Abstract
Cortical spreading depression (SD) is propagating neuronal and glial depolarization and is thought to underly the pathophysiology of migraine. We have reported that cortical SD facilitates the proliferative activity of NG2-containing progenitor cells (NG2 cells) that give rise to oligodendrocytes and immature neurons under the physiological conditions in the adult mammalian cortex. Astrocytes have an important role in the maintenance of neuronal functions and alleviate neuronal damage after intense neuronal excitation, including SD and seizures. We here investigated whether SD promotes astrocyte generation from NG2 cells following SD stimuli. Spreading depression was induced by epidural application of 1 mol/L KCl solution in adult rats. We investigated the cell fate of NG2 cells following SD-induced proliferation using 5′-bromodeoxyuridine labeling and immunohistochemical analysis. Newly generated astrocytes were observed only in the SD-stimulated cortex, but not in the contralateral cortex or in normal cortex. The astrocytes were generated from proliferating NG2 cells. Astrogenesis depended on the number of SD stimuli, and was accompanied by suppression of oligodendrogenesis. These observations indicate that the cell fate of NG2 cells was shifted from oligodendrocytes to astrocytes depending on SD stimuli, suggesting activity-dependent tissue remodeling for maintenance of brain functions.
Keywords: astrocyte, brain recovery, glial cells, neural stem cells, spreading depression
Introduction
Spreading depression (SD) is characterized by intense neuronal and glial depolarization that propagates through gray matter in the brain at the speed of 2 to 5 mm/min. It is accompanied by reversible suppression of neuronal activity, failure of ion homeostasis and changes in the cerebral blood flow (CBF) without neuronal degeneration. Spreading depression was reported to be generated in several neurological disorders, including migraine (James et al, 2001), brain trauma (Strong et al, 2002; Fabricius et al, 2006), and ischemic stroke (Strong et al, 2002; Fabricius et al, 2006). The initiation and propagation of SD are known to trigger disruption of membrane ionic gradients, massive potassium efflux, and glutamate release in neurons as well as glial cells.
Astrocytes are believed to play a major role in the regulation of the extracellular potassium ([K+]o), calcium ([Ca2+]o), and glutamate levels during and after neuronal excitation (Xiong and Stringer, 1999; Lian and Stringer, 2004a; Larrosa et al, 2006). It was reported that dysfunction of astrocytes in the brain led to epileptiform activity and occasionally to convulsive seizures (Willoughby et al, 2003; Lian and Stringer, 2004b). Moreover, it has been also suggested that inhibition of metabolism in astrocytes increases neuronal vulnerability to SD (Lian and Stringer, 2004c; Canals et al, 2008). Although astrocytes are thought to play a crucial role in the maintenance of neuronal activity as well as relieving neuronal damage during intense neuronal excitation, involving SD and seizures, the regulatory system for supplying astrocytes by neural activity remains unknown.
In a previous study, we showed that SD facilitated the proliferation of progenitor cells, which were immunopositive for NG2 (NG2 cells) in the cerebral cortex and that the extent of proliferation depended on the number of induced SD (Tamura et al, 2004; Kataoka et al, 2006). The NG2 cells are widely and abundantly present in the adult brain (Dawson et al, 2000) and can give rise to oligodendrocytes and neurons, but not to astrocytes under the same physiological conditions (Dawson et al, 2003; Dayer et al, 2005; Tamura et al, 2007a). On the other hand, the NG2 cells were reported to generate astrocytes during perinatal development (Zhu et al, 2008; Guo et al, 2009) and in the adult brain and spinal cord which underwent acute injury with neuronal death or gliosis (Hampton et al, 2004; Magnus et al, 2007; Zhao et al, 2009; White et al, 2010). Based on these findings, we investigate here whether astrogenesis occurs in the cerebral cortex following cortical SD, which is a potent stimulus for NG2 cell proliferation without cell death. The present study demonstrated differentiation of NG2 cells after cell division in the SD-induced cortex, and evaluated the effect of the extent of SD stimuli on cell fate using immunohistochemical techniques.
Materials and methods
Animals and Induction of Spreading Depression
Adult male Wistar rats (SLC, Hamamatsu, Japan; 8 to 9 weeks old) were used. All experimental protocols were approved by the Ethics Committee on Animal Care and Use of RIKEN and were performed in accordance with the Principles of Laboratory Animal Care (NIH publication No. 85-23, revised 1985). Rats were housed at a constant temperature (20°C) and humidity (50%) and maintained under a 12-hour light/dark cycle with free access to food and water.
Rats were anesthetized throughout surgery for induction of SD with isoflurane (4% for induction, 1% for maintenance) in a mixture of N2O and O2 (7:3). Rectal temperature was maintained at 37.0°C±0.4°C using a thermostatic heating pad.
Rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). The skull was exposed and four holes were drilled symmetrically into the skull overlying the right and left hemispheres of the cortex using a saline-cooled drill. Two holes (1.0 mm posterior and 1.5 mm lateral to bregma) were placed for applications of 1 mol/L KCl and 1 mol/L NaCl, respectively, and the other two holes (3.0 mm posterior and 1.5 mm lateral to bregma) were placed for recording the CBF. The dura covering the occipital cortex was gently removed and care was taken to prevent dehydration. Each probe for laser Doppler flowmetry (type FLO-N1; ω-wave) was positioned on the dural surface of the occipital cortex for continuous recordings of CBF. It is known that transient hyperperfusion of CBF is accompanied by temporal negative shift of direct current potential (Fabricius et al, 1995; Cui et al, 2003; Ayata et al, 2004). The number of SD events was evaluated by the transient CBF hyperperfusion. Rats were divided into three groups: (1) no application of KCl or NaCl solution (normal group; N=4); (2) single application of these solutions (mildly stimulated group; N=4); and (3) four applications of KCl or NaCl (highly stimulated group; N=4). In the mildly stimulated group, each cotton pad soaked with KCl or NaCl solution was placed together on the pial surface through each drilled hole for 10 minutes, and then each cotton pad was replaced with one soaked with physiological saline for 20 minutes. The total number of KCl-induced SD was recorded during 30 minutes after KCl application. In highly stimulated rats, a series of these procedures were repeated four times and the total number of SD episodes was monitored during 120 minutes. The operation for SD induction was conducted between 14:00 to 17:00.
5′-Bromodeoxyuridine Administration and Tissue Preparation
All animals were injected intraperitoneally with 5′-bromodeoxyuridine (BrdU; 50 mg/kg). 5′-Bromodeoxyuridine was injected 5 hours after SD induction, and then injected three times a day for 3 days (10:00, 15:00, 20:00). At 28 days after SD induction, animals were deeply anesthetized with diethyl ether and perfused transcardially with 4% paraformaldehyde buffered with 0.1 mol/L phosphate-buffered saline (PBS; pH 7.4). The brains were removed, postfixed in 4% paraformaldehyde buffered with 0.1 mol/L PBS at 4°C overnight and then immersed in 30% (w/v) sucrose solution. Coronal brain sections (30-μm thickness) were prepared using a cryostat and were collected as free-floating sections.
Immunohistochemical Staining
For BrdU staining, the brain sections were preincubated in 2 N HCl at 37°C for 30 minutes and rinsed in 0.1 mol/L boric acid (pH 8.5) at 25°C for 10 minutes. The sections were incubated with 1% hydrogen peroxide for 30 minutes to eliminate endogenous peroxidase. After washing, they were incubated with blocking solution (3% normal rabbit serum in PBS containing 0.3% Triton X-100) for 30 minutes. Then, sections were incubated with monoclonal rat anti-BrdU IgG (1:1,000; Abcam, Cambridge, MA, USA) with blocking solution at 4°C for 12 hours followed by incubation with biotinylated anti-rat IgG antibody (1:200; Vector Lab. Inc., Burlingame, CA, USA). Immunohistochemical detection was performed using the avidin–biotin–peroxidase complex method (PK-4000, Vector Lab. Inc.) with 3,3′-diaminobenzidine-reaction solution. For triple immunostaining, coronal sections were preincubated in 2 N HCl at 37°C for 30 minutes and rinsed in 0.1 mol/L boric acid (pH 8.5) at 25°C for 10 minutes and were incubated with several primary antibodies at 4°C for 12 to 15 hours. The primary antibodies used in the present study included monoclonal rat anti-BrdU IgG (1:1,000; Abcam), monoclonal mouse anti-NG2 IgG (1:200; Millipore, Billerica, MA, USA), polyclonal rabbit anti-NG2 IgG (1:200; Millipore), monoclonal mouse anti-glial fibrillary acidic protein (GFAP) IgG (1:200; Sigma, St Louis, MO, USA), polyclonal rabbit anti-GFAP IgG (1:200; Sigma), monoclonal mouse anti-S100β IgG (1:200; Sigma), polyclonal rabbit anti-glutathione S-transferase (GST)-pi IgG (1:500; Medical & Biological Laboratories, Nagoya, Japan), polyclonal rabbit anti-Iba1 IgG (1:300; Wako Pure Chemical Industries, Osaka, Japan), goat polyclonal rat anti-nestin IgG (1:60; R&D Systems, Minneapolis, MN, USA), mouse monoclonal rat anti-nestin IgG (1:300; Millipore). After washing for 30 minutes (three washes of 10 minutes) with 0.3% Triton X-100 in phosphate-buffered saline (PBST), the brain sections were incubated in the appropriate secondary antibodies conjugated with either Cy2, Cy3, or Cy5 (1:200; Jackson ImmunoResearch, West Grove, PA, USA) at 4°C for 4 hours and washed with PBST for 30 minutes. Some stained sections were mounted with Hoechst dye 33258 (Nacalai Tesque Inc., Kyoto, Japan) or TO-PRO3 (1:1,000; Molecular Probes, Eugene, OR, USA) -containing solution and were examined using a confocal laser microscope (Digital Eclipse C1; Nikon, Tokyo, Japan). Twenty-six confocal images were taken at 1 μm steps along the Z-stacks in each area. The first three and last three images of each image stack were excluded to avoid inclusion of truncated cells in the data.
