EMBO reports (2012) 13, 909–915; doi:; DOI: 10.1038/embor.2012.128
Mitochondria are dynamic organelles that undergo fission and fusion events, but we are only beginning to understand some of the reasons and the machineries involved in these processes [1]. Fission allows distribution of mitochondria to daughter cells following mitosis. Small mitochondria are more efficiently tracked along the cytoskeleton, which also allows a quality control mechanism to exist where fragmented ‘old’ mitochondria can be turned over by autophagy [2]. Fusion enables mitochondrial contents to be mixed between neighbouring organelles. Fission and fusion events must be tightly regulated, and whilst they are opposing processes, they seem to act in concert together—for example, fusion events often lead to a subsequent fission event at the same site. In this issue of EMBO reports, McBride and colleagues [3] identify a new mechanism by which mitofusins can be activated to initiate mitochondrial fusion under conditions of oxidative stress.
…GSSG stimulates mitochondrial fusion whereas GSH inhibits it
Fission involves surface receptors and adaptors (Fis1, Mff, MiD49 and MiD51/MIEF1) and the cytosolic dynamin-related protein Drp1. Fusion involves the dynamin GTPases mitofusin (Mfn) 1 and 2 at the outer and Opa1 at the inner membrane. Mitofusins form homo- and hetero-oligomers to tether adjacent mitochondria together. How the outer membranes fuse before Opa1 acts to fuse the inner membranes is not known. Moreover, the mechanisms regulating their fusion activity are not well-understood. Mitochondrial fission and fusion are important in developmental and physiological processes. Mitochondrial fragmentation occurs during apoptosis and necrosis, where the loss of the network facilitates cell death. However, under conditions of less severe stress, mitochondria can undergo hyperfusion, seemingly as a mechanism by which they can protect the cell from dying [4]. During nutrient starvation, which leads to the induction of macro-autophagy, mitochondria also undergo hyperfusion to prevent their encapsulation by autophagosomes. Hyperfusion is also important during the G1 to S transition of the cell cycle. Until now, the mechanisms regulating stress-induced mitochondrial hyperfusion have remained elusive.
McBride and colleagues used an in vitro fusion assay, which consists of mixing mitochondria isolated from cell cultures expressing either an amino-terminal or a carboxy-terminal domain of luciferase, targeted to the mitochondrial matrix. Both luciferase domains contained a leucine zipper that leads to dimerization upon mitochondrial fusion thereby generating a functional enzyme. The authors found that in vitro, mitochondrial fusion was stimulated by oxidative stress including hydrogen peroxide treatment, whereas anti-oxidants inhibited the process. They then tested whether fusion might be responsive to reactive oxygen species or cellular oxidants. Glutathione (GSH), found in all parts of the cell, provides the main redox buffer for cells. GSH contains a free thiol group, and the formation of a disulphide bond between two GSH molecules gives rise to oxidized GSH (GSSG). GSH reductase recycles GSSG to GSH thus maintaining the cellular redox state. During oxidative stress, GSSG accumulates and can interact with other proteins, and induces either glutathionylation or the generation of a disulphide bond known as ‘disulphide switching’. McBride and colleagues found that GSSG stimulates mitochondrial fusion whereas GSH inhibits it. The fusion reaction could be stimulated by adding GSSG directly to isolated mitochondria whilst it was inhibited by the cysteine alkylating agent iodoacetate. This implies that mitochondrial proteins with free thiol groups are direct substrates of GSH-mediated oxidation and that this regulates mitochondrial fusion. Analysis of Mfn1 and Mfn2 revealed that GSSG treatment induces oligomer formation, which could be resolved on non-reducing SDS–PAGE as four distinct species of 160–220 kDa. Addition of a reductant led to the loss of the oligomeric species on SDS–PAGE, suggesting that the mitofusins might indeed form disulphide bonds. The presence of discrete oligomeric species on non-reducing gels suggests that intermolecular disulphides might occur and that the mitofusins interact with additional proteins. Interestingly, two proteins shown to regulate mitofusin activity directly, SLP2 and non-apoptotic Bax, were not modified by oxidant. Similarly, Drp1 was unaffected, pointing to a direct activation of fusion rather than a decrease in fission activity. However, it remains possible that fission might also be downregulated by oxidation, and inactivation, of mitochondrial fission receptors such as Fis1, Mff and MiD49/MiD51 or by a different post-translational modification of Drp1. The authors validated their work by incubating cultured cells with agents that cause an increase in the levels of GSSG. Such treatment induced mitochondrial hyperfusion as expected, but it also resulted in the increase of Mfn2 oligomers.
