Abstract
Excessive amounts of salt in food, as usually consumed worldwide, affect the vascular system, leading to high blood pressure and premature disabilities. Salt entering the vascular bed after a salty meal is transiently bound to the endothelial glycocalyx, a negatively charged biopolymer lining the inner surface of the blood vessels. This barrier protects the endothelium against salt overload. A poorly-developed glycocalyx increases the salt permeability of the vascular system and the amount of salt being deposited in the body, which affects organ function. A simple test system is now available that evaluates vascular salt sensitivity in humans and identifies individuals who are at risk of salt-induced hypertension. This short review aims to discuss how the underlying basic research can be translated into medical practice and, thus, meaningful health outcomes.
Introduction
For millions of years, daily salt (sodium chloride) intake in man was about 1g. Then recently, about 10,000 years ago, salt intake increased by about ten-fold [1,2] because of the practice of using salt as a food preservative. It allowed former nomads to settle, grow grain and preserve foodstuffs over long periods. Over the last few millennia, humans got used to the taste of salt and enjoyed the benefits of non-perishable food [3]. However, the human genome could not adapt so quickly. Genetically, humans are well-equipped with mechanisms that retain even tiny amounts of salt, a prerequisite for survival at those times when salt was scarce and intake was low. In keeping with this background, humans have less efficient excretory mechanisms when challenged with large salt loads, and the limiting factor is the rate of renal salt excretion [4]. If salt intake exceeds the kidneys’ ability for salt excretion, then salt is deposited in the body, which, in synergy with aldosterone, affects heart [5], blood vessels [6] and kidneys [7]. Arterial hypertension, stroke and cardiac infarction are often the end result.
A paradigm shift
Not everybody is sensitive to salt. It is estimated that at least 30% of the world's population develop hypertension (elevated blood pressure) when exposed to a high salt diet [8]. In the past, salt sensitivity was thought to be the result of kidney malfunction, i.e. on the imbalance between salt input and salt output [9]. However, recent observations suggest that the vascular system may also play an important role in this imbalance [10]. More than 20 years ago, it was shown that the vascular endothelium expresses sodium channels similar to those in renal epithelia [11]. Some years later, it was demonstrated that sodium channel function in endothelium was regulated, much as it is in the kidney, by the mineralocorticoid hormone aldosterone [12]. The fact that the vascular system is also a potential target for aldosterone led to a paradigm shift in so far as the attention was no longer directed solely to the kidney but also to the vascular system [13-20].
Endothelium senses salt
Currently, there are far more data on the pathophysiology of aldosterone affecting vascular function than on the “normal” vascular physiology of this steroid hormone [21-33]. Sodium and aldosterone synergistically act on the endothelium. At cellular level, small changes in plasma sodium concentration can have a large impact on endothelial function as long as aldosterone (or aldosterone receptor function) is available [34]. Even a 5% increase in plasma sodium concentration mechanically stiffens endothelial cells by about 25%, leading to cellular dysfunction (decreased nitric oxide release/increased vascular smooth muscle tone). A major component of this high sodium sensitivity is the sodium channel in the endothelial plasma membrane [11,12,35], which is identical to the epithelial sodium channel cloned from renal tissue [36]. This channel allows sodium to enter the endothelial cells [37] and, by yet unknown mechanisms, turn off endothelial nitric oxide synthase activity [38].
Do these in vitro experiments translate into the in vivo setting and explain how plasma sodium as such affects blood pressure? This question is not easy to answer since changes in plasma sodium are usually accompanied by changes in osmolality, which may mask any direct action of sodium. However, a recent study properly corrected for any changes in osmolality, shows that there is indeed a marked alteration in blood pressure observable when plasma sodium is manipulated [39]. Similarly, blood pressure in dialysis patients is known to decrease when sodium concentration in the dialysate is lowered [40]. Furthermore, small but significant changes in plasma sodium, paralleled by concomitant changes in blood pressure, are known to occur in humans during acute or chronic salt intake [41,42]. Finally, there is experimental evidence that the brain may be involved in the sodium-triggered increase of blood pressure [41,43]. Blaustein and colleagues postulated an interesting hypothesis, namely that high sodium in the cerebrospinal fluid triggers the secretion of endogenous ouabain in the hypothalamus and suprarenal glands [44]. Endogenous ouabain acts in the brain, increasing sympathetic nerve activity, but also acts on blood vessels. Both endogenous ouabain actions lead to vasoconstriction and increase in blood pressure [45]. For obvious reasons, this more complex mechanism, involving different organs, cannot be used to explain the in vitro effect of sodium.