Cell Counting Procedure and Statistical Analysis
For cell counting, three to five coronal sections were randomly selected at 80-μm intervals from 0.00 to 3.80 mm posterior to the bregma in each animal. The numbers of all BrdU-labeled cells and the cells expressing each cellular marker (NG2, GFAP, S100β, GST-pi, and Iba1) were evaluated in each rectangular area (1.27 × 1.59 mm2) within the cortical layers I to VI of the cerebral cortex (insular, rhinal, and temporal association cortex) at the rhinal fissure in each cortical hemisphere. These cortical areas were determined in accordance with our previous study (Tamura et al, 2004) and were apart from the parietal cortex to avoid direct effect of KCl-application. Data from each animal were shown as mean values±s.e. Statistical analysis was performed using one-way analysis of variance followed by post hoc Tukey's test for comparison among groups. Data were analyzed using paired t-test for comparison between the ipsilateral and contralateral cortices. Significance threshold was assumed at P<0.05. Coefficients of correlation were analyzed by Pearson's test using SPSS Statistics Student software.
Results
Induction of Spreading Depression in Adult Cortex
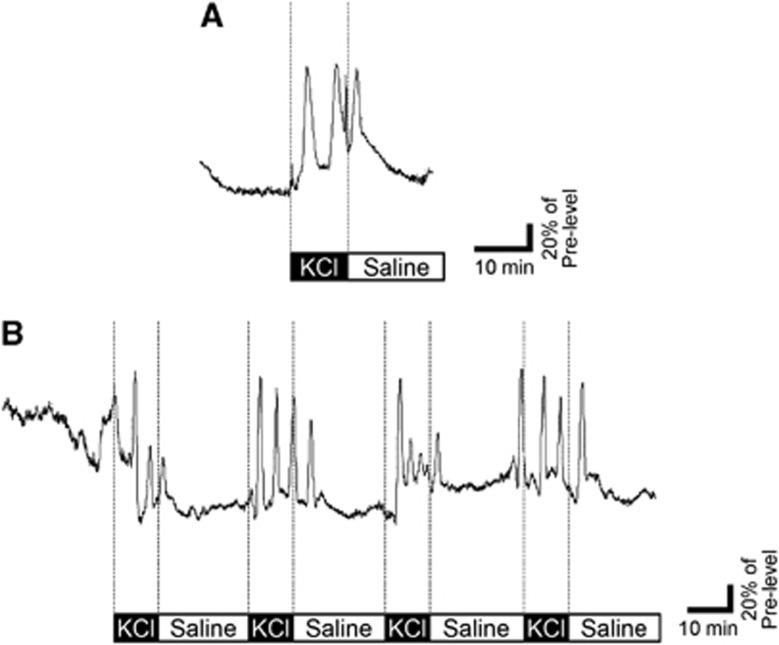
Placing a cotton absorbent soaked with 1 mol/L KCl on the surface of parietal cortex induced repetitive SD events, which were identified by transient elevations of CBF; however, 1 mol/L NaCl did not show any events. Single application of the KCl-containing cotton for 10 minutes induced 2 to 3 SD events for a duration of 30 minutes (Figure 1A, mildly stimulated group). Four applications of the KCl cotton evoked 10 to 18 SD episodes during 120 minutes (Figure 1B, highly stimulated group).
Figure 1.
Induction of spreading depression (SD) events in the mildly or highly stimulated rat cortex. Representative tracings of cortical blood flow (CBF) recorded from parietal cortex. Topical application of KCl solution induced 3 SD events in the mildly stimulated rat cortex (A). Repetitive application of KCl evoked 15 SD episodes in the highly stimulated rat cortex (B). Black boxes show timing of KCl application for 10 minutes and white boxes indicate application of saline for 20 minutes.
