Abstract
HIV infection of the CNS can result in neurologic dysfunction in a significant number of infected individuals. NeuroAIDS is characterized by neuronal injury and loss, yet there is no evidence of HIV infection in neurons. Thus, neuronal damage and dropout are likely due to indirect effects of HIV infection of other CNS cells, through elaboration of inflammatory factors and neurotoxic viral proteins, including the viral transactivating protein tat. We and others demonstrated that tat induces apoptosis in differentiated mature human neurons. We now demonstrate that the high level of tat toxicity observed in human neurons involves specific developmental stages that correlate with N-Methyl-D-Aspartate receptor (NMDAR) expression, and that tat toxicity is also dependent upon the species being analyzed. Our results indicate that tat treatment of primary cultures of differentiated human neurons with significant amounts of NMDAR expression induces extensive apoptosis. In contrast, tat treatment induces only low levels of apoptosis in primary cultures of immature human neurons with low or minimal expression of NMDAR. In addition, tat treatment has minimal effect on rat hippocampal neurons in culture, despite their high expression of NMDAR. We propose that this difference may be due to low expression of the NR2A subunit. These findings are important for an understanding of the many differences among tissue culture systems and species used to study HIV-tat-mediated toxicity.
Keywords: HIV-1, NeuroAIDS, Glutamate, NMDA, Dementia, HAND
Introduction
Human immunodeficiency virus (HIV) enters the CNS early after infection, and viral persistence within the brain can result in many neurological abnormalities. As the prevalence of HIV-associated neurocognitive disorders (HAND) increases (Sacktor 2002; McArthur 2004; Joseph and Prasad 2005), studies of the mechanisms mediating the pathogenesis of NeuroAIDS become even more important (Nath and Geiger 1998; Albright et al. 2003).
HIV cannot directly infect neurons; thus, neuronal cell damage and death must be due to indirect mechanisms, such as the elaboration of neurotoxins from HIV-infected cells. One of these neurotoxins is the viral transactivator, tat, that can be secreted from HIV-infected cells and taken up by infected and uninfected cells (Sabatier et al. 1991; Ensoli et al. 1993; Liu et al. 2000). While combined anti-retroviral treatment (cART) targets several steps of viral replication, it does not prevent the transcription and translation of the tat protein. Tat uptake by uninfected cells has many effects, depending on cell type. These effects include secretion of cytokines and chemokines (McManus et al. 2000; Rappaport et al. 1999; D’Aversa et al. 2005), activation of transcription factors (Kumar et al. 1998), alterations in gene transcription (Kumar et al. 1999), expression and activation of matrix metalloproteinases (Conant et al. 2004; Kumar et al. 1999; Toschi et al. 2001), dysregulation of glutamate metabolism within the CNS (Eugenin et al. 2003b), activation of N-Methyl-D-Aspartate receptors (NMDAR) in neurons (Bonavia et al. 2001; Haughey et al. 1999; Haughey et al. 2001; Kruman et al. 1998; Nath et al. 2000; Eugenin et al. 2007), activation and expression of other receptors (Rappaport et al. 1999; Eugenin et al. 2003b, 2007), and expression of proapoptotic proteins in neurons (Bartz and Emerman 1999; Kumar et al. 1999). Although the exact mechanisms of neuronal apoptosis are not known, there is a consensus that glutamate and its receptors can be involved in this process.
Our results obtained using human differentiated neurons in culture indicated that tat induces high levels of neuronal apoptosis (60–90%) by a mechanism dependent on the formation of a cell membrane macromolecular complex comprised of low-density lipoprotein receptor-related protein (LRP), postsynaptic density protein-95 kDa (PSD-95), NMDAR, and neuronal nitric oxide synthase (nNOS) (Eugenin et al. 2007). In rat and human non-mature neuronal culture systems, tat also induces neuronal apoptosis, but to a lesser extent, approximately 5–40% (Kruman et al. 1998; Haughey et al. 1999; Nath et al. 2000; Bonavia et al. 2001; Eugenin et al. 2003b, 2007). These differences may be due to developmental stages of the neurons as well as to the species being utilized. Our results demonstrate that the extent of neuronal differentiation, NMDAR expression and the species of origin are major factors that contribute to the differences observed in tat-mediated neuronal toxicity.
Glutamate and its receptors, including NMDAR, are the major transmitter/receptors at excitatory synapses within the CNS. Altered activation of NMDAR contributes to many pathological conditions, including NeuroAIDS, and results in neuronal damage and death (Gonzalez-Scarano and Martin-Garcia 2005). Our studies and those of others indicate that blocking this receptor abolishes the apoptosis caused by tat (Bonavia et al. 2001; Haughey et al. 1999, 2001; Kruman et al. 1998; Nath et al. 2000; Eugenin et al. 2003b, 2007). However, it is unknown whether the levels of apoptosis induced by tat correlate with NMDAR expression in the different culture models of neuronal tissue.
A critical point in the development of the human fetal brain is the period between 18 and 24 weeks, due to the changes in expression of glutamate receptors, including NMDA and AMPA/kainite, in areas including the brainstem, hippocampus, and cortex (de Graaf-Peters and Hadders-Algra 2006; Herlenius and Lagercrantz 2001; Herlenius and Lagercrantz 2004). In rat and mouse cultures, in vitro differentiation drives the expression of these receptors despite their low levels in the early stages of development (Herlenius and Lagercrantz 2001, 2004). A major problem for the studies of the human nervous system is the lack of information about the molecular development of human neurons in culture and optimal methods of culture, as well as how to compare cells obtained from humans to those from animal models.
