Abstract
Purpose
Docetaxel is extensively metabolized by CYP3A4 in the liver, but mechanisms by which the drug is taken up into hepatocytes remain poorly understood. We hypothesized that (i) liver uptake of docetaxel is mediated by the polymorphic solute carriers OATP1B1 and OATP1B3, and (ii) that inherited genetic defects in this process may impair systemic drug elimination.
Methods
Transport of docetaxel was studied in vitro using various cell lines stably transfected with OATP1B1*1A (wildtype), OATP1B1*5 [c.521T>C (V174A); rs4149056], OATP1B3, or the mouse transporter Oatp1b2. Docetaxel clearance was evaluated in wildtype and Oatp1b2-knockout mice, as well as in 141 white patients with multiple variant transporter genotypes.
Results
Docetaxel was found to be a substrate for OATP1B1, OATP1B3, and Oatp1b2, but was not transported by OATP1B1*5. Deficiency of Oatp1b2 in mice was associated with an 18-fold decrease in docetaxel clearance (P=0.0099), which was unrelated to changes in intrinsic metabolic capacity in mouse liver microsomes. In patients, however, none of the studied common reduced-function variants in OATP1B1 or OATP1B3 were associated with docetaxel clearance (P>0.05).
Conclusions
The existence of at least two potentially redundant uptake transporters in the human liver with similar affinity for docetaxel supports the possibility that functional defects in both of these proteins may be required to confer substantially altered disposition phenotypes. In view of the established exposure-toxicity relationships for docetaxel, we suggest that extreme caution is warranted if docetaxel has to be administered together with agents that potently inhibit both OATP1B1 and OATP1B3.
Keywords: OATP1B1, OATP1B3, docetaxel, pharmacokinetics, pharmacogenetics
Introduction
The antimicrotubular agent docetaxel is a widely used chemotherapeutic agent that has been approved for the treatment of multiple malignant diseases, including cancers of the breast, lung, head and neck, stomach, and prostate. The disposition properties of docetaxel are characterized by up to 10-fold differences in drug clearance between patients receiving the same therapeutic regimen (1). The high degree of interindividual pharmacokinetic variability observed with docetaxel has important toxicological ramification. In particular, it was previously demonstrated that a mere 50% decrease in docetaxel clearance is associated with a more than 4-fold increase in the odds of developing severe neutropenia, the dose-limiting toxicity (2-3).
Despite the established exposure-pharmacodynamic relationships for docetaxel, the mechanisms underlying the agent’s unpredictable pharmacokinetics remain largely unexplained. It has been speculated that a critical determinant of docetaxel’s pharmacokinetic variability is associated with differential expression of polymorphic drug-metabolizing enzymes and/or transporters at sites of elimination. However, several recent analyses indicated that the contribution of genetic variants in obvious candidate genes encoding enzymes or ATP-binding cassette transporters to explaining pharmacokinetic variability of docetaxel is rather limited (4-11).
The mechanisms by which docetaxel is taken up into human liver cells are still largely unknown. Previous in vitro screens have provided evidence that cellular uptake of the related compound paclitaxel may be regulated, in part, by the polymorphic organic anion transporting polypeptides OATP1B1 (gene name, SLCO1B1) and/or OATP1B3 (gene name, SLCO1B3) (12-13). These transporters are expressed at high levels in the liver, where their localization is restricted to the basolateral membrane of hepatocytes, and they have been implicated in the liver uptake of multiple structurally diverse endogenous molecules and xenobiotics (14). In the current study, we tested the hypothesis that inherited variation in OATP1B1 and OATP1B3 is associated with the disposition of docetaxel, and that these transporters collectively contribute to interindividual differences in the clearance of docetaxel in cancer patients.
Materials and Methods
In Vitro transport studies
Xenopus laevis oocytes injected with OATP1B1, OATP1B3, or rat Oatp1b2 cRNA along with water-injected controls were obtained from BD Biosciences. Chinese hamster ovary (CHO) cell lines stably expressing OATP1B1 or OATP1B3 (15) and Flp-In T-Rex293 cells transfected with OATP1B1*1A (wildtype), OATP1B1*1B [c.388A>G (N130D); rs2306283], OATP1B1*5 [c.521T>C (V174A); rs4149056], or OATP1B1*15 (N130D, V174A) have been described previously (16). OATP1B1 or OATP1B3 overexpressing human embryonal kidney (HEK293) cells were created by stably transfecting the respective cDNA fragments spliced from TrueClone plasmids (OriGene Technologies), cloned into a pIRES2-EGFP vector (BD Biosciences). Mouse Oatp1b2 overexpressing HEK293 cells were created similarly from a commercial cDNA cloned into a pDream2.1/MCS vector (GenScript). Overexpression of transporters in HEK293 cells was confirmed using TaqMan probes (Applied Biosystems).