The Number of Newly Generated Cells Increased Depending on the Spreading Depression Stimulus
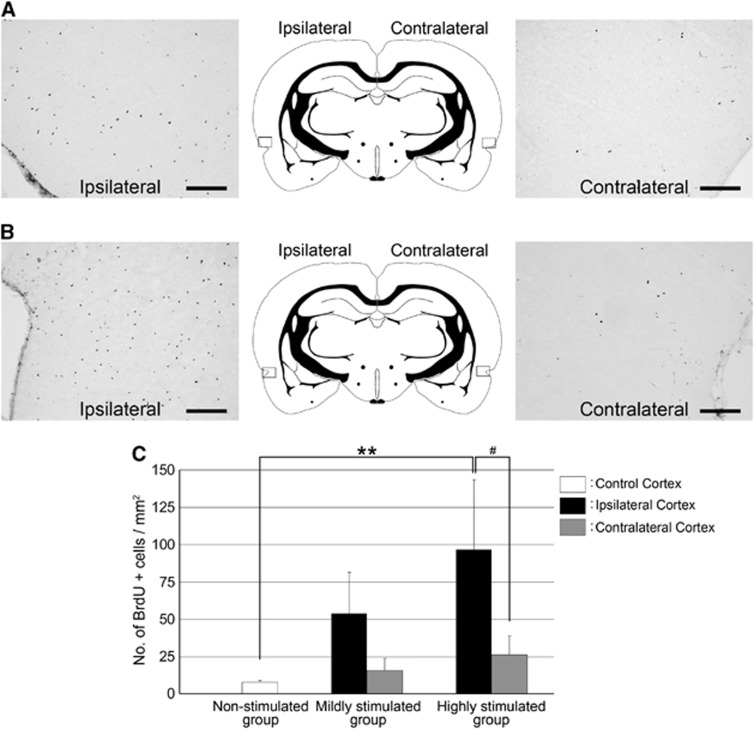
A previous study showed that the number of proliferative cells was dramatically increased in the SD-induced cortex for 3 days after the SD stimulus (Tamura et al, 2004). At 28 days after SD, a lot of BrdU-labeled cells were observed in the SD-induced cortex compared with the contralateral cortex of both the mildly stimulated group (Figure 2A) and the highly stimulated group (Figure 2B). The number of BrdU-incorporated cells in the highly stimulated cortex showed a trend to be more than that in the mildly or nonstimulated cortex (Figure 2C).
Figure 2.
Spreading depression (SD) induction increased the number of proliferating cells in the cerebral cortex depending on the number of SD events. The number of 5′-bromodeoxyuridine (BrdU)-labeled cells (black) in the ipsilateral cortex was higher than that in the contralateral cortex of the mildly stimulated group (A) and highly stimulated group (B). The locations of photographs are shown as rectangles in the schematic illustrations of the brain. Scale bar, 200 μm. (C) The numbers of BrdU-labeled cells in the SD-induced cortex (black bars) and in the contralateral cortex (gray bars) of mildly stimulated and highly stimulated groups, and the number of labeled cells in the control cortex (white bar) of nonstimulated group. The data are shown as mean±s.e. (n=4 animals); **P<0.01, #P<0.05.
Phenotypes of Newly Generated Cells Following Spreading Depression Induction
To determine characterization of the newly generated cells, we performed immunofluorescent staining using antibodies against each cellular marker and BrdU. In the ipsilateral cortex to SD induction, BrdU-labeled cells were composed of Iba1-immunopositive (Iba1+) microglia, NG2 cells, GST-pi–immunopositive (GST-pi+) oligodendrocytes, and GFAP-immunopositive (GFAP+) astrocytes, whereas in the contralateral cortex, the newly generated cells consisted of Iba1+ microglia, NG2 cells and GST-pi+ oligodendrocytes in both stimulated groups (Table 1).
Table 1. Absolute number of cells immunopositive for various cell differentiation markers in all BrdU-labeled cells at 28 days after SD.
| Nonstimulated group |
Mildly stimulated group |
Highly stimulated group |
|||
|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | ||
| BrdU+ GFAP+ cells | 0 | 19±9 | 0 | 57±19* | 0 |
| BrdU+ GST-pi+ cells | 74±13 | 106±40 | 102±28 | 41±10# | 105±26 |
| BrdU+ NG2+ cells | 591±64 | 686±310 | 732±135 | 692±89 | 771±139 |
| BrdU+ Iba1+ cells | 97±19 | 1463±785 | 141±58 | 4092±1480 | 678±75* |
BrdU, 5′-bromodeoxyuridine; GFAP, glial fibrillary acidic protein; GST-pi, glutathione S-transferase-pi; SD, spreading depression.
Data are presented as mean±s.e. (absolute number/mm3), n=4 animals at each group. *P<0.05: significant difference from the control cortex of nonstimulated group. #P<0.05: significant difference between the ipsilateral cortex and contralateral cortex within the same group.
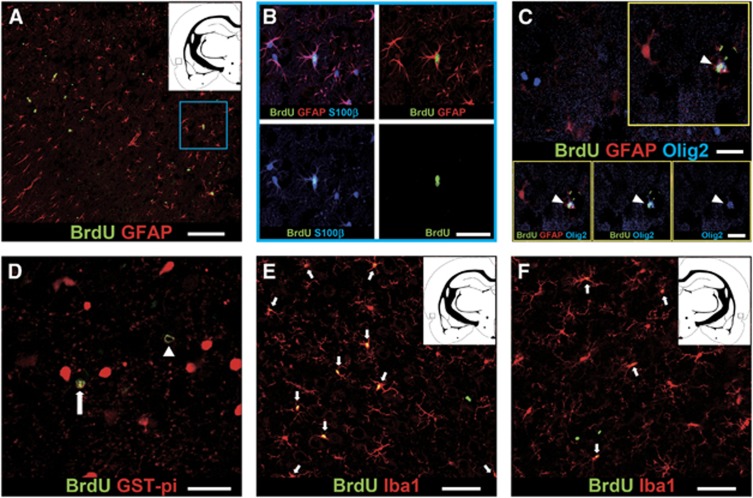
As shown in Figure 3A, BrdU+ GFAP+ astrocytes were found only in the ipsilateral cortex but were not observed in the contralateral cortex. The cells were also immunopositive for S100β and were often observed in doublets (Figure 3B). The newly generated astrocytes had complex bushy processes and appeared to be protoplasmic astrocytes in the gray matter (Bushong et al, 2004). The absolute number of these cells in the highly stimulated group was more than that in the mildly stimulated group (Table 1). These observations indicated that newborn astrocytes were produced by SD stimulation. Next, we confirmed whether the newly generated astrocytes were produced following proliferation of NG2 cells in the SD-induced cortex. Recently, the NG2 cells can give rise to a subset of astrocytes in response to acute brain injury by translocation of oligodendrocyte transcription factor 2 (Olig2), which is located in the nuclei of NG2+ progenitor cells, to the cytoplasm (Cassiani-Ingoni et al, 2006; Zhao et al, 2009). In our study, Olig2 was occasionally found only in the cytoplasm of the BrdU-labeled S100β+ astrocytes (Figure 3C). Therefore, some newborn astrocytes might be produced from NG2 cells following the SD stimulus.
