Abstract
Consumption of diets rich in fruits and vegetables is often associated with a reduced risk of developing cancer, particularly breast cancer. Considering that 1 in 8 women in the United States will develop breast cancer in the course of her lifetime, dietary manipulation could have a major impact on the incidence of breast cancer. We report here that fresh extracts of garlic (not boiled) arrested the growth and altered the morphology of MCF7 breast cancer cells. Deregulated levels of E-cadherin, cytokeratin8/18, and β-catenin correlated with the altered phenotype. We propose that early down-regulation of cyclin D1, reduced phosphorylation of ERK1, and increased phosphorylation of eIF2-α triggered the phenotypical changes. Reduced expression of hsp27 and sam68 and elevated levels of Rb and p21 further contributed to the sustained growth reduction. These findings provide a better understanding of the cellular responses to dietary supplements and provide potential options to treat breast cancer.
Keywords: garlic extracts, MCF7 cells, differentiation, cell cycle regulation, phosphorylation
Cancer accounts for nearly 25% of deaths in the United States. Unlike some cancers, the incidence and severity of breast cancer are constantly increasing. Currently, 1 of 8 U.S. women are diagnosed with breast cancer. In 2011, nearly a quarter of a million new cases of invasive breast cancer occurred, and 40,000 breast cancer deaths were expected. These staggering statistics call for effective strategies to reduce the incidence and development of breast cancer. Limited consumption of plant products and excessive intake of meat may contribute to the high incidence of cancer.1-5 In this context, several studies document that consumption of plant-based dietary products offers protection from cancer and that the intake of vegetables is often associated with a reduced risk of cancer.3-5 Accordingly, it may be possible to reduce the incidence and development of cancer with dietary supplements, particularly those rich in antioxidants, immune-strengthening and anticancerous ingredients.6-9 Although extensive efforts have been made to understand the molecular mechanism of cancer reduction, a specific approach to reduce breast cancer has not yet been identified.
Among the dietary products studied, garlic and brightly colored fruits and vegetables are well known for their anticancerous potential.10,11 Garlic possesses many medicinal properties, and its constituents exhibit antidiabetic, immune boosting, antioxidant, cardioprotective, and anticancerous functions.6-9,11,12 For example, garlic reportedly inhibited the growth of tumors in mice,13 aged garlic extract (AGE) reduced the risk of cancer and prevented the decline of natural killer (NK) cells in patients with advanced cancer.14,15 Garlic constituents have been studied extensively, and various derivatives of garlic were reported to inhibit the growth of several cancer cell types.16-18 However, limited research has focused on the use of fresh garlic to prevent or treat breast cancer.19-21 The most desirable approach to treat cancer would be to identify the molecular signals that induce growth arrest and trigger terminal differentiation of cancerous cells. Existing evidence suggests that pomegranate pericarp, garlic, and onion oils promote differentiation of prostate cancer and leukemic cells, respectively.22,23 However, the specific mechanism of differentiation and the crucial molecular markers have not been identified. Therefore, we investigated the effect of various dietary products on morphological differentiation of the breast cancer cell line MCF7 and found that 2-3 hours of exposure to garlic extract (fresh but not boiled) was sufficient to arrest the growth and alter the morphology of MCF7 cells. We propose that reduced levels of cyclin D1 and decreased phosphorylation of ERK1 triggered the morphological differentiation followed by the altered expression of E-cadherin, β-catenin, and keratin 8/18. Altered expression of p21, retinoblastoma (Rb), heat shock protein 27 (hsp27), and Src-associated in mitosis, 68 kDa (sam68) may have also contributed to the sustained growth reduction.
Results and Discussion
Garlic treatment alters the morphology and inhibits the growth of MCF7 cells
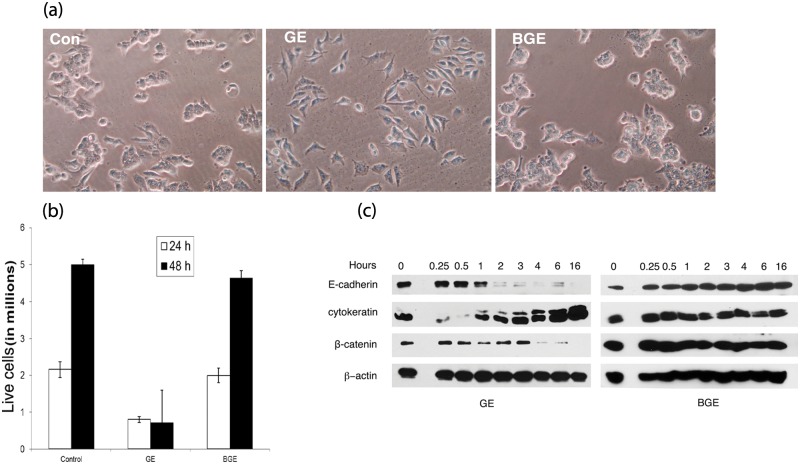
MCF7 cells were treated with fresh extracts of various plant products (bitter melon fruit and seed, neem leaf and seed, mint leaf, spring onions, and garlic) to assess their efficacy on breast cancer cell differentiation. We observed that a majority of the extracts exhibited cytolytic properties, with the exception of garlic. When MCF7 cells were exposed to fresh garlic extract (GE), within 1 hour, the cells began to alter their morphology. After 2-4 hours, GE-treated MCF7 cells became morphologically distinct, attained mesenchyme like phenotype, and lost cell-to-cell contact (Fig. 1a). In contrast, boiled garlic extract (BGE) failed to alter their morphology (Fig. 1a). These results were confirmed multiple times with garlic bulbs obtained from various sources, and the results were reproduced using 2 independent sources of MCF7 cells (ATCC and Dr. Kaladhar Reddy, Wayne State University, Detroit, MI).
