural deficit were determined by an observer blinded to treatment group.
Drug Treatment Groups
Mice subjected to MCAO were divided into three treatment groups: (1) saline, (2) etanercept (1 mg/kg), and (3) cTfRMAb-TNFR (1 mg/kg). Drug treatment was administered intravenously via the tail vein with a 0.3-mL syringe and a 29-g needle at 45 or 90 minutes after arterial occlusion. The protein concentration in the intravenous injection solution was 0.25 mg/mL in ABS buffer and a total volume of 100 μL was injected (25 μg protein/mouse). To determine the effect of delayed administration of the cTfRMAb-TNFR fusion protein on stroke outcome, a fifth group of mice was treated with the cTfRMAb-TNFR fusion protein (1 mg/kg intravenously) at 90 minutes after arterial occlusion. These mice were euthanized at 23 hours after the 1-hour MCAO. In a separate series, mice were euthanized at 7 days after reversible MCAO. The mice subjected to 60 minute of MCAO were divided into the following treatment groups: (1) saline and (2) cTfRMAb-TNFR (1 mg/kg), injected intravenously at 45 minutes after arterial occlusion, followed by euthanasia at 7 days after occlusion.
Neurologic Deficit Scoring
Before euthanasia at 1 day or 7 days after arterial occlusion, animals were monitored for neurologic deficit, and scored as follows: 0, no deficit; 1, failure to extend the contralateral forepaw fully; 2, intermittent circling; 3, sustained circling without moving forward; 4, walks only when stimulated with decreased level of consciousness.
Infarct Volume Calculation
After euthanasia, the brain was removed and cut into 2-mm coronal sections with a mouse brain matrix (Kent Scientific Co., Torrington, CT, USA). The brains were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich Inc., St Louis, MO, USA) and fixed overnight in 10% buffered formalin (Sigma-Aldrich Inc.). The sections were scanned with a UMAX PowerLook III flatbed scanner (UMAX Data Systems, Inc., Hsinchu, Taiwan) and the images processed in Adobe Photoshop. The area of either hemispheric or cortical infarct in each brain section was computed from the difference between the area of the contralateral hemisphere and the area of ipsilateral noninfarcted brain using NIH Image J software 1.62 (National Institutes of Health, Bethesda, MD, USA). Total infarct volume (mm3) was computed from the sum of infarct area for all sections from the same brain multiplied by the total section thickness (8 mm). Subcortical infarct volume was calculated as the difference between the hemispheric and cortical infarct volumes.
Statistical Analysis