Endothelial glycocalyx, sodium buffer and barrier
Most recently, the endothelial surface layer facing the blood stream has become a focus of interest [46-54]. This soft layer, termed endothelial glycocalyx, is a negatively charged biopolymer known to preferentially bind sodium [55]. It has been calculated that about 700 mg of sodium can be transiently bound to the endothelial glycocalyx in the human body, which is about the amount contained in a single meal [10]. Interestingly, the sodium buffering capacity of the endothelial glycocalyx is severely damaged by excessive sodium intake over time, leading to a significant reduction of the negatively charged heparan sulphate residues in the endothelial glycocalyx [56].
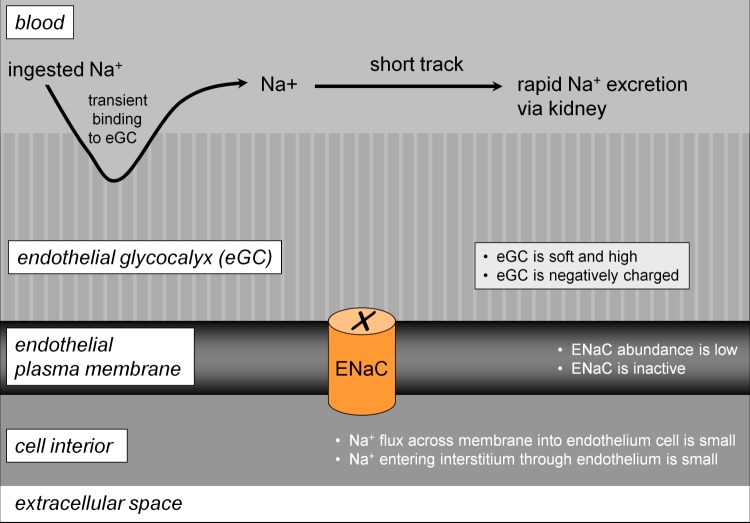
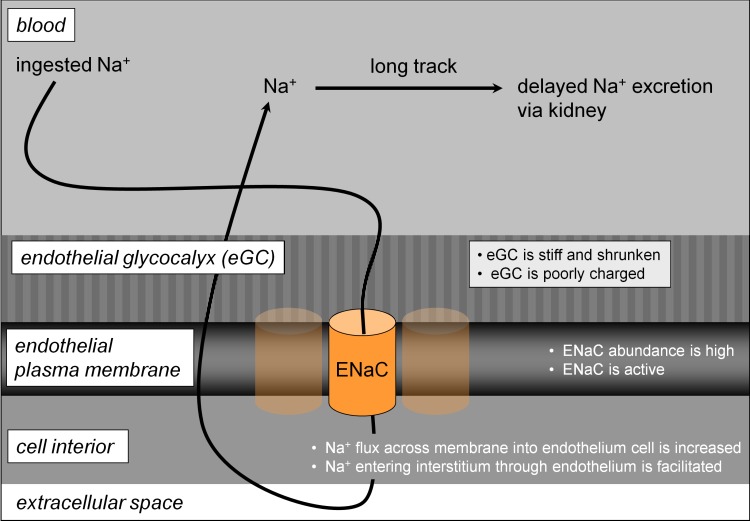
These observations led to a new concept of vascular sodium permeability, namely that two (more or less) permeable barriers determine the rate of sodium elimination after a salty meal [10]. One barrier is the endothelial plasma membrane, with a variable sodium permeability depending on the abundance of epithelial sodium channels. The other barrier, located on the surface of the endothelium, is the endothelial glycocalyx that transiently buffers ingested sodium [55,57,58] and, thus, controls access to the epithelial sodium channels (Figure 1). Sodium channel activity and glycocalyx function are inversely related to each other. A plasma sodium concentration in the high-physiological range (>140 mM) reduces the negatively charged heparan sulphate residues of the endothelial glycocalyx [56], increasing the amount of sodium reaching the endothelial sodium channels, which in turn makes the channels more active [34,37]. The overall effect is that the barrier against sodium entry fails under these conditions and the endothelium becomes more permeable to sodium (Figure 2).