Figure 1.
Effect of GE on the morphology and proliferation of MCF7 cells. (a) Morphology of control and GE- or BGE-treated cells. MCF7 cells were exposed to garlic extracts for 3 hours, and pictures were taken at 20× magnification under light microscope. (b) Cell proliferation. MCF7 cells were treated with fresh and boiled extracts of garlic for 3 hours, and cells were harvested at 24- and 48-hour intervals. Each bar represents the average ± SD of 3 independent experiments. (c) Effect of GE on proteins associated with morphology. GE- or BGE-treated MCF7 cell extracts were subjected to immunoblot analysis for the detection of E-cadherin, cytokeratin, and β-catenin as described in the Materials and Methods.
Since altered phenotypes are often associated with reduced growth rates,24-27 we monitored the effect of GE on the proliferation of MCF7 cells. We observed that a 3-hour exposure to garlic extracts (corresponding to 4 mg/mL dry mass) resulted in complete growth arrest and reduced proliferation (Fig. 1b). The estimated IC50 value of garlic extracts was 2.5 mg/mL plant dry mass (data not shown). In contrast, untreated and BGE-treated cells continued their multiplication (Fig. 1b). Our results suggest that boiling destroys the growth inhibitory properties of the garlic extract. In support of our observations, microwave/oven heating also was shown to inhibit the anticarcinogenic properties of garlic.28 Heating is known to destroy the enzymatic activities of allinase, which converts allin (an inactive form) into an active form, allicin.28 Importantly, the garlic extract used in the present investigation mostly comprised water-soluble constituents and negligible amounts of oil-soluble constituents. Hirsch et al. 16 reported that pure allicin, a major water-soluble chemical constituent of garlic, as well as aqueous extracts of garlic (with a similar concentration of allicin content) exhibited similar growth inhibitory potency, suggesting that garlic’s growth inhibitory properties are attributed to the water-soluble constituent, allicin. Accordingly, it is possible that allicin, present in the fresh garlic extracts, contributed to the altered morphology and growth reduction. The specificity of garlic in fighting malignant cancer cells was further demonstrated by Ghazanfari et al.,21 who reported that garlic extract specifically inhibits the growth of MCF7, colon, and gastric cancer cells and did not affect the growth of a nonmalignant cell line, L929.
Twenty-four hours of posttreatment with GE also resulted in cell death (15%-20%), as evidenced by trypan blue exclusion (data not shown); whereas untreated or BGE-treated cells were (100%) viable. The specificity of GE in inducing morphological differentiation of MCF7 cells was further validated by the fact that GE failed to alter the phenotype of MDA-MB-231 (metastatic breast cancer cell), glioblastoma (U-87MG), or monocytic leukemia (U-937) cells (data not shown). Previously, water extracts as well as oil-soluble constituents of garlic were reported to inhibit the growth of several types of cancer cells,16-21 and in no case did these derivatives alter their morphology. Several differentiation-inducing agents were reported to require prolonged exposures to attain the altered phenotype/growth inhibition. In some cases, the inducers had to remain in contact with the cells during the entire process of differentiation.24,25 In other cases, morphological differentiation of breast cancer cells induced by suberoylanilide hydroxamic acid (SAHA) was shown to be reversible.24 Specifically, soon after its removal, cells restored their original phenotype. Conversely, our data suggest that the garlic-mediated phenotypical alteration is irreversible. Moreover, 2-4 hours of exposure to GE resulted in an irreversible phenotype and permanent growth arrest. Altered morphology and growth reduction of the garlic-treated MCF-7 cells were persistent and were observed over 2-3 weeks even after the removal of GE (data not shown).
GE-mediated morphological differentiation and growth reduction are associated with an altered expression of epithelial cadherin (E-cadherin), cytokeratin 8/18, and β-catenin
To understand the role of the cell adhesion molecule (E- cadherin), cytoskeletal proteins (cytokeratins), and morphology-associated signaling molecule (β-catenin) on GE-mediated morphological alterations, garlic-treated MCF7 cellular extracts were subjected to immunoblotting with their respective antibodies. Immunoblot analysis revealed that E-cadherin levels initially declined during the morphological differentiation (1-6 hours), and by 16 hours the protein signal was completely lost (Fig. 1c), suggesting that reduced expression of E-cadherin correlates with the loss of epithelial morphology and cell-to-cell contact. In support of our observations, oncostatin-M-mediated phenotypical alteration of breast cancer cells was also shown to be associated with the down-modulation of E-cadherin.29 GE treatment also altered the levels of cytokeratins. A marginal reduction of keratin 8 (K8) and keratin 18 (K18) expression was observed between 15- and 30-min exposures to GE. However, a moderate up-regulation of K8/18 was observed after 6-16 hours post exposures (Fig. 1c). A possible explanation is that the initial down-regulation of epithelial keratins (K8/18) may be attributable to the induction of altered morphology (loss of epithelial phenotype), whereas K8/18 up-regulation at the later time intervals (6-16 hours) correlates with growth reduction. Similarly, doxorubicin-mediated growth reduction and morphological differentiation of MCF7 cells were shown to be associated with the up-regulation of K8/18.26 GE treatment also down-modulated the levels of β-catenin; its reduction was observed soon after the cells attained their altered phenotype (3 hours post exposure), and it vanished by 16 hours (Fig. 1c). These results suggest that the initial down-regulation of β-catenin did not contribute to the induction of altered morphology; rather, its initial reduction correlates with the altered phenotype. A complete loss/degradation of β-catenin also contributed to the GE-mediated sustained growth reduction. In agreement with our results, decursin, an anticancer agent, was reported to inhibit the proliferation of androgen-independent prostate cancer cells by targeting β-catenin to proteasome degradation.30 Moreover, BGE, which failed to alter the morphology and inhibit the growth of MCF cells, did not affect the expression of E-cadherin, k8/18, and β-catenin (Fig. 1c). Collectively, these data suggest that deregulation of E-cadherin, keratin 8/18, and β-catenin contributes to the GE-mediated altered phenotype and growth reduction.