Uptake experiments were performed as described previously (3), with results normalized to uptake values in cells transfected with an empty vector. Preliminary experiments indicated that Phenol Red, a pH indicator in trypsin used to re-suspend cultured cells, influenced OATP1B-mediated uptake of docetaxel in Flp-In T-Rex293 cells (Supplementary Fig. S1), and therefore these studies were performed in Phenol Red free conditions.
Animal experiments
Adult male Oatp1b2 knockout mice (17) and age-matched wildtype mice (Taconic), both on a DBA1/lacJ background, were housed in a temperature-controlled environment with a 12-hour light cycle, and given a standard diet and water ad libitum. Experiments were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital. Docetaxel, formulated in polysorbate 80 (Taxotere) and diluted in normal saline, was administered by tail vein injection at a dose of 10 mg/kg, and plasma, liver, and kidney from each animal were collected at 5, 15, 30, 60, 120, 240, and 480 min. Urine was collected from animals housed in metabolic cages for 48 hours after docetaxel administration. Samples were analyzed by liquid chromatography-tandem mass spectrometry (see Supplementary Methods for details) (18), and non-compartmental parameters calculated using PK Solutions 2.0 (Summit Research Services). Tissue concentrations of docetaxel were corrected for contaminating plasma (19). Gene expression patterns in livers were assessed using the Mouse 430v2 GeneChip array (Affymetrix). Microsomal metabolism of docetaxel in liver samples from wildtype and Oatp1b2-knockout mice was performed as described (20) in the presence or absence of the Cyp3a inhibitor, ketoconazole.
Patient studies
Patients were enrolled onto a prospective pharmacokinetic study (Dutch trial registry: NTR2311). Inclusion criteria included a confirmed diagnosis of a solid tumor for which docetaxel (formulated in polysorbate 80; Taxotere) was a reasonable therapeutic option, age 18 years or older, WHO performance score of 0 or 1, and adequate hematopoietic, hepatic and renal functions, as described (21). Concurrent use of agents known to induce or inhibit CYP3A4 was not allowed. The study was approved by the Erasmus University Medical Center review board, and all patients provided written informed consent.
Blood collection for pharmacokinetic analyses was performed using a limited-sampling strategy where 4 or 5 samples were obtained over a 24-h period after the end of infusion. Docetaxel concentrations in plasma were determined as described (Supplementary Methods). Pharmacokinetic parameters were estimated using a previously developed population model,(22) in NONMEM version 7 (Icon Development Solutions). There was no statistically significant influence of sex, administered dose, or tumor type on the clearance of docetaxel (P>0.05), and thus pharmacokinetic data of all patients were pooled in subsequent correlation studies without further correction or consideration of subgroup analyses.
Genomic DNA was isolated from whole blood using MagnaPure LC (Roche Diagnostics GmbH). Allelic discrimination analysis was performed for the determination of several variants in OATP1B1 and OATP1B3 that were selected from the literature on the basis of their relatively high predicted allelic frequency and/or the known or suspected influence on functional properties of the encoded proteins (Supplementary Table S1) (16). The analyses were performed using TaqMan assays on an ABI PRISM 7500 system (Applied Biosystems) according to the manufacturer’s instructions.
Statistical considerations
Data are presented as mean with standard deviation, unless stated otherwise. Statistical calculations were done using analysis of variance or Student’s -t test in SPSS version 17 (SPSS Inc), depending on the number of groups, and P<0.05 was considered significant.
Results
Docetaxel transport in vitro
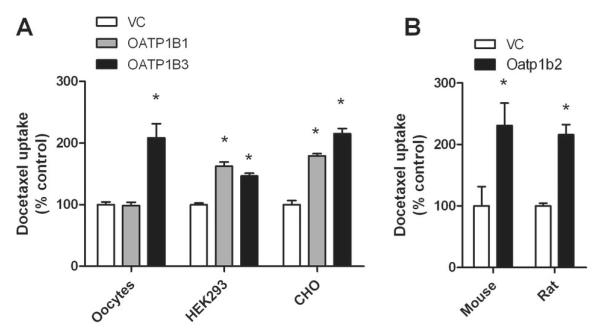
Experiments assessing the interaction of docetaxel with human OATP1B1 and OATP1B3 indicated that drug uptake is dependent on cell context, with both proteins being able to take up docetaxel when expressed in HEK293 cells or CHO cells, but no noticeable transport occurring by OATP1B1 when expressed in Xenopus laevis oocytes (Fig. 1A). Docetaxel was also found to be transported into cells expressing the mouse mOatp1b2 or rat rOatp1b2 transporters (Fig. 1B).