Figure 1. Model explaining low vascular sodium sensitivity.
This state of vascular function is associated with low daily sodium intake, and/or low aldosterone and/or favorable genetics. Abbreviations: ENaC, epithelial sodium channel.
Figure 2. Model explaining high vascular sodium sensitivity.
This state of vascular function is associated with high daily sodium intake, and/or high aldosterone and/or unfavorable genetics. Abbreviations: ENaC, epithelial sodium channel.
As indicated above, evidence for a salt-sensitive endothelial glycocalyx and its relationship to endothelial epithelial sodium channels is based on in vitro experiments and, at best, on ex vivo studies in human tissue (e.g. human umbilical veins). To our knowledge, there are no direct studies in humans. However, if aldosterone is viewed as a hormone that facilitates sodium retention and epithelial sodium channel expression in the vascular endothelium [14], then a link between salt and glycocalyx, albeit indirect, becomes visible. Clinical research often describes potentially important phenomena in the human without a mechanistic model. In this case, a mechanistic model based on in vitro experiments is available first, and it will be up to clinical research to test it in the human.
Salty food and sodium balance
After a salty meal, the translocation of sodium from the blood into the interstitium is delayed by the significant buffering capacity of the endothelial glycocalyx. Sodium will reversibly bind to/dissociate from the endothelial glycocalyx binding sites and, thus, can be readily excreted via the kidneys. Excessive sodium intake over time will damage the endothelial glycocalyx and lead to a decrease in its sodium buffering capacity because of the loss of negatively charged heparan sulphate residues. Following that, sodium gains direct access to the "unprotected" epithelial sodium channels. Thus, in addition to the paracellular transport route (i.e. sodium transport between endothelial cells along its chemical gradient), sodium uses the transcellular pathway for entering the large extracellular space (about 30% of body weight). There, sodium is bound reversibly to the extracellular matrix [59-63].
After the ingested sodium has spread throughout the body, the plasma sodium concentration decreases. Now, sodium starts diffusing back into the vascular bed (along its chemical gradient directed from interstitium to blood) and will finally be excreted by the kidneys (Figure 2). This “detour of sodium" through the whole organism delays renal sodium excretion significantly. In the meantime, a sodium load from the next salty meal may arrive and so on, leading over time to sodium accumulation in the organism.
Salt provocation test
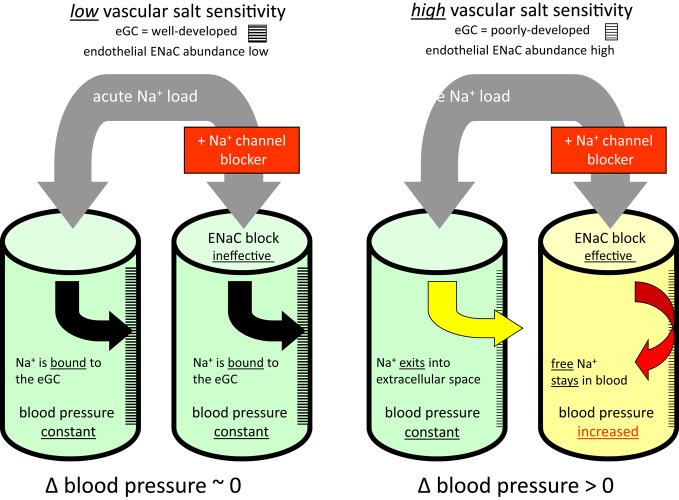
Vascular salt sensitivity can be defined as the ratio of "endothelial sodium channel activity over endothelial glycocalyx sodium buffer capacity". It should be noted that vascular salt sensitivity is not thought to be exclusively “congenital” [8,64-66] but could most likely be influenced (among other yet-unknown factors) by the amount of ingested salt and by endogenous aldosterone [67]. As salt sensitivity correlates positively with sodium channel activity in the endothelium, the blockade of these channels by amiloride analogues should help in identifying individuals with a high sensitivity to salt. Based on this concept, a salt provocation test has recently been published [68]. This test evaluates salt sensitivity in human subjects in quantitative terms. The test is described in general terms in Figure 3 (see figure legend and, for more details, see [68]).