GE exposure alters the levels of cell cycle regulatory proteins
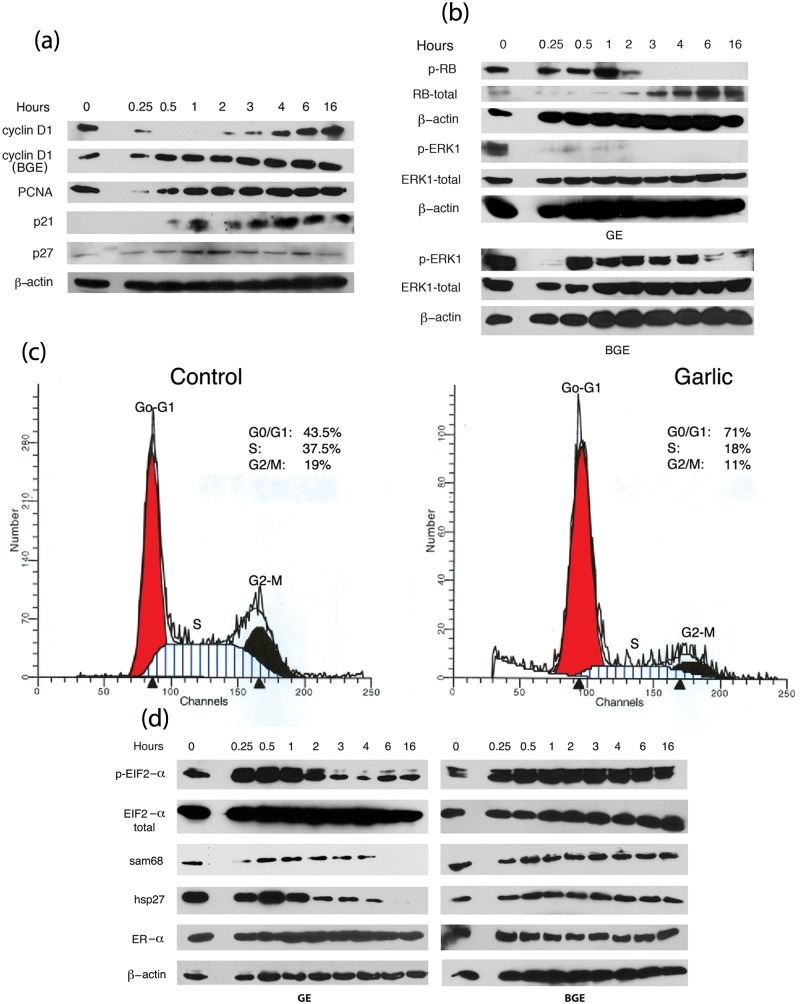
GE-mediated growth reduction prompted us to further investigate the involvement of the key cell cycle regulators, that is, cyclin D1, proliferating cell nuclear antigen (PCNA), and cell cycle inhibitors (p21 and p27). Immunoblot analysis of GE-treated MCF7 cell extracts with cell cycle– associated proteins revealed that the expression levels of cyclin D1 were initially down-modulated between 15 minutes and 2 hours of exposure and gradually returned to original levels (Fig. 2a). A dramatic down-regulation of cyclin D1 prior to morphological differentiation suggests that its deregulation would induce the phenotypical differentiation. Previously, down-regulation of cyclin D1 was shown to be required for the initiation of keratinocyte differentiation.31 In contrast, diallyl trisulfide (oil-soluble constituent of garlic) was reported to inhibit the growth of MCF7 cells through the up-regulation of cyclin D1 (mRNA levels) and down-regulation of cyclin B1 levels,18 suggesting that aqueous extracts and oil-soluble components of garlic exert distinct effects on cell cycle regulation. Failure of the BGE to inhibit the expression of cyclin D1 (Fig. 2a) further suggests that down-regulation of cyclin D1 is critical for the induction of morphological differentiat and growth suppression. We also observed that the levels of PCNA were marginally reduced after 15-30 minutes of exposure to GE, while p27 expression was moderately up-regulated after 1-3 hours of exposure (Fig. 2a). The levels of p21 were barely detectable in the untreated cells, while in the GE-treated cells p21 levels were readily detectable by 30 minutes, peaked after 1 hour of exposure, and remained high during the entire treatment period (Fig. 2a). However, BGE treatment did not alter the expression of PCNA, p21, and p27 (data not shown). Taken together, our data demonstrate that GE inhibits cell cycle progression and contributes to growth reduction by modulating the levels of cyclin D1, PCNA, p21, and p27.
Figure 2.
Effect of GE on the expression of various proteins associated with growth and differentiation. (a) Cell cycle regulatory proteins (cyclin D1, PCNA, p21, and p27) and (b) phosphorylation of Rb and ERK1. Cell extracts from GE-treated or control cells were subjected to Western analysis. (c) Cell cycle analysis. Exponentially growing control (untreated) and GE-treated cells were stained with propidium iodide, and cell cycle progression was analyzed as described in the Materials and Methods. (d) Expression levels of eIF2-α, hsp27, sam68, and ER-α and phosphorylation of eIF2-α. Control and GE-treated MCF7 cell extracts were subjected to Western blot analysis for the detection of various proteins using the respective antibodies as indicated in the figure.