In the current manuscript we demonstrate that differences in NMDAR expression, neuronal maturation, and species of origin significantly affect the levels of apoptosis induced by HIV-tat treatment. In addition, our data indicate that human undifferentiated progenitor and primary neuronal cultures, differentiated neuronal cultures, and rat cultures may have different levels of sensitivity and therefore, depending upon the question being addressed, certain models or cell types may be more useful than others.
Materials and Methods
Materials
Neurobasal media, DMEM, fetal bovine serum (FBS), penicillin/streptomycin (P/S), and trypsin–EDTA were purchased from GibcoBRL (Grand Island, NY). MK801, NMDA, monoclonal antibody to GFAP, polyclonal antibody to NR1, FITC or Cy3-conjugated anti-rabbit IgG, and Cy3 or FITC-conjugated anti-mouse IgG antibodies were from Sigma (St. Louis, MO). Monoclonal antibodies to NR2A and NR2B were from Invitrogen (San Francisco, CA). Polyclonal negative control antibodies were all from Santa Cruz Biotechnology (Santa Cruz, CA) and purified mouse IgG2B and IgG1 myeloma proteins were from Cappel Pharmaceuticals, Inc. LRP monoclonal antibodies were from Progen-Biotechnik GmbH (Heidelberg, Germany). In situ cell death detection kit-TMR red (TUNEL) was from Roche (Mannheim, Germany).
Tat Preparation
Tat protein was purchased from the University of Kentucky or was a gift from Dr. Avindra Nath (The Johns Hopkins Medical Center, Baltimore). Tat protein was expressed from the tat gene derived from the HIV-BRU strain encoding the first 72 amino acids (first exon) inserted into an Escherichia coli vector, PinPoint Xa-2, and expressed as a fusion protein. Tat1–72 was enzymatically cleaved from the fusion protein and purified as described (Conant et al. 1996; Ma and Nath 1997). Purification was >95% as determined by SDS-PAGE followed by Coomassie blue staining and Western blot analysis using polyclonal antibody to tat (AIDS Repository, NIH). Endotoxin contamination was not detected in these preparations.
Human Neuronal Cultures
Human fetal cortical tissue was used as part of an ongoing research protocol approved by the Albert Einstein College of Medicine. Dissociation, isolation, and culture of mixed population of cortical cells were as described (Eugenin et al. 2003b, 2007). Neuronal cultures were obtained from mixed cortical cells after 7–10 days in culture and plated in Neurobasal media supplemented with N2 neurosurvival factor and 1–5% FBS. Experiments were performed 6–8 days after splitting of cells obtained from 23 week tissue or after 6–10 weeks in culture for the immature neurons from 22 week tissue or earlier. This long-term culture facilitated expression of NMDAR, although it was still at low levels. In our primary neuron population, 25–35% of neurons express NMDAR. We did not detect contamination with microglia using the macrophage markers CD14 and CD68 (Abcam, Cambridge, MA). We define neurons derived from 23 week tissue as mature and those from 22 week tissue or younger as immature.
Culture and Differentiation of Neuronal Progenitor Cells
Human progenitor cells were isolated from telencephalon of an 8 week gestation brain (Messam et al. 2003). After dissection and dissociation, the cells were grown and expanded into serum-free Neurobasal medium supplemented with 0.5% bovine albumin, Neurosurvival factor (Clonetics1:50), N2 factor (Invitrogen, 1:50), bFGF (Preprotech, 25 ng/ml), EGF (Peprotech, 20 ng/ml), gentamycin (Life Technologies, 50 ug/ml), and glutamine (Quality Biologicals, 2 mM). For differentiation of progenitor cells into neurons (MAP-2 and NeuN positive, and nestin negative) the above medium was replaced with medium containing Neurobasal media, Neurosurvival factor (Clonetics, 1:50), N2 factor (Invitrogen, 1:50), PDGF (Sigma, 10 ng/ml), BDNF (Sigma, 10 ng/ml), gentamycin (Life Technologies, 50 ug/ml), and glutamine (Quality Biologicals, 2 mM) for 20 days, the time required to reach full differentiation as described previously (Hou et al. 2006). These cultures were fed by replacing 50% of the medium.
Culture of Rat Hippocampal Neurons
Cultures were generated using rat embryos and isolated hippocampus. Hippocampi were mechanically and enzymatically dissociated using trypsin/EDTA. Cells obtained after this treatment were maintained in BME medium supplemented with 10% FBS, 1% glutamax, and 1% P/S, for 2 h. BME medium was replaced by NB1.15 medium containing Neurobasal media, B27 supplement, and 1% glutamax-1 and 1% P/S. Cultures were fed once a week by replacing 50% of the NB1.15 medium.
RT-PCR Detection of NMDAR Subunits
Total RNA was isolated using Trizol reagent. Potential contamination with DNA was avoided by DNase treatment using Ambion’s DNA free kit. We used a previous published protocol (Eugenin et al. 2003a). Primers for the human NR1 subunit sequence, forward 5′-AAGCCTCGAGGGTACCA GAT-3′ and reverse 5′-AGCTTGATGAGCAGGTCGAT-3′, were used with an amplicon size of 236 bp. Primers of the human NR2A subunit were forward 5′-TGTGAAGAAATG CTGCAAGG-3′ and reverse 5′-ACTGCCCGTTGATAG ACCAC-3′ with an amplicon of 165 bp. An internal positive control was included in each experiment using human β-actin-specific primers (foward 5′-CGG AAC CGC TCA TTG CC-3′; reverse 5′-ACC CAC ACT GTG CCC ATC T A-3′; data bank Accession Number XM 004814). The NMDAR subunits and β-actin amplified cDNA fragments were resolved in 1.5% agarose gels. Ethidium bromide staining showed bands of NR1, NR2A, and β-actin amplified PCR products.
