Abstract
Mitochondrial hyperfusion has recently been shown to function as a cellular stress response, providing transient protection against apoptosis and mitophagy. However, the mechanisms that mediate this response remain poorly understood. In this study, we demonstrate that oxidized glutathione (GSSG), the core cellular stress indicator, strongly induces mitochondrial fusion. Biochemical and functional experiments show that GSSG induces the generation of disulphide-mediated mitofusin oligomers, in a process that also requires GTP hydrolysis. Our data outline the molecular events that prime the fusion machinery, providing new insights into the coupling of mitochondrial fusion with the cellular stress response.
Keywords: mitochondria, fusion, glutathione, stress, mitofusin
Introduction
Mitochondrial plasticity is requisite for mitochondrial function, where the positioning and shape of the organelles within a cell will have a profound impact on metabolism and cell survival [1]. Fusion is antagonistic to mitochondrial division, and the extent of interconnectivity among the mitochondrial reticulum is dependent on the relative contribution of these processes. The last decade has seen progress in our understanding of the machinery required for mitochondrial fusion through the use of genetic, cell biological and cell-free systems of analysis [2]. In mammalian cells, there are three integral membrane GTPases required for fusion, Mitofusin 1 (Mfn1), Mitofusin 2 (Mfn2) and autosomal dominant Optical Atrophy 1 (Opa1). In addition, non-apoptotic Bax stimulates mitochondrial fusion both in vitro and in vivo [3], supporting the links between mitochondrial dynamics and the cell death machinery [4].
Mitochondrial fusion has been shown to be triggered as an early response to cellular stress responses [5–7]. Here we investigate the contribution of the glutathione redox ratio in the control of mitochondrial fusion. The cellular level of reduced glutathione (GSH) ranges between 8 and 15 mM, which is used to neutralize oxidized lipids and toxins within the cell [8]. The result is an accumulation of oxidized glutathione (GSSG), which increases from 1% of the total glutathione to upwards of 10% during cellular stress. GSSG is highly reactive and functions as a core sensor of cellular stress. GSSG reacts with cysteines on target proteins, resulting in either glutathionylation [9] or in the generation of new disulphide bonds, altering protein conformation in a process called disulphide switching. This process is increasingly studied within the mitochondrial homeostasis, including import [10], control of proton leak [11] and calcium flux [12]. These modifications are directly correlated to increased levels of cellular stress, prompting us to investigate the potential role of glutathione in regulating mitochondrial fusion. We demonstrate that the glutathione redox status is a core determinant of mitochondrial fusion, leading to profound molecular transitions within the fusion machinery, effectively ‘priming’ them for fusion.