GE treatment causes down-modulation of Rb and ERK1 phosphorylation
Since cyclin-dependent kinase1 (CDK1) and cyclin D1–mediated Rb phosphorylation events inactivate Rb and contribute to cell cycle arrest and subsequent growth inhibition, we hypothesized that diminished levels of cyclin D1 in GE-treated cells would reduce the phosphorylation of Rb. In support of our hypothesis, garlic treatment inhibited the phosphorylation of Rb. A gradual decline of Rb phosphorylation was observed during 2-6 hours of exposure to GE, and by 16 hours, Rb was completely dephosphorylated. However, the levels of Rb protein were gradually increased between 4 and 16 hours post treatment (Fig. 2b). These data suggest that GE-mediated growth suppression can be attributed to the inactivation and stabilization of Rb protein. Besides the role of Rb in growth reduction, hypophosphorylation of Rb was implicated in differentiation.32-34 Consistent with our observations, Yen et al. 35 reported that reduced phosphorylation of Rb and increased expression of Rb proteins results in differentiation of megakaryocytic leukemic cells.35 Finally our data suggest that garlic’s growth-inhibitory properties are ascribed to its capacity to preserve and protect the function of Rb, presumably by the processes of dephosphorylation and stabilization.
Since ERK1 phosphorylation plays a crucial role in governing cellular proliferation and morphological differentiation, we also tested the levels of phosphorylated ERK1 in GE-treated MCF7 cellular extracts. We observed that 15 minutes to 2 hours of exposure to GE markedly inhibited the phosphorylation of ERK1 and that prolonged exposures (6-16 hours) resulted in a complete loss of its phosphorylation (Fig. 2b). However, β-actin and total ERK1 protein levels remained unaltered (Fig. 2b), suggesting that down-regulation of ERK1 phosphorylation is a result of its reduced phosphorylation rather than the reduction of protein levels. A modest decline in the phosphorylation of ERK1 was also observed in the BGE-treated MCF7 cells. This reduction was detected initially after 15 minutes of exposure to BGE and later after 6-16 hours post exposure (Fig. 2b). However, during the commitment of morphological alteration (1-3 hours), ERK1 phosphorylation was not inhibited. BGE also failed to alter the expression levels of total ERK1 and β-actin (Fig. 2b). Therefore, our results suggest that sustained reduction of ERK1 phosphorylation is crucial for GE-mediated commitment of morphological differentiation and subsequent growth reduction. Depending on the cell type, ERK1 phosphorylation either positively or negatively regulates the growth and differentiation of cancer cells.18,19,24,36 Consistent with our results, diallyl disulfide (oil soluble constituent of garlic)–induced differentiation of a gastric cancer cell line, MGC803, was shown to be associated with the initial down-regulation of ERK1 phosphorylation.36 In contrast, ERK1 phosphorylation was reportedly up-regulated in GE-treated MDA-MB-435 cells.19 It is possible that the effect of garlic extracts on ERK1 phosphorylation is cell type specific. Our data suggest that early down-regulation of ERK1 phosphorylation is critical for the induction of GE-mediated morphological differentiation and that its complete dephosphorylation correlates with growth reduction.
GE-treated MCF7 cells were arrested at the G0-G1 phase
Garlic-induced growth inhibition and altered expression of cell cycle–associated proteins prompted us to investigate its effect on the regulation of the cell cycle progression. FACS (fluorescence activated cell sorting) analysis of GE-treated cells revealed that a major proportion of the GE-treated cells were arrested at various phases of the cell cycle (Fig. 2c). We observed that 71% of the GE-treated MCF7 cells were arrested at the G0-G1 phase, while only 43% of the untreated cells remained in the G0/G1 phase (Fig. 2c). Furthermore, 18% of the GE-treated cells progressed to the S phase, and few cells (11%) remained in the G2/M phase. In contrast, a higher percentage (37%) of the untreated cells progressed to the S phase, and 19% of the cells were maintained in the G2/M phase (Fig. 2c, lower panel), indicating their active participation in cell division. Taken together, FACS analysis of the GE-treated cells suggests that a majority of the garlic-treated MCF7 cells were arrested in the G1 phase and failed to progress toward the S and G2-M phases, thus resulting in G0/G1 arrest. A possible explanation is that a cascade of events, such as an early down-regulation of cyclin D1 (Fig. 2c), decreased phosphorylation of Rb and a subsequent up-regulation of CDK inhibitors (p21 and p27) contributed to the G0/G1 arrest reduced their entry to the S and G2-M phases, eventually leading to the cell cycle exit. Consistent with our results, fangichinoline-mediated G1/S arrest in prostate cancer cells was accompanied by an altered expression of cyclin D1, PCNA, and p27.37 Furthermore, by modulating the levels of cyclin D1, p21, and Rb phosphorylation, quinidine treatment resulted in G1 arrest and contributed to the induction of differentiation.38
Up-regulation of eIF2-α phosphorylation initiates morphological alterations and growth inhibition
Cellular differentiation is a complex process that involves differential regulation of gene transcription and translation. We assessed the effect of GE on the phosphorylation of eukaryotic initiation factor (eIF2-α), since it is implicated in the translational regulation of various genes involved in cell proliferation and differentiation.39-42 Garlic treatment specifically up-regulated eIF2-α phosphorylation, while the levels of total eIF2-α protein were unaltered (Fig. 2d). GE-treated MCF7 cells exhibited an increased phosphorylation of eIF2-α between 15 and 60 minutes of exposure (Fig. 2d). However, soon after the cells attained their altered phenotype (3 hours post exposure), eIF2-α phosphorylation returned to control levels (Fig. 2d), suggesting that increased phosphorylation of eIF2-α is required for the induction of altered morphology. Furthermore, BGE, which did not contribute to the altered morphology, failed to alter the phosphorylation of eIF2-α (Fig. 2d, right panel). Since increased phosphorylation of eIF2-α will result in its inactivation and contribute to the inhibition of protein translation, we reasoned that the initial down-regulation of eIF2-α is sufficient to inhibit the expression of crucial cell cycle/proliferation–associated proteins, thus resulting in cell cycle exit and growth arrest. Increased phosphorylation of eIF2-α was also reported to be associated with the differentiation of erythroleukemic cells (K562) and osteoblasts39,42, thus directly connecting its phosphorylation status to the induction of differentiation. Overall, our results suggest that early down-regulation of eIF2-α activity is a necessary step required to induce the GE-mediated morphological differentiation and growth reduction.