Treatment of Human and Rat Neuronal Cultures
Tat treatment (10–500 ng/ml), was in Neurobasal medium supplemented with the appropriate factors for each cell type. The NMDAR antagonist blocker, MK801 (5 μM), was added 15 min before tat treatment. This concentration was optimized previously (Eugenin et al. 2007).
Immunofluorescence and Apoptosis Assay
Human/rat neuronal and progenitor cultures were grown on coverslips, fixed and permeabilized in 70% ethanol, washed, and incubated in TUNEL reaction mixture at 37°C for 1 h in case of quantification of apoptosis and/or washed and incubated in blocking solution at room temperature. Cells were then incubated overnight in primary antibody: anti-GFAP (1:800), anti-MAP2 (1:300), anti-LRP (1:300), anti-NR1 (monoclonal, 1:500), anti-NR1 (rabbit polyclonal, 1:300), or anti-NR2A (1:500). Cells were washed and incubated with FITC-conjugated goat anti-rabbit IgG, Cy3-conjugated sheep anti-rabbit IgG, FITC-conjugated sheep anti-mouse IgG, and/or Cy3-conjugated goat anti-mouse IgG (1:300) for 1 h at room temperature, followed by a 1 h PBS wash. Coverslips were mounted using Prolong Gold with DAPI (Molecular Probes) and examined by confocal microscopy. The percentage of apoptotic neurons was determined by triple immunofluorescence staining (TUNEL, DAPI, and anti-MAP-2) as described (Eugenin et al. 2003b, 2007). The total number of neurons, as well as the number of cells that were TUNEL positive in 10 fields per coverslip, were determined (2–4 coverslips per condition), and the data were expressed as percent apoptotic neurons.
Co-immunoprecipitation for NR2A, NR2B, and LRP
Post-treatment, cells were washed twice with cold PBS and harvested in PBS plus 2 mM PMSF, and the cell suspension was sonicated in RIPA buffer with protease inhibitors. The protein was quantified by Bradford assay. Before the addition of specific antibodies, 200 μg of protein were pre-cleared with 50 μl Pansorbin (Calbiochem). The supernatant was then incubated with the appropriate antibody or control antibody for 1.5 h, followed by 50 μl of PAN-SORBIN for 30 min and pelleted. The pellets were washed with RIPA buffer plus 2 mM PMSF, resuspended in sample buffer, and then separated by SDS-PAGE and electro-transferred to nitrocellulose for analysis of interacting proteins by Western blotting (Eugenin et al. 2007).
Western Blot Analysis
Cells were lysed and protein content was determined by Bradford assay. The Western blot analyses were performed as described previously (Eugenin and Berman 2003; Eugenin et al. 2007).
Statistical Analysis
Mean differences were tested by non-parametric Kruscal–Wallis analysis to analyze percentage of apoptotic cells and calcium imaging. If a significant F-value was obtained, means were compared with Bonferonni–Dunn multiple comparison test. Results were considered significant when P <0.05.
Results
Characterization of NMDAR Expression During Human Cortex Development
Many differences in the levels of tat-induced neuronal apoptosis have been reported, depending upon the system being used. We propose that tat triggers varying amounts of apoptosis due to the differential expression of NMDAR and to interspecies differences between human and mouse/rat systems. The existing literature using rat/mouse/human systems indicates that tat requires NMDAR activity to mediate apoptosis (Bonavia et al. 2001; Eugenin et al. 2003b, 2007; Johnston et al. 2001; Karn 1999; Kruman et al. 1998; Macho et al. 1999; Nath et al. 2000; Perez et al. 2001; Prendergast et al. 2002; Wang et al. 1999). However, in vivo, significant amounts of NMDAR expression have been detected only after a critical period of human brain development, starting at 18–24 weeks (de Graaf-Peters and Hadders-Algra 2006; Herlenius and Lagercrantz 2001, 2004; Ritter et al. 2001). Thus, we hypothesized that the amount of NMDAR expressed by cultured human neurons, according to their developmental stages, was a major reason for the differences in tat-induced neuronal cell death.
Using human neuronal cultures obtained from CNS tissue before and during the critical period of NMDAR expression, we characterized the mRNA and protein expression of the NMDAR subunit 1 (NR1) and subunit 2A (NR2A), as our data demonstrated their involvement in HIV-tat-induced apoptosis (Eugenin et al. 2007). We chose subunit NR2A because we previously demonstrated that this subunit is present in the tat-induced complex necessary for apoptosis (Eugenin et al. 2007). However, subunit NR2B is expressed in the human cultures, but is not recruited to the macromolecular complex induced by tat treatment (Eugenin et al. 2007). RT-PCR for NR1, NR2A, and actin as a loading control, using RNA isolated from immature and mature cultures of human neurons indicated that both immature and mature neurons expressed NR1 and NR2A mRNAs (Fig. 1a). Western blot analyses were used to evaluate expression of NR1 and NR2A proteins in these cultures (Fig. 1b). Immature neurons did not show significant amounts of NR1 or NR2A protein even after 6–10 weeks in vitro, whereas mature neurons showed readily detectable expression of NR1 and NR2A protein (Fig. 1b). NR2B was also detected in the mature neurons (data not shown). Only minimal NMDAR expression can be induced in cells derived from early stages of development, even after 1–3 months in culture. This low expression may be mediating the weak response of these neurons to tat toxicity.