Figure 3.
Phenotype characterizations of 5′-bromodeoxyuridine (BrdU)-incorporated newly generated cells at 28 days after spreading depression (SD) stimulus. (A) Confocal image of BrdU-labeled (green) glial fibrillary acidic protein (GFAP+) (red) cells in the highly stimulated rat. Scale bar, 100 μm. (B) Magnified views of the area indicated by a blue square are shown in panel A. Immunostaining for BrdU (green), GFAP (red), and S100β (blue) in the SD-induced cortex of the highly stimulated rat. Scale bar, 50 μm. (C) Newly generated astrocytes were generated from proliferating NG2 cells with Olig2 expression. Triple immunostaining for BrdU (green), S100β (red), and Olig2 (blue) was performed in the SD-induced cortex of highly stimulated rat. Arrowhead, BrdU+ S100β+ mature astrocyte was immunoreactivity with Olig2 in the cytoplasm. Scale bar, 20 μm. (D) Confocal image of BrdU (green) and glutathione S-transferase (GST)-pi (red) staining. Arrowhead, BrdU+ mature oligodendrocyte with immunoreactivity for GST-pi in the cytoplasm. Arrow, BrdU+ cell expressing GST-pi in the nucleus is NG2 cell (referred to in Tamura et al, 2007b). Scale bar, 50 μm. Immunostaining for BrdU (green) and Iba1 (red) in the SD-induced cortex (E) and in the contralateral cortex (F) of highly stimulated rat. Arrows, newborn microglia are immnoreactive for BrdU (green) and Iba1 (red). Scale bar, 30 μm. The locations of photographs are shown as squares in the insets in (A, E, F).
In the physiological condition, almost all newly generated cells are NG2 cells and approximately 10% of those cells differentiate into GST-pi+ oligodendrocytes (Dawson et al, 2003, Tamura et al, 2007a). It was also reported that GST-pi protein translocates from the cell nuclei to the cytoplasm during the oligodendrocyte-lineage: NG2+ oligodendrocyte progenitor cells show GST-pi protein in the cell nuclei, and RIP+ mature oligodendrocytes show the protein mainly in the cytoplasm (Tamura et al, 2007b). At 28 days after SD stimulation, newly generated mature oligodendrocytes with immunoreactivity for GST-pi in the cytoplasm were found both in the ipsilateral and in the contralateral cortices (Figure 3D). In the highly stimulated group, however, the absolute number of BrdU+ GST-pi+ oligodendrocytes in the ipsilateral cortex was less than those in the contralateral cortex and in normal (nonstimulated) cortex (Table 1). Furthermore, the proportion of newly generated oligodendrocytes in all the BrdU-labeled cells reached only 1.8%±0.3% in the ipsilateral cortex of the highly stimulated group, whereas the proportion was ∼10% in the contralateral cortex (Supplementary Table 1). These data suggest that oligodendrogenesis may be suppressed by SD stimuli, in contrast to astrogenesis.
Previously, we reported that almost all proliferating cells were NG2 cells and OX-42 expressing microglia in the cortex within 3 days following SD stimuli (Tamura et al, 2004). At 28 days after SD induction, most BrdU-labeled cells were also composed of NG2 cells and Iba1+ microglia both in the ipsilateral and in the contralateral cortices. Immunostaining showed that the absolute number of newborn NG2 cells did not show significant difference between both cortices of all groups (Table 1).
In the SD-induced cortex, BrdU-labeled Iba1+ microglia were a major population of newly generated cells at 28 days after SD. The absolute numbers of newly generated Iba1+ microglia in both cortices of both mildly and highly stimulated groups were much higher than those of nonstimulated group, and the numbers of newborn microglia increased with the number of SD (Table 1). Furthermore, the proportion of newly generated Iba1+ microglia in all the BrdU-labeled cells were 51.2%±24.0% and 80.9%±3.3% in the ipsilateral side and 20.9%±4.6% and 52.5%±7.7% in the contralateral side of the mildly and highly stimulated rats, respectively (Figures 3E and 3F; Supplementary Table 1). Thus, increases in newly generated microglia were not specific phenomena in the SD-induced cortex.