Reduced expressions of hsp27 and sam68 correlate with GE-mediated growth suppression
Our objective here was to investigate whether GE-mediated growth inhibition alters the levels of crucial signaling associated proteins, sam68 and hsp27, which play a significant role in the proliferation of cancer cells.43-46 We observed that 4-6 hours of GE treatment down-modulated the levels of sam68 and hsp27, and subsequently their expression levels were completely lost by 16 hours (Fig. 2d, left panel). Moreover, BGE, which failed to inhibit the growth of MCF7 cells, did not alter their expression (Fig. 2d, right panel). Our data further suggest that down-regulation of sam68 and hsp27 is coupled to GE-mediated growth reduction rather than cytoskeletal reorganization (altered morphology). However, GE did not alter the expression levels of estrogen receptor α (ER-α), which is implicated in the proliferation of ER positive breast cancer cells, suggesting that ER-α has no role on the GE-mediated responses (Fig. 2d).
Garlic treatment alters lipid metabolism and results in a differential lipid accumulation
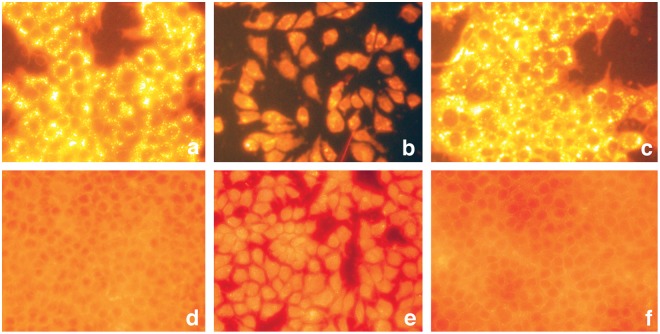
Cellular proliferation and morphological differentiation often result in the accumulation of lipid droplets.24,25,27,47,48 Lipid droplets, which consist of neutral lipids, triacyl glycerols (energy reserves), or cholesteryl esters, serve as storage deposits. The fluorescent dye, Nile red, selectively stains lipid droplets49; neutral lipids display golden yellow staining when visualized under green fluorescent light (excitation at 450-500 nm and emission at 528 nm), and polar lipids (present in the membranes) stain red under a red fluorescent light (excitation 515-560 nm and emission at 590 nm). Since highly proliferating cancer cells require excess amount of lipids to sustain their constant multiplication, we investigated the effect of GE on lipid accumulation and observed that GE treatment resulted in a differential lipid staining (Fig. 3). When visualized under green fluorescent light, GE-treated MCF7 cells exhibited less intensive lipid staining and accumulated few lipid droplets (Fig. 3b), suggesting their reduced proliferation. In contrast, untreated (control) and BGE-treated cells displayed highly intensive yellow gold fluorescence, and these cells accumulated numerous yellow gold lipid droplets in the cytoplasm, suggesting their higher proliferation rate (Fig. 3a and 3c). Consistent with our results, proliferating 3T3 cells (but not quiescent cells) displayed numerous golden yellow cytoplasmic lipoid droplets.48 Furthermore, inhibition of fatty acid synthase reportedly inhibited the growth and reduced the accumulation of lipid droplets,47 suggesting that growth reduction is associated with the inhibition of lipid synthesis. When visualized under red fluorescent light, control cells and BGE-treated cells exhibited diffused and less intensive lipid staining in the cytoplasm (Fig. 3d and 3f). However, GE-treated cells displayed very intense reddish orange/yellow staining, and the entire cell was stained positive for lipid accumulation (Fig. 3e). Consistent with our observations, a differential regulation of the membrane lipids was also observed during the growth arrest and differentiation of leukemic cells,50 and spot14-induced differentiation of breast cancer cells was also associated with the accumulation of neutral lipids.27 Taken together, our results suggest that garlic treatment alters lipid metabolism and composition. It contributes to the differential distribution/deposition of lipids, resulting in reduced cell proliferation and presumably facilitating the cell differentiation.
Figure 3.
Effect of GE on lipid accumulation. Untreated and GE- and BGE-treated MCF7 cells were subjected to lipid staining using Nile red as described in Materials and Methods. Upper panel (a-c) represents the lipid accumulation of MCF7 cells visualized under a green fluorescent filter. (a) Untreated, (b) GE-treated, and (c) BGE-treated. Lower panel (d-f) represents the lipid staining of MCF7 cells under a red fluorescent filter. (d) Untreated, (e) GE-treated, and (f) BGE-treated.