Results
GSSG stimulates mitochondrial fusion
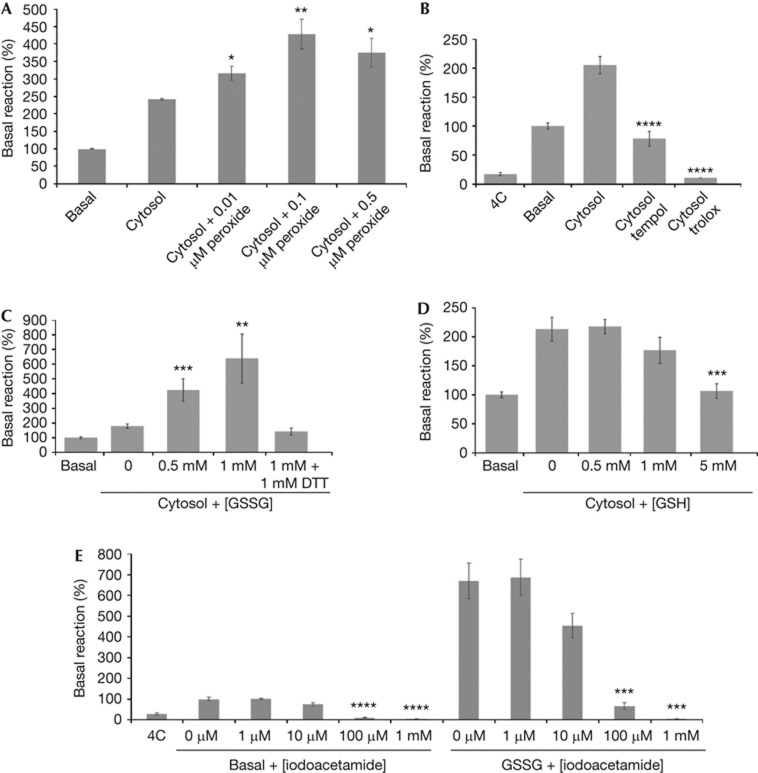
Given the evidence that mitochondrial fusion is a stress response [5], we tested whether we could observe this in our isolated fusion assay [13]. Mitochondrial fusion occurs efficiently without exogenous cytosol, termed the ‘basal’ reaction; however, the addition of cytosol further stimulates fusion by approximately two- to threefold (Fig 1A) [13]. Consistent with previous in vivo experiments, where treatment with sublethal doses of hydrogen peroxide led to a hyperfused state [14], we also observe that low doses of hydrogen peroxide stimulated mitochondrial fusion (Fig 1A). Conversely, addition of two different antioxidants, Tempol and Trolox, led to a strong inhibition of mitochondrial fusion (Fig 1B), suggesting that the activation of fusion might require some level of reactive oxygen species (ROS) or oxidation. We then tested the effects of glutathione, the cell’s primary sentinel of cellular redox stress, in our assay. Addition of physiological levels of GSSG mimicking the levels seen during cellular stress (0.5–1 mM) led to a dose-dependant stimulation of mitochondrial fusion (Fig 1C). Including dithiothreitol in the reaction to reduce the GSSG abolished the stimulation. In contrast, the addition of 5 mM GSH inhibited fusion (Fig 1D). Addition of GSSG directly to mitochondria in the absence of cytosols also stimulated mitochondrial fusion (Fig 1E), consistent with a mitochondrial-associated target for GSSG. Addition of iodoacetate (which binds and blocks free cysteine residues) inhibited both basal and GSSG-stimulated mitochondrial fusion (Fig 1E), confirming that free cysteines are essential in the reaction.
Figure 1.
Oxidants and antioxidants have opposing effects on mitochondrial fusion. (A) Addition of H2O2 to the fusion reaction containing HeLa cytosols stimulates fusion. (B) Addition of 10 mM of the antioxidants Tempol (a superoxide scavenger) and Trolox (a water-soluble vitamin E analogue) block mitochondrial fusion in the presence of stimulatory HeLa cytosols. (C) Addition of GSSG to the fusion reaction containing HeLa cytosols stimulates fusion, but this stimulation is abrogated by the addition of DTT. (D) Addition of GSH blocks mitochondrial fusion at higher concentrations. (E) In the absence of cytosols, 0.5 mM GSSG still stimulates fusion. Addition of iodoacetate inhibits both basal and GSSG-stimulated fusion. Amount of fusion, as determined by luciferase activity, is normalized to the basal reaction (without cytosol addition) set at 100%. Error bars show mean+s.e. from the replicates of at least three independent experiments, and the statistical significance indicated on the graphs were determined by paired t-test analysis; *P<0.05, **P<0.005, ***P<0.0005 and ****P<0.0001. DTT, dithiothreitol; GSH, reduced glutathione; GSSG, oxidized glutathione.