The Relation Between the Number of Spreading Depression Events and Astrogenesis or Oligodendrogenesis
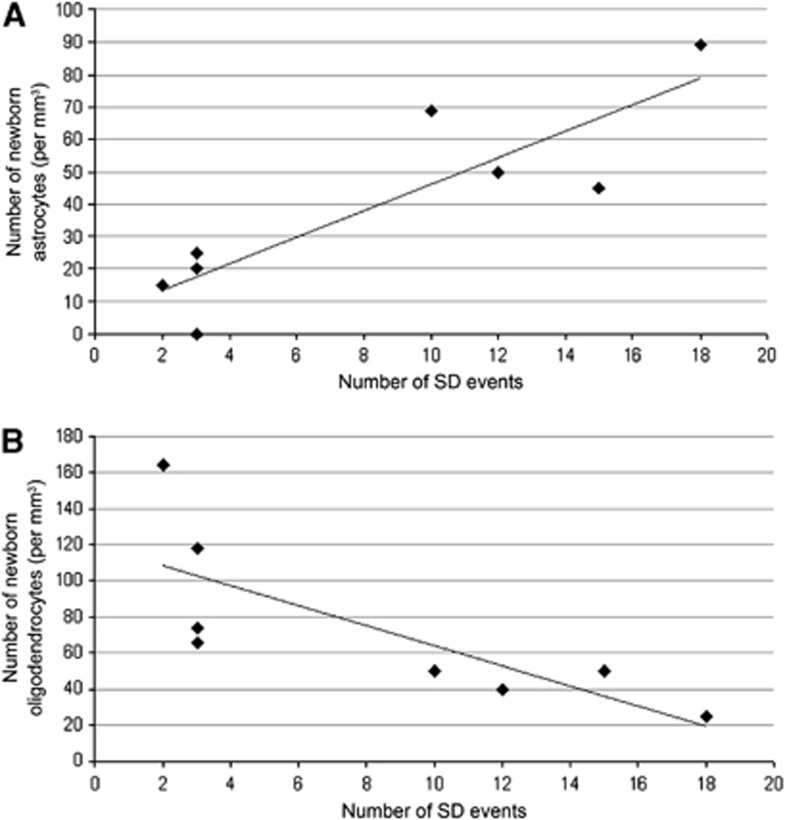
A cell counting study of newly generated astrocytes and oligodendroytes revealed enhancement of astrogenesis and suppression of oligodendrogenesis in the SD-induced cortex. To examine the relationship between gliogenesis and the number of SD events in more detail, we performed Pearson correlation coefficient analysis. The analysis showed a significant positive correlation between the number of SD events and newly generated astrocytes (r=0.873; P<0.01), and a significant negative correlation between the number of SD events and newly generated oligodendrocytes (r=−0.769; P<0.05) (Figure 4). These results suggest that the fate decision of NG2 cells shifts from oligodendrocytes to astrocytes depending on the SD stimuli.
Figure 4.
Correlation between the number of spreading depression (SD) events and the number of astrocytes (A) or oligodendrocytes (B) being generated. The number of newly generated astrocytes was proportional to the increase in the number of SD events (A), whereas the production of oligodendrocytes tended to be inversely related to the number of SD events (B).
Discussion
In the present study, we showed that SD-induced astrogenesis occurred only in the stimulated cortex, but not in the contralateral or in the normal cortex (Figures 3A and 3B; Table 1). Furthermore, the efficacy of astrogenesis depended on the number of SD episodes (Figure 4) and the newly generated astrocytes were derived from NG2 cells following their proliferation (Figure 3C). Contrary to astrogenesis, the production of oligodendrocytes from NG2 cells was reduced depending on the number of SD episodes (Figure 4; Table 1). Therefore, SD shifts the cell fate determination of NG2 cells from oligodendrogenesis to astrogenesis.