Proposed mechanism of GE-mediated growth inhibition/morphological alteration
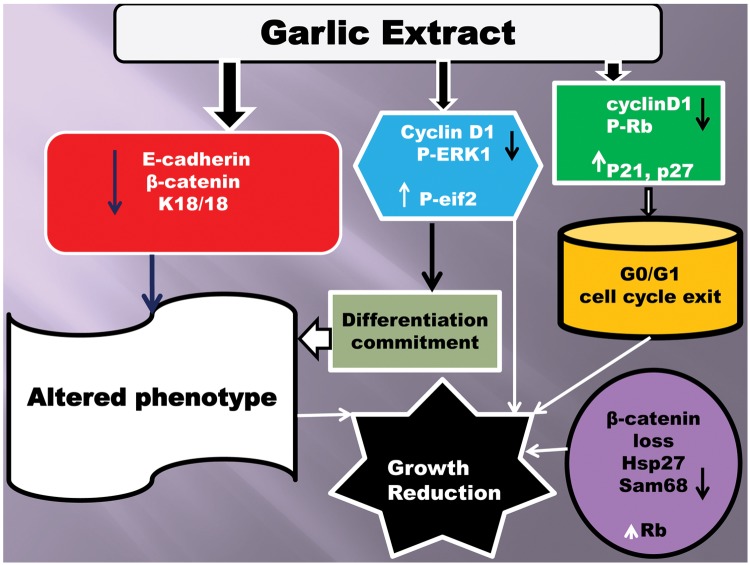
Our data provide evidence that fresh extract of garlic specifically inhibits the growth and alters the phenotype of MCF7 cells. As depicted in Fig. 4, we propose that the following sequence of molecular events contributes to the garlic mediated responses: (1) Early down-regulation of ERK1 phosphorylation, up-regulation of eIF2-α phosphorylation, and initial reduction of cyclin D1 expression contributed to the induction of the altered phenotype. (2) Decreased levels of E-cadherin and β-catenin and up-regulation of epithelial keratins (K8/18) correlated with the loss of epithelial phenotype with a simultaneous gain of mesenchyme like morphology. (3) Down-regulation of cyclin D1, up-regulation of CDK inhibitors (p21 and p27), and reduced phosphorylation of Rb resulted in G0/G1 arrest by preventing the subsequent cell cycle progression followed by the cell cycle exit. (4) Loss of ERK1 and Rb phosphorylation; reduced levels of β-catenin, hsp27, and sam68; and up-regulation of retinoblastoma and K8/18 proteins at late time points (6-16 hours exposures) are further required to maintain the sustained growth reduction.
Figure 4.
Proposed mechanism of GE-mediated morphological differentiation and growth inhibition. Figure represents the differential regulation of various proteins by GE and their implication on the growth suppression and morphological differentiation of MCF7 cells.
Conclusions
Our results demonstrate that a short exposure to fresh but not boiled garlic extract is sufficient to permanently alter the morphology and trigger the growth arrest of MCF7 cells. These processes are tightly linked to the altered regulation of several proteins as indicated in Fig. 4. Although many constituents of garlic reportedly inhibited the growth and induced apoptosis of several types of cancer cells, none of these compounds altered the morphology of MCF7 cells. Moreover, garlic derivatives require longer exposures to obtain growth inhibition. Collectively, our data offer molecular insight on garlic-mediated gene regulation in relation to growth arrest (MCF-7 cells) and shed light on the intervention of dietary products in combating breast cancer. Our future studies will be directed towards investigating the effect of GE on the modulation of several signaling cascades involving the STAT, JNK, and SAPK pathways.
Materials and Methods
Chemicals, reagents, and antibodies
Sam68, β-actin, cyclin D1, β-catenin, and ER-α antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). E-cadherin antibodies were obtained from BD Biosciences (San Jose, CA). PCNA, P21, P27, hsp27, and pan keratin antibodies and secondary antibodies (anti-rabbit and anti-mouse) were procured from Cell Signaling (Danvers, MA). EIF2-α antibodies (total and phosphorylated) were from Abcam (Cambridge, MA). ERK1 and phospho ERK1 antibodies were obtained from Invitrogen (San Diego, CA). Propidium iodide, Nile red, and Hoechst 33258 were purchased from Sigma (St. Louis, MO), and enhanced chemiluminescence reagent (Femtoglow) was from Michigan Diagnostics (Royal Oak, MI).
Cell lines and media
MCF7, MDA-MB-231, U937, and U-87 MG cells were purchased from ATCC (Manassas, VA). Dulbecco’s modified eagle medium (DMEM-F12), penicillin, streptomycin, glutamine, and trypsin EDTA were purchased from Mediatech (Manassas, VA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). All cell lines were maintained in DMEM-F12 medium supplemented with 10% heat- inactivated FBS, penicillin, streptomycin, and glutamine.
Garlic treatment and immunoblotting
Garlic cloves were purchased from a local grocery store. Ten grams of freshly peeled cloves were finely chopped with a sterilized razor and ground in a mortar with a pestle in the presence of 5 ml of cold DMEM-F12 medium and filtered through sterile cheesecloth. The extracts were spun at 1,000 rpm for 5 minutes at 4°C, and the supernatants corresponding to 4 mg/mL (plant dry mass) were used. The garlic extracts were used within 1-2 hours of preparation. During this time the extracts were kept at 4°C. For long-term storage, the garlic extract aliquots were stored at −70°C until further use. To test whether boiling destroys the anticancerous properties of garlic, boiled extracts were prepared by boiling the garlic extract in a water bath for 30 minutes and cooling on ice. MCF7 cells were plated in DMEM-F12 with 10% FBS in 6-well tissue culture dishes. After 24 hours, cells were replaced with plain DMEM-F12 medium. Cells were exposed to fresh and boiled extracts of the garlic (4 mg/mL plant dry mass) for various time periods (15 and 30 minutes, 1, 2, 3, 4, 6, and 16 hours). For the preparation of cellular extracts, cells were washed with ice-cold PBS and lysed by directly adding boiling SDS sample buffer. Samples were boiled for 3 minutes and stored at −70°C until use. Control and garlic treated cellular extracts (5-25 µg of protein) were loaded on 10% SDS poly acrylamide gels. A minimal amount of protein (5 µg) was sufficient for the detection of cyclin D1, PCNA, keratin 8/18, E-cadherin, β-catenin, ER-α, hsp27, and sam68, whereas a higher amount of protein (25 µg) was required to detect the levels of ERK1, Rb, p21, p27, and elF2-α (protein and phosphorylation). Western blotting was performed as previously described.46
Cell proliferation
MCF7 cells were seeded in 6-well cell cuture dishes and replaced with DME F12. After 16 hours, cells were treated with fresh or boiled extracts of garlic (4 mg/mL dry mass) for 3 hours. Cells were harvested at 24- and 48-hour intervals and stained with trypan blue. Cells that stained negative for blue staining (intact, live cells) were counted, and cell density was represented as the number of living cells present in a single well of a 6-well cell culture dish.