Data are represented as mean±s.e.m., and statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software San Diego, CA, USA). One-way analysis of variance with Bonferroni's correction (for more than two groups) or Student's t-test (for two groups) was used to compare the means of infarct volume. Kruskal–Wallis or Mann–Whitney U-test was used to determine a statistical difference between the neurologic deficit scoring. Statistical significant differences in the mortality rate were determined using χ2 or Fisher's exact test. A P<0.05 was considered to be statistically significant.
Results
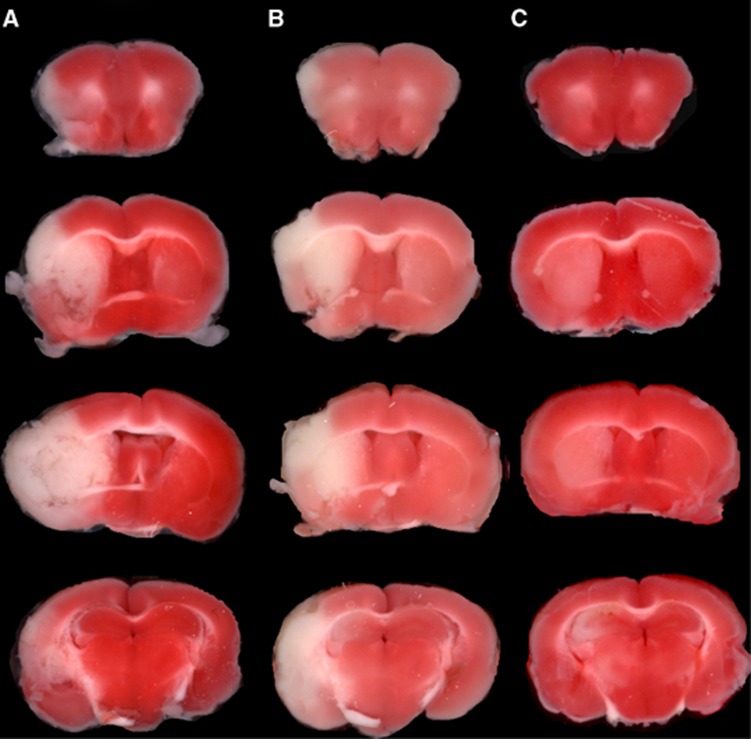
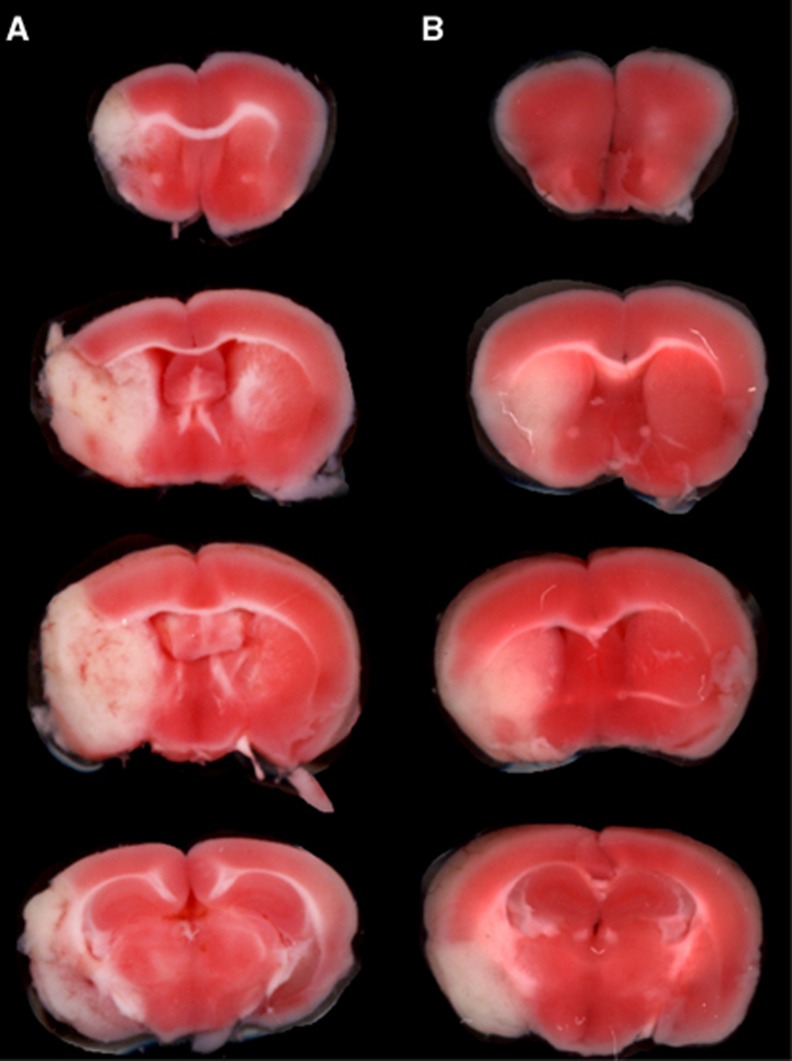
The TTC stains of coronal sections of mouse brain removed 24 hours after a 1-hour reversible MCAO are shown in Figure 1. The scans from a representative mouse in the saline treatment group, the etanercept treatment group, and the cTfRMAb-TNFR fusion protein treatment group are shown in Figures 1A, 1B, and 1C, respectively. The mean±s.e.m. of the stroke volume in the hemisphere, the cortex, and the subcortex for each of the treatment groups is given in Table 1, along with the mean±s.e.m. of the neural deficit scores. Treatment of mice with 1 mg/kg cTfRMAb-TNFR fusion protein at 45 minutes after the arterial occlusion caused a statistically significant 45% reduction in hemispheric stroke volume, a 48% reduction in cortical stroke volume, and a 42% reduction in subcortical stroke volume, which was associated with a 54% reduction in neural deficit score (Table 1). Conversely, treatment of mice with 1 mg/kg etanercept at 45 minutes after the arterial occlusion caused no significant changes in either stroke volumes or neural deficit scores (Table 1). If the time between occlusion and treatment with 1 mg/kg cTfRMAb-TNFR fusion protein was prolonged from 45 to 90 minutes after arterial occlusion, then a statistically significant 45% reduction in hemispheric stroke volume and a 62% reduction in cortical stroke volume was observed; however, the reduction in subcortical stroke volume or neural deficit score was not significant in this group (Table 1). In a separate series of studies, the duration between the 60-minute arterial occlusion and euthanasia was prolonged from 1 day to 7 days. Mice were treated with either saline or 1 mg/kg cTfRMAb-TNFR fusion protein at 45 minutes after arterial occlusion, and were euthanized at 7 days after the 1-hour MCAO. The TTC stain for a representative mouse in each group is shown in Figure 2. The treatment of mice with 1 mg/kg cTfRMAb-TNFR fusion protein at 45 minutes after arterial occlusion caused a statistically significant 32% reduction in hemispheric stroke volume, and a 38% reduction in subcortical stroke volume, which was correlated with 40% reduction in neural deficit score (Table 1).