During mammalian development, NG2 cells can generate a subpopulation of protoplasmic astrocytes in the brain and spinal cord (Zhu et al, 2008; Komitova et al, 2009; Dimou et al, 2008; Guo et al, 2009). In the adult brain, they can also give rise to astrocytes in response to several brain injury with cell death (Magnus et al, 2007; Tripathi et al, 2010; White et al, 2010); however, the cells do not appear to generate astrocytes under normal conditions (Tamura et al, 2007a; Dimou et al, 2008; Rivers et al, 2008). In the present study, astrogenesis occurred without cell death in the ipsilateral cortex of adult rats following SD, neuronal and glial depolarization (Figures 3A and 3B). None of apoptotic cells was found in the observed cortices of both ipsilateral and contralateral sides using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) for detecting apoptotic cells. Other research groups also reported that cortical SD did not induce cell death (Nedergaard and Hansen, 1993; Yanamoto et al, 2005). Statistical analysis indicated a significantly positive correlation between the number of SD events and astrogenesis (P=0.005). It has been reported that cytokines, including LIF (leukemia inhibitory factor), bone morphogenetic proteins (BMPs), and IL-6 (interleukin-6), facilitated differentiation of neural stem/progenitor cells into astrocytes (Bonaguidi et al, 2005; Nakanishi et al, 2007). In our study, reverse transcriptase polymerase chain reaction analysis of cortical tissues indicated the upregulation of those gene expressions (LIF, 1.5 to 2.9 times; BMP4, 1.1 to 1.5 times; IL-6, 1.1 to 1.3 times) in the ipsilateral cortex compared with the contralateral side at 24 and 48 hours after SD stimuli, although the expression of BMP2 did not change. Thus, it indicated the possibility that these cytokines induced by SD contributed to astrogenesis. Furthermore, we suggested that the newly generated astrocytes were derived from the proliferating NG2 cells. The source of newly generated astrocytes is thought to be dedifferetiated GFAP+ astrocytes or NG2 cells that are present in the parenchyma. In the present study, we observed that 55% and 45% of BrdU-labeled cells were NG2+ cells and Iba1+ microglia (including OX-42+ microglia), respectively, but not GFAP+ cells in the SD-induced cortex within 3 days after induction of SD (unpublished data). These observations were consistent with our previous report (Tamura et al, 2004). At 3 days after SD induction, only NG2+ cells contained Olig2 in the nuclei, but not Iba1+ microglia in BrdU-labeled cells. At 28 days, Olig2 expression was observed in the nuclei of NG2 cells and in the cytoplasm of S100β+ astrocytes (Figure 3C) in BrdU-labeled cells. These findings were supported by previous reports that Olig2-expressing NG2 cells can differentiate into S100β+ astrocytes accompanied by the translocation of Olig2 from the nuclei into the cytoplasm (Cassiani-Ingoni et al, 2006; Zhao et al, 2009). It has been reported that expression of Olig2 was also observed in reactive astrocytes (Tatsumi et al, 2008). In the present study, however, BrdU-labeled astrocytes did not express nestin, a marker for reactive astrocytes (Supplementary Figure 1). Moreover, never Iba1+ microglia contained Olig2 at 3 days and 28 days after SD induction (unpublished data). These observations suggested that the newborn astrocytes were derived from NG2 cells. Although progenitor cells migrating from the SVZ (subventricular zone) were also a candidate for newborn astrocytes in the cerebral cortex. However, the BrdU-labeled cell density in the SVZ was not affected by SD induction (Yanamoto et al, 2005). This finding suggests that origin of the newborn cells in the cortex was unlikely to be progenitor cells in the SVZ.
Oligodendrocytes have been reported to be produced from NG2 cells (Dawson et al, 2003, Tamura et al, 2007a). In the ipsilateral cortex, the absolute number of newly generated oligodendrocytes in the highly stimulated group was much less than the numbers in the mildly and the nonstimulated groups (Table 1). In the present study, the proportion of newly generated oligodendrocytes in all newborn cells was reduced depending on the extent of SD stimuli in the highly stimulated group (Supplementary Table 1). Taken together, the production of oligodendrocytes showed a trend to be reduced depending on the number of SD. Indeed, statistical analysis indicated a significantly negative correlation between the number of SD episodes and oligodendrogenesis (P=0.027). The decreased oligodendrogenesis is not thought to be attributed to cell death following SD, since none of TUNEL-positive cells was found in the cerebral cortex. It has been known that BMPs are concerned with regulating the production of both oligodendrocytes and astrocytes. Application of BMPs to cultured cells inhibited the production of oligodendrocytes and facilitated the generation of new astrocytes in vitro (Grinspan et al, 2000; See et al, 2004). During development, overexpression of BMP4 showed both the decrease in oligodendrogenesis and the facilitation of astrogenesis in vivo (Gomes et al, 2003). In addition to BMP4, we observed upregulation of BMP type II receptor (BMPRII) expression in the SD-induced cortex at 48 hours after induction of SD (1.5 times versus contralateral side), suggesting the involvement of BMP signaling in the shift of differentiation of NG2 cells from oligodendrogenesis to astrogenesis.
Astrocytes have been postulated to protect neurons undergoing intense neuronal excitation, including seizures and SD. The neuroprotective activity of astrocytes is thought to be associated with regulation of extracellular concentrations of ions and glutamate (Xiong and Stringer, 1999; Lian and Stringer, 2004a; Larrosa et al, 2006). In fact, recovery of the [K+]o and [Ca2+]o was slowed in the SD-induced cortex, when the astrocyte function was selectively suppressed by reversible glial toxins (Lian and Stringer, 2004a). Moreover, the functional disruption of astrocytes facilitates the susceptibility to chemical convulsants (Lian and Stringer, 2004b) and leads to convulsive seizures (Willoughby et al, 2003). Thus, the shift of NG2 cell fate from oligodendrogenesis to astrogenesis following SD stimuli shown in the present study might be an important adaptive system for preventing neuronal damage and building up tolerance for subsequent neuronal hyperexcitation.