Fig. 1.
Expression of NMDAR during the development of human neurons. Cultures of human neurons obtained from different stages of gestation, immature and mature human neurons characterized by RT-PCR (a) and Western blot (b). a Relative levels of NR1, NR2A, and actin mRNA were measured by semiquantitative RT-PCR as described in the “Materials and Methods”. Immature and mature neuronal cultures were evaluated for expression of those genes. Both cultures expressed the mRNA subunits. Lane 1 is a 100 bp DNA ladder as a molecular weight marker. Lane 2 is a negative control without enzyme. Lane 3 is the product with primers for the NR1 subunit mRNA. Lane 4 is the product with primers for the NR2A subunit mRNA. Lanes 5 and 6 are actin loading controls. b Levels of NR1 and NR2A were determined by Western blot analysis of homogenates of neuronal cultures (150 μg of protein per lane) prepared from immature human brain tissue or mature tissue and probed with NR1 and NR2A antibodies. (+) is rat brain lysate as a positive control. N is lysate of human neuronal cultures. Arrows indicate NR1 and NR2A bands (n = 5)
HIV-Tat-induced Activation of NMDAR Is Dependent Upon the Levels of NMDAR Expression
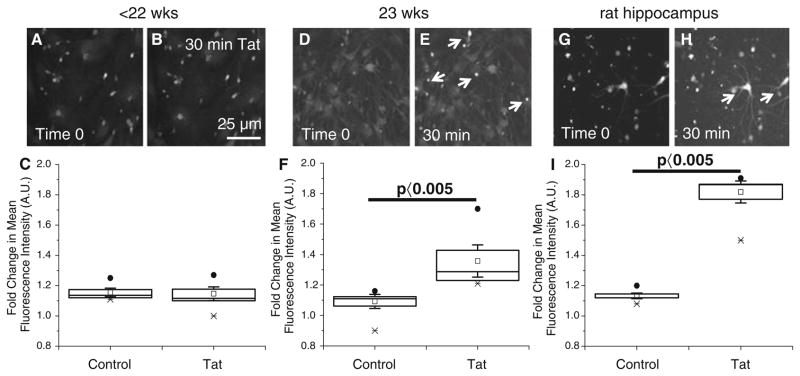
Tat induces a rise in cytoplasmic free calcium through IP3-mediated release from the endoplasmic reticulum and influx through glutamate receptors on the neuronal cell membrane (Haughey et al. 1999). To determine the effect of tat on intracellular calcium levels in the neurons in our system, we performed time-lapse calcium imaging. Neurons were loaded with 1 μM Fluo-4 at room temperature for 15–30 min. Tat (10–500 ng/ml) or NMDA (60 μM) and glycine (10 mM) treatment of human neuronal cultures indicated that neither treatment significantly increased intracellular calcium in neuron cultures obtained before the NMDAR maturation (Fig. 2a–c for tat treatment. NMDA/ Gly treatment, data not shown). Calcium imaging after tat or NMDA plus glycine treatment of mature, differentiated human neuronal cultures with high-NMDAR expression resulted in increased intracellular calcium in approximately 30% of the neurons (Fig. 2d–f). This increase in calcium correlated with the significant expression of NR1 and NR2A subunits of the NMDAR described in Fig. 1. In these imaging studies, three populations of neurons were detected, classified by their calcium response to tat treatment (data not shown). One subset of neurons did not respond to tat treatment with any change in intracellular calcium above vehicle treatment. A second group had a slow, prolonged increase in intracellular calcium that, by the end of 15 min, had resulted in a 20–30% increase in intracellular calcium. The third group responded more vigorously to tat treatment, resulting in a 60–70% increase in intracellular calcium in 15 min. We hypothesize that the strong responders were NMDAR positive cells.
Fig. 2.
Neurons isolated from different stages of development respond differently by calcium imaging to tat treatment. Neurons were preloaded with the calcium indicator dye, fluo-4, for 15–30 min at room temperature before time lapse imaging. Baseline mean fluorescence was determined for each individual neuron by averaging the mean fluorescence for the first four frames of the time lapse, before treatment with tat or vehicle. The response to tat treatment (100 ng/ml) varied depending on the cell culture utilized. Cultures of immature neurons (a–c), mature neurons (d–f), and rat hippocampal neurons were analyzed (g–h). a and b, d and e, and g and h, correspond to photographs before and after tat treatment. Arrows denote neurons that respond by increasing calcium after 30 min of tat treatment. (c, f, i). Bar: 25 μm. Quantification of the increase in calcium in response to tat treatment. In these graphs bars show the means ± SD. Filled circles, x’s, and open circles indicate high, low, and mean results (P <0.005, control vs. tat, n = 4)
Cultures of immature human neuronal cells did not respond to tat or NMDA/Gly treatments by calcium imaging (Fig. 2a–c). However, after 1–3 months in tissue culture, these cultures shifted from no NMDAR expression to low levels of expression, resulting in a minimal calcium response to tat or NMDA/Gly treatment (data not shown). These results indicate that culturing immature human neurons for a long time enables these cells to express low levels of functional NMDARs. These results correlated with the low expression of subunits NR1 and NR2A of the NMDAR in immature neurons described in Fig. 1.