Figure 1.
TTC stains of coronal sections from a representative mouse from each of three treatment groups: (A) saline, (B) etanercept (1 mg/kg), and (C) cTfRMAb-TNFR (1 mg/kg). The most rostral brain section is at the top. Drug treatments were administered 45 minutes after the arterial occlusion, and mice were euthanized at 24 hours after the 1-hour arterial occlusion. cTfRMAb, chimeric MAb against mouse TfR; cTfRMAb-TNFR, fusion protein of cTfRMAb and TNFR ECD; TTC, 2,3,5-triphenyltetrazolium chloride; TNFR, tumor necrosis factor receptor.
Table 1. Stroke volumes and neural deficit scores.
| Treatment (mice per group) | Time after MCAO | Hemispheric stroke volume (mm3) | Cortical stroke volume (mm3) | Sub-cortical stroke volume (mm3) | Neural deficit score |
|---|---|---|---|---|---|
| Saline at 45 minutes (10) | 1 day | 70.9±4.5 | 43.6±4.0 | 27.9±3.2 | 2.4±0.3 |
| cTfRMAb-TNFR at 45 minutes (8) | 1 day | 39.2±7.3** | 22.8±5.6* | 16.2±2.3** | 1.1±0.3** |
| Etanercept at 45 minutes (9) | 1 day | 57.7±9.2 | 31.8±7.2 | 25.9±3.1 | 2.4±0.2 |
| cTfRMAb-TNFR at 90 minutes (8) | 1 day | 39.3±8.0** | 16.8±6.9** | 22.6±1.9 | 2.1±0.1 |
| Saline at 45 minutes (11) | 7 days | 67.7±7.4 | 38.7±6.7 | 29.2±2.4 | 2.0±0.1 |
| cTfRMAb-TNFR at 45 minutes (11) | 7 days | 46.6±4.9* | 28.6±4.0 | 18.1±1.4** | 1.2±0.3* |
cTfRMAb, chimeric MAb against mouse TfR; cTfRMAb-TNFR, fusion protein of cTfRMAb and TNFR ECD; MCAO, middle cerebral artery occlusion; TNFR, tumor necrosis factor receptors.
*P<0.05;
**P<0.01;
***P<0.005.
Figure 2.
TTC stains of coronal sections from a representative mouse from each of two treatment groups: (A) saline, (B) cTfRMAb-TNFR (1 mg/kg). The most rostral brain section is at the top. Drug treatments were administered 45 minutes after the arterial occlusion, and mice were euthanized 7 days after the 1-hour arterial occlusion. cTfRMAb, chimeric MAb against mouse TfR; cTfRMAb-TNFR, fusion protein of cTfRMAb and TNFR ECD; TTC, 2,3,5-triphenyltetrazolium chloride; TNFR, tumor necrosis factor receptor.
Discussion
The findings of this study show that the intravenous injection of a brain-penetrating TNFI, the cTfRMAb-TNFR fusion protein, is therapeutic in experimental stroke with a reduction in stroke volume that correlates with a reduction in the neural deficit (Table 1). The therapeutic effect is long lasting and persists at 7 days after the acute ischemia (Figure 2). In contrast, a nonbrain-penetrating TNFI, etanercept, has no effect on either infarct volume or neural deficit after intravenous administration (Table 1).