In summary, we demonstrated for the first time that neural excitation-facilitated astrocyte production occurs only in the stimulated cortex and the cell fate of NG2 cells is shifted from oligodendrogenesis to astrogenesis. The newly generated astrocytes will be incorporated into circuits underlying the maintenance of brain functions by tissue remodeling.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported in part by JST, CREST, and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and the Japanese Government.
Supplementary Material
References
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, Moskowitz MA. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Canals S, Larrosa B, Pintor J, Mena MA, Herreras O. Metabolic challenge to glia activates an adenosine-mediated safety mechanism that promotes neuronal survival by delaying the onset of spreading depression waves. J Cereb Blood Flow Metab. 2008;28:1835–1844. doi: 10.1038/jcbfm.2008.71. [DOI] [PubMed] [Google Scholar]
- Cassiani-Ingoni R, Coksaygan T, Xue H, Reichert-Scrivner SA, Wiendl H, Rao MS, Magnus T. Cytoplasmic translocation of Olig2 in adult glial progenitors marks the generation of reactive astrocytes following autoimmune inflammation. Exp Neurol. 2006;201:349–358. doi: 10.1016/j.expneurol.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Cui Y, Kataoka Y, Li QH, Yokoyama C, Yamagata A, Mochizuki-Oda N, Watanabe J, Yamada H, Watanabe Y. Targeted tissue oxidation in the cerebral cortex induces local prolonged depolarization and cortical spreading depression in the rat brain. Biochem Biophys Res Commun. 2003;300:631–636. doi: 10.1016/s0006-291x(02)02906-6. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors. J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius M, Akgoren N, Lauritzen M. Arginine-nitric oxide pathway and cerebrovascular regulation in cortical spreading depression. Am J Physiol. 1995;269:H23–H29. doi: 10.1152/ajpheart.1995.269.1.H23. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- James MF, Smith JM, Boniface SJ, Huang CL, Leslie RA. Cortical spreading depression and migraine: new insights from imaging. Trends Neurosci. 2001;24:266–271. doi: 10.1016/s0166-2236(00)01793-8. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Tamura Y, Takamori Y, Cui Y, Yamada H. Perineuronal germinal cells in the rat cerebral cortex. Med Mol Morphol. 2006;39:28–32. doi: 10.1007/s00795-006-0307-x. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhu X, Serwanski DR, Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol. 2009;512:702–716. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrosa B, Pastor J, Lopez-Aguado L, Herreras O. A role for glutamate and glia in the fast network oscillations preceding spreading depression. Neuroscience. 2006;141:1057–1068. doi: 10.1016/j.neuroscience.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res. 2004a;1012:177–184. doi: 10.1016/j.brainres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Inhibition of aconitase in astrocytes increases the sensitivity to chemical convulsants. Epilepsy Res. 2004b;60:41–52. doi: 10.1016/j.eplepsyres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Energy failure in astrocytes increases the vulnerability of neurons to spreading depression. Eur J Neurosci. 2004c;19:2446–2454. doi: 10.1111/j.0953-816X.2004.03289.x. [DOI] [PubMed] [Google Scholar]
- Magnus T, Coksaygan T, Korn T, Xue H, Arumugam TV, Mughal MR, Eckley DM, Tang SC, Detolla L, Rao MS, Cassiani-Ingoni R, Mattson MP. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85:2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:568–574. doi: 10.1038/jcbfm.1993.74. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun SB, Mamontov P, Golden JA, Grinspan JB. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–492. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007a;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Intracellular translocation of glutathione S-transferase pi during oligodendrocyte differentiation in adult rat cerebral cortex in vivo. Neuroscience. 2007b;148:535–540. doi: 10.1016/j.neuroscience.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Yamada H. Cellular proliferation in the cerebral cortex following neural excitation in rats. Neurosci Res. 2004;50:129–133. doi: 10.1016/j.neures.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, Wanaka A. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res. 2008;86:3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ. Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells. 2010;28:297–307. doi: 10.1002/stem.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L, Broberg M, Thoren AE, Medvedev A, Sims NR, Nilsson M. Fluorocitrate-mediated astroglial dysfunction causes seizures. J Neurosci Res. 2003;74:160–166. doi: 10.1002/jnr.10743. [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Stringer JL. Astrocytic regulation of the recovery of extracellular potassium after seizures in vivo. Eur J Neurosci. 1999;11:1677–1684. doi: 10.1046/j.1460-9568.1999.00587.x. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Miyamoto S, Tohnai N, Nagata I, Xue JH, Nakano Y, Nakajo Y, Kikuchi H. Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke. 2005;36:1544–1550. doi: 10.1161/01.STR.0000169903.09253.c7. [DOI] [PubMed] [Google Scholar]
- Zhao JW, Raha-Chowdhury R, Fawcett JW, Watts C. Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice. Eur J Neurosci. 2009;29:1853–1869. doi: 10.1111/j.1460-9568.2009.06736.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.