Figure 3. Salt provocation test.
The four cylinders symbolize the vascular system. After an acute Na+ load (5 g NaCl orally), with and without addition of an epithelial sodium channel blocker (two separate sessions), blood pressure is measured over a period of one hour. The difference between these two measurements (∆ blood pressure) is evaluated. Low vascular sensitivity is indicated by a ∆ ~ zero, high vascular sensitivity is indicated by a ∆ > zero. Abbreviations: eGC, endothelial glycocalyx; ENaC, epithelial sodium channel.
Conclusions
The endothelial glycocalyx appears to be a key structure for regulating body sodium. In vitro studies show that the quality of this biopolymer layer is determined by the abundance of its electrical negative charges. Loss of these surface charges renders the endothelial surface vulnerable to unwanted intruders, among them excessive sodium. We look forward to clinical research that will hopefully confirm these results in vivo in humans and substantiate the mechanism behind the protective effect of a low salt diet on the cardiovascular system.
Acknowledgments
We thank the Endothelial Research Group in the Institute of Physiology II, Münster, and Prof. Hugh deWardener, London, for great support over the past five years, and COST Action TD1002 for supporting networking activities.
Competing interests
The authors declare that they have no competing interests. The salt provocation test is covered by the authors pending patent number EP12154853.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/4/20
References
- 1.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]; F1000 Factor 10Evaluated by Hans Oberleithner 12 Sep 2012
- 2.MacGregor G. Salt--Neptune's poisoned chalice. Trans Med Soc Lond. 2002;119:13–4. [PubMed] [Google Scholar]
- 3.Ritz E. Salt--friend or foe? Nephrol Dial Transplant. 2006;21:2052–6. doi: 10.1093/ndt/gfi256. [DOI] [PubMed] [Google Scholar]
- 4.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 5.Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–8. [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 6.Endemann DH, Touyz RM, Iglarz M, Savoia C, Schiffrin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2004;43:1252–7. doi: 10.1161/01.HYP.0000128031.31572.a3. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 7.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 8.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.HYP.27.3.481. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 9.Guyton AC, Coleman TG, Cowley AV, Jr., Scheel KW, Manning RD, Jr., Norman RA., Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–94. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 10.Oberleithner H. Two barriers for sodium in vascular endothelium? Ann Med. 2012 doi: 10.3109/07853890.2011.653397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigne P, Champigny G, Marsault R, Barbry P, Frelin C, Lazdunski M. A new type of amiloride-sensitive cationic channel in endothelial cells of brain microvessels. J Biol Chem. 1989;264:7663–8. [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 12.Golestaneh N, Klein C, Valamanesh F, Suarez G, Agarwal MK, Mirshahi M. Mineralocorticoid receptor-mediated signaling regulates the ion gated sodium channel in vascular endothelial cells and requires an intact cytoskeleton. Biochem Biophys Res Commun. 2001;280:1300–6. doi: 10.1006/bbrc.2001.4275. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 13.Wehling M, Spes CH, Win N, Janson CP, Schmidt BM, Theisen K, Christ M. Rapid cardiovascular action of aldosterone in man. J Clin Endocrinol Metab. 1998;83:3517–22. doi: 10.1210/jc.83.10.3517. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen Dinh CA, Jaisser F. Extrarenal effects of aldosterone. Curr Opin Nephrol Hypertens. 2012;21:147–56. doi: 10.1097/MNH.0b013e32834fb25b. [DOI] [PubMed] [Google Scholar]
- 15.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–8. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 16.Oberleithner H, Ludwig T, Riethmüller C, Hillebrand U, Albermann L, Schäfer C, Shahin V, Schillers H. Human endothelium: target for aldosterone. Hypertension. 2004;43:952–6. doi: 10.1161/01.HYP.0000123572.45556.a5. [DOI] [PubMed] [Google Scholar]