GSSG and GTP hydrolysis ‘Prime’ the fusion machinery
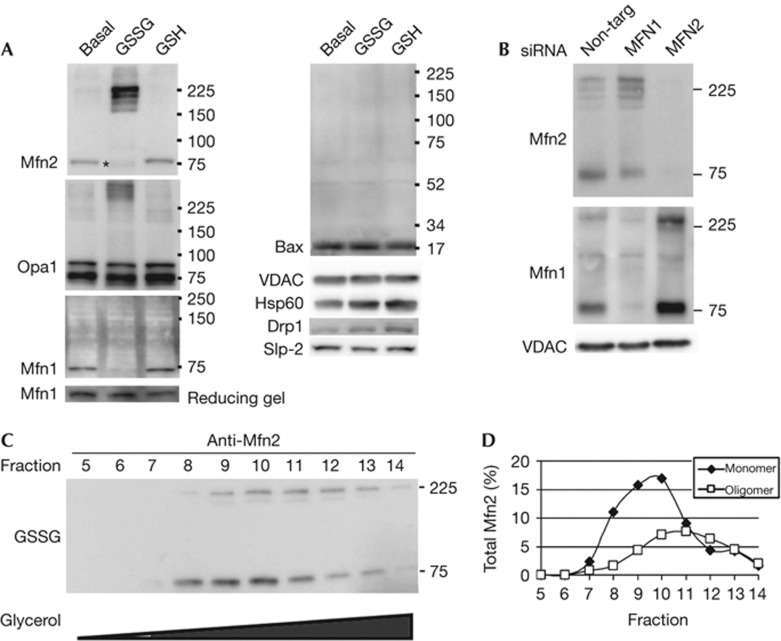
Given the likelihood of GSSG treatment to alter cysteine residues, either through glutathionylation or disulphide transitions, we examined the mobility of the fusion machinery in non-reducing gels, which preserve disulphide linkages. We observed a significant GSSG-dependent shift of Mfn2 immunoreactivity into four distinct oligomeric species running between 160 and 225 kDa (Fig 2A). Additionally, we note the more rapid migration of the Mfn2 monomer with GSSG treatment, most likely due an intramolecular disulphide bond. We also observed a significant accumulation of oligomeric forms of Opa1 (Fig 2A), which were sensitive to reducing conditions (supplementary Fig S1A online). Mfn1 immunoreactivity was lost on GSSG treatment, although the protein levels were unchanged when the reactions are separated using a reducing gel (Fig 2A). This indicates that Mfn1 likely undergoes a conformational change masking the epitope within the second heptad repeat that was recognized by the monoclonal anti-Mfn1 antibody. We were able to confirm a larger oligomeric form of Mfn1 with GSSG treatment by soaking the membrane in reducing buffer containing β-mercaptoethanol before immunoblotting (Fig 2B).
Figure 2.
Electrophoretic mobility of the fusion machinery is altered on treatment with GSSG. (A) Western blots from samples run under non-reducing conditions with proteins isolated from mitochondria incubated under basal fusion conditions either untreated, or treated with 0.5 mM GSSG or 0.5 mM GSH. Asterisk indicates the more rapid migration of the Mfn2 monomer with GSSG treatment. (B) Mitochondria were isolated from cells treated with control siRNA, Mfn1 siRNA, or Mfn2 siRNA. Following the basal fusion reaction in the presence of 0.5 mM GSSG, proteins were isolated and analysed by western blotting from a gel run under non-reducing conditions. The membrane was reduced in buffer containing β-mercaptoethanol prior immunoblotting with Mfn1, allowing the reduced form of the protein to be recognized. Western blots are representative of at least three independent experiments. (C) Proteins from digitonin-permeabilized GSSG-treated mitochondria were separated by a 5–24% glycerol gradient. Fractions 5–14 (9–18%) were analysed under non-denaturing conditions and probed for Mfn2. (D) Quantification of the monomeric and oligomeric Mfn2 bands. The oligomeric form migrates further into the gradient than the monomeric form, indicating they are larger protein complexes. GSH, reduced glutathione; GSSG, oxidized glutathione; Mfn1, Mitofusin 1; Mfn2, Mitofusin 2; Opa1, Optical Atrophy 1; siRNA, short interfering RNA.
Although Bax has been shown to have a regulatory role in mitochondrial fusion, it did not engage in any disulphide-based oligomers in response to GSSG (Fig 2A). Nor did we observe any change in the mobility of Drp1 or Slp2 other proteins that interact with Mfns and regulate morphology (Fig 2A, full-length blots are available in supplementary Fig S1B online). Importantly, we observed no change in the mobility of two control mitochondrial proteins, VDAC and Hsp60, demonstrating the specificity of the effect. The appearance of oligomerized forms of Mfn2, Mfn1 and Opa1 directly correlates to the activation of mitochondrial fusion, prompting us to consider that this might represent a conformation that is activated for fusion.
Previous work shows that mitofusin interactions can be homotypic or heterotypic [3]. To address whether the formation of GSSG-induced oligomers require both Mfns, we probed for the oligomeric assembly of each Mfn in the absence of the other. The data clearly show that the oligomeric forms do not require the presence of both Mfns, as Mfn2 oligomers continue to form on GSSG treatment when Mfn1 is silenced, and vice versa (Fig 2B). Although Mfn heterodimers are not a requisite, the appearance of several oligomeric species could represent multimers of Mfns with altered mobility due to distinct disulphide species, or could reflect the presence of additional, unknown partners within these complexes. To further examine the properties of the Mfn2 oligomers, we examined their migration in a glycerol gradient using a non-reducing gel (Fig 2C). With GSSG treatments, we observe both the monomeric and oligomeric forms of Mfn2, and the oligomeric complexes migrate further into the gradient, peaking 2 fractions later than the monomer (Fig 2D). This further confirms the presence of distinct protein complexes.