The lack of a therapeutic effect of intravenous etanercept in acute cerebral ischemia parallels similar observations in acute traumatic brain injury. The intravenous administration of 5 mg/kg etanercept is not therapeutic in traumatic brain injury, whereas the intracerebroventricular injection of etanercept is therapeutic in acute brain injury (Knoblach et al, 1999). The lack of a therapeutic effect of intravenous etanercept in acute stroke is consistent with the dual observations that etanercept does not cross the intact BBB (Boado et al, 2010), and that the BBB is intact in the early hours after stroke (Menzies et al, 1993; Belayev et al, 1996), when neuroprotection is still possible in stroke (Zivin, 1998). The maintenance of an intact BBB during the period after acute stroke when neuroprotection is possible means that biologic neuroprotective agents for stroke must be reengineered to penetrate the BBB. Etanercept is a fusion protein of the ECD of the type II human TNFR and the Fc domain of human IgG1 (Peppel et al, 1991). The TNFR ECD of etanercept can be reengineered for brain penetration by genetic fusion to a BBB molecular Trojan horse. The latter is a peptidomimetic MAb against an endogenous BBB receptor, such as the human insulin receptor (HIR). The HIRMAb (MAb against HIR) binds the endogenous BBB insulin receptor, and this binding triggers receptor-mediated transport across the BBB (Pardridge et al, 1995). A fusion protein of the genetically engineered HIRMAb and the human type II TNFR ECD has been engineered, and shown to bind both TNFα and the HIR with high affinity (Hui et al, 2009). The HIRMAb-TNFR fusion protein is rapidly taken up by brain in the Rhesus monkey, and the brain uptake is 3% of injected dose/brain after intravenous administration (Boado et al, 2010). However, the HIRMAb is only active in humans and Old World primates such as the Rhesus monkey (Pardridge et al, 1995). There is no known MAb against the mouse or rat insulin receptor that can be used as a BBB Trojan horse. Therefore, a surrogate Trojan horse has been engineered for the mouse, which is a genetically engineered chimeric MAb against the mouse TfR, and designated as cTfRMAb (Boado et al, 2009). A fusion protein of the cTfRMAb and the human TNFR, designated as cTfRMAb-TNFR fusion protein, has been engineered and shown to bind the mouse TfR and human TNFα with high affinity (Zhou et al, 2011a). The affinity of the cTfRMAb-TNFR fusion protein for binding to human TNFα is equal to the affinity of etanercept binding of human TNFα (Zhou et al, 2011b). Etanercept binds mouse and human TNFα with the same high affinity (Scallon et al, 2002), which enables the use of the human TNFR as a TNFI in mouse models. The brain uptake of the cTfRMAb-TNFR fusion protein in the mouse is high, 2.8% injected dose/g (Zhou et al, 2011a), which is comparable to, or even greater than the brain uptake of diazepam in the mouse, which is 1.8% injected dose/g (Greenblatt and Sethy, 1990). Prior work has shown that the cTfRMAb-TNFR fusion protein is neuroprotective after chronic intravenous administration in experimental Parkinson's disease in the mouse (Zhou et al, 2011b). The present work shows an acute, single injection of the cTfRMAb-TNFR fusion protein is neuroprotective in experimental ischemia in the mouse. The TfRMAb alone has no neuroprotective effect in experimental stroke (Zhang and Pardridge, 2001).
The present investigation assesses neuroprotection in stroke after a 60-minute reversible occlusion of the middle cerebral artery in the mouse. The 60-minute occlusion in the mouse may correlate with a more prolonged occlusion in humans, since the evolution of an infarction in the mouse MCAO model is faster than in other species (Carmichael, 2005). In the present study, the cTfRMAb-TNFR or etanercept fusion proteins were administered at a dose of 1 mg/kg intravenously. This therapeutic dose of the cTfRMAb-TNFR fusion protein produces a brain level of TNFR decoy receptor that saturates cerebral TNFα (Zhou et al, 2011a), and is neuroprotective in a mouse model of Parkinson's disease (Zhou et al, 2011b). The finding that a single intravenous injection of the cTfRMAb-TNFR fusion protein given within 45 to 90 minutes of the MCAO is neuroprotective is consistent with prior work showing that the transcranial delivery of biologic TNFIs is neuroprotective in acute stroke. The intracerebroventricular injection of either a TNFα neutralizing antibody or a TNFR decoy receptor is neuroprotective in stroke (Barone et al, 1997), as is the topical application of a TNFR decoy receptor in regional brain ischemia (Nawashiro et al, 1997). The proinflammatory role of TNFα in acute stroke is supported by other findings that TNFα mRNA and protein are elevated within an hour of an acute stroke (Liu et al, 1994). Immunocytochemistry of brain in the mouse MCAO model shows an increase in neuronal TNFα in the infarct zone, which peaks by 12 hours, and returns toward baseline by 24 hours after the infarct (Yang et al, 1999). Over production of TNFα in stroke mediates neural damage by accelerating neuronal apoptosis (Pettigrew et al, 2008), and increases NF-κB production in cerebral ischemia (Schwaninger et al, 2006). Overproduction of the NF-κB transcription factor enhances neuronal apoptosis in both acute and chronic brain disorders (Mattson and Camandola, 2001). The neuroprotective effect of the cTfRMAb-TNFR fusion protein is prolonged and persists at 7 days after infarction, based on TTC stains of brain (Table 1; Figure 2). Neural loss can also be assessed with Fluoro-Jade B staining. However, Fluoro-Jade B staining of neural loss peaks at 24 hours and decreases to baseline values by 7 days after stroke in mice (Liu et al, 2009).