- 17.Funder JW. Minireview: aldosterone and the cardiovascular system: genomic and nongenomic effects. Endocrinology. 2006;147:5564–7. doi: 10.1210/en.2006-0826. [DOI] [PubMed] [Google Scholar]
- 18.Fels J, Oberleithner H, Kusche-Vihrog K. Menage a trois: aldosterone, sodium and nitric oxide in vascular endothelium. Biochim Biophys Acta. 2010;1802:1193–202. doi: 10.1016/j.bbadis.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Jeong Y, Chaupin DF, Matsushita K, Yamakuchi M, Cameron SJ, Morrell CN, Lowenstein CJ. Aldosterone activates endothelial exocytosis. Proc Natl Acad Sci U S A. 2009;106:3782–7. doi: 10.1073/pnas.0804037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusche-Vihrog K, Callies C, Fels J, Oberleithner H. The epithelial sodium channel (ENaC): Mediator of the aldosterone response in the vascular endothelium? Steroids. 2010;75:544–9. doi: 10.1016/j.steroids.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 22.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–43. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–73. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 24.Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 25.Funder JW. Mineralocorticoid receptors and cardiovascular damage: it's not just aldosterone. Hypertension. 2006;47:634–5. doi: 10.1161/01.HYP.0000203732.03784.3b. [DOI] [PubMed] [Google Scholar]
- 26.Funder JW, Reincke M. Aldosterone: a cardiovascular risk factor? Biochim Biophys Acta. 2010;1802:1188–92. doi: 10.1016/j.bbadis.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Lang F. Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension. 2011;57:146–7. doi: 10.1161/HYPERTENSIONAHA.110.164558. [DOI] [PubMed] [Google Scholar]
- 28.Waanders F, de Vries LV, van Goor H, Hillebrands JL, Laverman GD, Bakker SJ, Navis G. Aldosterone, From (Patho)Physiology to Treatment in Cardiovascular and Renal Damage. Curr Vasc Pharmacol. 2011;9:594–605. doi: 10.2174/157016111796642689. [DOI] [PubMed] [Google Scholar]
- 29.Gekle M, Grossmann C. Actions of aldosterone in the cardiovascular system: the good, the bad, and the ugly? Pflugers Arch. 2009;458:231–46. doi: 10.1007/s00424-008-0616-0. [DOI] [PubMed] [Google Scholar]
- 30.Safar ME. Systolic hypertension in the elderly: arterial wall mechanical properties and the renin-angiotensin-aldosterone system. J Hypertens. 2005;23:673–81. doi: 10.1097/01.hjh.0000163130.39149.fe. [DOI] [PubMed] [Google Scholar]
- 31.Duprez D, De Buyzere M, Rietzschel ER, Clement DL. Aldosterone and vascular damage. Curr Hypertens Rep. 2000;2:327–34. doi: 10.1007/s11906-000-0017-z. [DOI] [PubMed] [Google Scholar]
- 32.Fiebeler A, Muller DN, Shagdarsuren E, Luft FC. Aldosterone, mineralocorticoid receptors, and vascular inflammation. Curr Opin Nephrol Hypertens. 2007;16:134–42. doi: 10.1097/MNH.0b013e32801245bb. [DOI] [PubMed] [Google Scholar]
- 33.Bussemaker E, Hillebrand U, Hausberg M, Pavenstadt H, Oberleithner H. Pathogenesis of hypertension: interactions among sodium, potassium, and aldosterone. Am J Kidney Dis. 2010;55:1111–20. doi: 10.1053/j.ajkd.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104:16281–6. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch. 2008;455:849–57. doi: 10.1007/s00424-007-0341-0. [DOI] [PubMed] [Google Scholar]
- 36.Rossier BC, Canessa CM, Schild L, Horisberger JD. Epithelial sodium channels. Curr Opin Nephrol Hypertens. 1994;3:487–96. doi: 10.1097/00041552-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. 2012;463:269–78. doi: 10.1007/s00424-011-1038-y. [DOI] [PubMed] [Google Scholar]
- 38.Li J, White J, Guo L, Zhao X, Wang J, Smart EJ, Li XA. Salt inactivates endothelial nitric oxide synthase in endothelial cells. J Nutr. 2009;139:447–51. doi: 10.3945/jn.108.097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman SM, McIndoe RA, Tanaka M. The relation of blood sodium concentration to blood pressure in the rat. J Hypertens. 1990;8:61–6. doi: 10.1097/00004872-199001000-00010. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 40.Santos SF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:522–30. doi: 10.2215/CJN.03360807. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 41.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 42.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int. 2012;81:407–11. doi: 10.1038/ki.2011.369. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by David Pollock 10 Feb 2012