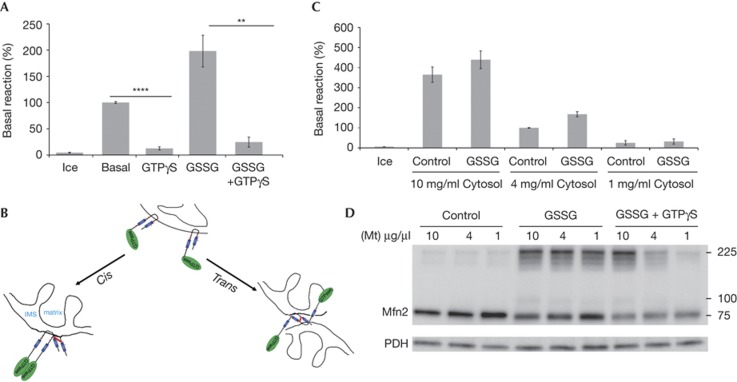
Addition of non-hydrolyzable GTP-γS has been shown to inhibit mitochondrial fusion [3, 13]. Consistent with this, the stimulation of fusion provided by GSSG was also abolished in the presence of GTP-γS (Fig 3A). However it was not clear at what stage in the fusion reaction GTP-γS and GSSG were having their effects. Given that Mfns are thought to mediate mitochondrial fusion through trans interactions with opposing mitochondria, we sought to determine whether the oligomeric forms of Mfn2 represented interactions in cis or in trans (Fig 3B). We reasoned that if the oligomers we observe are due to trans interactions, they should be dependent on the concentration of mitochondria within the fusion reaction. Our standard fusion reaction is performed in the presence of 4 mg/ml mitochondria and includes a preincubation step to promote tethering. To prevent spurious trans interactions in the experiment, we omitted the tethering step. Increasing the amount of mitochondria in the reaction to 10 mg/ml increases mitochondrial fusion, which is still further stimulated in the presence of GSSG (Fig 3C). In contrast, at 1 mg/ml of mitochondria, fusion is completely abolished, even in the presence of GSSG (Fig 3C). Separation of the reaction in non-reducing gels revealed that at all three concentrations of mitochondria, Mfn2 oligomers were similarly observed (Fig 3D). There were slightly fewer oligomers at the lowest concentration, suggesting that a minor fraction of the complexes might reflect trans interactions.
Figure 3.
GSSG-induced Mfn2 oligomers require GTP hydrolysis and occur primarily in cis. (A) Basal mitochondrial fusion reactions (without cytosol) were performed in the presence of the indicated combinations of 1 mM GSSG and/or 0.5 mM GTP-γS. Amount of fusion, as determined by luciferase activity, is normalized to the basal reaction set at 100%. Error bars show mean+s.e. from the replicates of three independent experiments, and statistical significance was determined by paired t-test analysis; **P<0.005, ****P<0.0001. (B) A model highlighting the different types of Mfn2 oligomers that might be occurring, either in cis or in trans. Blue regions highlight the heptad repeats, the GTP binding domain is shown in green, and a potential disulphide is shown as a red line. (C) Decreasing concentrations of mitochondria (10, 4, 1 μg/μl) were incubated under basal fusion conditions without an initial pelleting step and analysed for fusion as determined by luciferase activity. Data are normalized to the 4 μg/μl reaction set at 100%, with error bars showing mean+s.e. from the replicates of three independent experiments. (D) Western blot of Mfn2 oligomers from basal, 1 mM GSSG or 1 mM GSSG+0.5 mM GTP-γS-treated fusion reactions containing decreasing concentrations of mitochondria as in B. GSSG, oxidized glutathione; Mfn2, Mitofusin 2; Mt, mitochondria.
When GTP-γS was added to the GSSG within the dilution experiment, a clear dependence on GTP hydrolysis was revealed for the generation of stable Mfn2 oligomers in cis (Fig 3D). However, unlike GSSG treatment alone, this effect was absolutely dose dependent, with ablation of oligomers formed at the dilute concentrations of mitochondria where fusion was undetectable. This result indicates that nucleotide hydrolysis is required for the generation of the oligomers formed at 1 and 4 mg/ml mitochondria; however, at higher concentrations of mitochondria (10 mg/ml), trans oligomers might occur in the presence of GSSG even without GTP hydrolysis.