Cell cycle analysis
To monitor the distribution of MCF7 cells at various phases of the cell cycle, cells were exposed to garlic extract for 1 hour; then cells were harvested, and 1 × 106 cells from the control or GE-treated wells were processed and subjected to FACS analysis as described.46
Nile red staining for the lipid accumulation
MCF7 cells were treated with fresh or boiled garlic extracts for 4 hours; then the cells were washed and replaced with fresh DMEM-F12 with 10% FBS. After 4 days, cells were washed with PBS, stained with Nile red, and visualized under green and red fluorescent lights to detect cytoplasmic lipid droplets as described.49
Acknowledgments
We thank Dr. Gerry Boss (University of California, San Diego), for his helpful suggestions and critical review of the manuscript. We also thank Mary Olive and Carmel Harkins of the Wayne State University Microscopy and Imaging Resources Laboratory for assistance with the microscopy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported, in part, by the DOD grant W81XWH-05-1-0529 awarded to TRR. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center grant P30CA22453 to The Karmanos Cancer Institute, Wayne State University and the Perinatology Research Branch of the National Institutes of Child Health and Development, Wayne State University.
References
- 1. Lam TK, Cross AJ, Consonni D, et al. Intakes of red meat, processed meat, and meat mutagens increase lung cancer risk. Cancer Res. 2009;69:932-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linos E, Willett WC, Cho E, Colditz G, Frazier LA. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2146-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. 2012;131:201-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lam TK, Ruczinski I, Helzlsouer KJ, Shugart YY, Caulfield LE, Alberg AJ. Cruciferous vegetable intake and lung cancer risk: a nested case-control study matched on cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010; 19:2534-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang CX, Ho SC, Chen YM, Fu JH, Cheng SZ, Lin FY. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer. 2009;125:181-8 [DOI] [PubMed] [Google Scholar]
- 6. Kyo E, Uda N, Kasuga S, Itakura Y. Immunomodulatory effects of aged garlic extract. J Nutr. 2001;131:1075S-9S [DOI] [PubMed] [Google Scholar]
- 7. Lamm DL, Riggs DR. Enhanced immunocompetence by garlic: role in bladder cancer other malignancies. J Nutr. 2001;131:1067S-70S [DOI] [PubMed] [Google Scholar]
- 8. Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131:1010S-5S [DOI] [PubMed] [Google Scholar]
- 9. Rowe CA, Nantz MP, Nieves C, West RL, Percival SS. Regular consumption of concord grape juice benefits human immunity. J Med Food. 2011;14:69-78 [DOI] [PubMed] [Google Scholar]
- 10. Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyanins from fruits and vegetables—does bright colour signal cancer chemo preventive activity? Eur J Cancer. 2005;41:1931-40 [DOI] [PubMed] [Google Scholar]
- 11. Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem. 2011;11:249-53 [DOI] [PubMed] [Google Scholar]
- 12. Neil A, Silagy C. Garlic: its cardio-protective properties. Curr Opin Lipidol. 1994;5:6-10 [DOI] [PubMed] [Google Scholar]
- 13. Perchellet JP, Perchellet EM, Belman S. Inhibition of DMBA-induced mouse skin tumorigenesis by garlic oil and inhibition of two tumor- promotion stages by garlic and onion oils. Nutr Cancer. 1990;14:183-93 [DOI] [PubMed] [Google Scholar]
- 14. Tanaka S, Haruma K, Yoshihara M, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr. 2006;136:821S-826S [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa H, Saeki T, Otani T, et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr. 2006;136:816S-820S [DOI] [PubMed] [Google Scholar]
- 16. Hirsch K, Danilenko M, Giat J, et al. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer. 2000;38:245-54 [DOI] [PubMed] [Google Scholar]
- 17. El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr. 2006;136:864S-869S [DOI] [PubMed] [Google Scholar]
- 18. Malki A, El-Saadani M, Sultan AS. Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther. 2009;8:2175-85 [DOI] [PubMed] [Google Scholar]
- 19. Lund T, Stokke T, Olsen ØE, Fodstad Ø. Garlic arrests MDA-MB-435 cancer cells in mitosis, phosphorylates the proapoptotic BH3-only protein BimEL and induces apoptosis. Br J Cancer. 2005;92:1773-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su CC, Chen GW, Tan TW, Lin JG, Chung JG. Crude extract of garlic induced caspase-3 gene expression leading to apoptosis in human colon cancer cells. In Vivo. 2006;20:85-90 [PubMed] [Google Scholar]
- 21. Ghazanfari T, Yaraee R, Rahmati B, Hakimzadeh H, Shams J, Jalali-Nadoushan MR. In vitro cytotoxic effect of garlic extract on malignant and nonmalignant cell lines. Immunopharmacol Immunotoxicol. 2011;33:603-8 [DOI] [PubMed] [Google Scholar]
- 22. Kawaii S, Lansky EP. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human pro myelocytic leukemia cells. J Med Food. 2004;7:13-8 [DOI] [PubMed] [Google Scholar]
- 23. Seki T, Tsuji K, Hayato Y, Moritomo T, Ariga T. Garlic and onion oils inhibit proliferation and induce differentiation of HL-60 cells. Cancer Lett. 2000;160:29-35 [DOI] [PubMed] [Google Scholar]
- 24. Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM. The histone de acetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001;61:8492-7 [PubMed] [Google Scholar]