Another widely used model of CNS toxicity is rat hippocampal neurons in culture, due to their reproducible formation of synapses and high expression of NMDAR as compared to human immature neurons or neuronal cell lines. We used rat hippocampal neurons to examine whether tat alters intracellular calcium. Tat treatment strongly increased intracellular calcium in rat hippocampal neurons as compared to vehicle treated cells (Fig. 2g–i).
HIV Tat Protein Induces Extensive Apoptosis Only in Differentiated, Mature Cultures of Human Neurons
To examine whether differences in neuronal toxicity induced by tat treatment are due to change in NMDAR expression or to other developmental change, we determined the levels of apoptosis induced by tat treatment in primary neuronal cultures obtained prior to and during the critical period of NMDAR expression. We also determined whether human neuronal progenitor cells, undifferentiated and differentiated into neurons, as well as rat hippocampal neurons, were sensitive to tat treatment.
Tat treatment (10–500 ng/ml) for 24 h resulted in low levels of apoptosis, 5–8%, in neuronal cultures generated from immature human CNS tissue (Fig. 3a). This apoptosis induced by tat was blocked by pretreatment of the cultures with MK801 (5 μM) (Fig. 3a). If immature neurons were cultured for 6–10 weeks to induce expression of NMDAR, apoptosis induced by tat treatment reached 10–15% (Fig. 3b). This apoptosis induced by tat was NMDAR dependent, as MK801 blocked apoptosis.
Fig. 3.
Tat induces apoptosis in neuronal cultures that depends on their maturity and is prevented by MK801, implicating NMDAR mediation. The percentage of total neurons that are apoptotic, Neuronal apoptosis (%), is plotted for control, after 24 h of tat treatment(100 ng/ml), and after 24 h tat treatment following pretreatment with MK801 (5 μM). For all experiments, control apoptosis was low, 2–5%. Tat treatment increased apoptosis to varying degrees and the effect of tat was largely blocked by MK801 pretreatment, indicating mediation by NMDARs. a Tat treatment induced a low rate of apoptosis in neuronal cultures obtained before 22 weeks (immature human neurons). b Tat treatment induced only slightly, but not significantly, more apoptosis in immature neuronal cultures that were cultured for 6–10 weeks. c Mature human neurons showed a much greater incidence of apoptosis. d, e Undifferentiated and differentiated human neural progenitor cells exhibited a small increase in apoptosis in response to tat treatment. f Apoptosis of rat hippocampal neurons, which exhibit strong expression of NMDARs, was only moderately increased in response to tat. * P <0.05, tat compared to control, and # P <0.05, MK801 pretreatment compared to tat alone (n = 6 for each panel)
Mature neuronal cultures obtained after onset of NMDAR protein expression that were exposed to tat (10–500 ng/ml) underwent extensive apoptosis (Fig. 3c). Neuronal apoptosis reached 70–90% after 24 h of tat treatment and was blocked by pretreatment with MK801 (Fig. 3c).
Brain development involves differentiation of multipotent neural progenitor cells (de Graaf-Peters and Hadders-Algra 2006; Herlenius and Lagercrantz 2001, 2004; Ritter et al. 2001). These multipotent cells differentiate into different cell types in the brain, including glutamatergic neurons (de Graaf-Peters and Hadders-Algra 2006; Herlenius and Lagercrantz 2001, 2004; Ritter et al. 2001). To examine whether neurons differentiated from human neural progenitor cells are sensitive to tat-induced apoptosis, we examined human progenitor cells that can be differentiated into MAP-2 positive and nestin negative cells (Hou et al. 2006). These cells express low levels of NMDAR, subunits NR1 and NR2A, as evaluated by Western blot and immunofluorescence, both before and after differentiation into neurons. We did not detect significant changes in the amount of NMDAR protein or its cell distribution between undifferentiated and differentiated cells by Western blot and confocal microscopy (data not shown).
Tat treatment of undifferentiated and differentiated human neuronal progenitor cells resulted in low levels of apoptosis (5–10%, Fig. 3d, e). Apoptosis induced by tat in both cell types, undifferentiated and differentiated, was NMDAR dependent, as pretreatment with MK801 blocked this tat-induced apoptosis (Fig. 3d, e).
We also examined whether rat hippocampal neurons that are well characterized to have high expression of NMDAR and undergo apoptosis in response to many stimuli, would respond to tat treatment. Tat treatment of primary rat hippocampal neuronal cultures resulted in low levels of apoptosis, 12.5–18%, that were blocked with MK801 (Fig. 3f). These low levels of apoptosis occurred despite their high NMDAR expression as compared to human neuronal cultures. Thus, species differences as well as differences in NMDAR expression contribute to the extent of tat-mediated neurotoxicity.
Extensive Apoptosis Induced by Tat Is Correlated with the Formation of a Macromolecular Complex Between NMDAR and LRP in Human Neurons
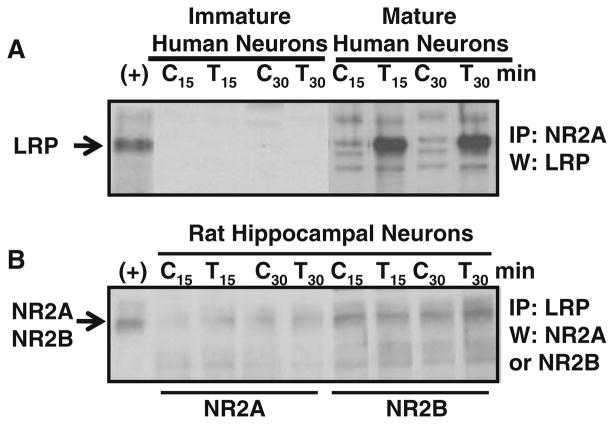
Tat-induced apoptosis in human differentiated neurons is dependent on the formation of a macromolecular complex on the surface of the neurons that includes low-density lipoprotein receptor-related protein (LRP), postsynaptic density protein-95 (PSD-95), NMDAR, and neuronal nitric oxide synthase (nNOS) (Eugenin et al. 2007). To demonstrate whether the differences in toxicity observed between mature and immature human neurons were associated with the formation of this macromolecular complex, we examined by immunoprecipitation the interaction between LRP and the NR2A subunit after tat treatment.