In vivo modulation of glutathione alters morphology
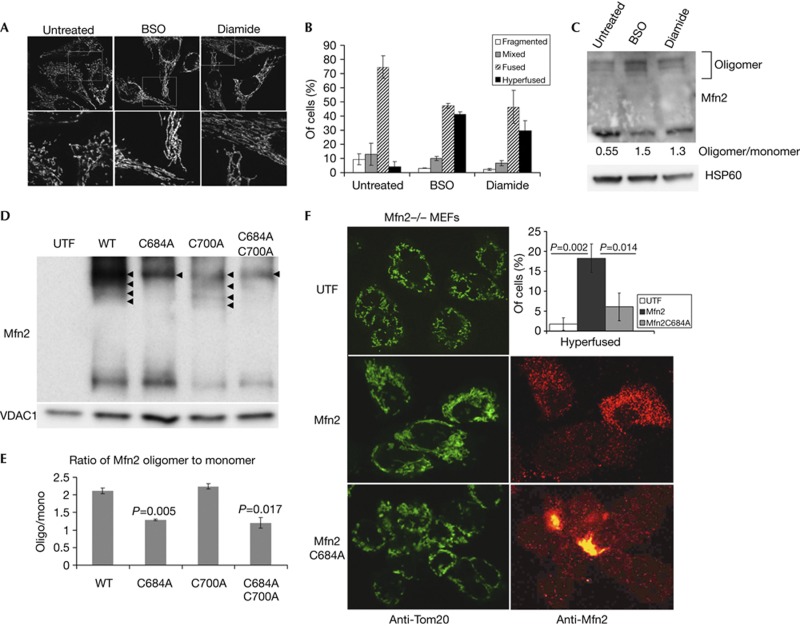
The results presented so far have used a staged mitochondrial fusion reaction in vitro. It was important to confirm the pro-fusion role of GSSG within intact cells. For this we used standard approaches to alter the GSH:GSSG ratio within intact HeLa cells. We incubated cells for 24 h with either L-buthionine-sulfoximine (BSO), which inhibits the synthesis of GSH, leading to a concomitant increase in the ratio of cellular GSSG, or diamide which directly converts GSH to GSSG. As expected from the cell-free fusion reactions, these treatments also led to a hyperfused mitochondrial reticulum (Fig 4A,B). This transition was accompanied by an increase in the ratio of oligomerized to monomeric Mfn2, where untreated cells are mostly monomeric (0.55), to mostly oligomeric (1.5 BSO and 1.3 diamide; Fig 4C). Therefore, the glutathione ratio is a core determinant of mitochondrial fusion, both in intact cells, and in our cell-free reaction.
Figure 4.
GSSG-induced Mfn2 oligomers through Cysteine 684 are required for fusion in vivo. (A) Treatment of HeLa cells with BSO (100 μM for 24 h) or Diamide (100 μM for 1 h), leads to increased fusion in vivo, as revealed by Tom20 staining. (B) Quantification of at least 30 cells from triplicate reactions treated as in A, error bars show mean+s.d. (C) Western blots and quantification of the increase in oligomeric Mfn2 on inhibition of glutathione synthesis. (D) Wild-type Mfn2, Mfn2C684A and/or Mfn2C700A constructs were transfected into Mfn2−/− MEFs as indicated. Mitochondria were isolated, treated with 0.5 mM GSSG and Mfn2 oligomers observed by western analysis following non-reducing electrophoresis. VDAC1 was probed as a load control. Arrowheads indicate the presence of oligomeric forms of Mfn2. (E) The ratio of oligomer:monomer was quantified from two blots and the P-value versus the wild type is indicated. (F) Confocal analysis of mitochondrial morphology within Mfn2−/− MEFs either untransfected (UTF), or transfected with Mfn2 or Mfn2C684A. Mitochondria are labelled with Tom20 (green), and transfected cells are confirmed using monoclonal anti-Mfn2 antibodies (red). The percentage of transfected cells showing hyperfused mitochondria in each condition is shown in the top right panel. Data were obtained from two experiments counting at least 50 cells for each condition; P-values were determined by Student’s T-test, error bars show mean+s.d. BSO, L-buthionine-sulfoximine; GSSG, oxidized glutathione; MEF, mouse embryonic fibroblast; Mfn2, Mitofusin 2; WT, wild-type.