This implies that mitochondrial proteins with free thiol groups are direct substrates of GSH-mediated oxidation and that this regulates mitochondrial fusion
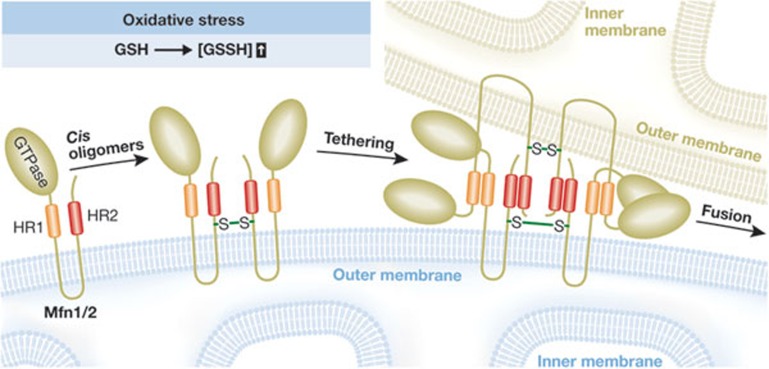
Mitofusins contain a cytosolically exposed N-terminal GTPase domain followed by a heptad repeat (HR1), and two transmembrane regions that form a hairpin in the mitochondrial outer membrane allowing the exposure of another heptad repeat (HR2) to the cytosol. The heptad repeats are involved in membrane-tethering events, and structural analysis of a HR2 dimer revealed that it forms an antiparallel coiled-coil [5]. When the authors added non-hydrolysable GTP to their in vitro assay, the GSSG-mediated fusion was blocked. When mitochondria were diluted to reduce tethering between mitochondria, GSSG still induced mitofusin oligomerization and this depended on GTP hydrolysis. This suggests that stress-induced oligomerization occurs largely between mitofusin molecules on the surface of the same mitochondria—that is, in cis. At higher concentrations of mitochondria where tethering events are enhanced, GTP hydrolysis did not seem to be required to induce oligomerization of oxidized mitofusins between adjacent mitochondria—that is, in trans. To narrow down the cysteine residues that are oxidized in mitofusins, the authors performed site-directed mutagenesis of Mfn2 and introduced the mutants into Mfn2−/− mouse embryonic fibroblasts. The authors found that mutation of Cys 684 in the hinge region of HR2 reduced the formation of oligomers and was less functional in rescuing mitochondrial fusion. The authors suggest that disulphide bond formation between this residue and cysteines in adjacent mitofusins might alter the coiled-coil interactions, thereby priming the proteins to activate fusion.
…disulphide bond formation between […] adjacent mitofusins might alter the coiled-coil interactions, thereby priming the proteins to activate fusion
Why is hyperfusion needed? Mitochondrial fragmentation is an important aspect of cell death pathways, and hyperfusion might open a window of time to enable the cell to activate proteins involved in responding to cellular insults. However, cells can also block fusion during stress-induced apoptosis. It was found that stress-activated c-Jun N-terminal kinase phosphorylates Mfn2, leading to its ubiquitination and degradation [6]. How cells decide whether to turn on either survival mechanisms that activate mitofusins, or death mechanisms that inactivate them, requires additional studies.
The work by McBride and colleagues points to the importance of intracellular redox conditions in regulating mitochondrial fusion. Changes in cellular GSH might also be important for regulating other members of the fission–fusion machinery. Ganglioside-induced differentiation associated protein 1 (GDAP1) functions in mitochondrial fission, and mutations in GDAP1 lead to the neurodegenerative disorder Charcot Marie-Tooth disease (CMT) 2A. GDAP1 contains GSH S-transferase domains, and loss of the protein has been found to decrease the levels of GSH [7]. Interestingly, mutations in Mfn2 lead to CMT4A, which is clinically almost indistinct from CMT2A. A study of a family with CMT revealed an asymptomatic mother with a mutation in GDAP1 and an asymptomatic father with a mutation in Mfn2. Their child who inherited both mutant alleles developed severe neuropathy [8]. The cumulative effects of inheriting both mutations points to an overlap in the function of these proteins. The new findings, pointing to the importance of GSH in mitochondrial fusion, warrant investigation into whether a closer connection between GDAP1 and mitofusins exists.
Figure 1.
Model for mitochondrial fusion induced by oxidative stress. Oxidation of cysteines in mitofusins induces oligomer formation in cis, probably through the formation of one or more disulphide bonds, which might cause a conformational change in the heptad repeat (HR) regions aiding in tethering to mitofusins in trans to enhance membrane fusion.
Footnotes
The authors declare that they have no conflict of interest.
References
- Palmer CS et al. (2011) Cell Signal 23: 1534–1545 [DOI] [PubMed] [Google Scholar]
- Liu X et al. (2008) EMBO J 28: 3074–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt T et al. (2012) EMBO Rep [Epub ahead of print] doi:; DOI: 10.1038/embor.2012.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D et al. (2009) EMBO J 28: 1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T et al. (2004) Science 305: 858–862 [DOI] [PubMed] [Google Scholar]
- Leboucher GP et al. (2012) Mol Cell 47: 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack R et al. (2012) Hum Mol Genet 21: 150–162 [DOI] [PubMed] [Google Scholar]
- Vital A et al. (2012) Neuromuscul Disord 22: 735–741 [DOI] [PubMed] [Google Scholar]