- 25. You H, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induces MDA-MB-435 and MCF-7 human breast cancer cells to undergo differentiation. Cell Growth Differ. 2001;12:471-80 [PubMed] [Google Scholar]
- 26. Kim J, Freeman MR. JNK/SAPK mediates doxorubicin-induced differentiation and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 2003;79:321-8 [DOI] [PubMed] [Google Scholar]
- 27. Sanchez-Rodriguez J, Kaninda-Tshilumbu JP, Santos A, Perez-Castillo A. The spot 14 protein inhibits growth and induces differentiation and cell death of human MCF-7 breast cancer cells. Biochem J. 2005;390:57-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song K, Milner JA. The influence of heating on the anticancer properties of garlic. J Nutr. 2001;131:1054S-7S [DOI] [PubMed] [Google Scholar]
- 29. Ng DC, Lim CP, Lin BH, Zhang T, Cao X. SCG10-like protein (SCLIP) is a STAT3-interacting protein involved in maintaining epithelial morphology in MCF-7 breast cancer cells. Biochem J. 2009;425: 95-105 [DOI] [PubMed] [Google Scholar]
- 30. Song GY, Lee JH, Cho M, Park BS, Kim DE, Oh S. Decursin suppresses human androgen-independent PC3 prostate cancer cell proliferation by promoting the degradation of beta-catenin. Mol Pharmacol. 2007;72:1599-606 [DOI] [PubMed] [Google Scholar]
- 31. Nishi K, Inoue H, Schnier JB, Rice RH. Cyclin D1 down regulation is important for permanent cell cycle exit and initiation of differentiation induced by anchorage-deprivation in human keratinocytes. J Cell Biochem. 2009;106:63-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinberg RA. The Rb gene and the negative regulation of cell growth. Blood.1989;74:529-32 [PubMed] [Google Scholar]
- 33. Thomas NS, Burke LC, Bybee A, Linch DC. The phosphorylation state of the retinoblastoma (RB) protein in G0/G1 is dependent on growth status. Oncogene. 1991;6:317-22 [PubMed] [Google Scholar]
- 34. Akiyama T, Toyoshima K. Marked alteration in phosphorylation of the RB protein during differentiation of human pro myelocytic HL60 cells. Oncogene. 1990;5:179-83 [PubMed] [Google Scholar]
- 35. Yen A, Varvayanis S, Platko JD. 12-O-tetradecanoylphorbol-13-acetate and staurosporine induce increased retinoblastoma tumor suppressor gene expression with megakaryocytic differentiation of leukemic cells. Cancer Res. 1993;53:3085-91 [PubMed] [Google Scholar]
- 36. Ling H, Zhang LY, Su Q, et al. Erk is involved in the differentiation induced by diallyl disulfide in the human gastric cancer cell line MGC803. Cell Mol Biol Lett. 2006;11:408-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang CD, Huang JG, Gao X, et al. Fangchinoline induced G1/S arrest by modulating expression of p27, PCNA, and cyclin D in human prostate carcinoma cancer PC3 cells and tumor xenograft. Biosci Biotechnol Biochem. 2010;74:488-93 [DOI] [PubMed] [Google Scholar]
- 38. Zhou Q, Melkoumian ZK, Lucktong A, Moniwa M, Davie JR, Strobl JS. Rapid induction of histone hyper acetylation and cellular differentiation in human breast tumor cell lines following degradation of histone deacetylase-1. J Biol. Chem. 2000;275:35256-63 [DOI] [PubMed] [Google Scholar]
- 39. Gerlitz G, Jagus R, Elroy-Stein O. Phosphorylation of initiation factor-2 alpha is required for activation of internal translation initiation during cell differentiation. Eur J Biochem. 2002;269:2810-9 [DOI] [PubMed] [Google Scholar]
- 40. Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064-73 [DOI] [PubMed] [Google Scholar]
- 41. Zhu K, Chan W, Heymach J, Wilkinson M, McConkey DJ. Control of HIF-1alpha expression by eIF2 alpha phosphorylation-mediated translational repression. Cancer Res. 2009;69(5):1836-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saito A, Ochiai K, Kondo S. et al. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286:4809-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mairesse N, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27 kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20: 205-12 [DOI] [PubMed] [Google Scholar]
- 44. Bielli P, Busà R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multi-functional player in human cancer. Endocr Relat Cancer. 2011;18(4):R91-R102 [DOI] [PubMed] [Google Scholar]
- 45. Song L, Wang L, Li Y, et al. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J Pathol. 2010;222(3):227-37 [DOI] [PubMed] [Google Scholar]
- 46. Modem S, Chinnakannu K, Bai U, Reddy GP, Reddy TR. Hsp22 (HspB8/H11) knockdown induces Sam68 expression and stimulates proliferation of glioblastoma cells. J Cell Physiol. 2011;226: 2747-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmid B, Rippmann JF, Tadayyon M, Hamilton BS. Inhibition of fatty acid synthase prevents pre adipocyte differentiation. Biochem Biophys Res Commun. 2005;328:1073-82 [DOI] [PubMed] [Google Scholar]
- 48. Diaz G, Batetta B, Sanna F, et al. Lipid droplet changes in proliferating and quiescent 3T3 fibroblasts. Histochem Cell Biol. 2008;129: 611-21 [DOI] [PubMed] [Google Scholar]
- 49. Diaz G, Melis M, Batetta B, Angius F, Falchi AM. Hydrophobic characterization of intracellular lipids in situ by Nile Red red/yellow emission ratio. Micron. 2008;39:819-24 [DOI] [PubMed] [Google Scholar]
- 50. Nathan I, Ben-Valid I, Henzel R, et al. Alterations in membrane lipid dynamics of leukemic cells undergoing growth arrest and differentiation: dependency on the inducing agent. Exp Cell Res. 1998;239:442-6 [DOI] [PubMed] [Google Scholar]