Tat treatment (10–500 ng/ml) of cultures of immature neurons cultured for 6–10 weeks did not result in formation of a complex containing LRP and NR2A as indicated by immunoprecipitation with NR2A antibody and probing with LRP antibody (Fig. 4), despite the fact that low levels of NR1 could be detected (Fig. 1). Tat treatment of mature neuronal cultures with high-NMDAR expression resulted in a robust increase in the interaction between LRP and NR2A (measured at 15 and 30 min after tat application, Fig. 4a), as well as pronounced apoptosis subsequently (Fig. 3c) (Eugenin et al. 2007). Association with NR2B was not detected (not shown).
Fig. 4.
Tat induces the formation of an LRP–NMDAR complex that is not formed in immature neuronal cultures, which express little NMDAR protein. a NR2A was immunoprecipitated (IP) from neuronal lysates of control (C) cultures or cultures treated with tat (T) for 15 and 30 min, and analyzed by Western blot (W) with antibodies to LRP. Tat treatment markedly increased the amount of LRP that was coimmunoprecipitated with NR2A in mature human neuronal cultures, but not in immature neuronal cultures. b In cultures of rat hippocampal neurons tat treatment did not increase association of LRP with NR2A or NR2B. LRP was immunoprecipitated from neuronal lysates of control (C) cultures or cultures treated with tat (T) and analyzed by Western blot (W) with antibodies to NR2A or NR2B as indicated. Total human fetal cortex lysate was used as a positive control for NR2A and NR2B (+). Tat treatment did not increase LRP/ NR2A or LRP/NR2B complex formation (n = 3)
In Rat Hippocampal Neurons, Association Between LRP and NMDAR Is Not Altered by Tat Treatment
Immunoprecipitation studies were performed to examine whether rat hippocampal neurons respond to tat treatment by the formation of the macromolecular complex between LRP and NMDAR. Untreated rat neuronal cultures have significant baseline interaction between LRP and NR2A as well as with NR2B containing receptors (Fig. 4b). Tat treatment (10–500 ng/ml) for 15 and 30 min of these rat hippocampal neuronal cultures did not result in changes in the interaction between LRP and NR2A or NR2B (Fig. 4b).
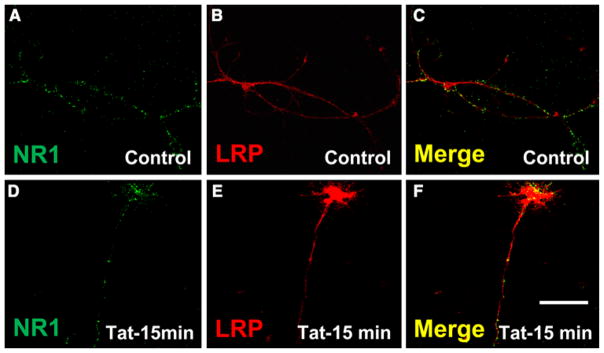
Immunofluorescence studies of the cultures of rat hippocampal neurons for LRP and NMDAR subunit 1 confirmed that the complex between LRP and NMDAR is preformed, as demonstrated by the clear colocalization between both proteins (Fig. 5a–c). After tat treatment for 15 min, there was still colocalization of both proteins (Fig. 5d–f). The staining of LRP and NMDAR colocalization on the surface of the rat hippocampal neurons looked similar to the staining we reported for mature human neuronal cultures after tat treatment (Eugenin et al. 2007), but this colocalization in rat cultures was not increased and did not result in high levels of apoptosis in response to tat treatment as observed in human differentiated neurons (Eugenin et al. 2007). These results indicate that in addition to complex formation, interspecies differences contribute to the differential toxicity of tat for human and rat neurons. One possibility is the differential presence of NR2A and NR2B in human and rat complexes, with NR2A being deleterious and/or NR2B being protective.
Fig. 5.
Rat hippocampal neurons express LRP/NMDAR complexes, but the distribution of these proteins is not altered by tat treatment. Confocal microscopy indicates that tat treatment (100 ng/ml, 15 min) did not alter the colocalization of LRP and NR1 on the surface of rat hippocampal neurons. Double immunofluorescence labeling for LRP (Cy3 staining, red) and NR1 (FITC staining, green) shows LRP and NR1 in control (a–c) and tat (d–f) treated cultures. Specificity was confirmed by replacing the primary antibody with a non-specific myeloma protein of the same isotype or the appropriate polyclonal reagent (data not shown). Bar: 20 μm. (n = 3)
Discussion
This report characterizes the differences in tat-mediated toxicity observed using human neurons obtained from early and later stages of human development, undifferentiated and differentiated human neuronal progenitor cells and rat hippocampal neurons. Our findings indicate that, in addition to the developmental stages of the neurons, significant expression of NMDAR, subsequent formation of a complex between LRP and NMDAR induced by tat treatment, and species analyzed contribute to the levels of toxicity induced by tat treatment.