Finally, we attempted to identify which cysteine residues were involved in the generation of the disulphide-dependent oligomers. Mfn1 and Mfn2 contain 14 and 13 cysteines, respectively. We mutated eight of the conserved cysteines in Mfn2 common to the Mfns. On transfection of these mutants into Mfn2−/− embryonic fibroblasts, only MfnC684A resulted in the loss of three of the four GSSG-induced oligomers (Fig 4D,E; supplementary Fig S2 online). Although the highest oligomeric species remained, transfection of Mfn2C684A showed a significantly reduced ability to rescue the mitochondrial morphology of the Mfn2−/− mouse embryonic fibroblasts (MEFs) compared with wild-type Mfn2, further demonstrating the critical role of this cysteine residue in the function of Mfn2 (Fig 4F). This cysteine residue is located at the hinge of the HR2 region, suggesting that disulphide transitions in this region could alter the stable coiled-coil interactions previously described for Mfns [15], acting as a ‘priming’ step for the Mfns in fusion.
Discussion
Our data are the first to indicate the requirement for disulphide-induced modifications of the mitochondrial fusion machinery. In contrast to the documented roles for posttranslational modifications of the fission GTPase Drp1 [16], there is limited understanding of how the mitofusins are regulated in fusion. Our data show that GTP hydrolysis is required to assemble and/or stabilize an oligomeric complex of the mitofusins. As GTP hydrolysis by the yeast Mfn homologue Fzo1 is also required for fusion [17, 18] and given the relatively low affinity of the Mfns for GTP [19, 20], we consider that futile cycles of hydrolysis and nucleotide exchange occur within these GTPases, and in the presence of GSSG, new disulphide linkages form following a cycle of hydrolysis. We have shown that this stabilizes an oligomeric conformation formed in cis, which is important for subsequent docking and fusion. The involvement of glutathione provides a direct link between the rates of mitochondrial fusion and the level of cellular stress. Neutralizing stress with either GSH or ROS scavengers effectively blocked mitochondrial fusion, indicating that this is a requisite event for fusion even in steady state. As a more global stress response, we note that increased GSSG levels have been reported in many of the physiological conditions associated with mitochondrial hyperfusion, including the transition between G1/S, to cellular senescence and starvation-induced autophagy [21–23].
It has been proposed that hyperfused mitochondria will render the cells resistant to cell death and mitophagy [5–7]. As a transient response, mitochondrial hyperfusion might provide a critical window of protection while the longer-term, transcriptional stress responses are completed. Taken together, the ubiquitous elevation of GSSG within the cell provides a common mechanism of disulphide conversion that will result in a staged response throughout the cell, and within all organelles. Future work will continue to focus on the structural details of this response, and the functional implications of mitochondrial fusion in cellular homeostasis.
Methods
Fusion assay. The in vitro mitochondrial fusion assay was performed as previously described [13]. Briefly, in a 25-μl reaction, 100 μg of mitochondria were pelleted and incubated on ice for 30 min to promote tethering, resuspended and incubated at 37 °C for 20–30 min to allow fusion followed by reading luciferase activity or analysis of proteins by western blot. Treatment of mitochondria during in vitro fusion with various drugs included: hydrogen peroxide (EMD), Tempol (EMD), Trolox (Sigma), iodoacetate (Sigma), GSSG (Sigma) and GSH (Sigma). Cytosols were prepared from suspension-adapted sHeLa cells, as previously described [24], and added at 1 mg/ml as indicated. Error bars show mean +/− s.e. from the replicates of at least three independent experiments. Student’s T-test was used to determine P-values between indicated conditions.
Western blot. Proteins were resolved by SDS–PAGE under reducing or non-reducing conditions, transferred to polyvinylidene difluoride membrane and probed with indicated antibodies; Bax (Millipore), Drp1 (BD Biosciences), HSP60 (Santa Cruz), Mitofusin 1 (Abcam), Mitofusin 2 (Abnova), Opa1 (BD Bioscience), PDH (MitoSciences), Slp2 (Enzo), VDAC1 (MitoSciences).