In summary, the present study shows that reengineering a biologic TNFI, the human type II TNFR decoy receptor, as an IgG-TNFR fusion protein that penetrates the BBB, enables therapeutic effects in acute experimental stroke in the mouse after intravenous administration. The role of TNFα in the acute response to stroke has been shown in human stroke (Sairanen et al, 2001). Therefore, BBB-penetrating biologic TNFIs, such as a Trojan horse-TNFR decoy receptor fusion protein, may offer a new approach for the intravenous treatment of acute ischemic stroke.
RJB is an employee of, and WMP is a consultant of ArmaGen Technologies, Inc.
References
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Stroke. 1997. pp. 1233–1244. [DOI] [PubMed]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol. 2010;146:84–91. doi: 10.1016/j.jbiotec.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102:1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R, Baumgartner SW, Weisman MH, Liu T, White B, Peloso P. Long term safety of etanercept in elderly subjects with rheumatic diseases. Ann Rheum Dis. 2006;65:379–384. doi: 10.1136/ard.2005.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DJ, Sethy VH. Benzodiazepine concentrations in brain directly reflect receptor occupancy: studies of diazepam, lorezepam, and oxazepam. Psychopharmacology. 1990;102:373–378. doi: 10.1007/BF02244106. [DOI] [PubMed] [Google Scholar]
- Hui EK, Boado RJ, Pardridge WM. Tumor necrosis factor receptor-IgG fusion protein for targeted-drug delivery across the human blood brain barrier. Mol Pharm. 2009;6:1536–1543. doi: 10.1021/mp900103n. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-á after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, Fluoro-Jade B and neuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Stroke. 1994. pp. 1481–1488. [DOI] [PubMed]
- Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer PH. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Diff. 2001;8:679–686. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. J Clin Invest. 2001. pp. 247–254. [DOI] [PMC free article] [PubMed]
- Menzies SA, Betz AL, Hoff JT. Contributions of ions and albumin to the formation and resolution of ischemic brain edema. J Neurosurg. 1993;78:257–266. doi: 10.3171/jns.1993.78.2.0257. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Martin D, Hallenbeck JM. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res. 1997;778:265–271. doi: 10.1016/s0006-8993(97)00981-5. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res. 1995;12:807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew LC, Kindy MS, Scheff S, Springer JE, Kryscio RJ, Li Y, Grass DS. Focal cerebral ischemia in the TNFalpha-transgenic rat. J Neuroinflamm. 2008;5:47–66. doi: 10.1186/1742-2094-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen T, Carpen O, Lindsberg MK, Paetau A, Turpeinen U, Kaste M, Lindsberg PJ. Stroke. 2001. pp. 1750–1758. [DOI] [PubMed]
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Inta I, Herrman O. Biochem Soc Trans. 2006. pp. 1291–1294. [DOI] [PubMed]
- Shimin L, Gehua Z, Meloni BP, Campbell K, Winn HR. Rodent stroke model guidelines for preclinical stroke trials. J Exp Stroke Transl Med. 2009;2:2–27. doi: 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G-Y, Schielke GP, Gong C, Mao Y, Ge H-L, Liu X-H, Betz AL. Expression of tumor necrosis factor-alpha and intercellular adhesion molecule-1 after focal cerebral ischemia in interleukin-1â converting enzyme deficient mice. J Cereb Blood Flow Metabol. 1999;19:1109–1117. doi: 10.1097/00004647-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood-brain barrier drug targeting system. Stroke. 2001;32:1378–1384. doi: 10.1161/01.str.32.6.1378. [DOI] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Hui EK, Lu JZ, Pardridge WM. Brain-penetrating TNFR decoy receptor in the mouse. Drug Metab Dispos. 2011a;39:71–76. doi: 10.1124/dmd.110.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Sumbria R, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection with a brain-penetrating biologic tumor necrosis factor factor inhibitor. J Pharmacol Exp Ther. 2011b;339:618–623. doi: 10.1124/jpet.111.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA. Factors determining the therapeutic window for stroke. Neurology. 1998;50:599–603. doi: 10.1212/wnl.50.3.599. [DOI] [PubMed] [Google Scholar]