- 43.de Wardener HE. The hypothalamus and hypertension. Physiol Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- 44.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–49. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Luke Janssen 28 Feb 2012
- 45.Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension. 2009;53:291–8. doi: 10.1161/HYPERTENSIONAHA.108.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 47.Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol. 2009;22:155–62. doi: 10.1097/ACO.0b013e328328d1b6. [DOI] [PubMed] [Google Scholar]
- 48.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–42. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 49.Nieuwdorp M, Meuwese MC, Vink H, Hoekstra JB, Kastelein JJ, Stroes ES. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol. 2005;16:507–11. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 50.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–66. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 51.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–93. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 52.Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci Signal. 2008;1:t8. doi: 10.1126/scisignal.140pt8. [DOI] [PubMed] [Google Scholar]
- 53.Van Teeffelen JW, Brands J, Stroes ES, Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17:101–5. doi: 10.1016/j.tcm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 55.Bevan JA. Flow regulation of vascular tone. Its sensitivity to changes in sodium and calcium. Hypertension. 1993;22:273–81. doi: 10.1161/01.HYP.22.3.273. [DOI] [PubMed] [Google Scholar]
- 56.Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011;462:519–28. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegel G, Walter A, Kauschmann A, Malmsten M, Buddecke E. Anionic biopolymers as blood flow sensors. Biosens Bioelectron. 1996;11:281–94. doi: 10.1016/0956-5663(96)88415-6. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 58.Siegel G, Malmsten M, Klussendorf D, Walter A, Schnalke F, Kauschmann A. Blood-flow sensing by anionic biopolymers. J Auton Nerv Syst. 1996;57:207–13. doi: 10.1016/0165-1838(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 59.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–17. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 60.Titze J, Krause H, Hecht H, Dietsch P, Rittweger J, Lang R, Kirsch KA, Hilgers KF. Reduced osmotically inactive Na storage capacity and hypertension in the Dahl model. Am J Physiol Renal Physiol. 2002;283:F134–41. doi: 10.1152/ajprenal.00323.2001. [DOI] [PubMed] [Google Scholar]
- 61.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schöfl C, Renz W, Santoro D, Niendorf T, Müller DN, Neininger M, Cavallaro A, Eckardt KU, Schmieder RE, Luft FC, Uder M, Titze J. (23)Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59:167–72. doi: 10.1161/HYPERTENSIONAHA.111.183517. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by John Lorenz 29 Dec 2011
- 62.Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens. 2010;19:385–92. doi: 10.1097/MNH.0b013e32833aeb3b. [DOI] [PubMed] [Google Scholar]
- 63.Titze J. Water-free sodium accumulation. Semin Dial. 2009;22:253–5. doi: 10.1111/j.1525-139X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 64.Luft FC, Miller JZ, Weinberger MH, Grim CE, Daugherty SA, Christian JC. Influence of genetic variance on sodium sensitivity of blood pressure. Klin Wochenschr. 1987;65:101–9. doi: 10.1007/BF01728599. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Hans Oberleithner 12 Sep 2012
- 65.Luft FC. Molecular genetics of salt-sensitivity and hypertension. Drug Metab Dispos. 2001;29:500–4. [PubMed] [Google Scholar]
- 66.Funder JW. The genetic basis of primary aldosteronism. Curr Hypertens Rep. 2012;14:120–4. doi: 10.1007/s11906-012-0255-x. [DOI] [PubMed] [Google Scholar]
- 67.Funder JW. Relative aldosterone excess: relative to what? Hypertension. 2005;46:643–4. doi: 10.1161/01.HYP.0000184227.75221.6e. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Hans Oberleithner 12 Sep 2012
- 68.Oberleithner H. A physiological concept unmasking vascular salt sensitivity in man. Pflugers Arch. 2012 doi: 10.1007/s00424-012-1128-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]