The NMDAR is one of the major glutamate-activated ionotropic receptors in the brain, and is permeable to sodium, potassium, and calcium ions after activation by glutamate and relief of magnesium block by depolarization. Compromised neuronal viability due to aberrant glutamate receptor activation has been described in several neurodegenerative diseases including HIV-dementia (Nishizawa 2001; Gonzalez-Scarano and Martin-Garcia 2005). Much less is known about the expression of ionotropic glutamate receptors in the human brain during development. Autoradiographic studies demonstrate [3H]glutamate binding starting after 18 weeks of gestational age in the hippocampus, thalamus, subthalamic nucleus, caudate-putamen, and cortex (Barks et al. 1988; Lee and Choi 1992; Ritter et al. 2001). We define the period of expression of the neurotransmitter glutamate and their receptors as the critical period of NMDAR expression in the human brain. Our characterization of this period indicates that NMDAR expression is low before this maturation period and increases during this period and is high at the end of this period for subunits NR1, NR2B, and NR2A in neurons in tissue culture.
The NR1 subunit is required for the formation of the channel with two additional NR2 subunits. The NR2 subunits of the NMDA receptor differ in their spatial and temporal expression (Monyer et al. 1994; Portera-Cailliau et al. 1996), as well as in their sequences, physiological characteristics, and intracellular binding partners. NR2D is expressed developmentally in the rodent brain in the diencephalon and brain stem, while NR2B is expressed both embryonically and postnatally throughout the fore-brain. NR2A and NR2C are both exclusively postnatal in the rodent CNS; however, NR2A is expressed in human neurons embryonically. Our data obtained in human neuronal cultures demonstrated that during the process of tat-induced apoptosis, the early formation of a macromolecular complex among tat–LRP–PSD-95–NMDAR containing subunit 2A and not 2B, and nNOS results in apoptosis in human neurons (Eugenin et al. 2007). However, despite the fact that immature human neurons express low levels of NMDAR after isolation and after long periods of culture, the formation of the complex still depends upon subunit 2A, suggesting a potential role of this subunit, rather that NR2B, in apoptosis.
NMDAR function depends on subunit composition. For example, NR2A receptors have faster deactivation kinetics than NR2B containing receptors (Cull-Candy and Leszkiewicz 2004), and they are differentially expressed during development (Cull-Candy and Leszkiewicz 2004). It is not yet clear whether the difference in subunit composition between the human and rat, pre-formation of the complex in rat neurons with NR2B being in the complex, and/or alternative signaling contributes to the interspecies differences in apoptosis after tat treatment.
We only detected significant amounts of LRP-NR2A complex formation in neuronal cultures obtained from mature human neurons. The formation of the complex was greatly increased by tat treatment and highly correlated with apoptosis as we previously published (Eugenin et al. 2007). In rat hippocampal neurons, the complex was pre-formed and the interaction between these proteins was not affected by tat treatment, despite the increase in calcium induced by tat and high expression of NMDAR. This suggests that in rat neurons, a key factor in the amplification system of toxicity is missing or altered. One of these alterations is the presence of the NR2B subunit in the complex between LRP and NMDAR. This subunit is not found in the complex induced by tat in human cells. We suggest that NR2B may protect from tat-induced apoptosis.
Despite the fact that rat hippocampal neurons express higher levels of functional NMDAR as compared to human neuronal cells, rat hippocampal neurons showed lower levels of apoptosis than human neuronal cells. Our and others’ data indicate that tat treatment induces apoptosis levels of approximately 5–15% in rat neurons (Haughey et al. 2001; Magnuson et al. 1995), as compared to the 65–90% apoptosis in mature human neurons (Eugenin et al. 2003b, 2007). Some of these interspecies differences can be due to differences in NMDAR. Nevertheless, in rat cells tat increases intracellular calcium, but does not cause extensive apoptosis.
Additional differences between rat and humans have been detected. For example, non-primate cells express low levels of cyclinT1, a protein required for binding of tat to TAR viral RNA (Wei et al. 1998). This suggests that additional tat effects in the nucleus as a transcription factor also can be a determinant in host gene expression. The human neuronal progenitor cells had low levels of NMDAR even after differentiation, and treatment with tat resulted in only low levels of apoptosis. These cells therefore resembled the neurons derived from tissue prior to or during the onset of NMDAR expression.
Interestingly, for all of the neuronal cell types examined in this report and independent of the level of apoptosis triggered by tat treatment, preincubation with MK801, an NMDAR blocker was protective, indicating that activation of NMDAR is essential for apoptosis induced by tat. Thus, this receptor is critical for tat-induced apoptosis, despite difference in NMDAR expression and extent of apoptosis in the different systems examined.
In summary, we demonstrated major differences between immature and mature primary human neurons, undifferentiated and differentiated human neural progenitor cells, and rat hippocampal neurons in their sensitivity to tat toxicity. Excessive activation of NMDA and non-NMDA glutamate receptors has been implicated in the pathophysiology of several brain diseases, including NeuroAIDS (Gonzalez-Scarano and Martin-Garcia 2005; Nishizawa 2001). However, developmental changes in HIV neuronal toxicity have not been described. Specific stage of synaptogenesis and expression of neurotransmitters and their receptors, especially NMDA receptors, may determine the response to tat-mediated cell death.