Glycerol gradient. Isolated mitochondria (1.5 mg) were incubated under fusion conditions, lysed in 1% digitonin in the presence of protease inhibitors, debris removed by centrifugation and 100 μl of proteins layered on a 2-ml glycerol gradient comprises of 100 μl steps from 5–24%. The gradient was run with a Beckman Optima TLX Ultracentrifuge using a TLS-55 rotor spun at 54,000 r.p.m. for 3.5 h.
Cell culture. HeLa cells, grown as previously described [13], were treated with 100 μM BSO (Sigma) for 24 h or 100 μM diamide (Sigma) for 1 h before collecting or immunofluorescence. Cells were transfected with 20 nM short interfering RNA (siRNA; ON-TARGETplus siRNA, Dharmacon) for 4 days
Mfn2−/− MEFs were obtained from ATCC and grown as recommended. MEFs were transfected with wild-type and mutant Mfn2 constructs in the pcDNA4/HisC plasmid (Invitrogen) for 24 h using Lipofectamine 2000 (Invitrogen) following the recommended protocol. Cells were collected from five 10-cm dishes, mitochondria isolated, and treated with 0.5 mM GSSG before analysis.
Immunofluorescence microscopy.HeLa cells, grown on glass coverslips and treated as indicated in triplicate, were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 and labelled with anti-Tom20 antibody (Santa Cruz) or Mfn2 (Abnova). Cells were then imaged with an Olympus IX81 inverted microscope with appropriate lasers, using Olympus FV1000 confocal scanning microscope fitted with a × 100 objective NA1.4. In blinded analysis, at least 30 cells from each condition were scored manually for morphology into the indicated classes.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This work was supported by CIHR#43935 to H.M.M. and by Juvenile Diabetes Research Foundation to H.M.M. and R.M., T.S. was the recipient of a University of Ottawa Cardiac Research Endowment Fellowship.
Author contributions: T.S. was the primary experimentalist and contributed to the study design and writing of the manuscript. M.G. contributed to the experiments. R.M. contributed to the experimental design and writing of the manuscript. H.M.M. conceived and directed experiments, and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Braschi E, McBride HM (2010) Mitochondria and the culture of the Borg: understanding the integration of mitochondrial function within the reticulum, the cell, and the organism. Bioessays 32: 958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J (2007) The machines that divide and fuse mitochondria. Annu Rev Biochem 76: 751–780 [DOI] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J (2011) The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell 41: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ (2011) Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21: 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D et al. (2009) SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28: 1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 108: 10190–10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208 [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A (2009) Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci 34: 85–96 [DOI] [PubMed] [Google Scholar]
- Koehler CM, Tienson HL (2009) Redox regulation of protein folding in the mitochondrial intermembrane space. Biochim Biophys Acta 1793: 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME (2011) Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem 286: 21865–21875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BJ et al. (2010) S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol 190: 391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauss AC et al. (2010) A novel cell-free mitochondrial fusion assay amenable for high-throughput screenings of fusion modulators. BMC Biol 8: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS et al. (2006) Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol 209: 468–480 [DOI] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862 [DOI] [PubMed] [Google Scholar]
- Wilson TJ, Slupe AM, Strack S (2012) Cell signaling and mitochondrial dynamics: Implications for neuronal function and neurodegenerative disease. Neurobiol Dis (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton F, Fres JM, Schauss A, Pinson B, Praefcke GJ, Langer T, Escobar-Henriques M (2011) Ugo1 and Mdm30 act sequentially during Fzo1-mediated mitochondrial outer membrane fusion. J Cell Sci 124: 1126–1135 [DOI] [PubMed] [Google Scholar]
- Cohen MM, Amiott EA, Day AR, Leboucher GP, Pryce EN, Glickman MH, McCaffery JM, Shaw JM, Weissman AM (2011) Sequential requirements for the GTPase domain of the mitofusin Fzo1 and the ubiquitin ligase SCFMdm30 in mitochondrial outer membrane fusion. J Cell Sci 124: 1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117: 6535–6546 [DOI] [PubMed] [Google Scholar]
- Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H (2005) Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem 280: 25060–25070 [DOI] [PubMed] [Google Scholar]
- Hoffman A, Spetner LM, Burke M (2008) Ramifications of a redox switch within a normal cell: its absence in a cancer cell. Free Radic Biol Med 45: 265–268 [DOI] [PubMed] [Google Scholar]
- Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP (2008) Real-time imaging of the intracellular glutathione redox potential. Nat Methods 5: 553–559 [DOI] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS (2003) Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med 35: 626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98: 377–386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.