Acknowledgments
We are grateful to Dr. Brad Poulos and The Human Fetal Tissue Repository at the AECOM. This work was supported by the National Institutes of Mental Health Grants MH070297, MH075679, and MH083497 to J. W. B. and by a KO1 grant from the National Institute of Mental Health (MH076679) to E.A.E., National Institute of Neurological Disorders and Stroke (NS055363 and NS045287 to M.V.L.B. and NS020752 to R.S.Z.), MSTP Training Grant, 5 T32 GM007288 (to JEK and JEH), and from the HIV AIDS and Opportunistic Infection Institutional Training Grant, T32 AI-007501 (to JEH). We thank the NIH Centers for AIDS Research Grant (CFAR) AI-051519 at the Albert Einstein College of Medicine.
Contributor Information
E. A. Eugenin, Department of Pathology, Albert Einstein College of Medicine, Forchheimer 727, 1300 Morris Park Avenue, Bronx, NY 10461, USA
J. E. King, Department of Pathology, Albert Einstein College of Medicine, Forchheimer 727, 1300 Morris Park Avenue, Bronx, NY 10461, USA
J. E. Hazleton, Department of Pathology, Albert Einstein College of Medicine, Forchheimer 727, 1300 Morris Park Avenue, Bronx, NY 10461, USA
E. O. Major, National Institute of Health (NINDS/NIH), Molecular Medicine and Virology Section, Bethesta, MD 20892, USA
M. V. L. Bennett, Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY 10461, USA
R. S. Zukin, Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY 10461, USA
Joan W. Berman, Email: joan.berman@einstein.yu.edu, berman@aecom.yu.edu, Department of Pathology, Albert Einstein College of Medicine, Forchheimer 727, 1300 Morris Park Avenue, Bronx, NY 10461, USA. Department of Microbiology/Immunology, Albert Einstein College of Medicine, Bronx, NY 10461, USA
References
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Barks JD, Silverstein FS, Sims K, Greenamyre JT, Johnston MV. Glutamate recognition sites in human fetal brain. Neurosci Lett. 1988;84:131–136. doi: 10.1016/0304-3940(88)90396-5. [DOI] [PubMed] [Google Scholar]
- Bartz SR, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Conant K, Ma M, Nath A, Major EO. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70:1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10:21–28. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- D’Aversa TG, Eugenin EA, Berman JW. NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J Neurosci Res. 2005;81:436–446. doi: 10.1002/jnr.20486. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood–brain barrier. Methods. 2003;29:351–361. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Branes MC, Berman JW, Saez JC. TNF-alpha plus IFN-gamma induce Connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J Immunol. 2003a;170:1320–1328. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003b;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190(Suppl 1):S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hou J, Seth P, Major EO. JC virus can infect human immune and nervous system progenitor cells: implications for pathogenesis. Adv Exp Med Biol. 2006;577:266–273. doi: 10.1007/0-387-32957-9_19. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Zhang K, Silva C, Shalinsky DR, Conant K, Ni W, Corbett D, Yong VW, Power C. HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann Neurol. 2001;49:230–241. doi: 10.1002/1531-8249(20010201)49:2<230::aid-ana43>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Joseph J, Prasad V. NeuroAIDS in the developing world. J Neurovirol. 2005;11(1):4–6. [PubMed] [Google Scholar]
- Karn J. Tackling Tat. J Mol Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kumar A, Manna SK, Dhawan S, Aggarwal BB. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J Immunol. 1998;161:776–781. [PubMed] [Google Scholar]
- Kumar A, Dhawan S, Mukhopadhyay A, Aggarwal BB. Human immunodeficiency virus-1-tat induces matrix metalloproteinase-9 in monocytes through protein tyrosine phosphatase-mediated activation of nuclear transcription factor NF-kappaB. FEBS Lett. 1999;462:140–144. doi: 10.1016/s0014-5793(99)01487-8. [DOI] [PubMed] [Google Scholar]
- Lee H, Choi BH. Density and distribution of excitatory amino acid receptors in the developing human fetal brain: a quantitative autoradiographic study. Exp Neurol. 1992;118:284–290. doi: 10.1016/0014-4886(92)90185-s. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A, Calzado MA, Jimenez-Reina L, Ceballos E, Leon J, Munoz E. Susceptibility of HIV-1-TAT transfected cells to undergo apoptosis. Biochemical mechanisms. Oncogene. 1999;18:7543–7551. doi: 10.1038/sj.onc.1203095. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Hou J, Gronostajski RM, Major EO. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann Neurol. 2003;53:636–646. doi: 10.1002/ana.10523. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 Tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7:1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. N-methyl-D-aspartate receptor proteins NR2A and NR2B are differentially distributed in the developing rat central nervous system as revealed by subunit-specific antibodies. J Neurochem. 1996;66:692–700. doi: 10.1046/j.1471-4159.1996.66020692.x. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Rogers DT, Mulholland PJ, Littleton JM, Wilkins LH, Jr, Self RL, Nath A. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002;954:300–307. doi: 10.1016/s0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol. 1999;65:458–465. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Unis AS, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor expression in human fetal brain. Brain Res Dev Brain Res. 2001;127:123–133. doi: 10.1016/s0165-3806(01)00126-2. [DOI] [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Toschi E, Barillari G, Sgadari C, et al. Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol Biol Cell. 2001;12:2934–2946. doi: 10.1091/mbc.12.10.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Barks JD, Silverstein FS. Tat, a human immunodeficiency virus-1-derived protein, augments excitotoxic hippocampal injury in neonatal rats. Neuroscience. 1999;88:585–597. doi: 10.1016/s0306-4522(98)00242-5. [DOI] [PubMed] [Google Scholar]
- Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]