Figure 1.
In vitro transport studies of docetaxel. (A) Transport of docetaxel by human OATP1B1 and OATP1B3 was evaluated with constructs transfected in Xenopus laevis oocytes (docetaxel concentration, 2 μM; 30-min incubations), HEK293 cells (2 μM; 30-min), or CHO cells (1 μM; 2-min). (B) Transport of docetaxel by mouse Oatp1b2 transfected in HEK293 cells (0.1 μM; 60-min) or rat Oatp1b2 transfected in Xenopus laevis oocytes (2 μM; 30-min). Data represent the mean of 2 to 32 observations, and are expressed as the average percent of uptake values in cells transfected with an empty vector (VC). Error bars represent the standard error. The star (*) denotes a significant difference from VC (P<0.05).
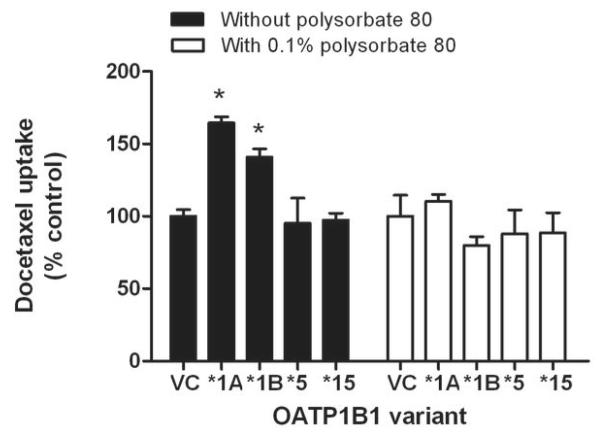
The transport of docetaxel into CHO cells transfected with OATP1B1 or OATP1B3 was found to be time-dependent and saturable with a Michaelis-Menten constant (Km) of 7.6±3.0 μM and 2.2±0.6 μM, respectively, and a maximum velocity (Vmax) of 30.7±5.7 pmol/mg/min and 27.2 ± 2.4 pmol/mg/min, respectively (Supplementary Fig. S2), and similar results were obtained for paclitaxel (Supplementary Fig. S3). Compared to cells overexpressing the wildtype OATP1B1 (OATP1B1*1A), in vitro transport activity of cells transfected with constructs carrying the c.521C substitution (OATP1B1*5 and OATP1B1*15) was completely lost (Fig. 2). Interestingly, the presence of the docetaxel excipient polysorbate 80 (Tween 80), at levels that can be achieved in patients,(23) abrogated the OATP1B1-genotype dependent transport of docetaxel (Fig. 2).
Figure 2.
Influence of OATP1B1 variants on docetaxel transport in vitro. Transport of docetaxel (concentration, 0.1 μM; 60-min incubations) was evaluated in Flp-In T-Rex293 cells transfected with OATP1B1*1A (wildtype), OATP1B1*1B [c.388A>G (N130D); rs2306283], OATP1B1*5 [c.521T>C (V174A); rs4149056], or OATP1B1*15 (N130D, V174A). Data represent the mean of 6 observations, and are expressed as the average percent of uptake values in cells transfected with an empty vector (VC) in the absence or presence of 0.1% of polysorbate 80. Error bars represent the standard error. The star (*) denotes a significant difference from VC (P<0.05).
Docetaxel pharmacokinetics in Oatp1b2-knockout mice
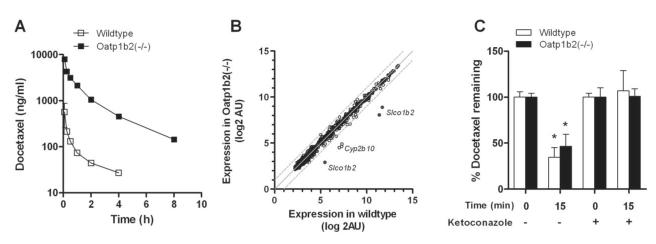
We next evaluated the possible importance of these transporters for docetaxel in mice with a genetic deletion of Oatp1b2 [Oatp1b2(−/−) mice]. The area under the curve (AUC) for docetaxel in these animals was dramatically increased compared with that observed in wildtype mice (8,826±845 vs 336±96.9 ng×h/ml; P=0.0066) as a result of a more than 18-fold decrease in systemic clearance (1.08±0.097 vs 19.9±7.08 l/h/kg; P=0.0099). The respective concentration-time profiles of docetaxel in mice (Fig. 3A) suggests that the slow clearance in the Oatp1b2(−/−) mice is due to a distribution defect rather than an event occurring in the terminal elimination phase. Indeed, the terminal half-lives of docetaxel were not significantly different in Oatp1b2(−/−) mice and wildtype mice (2.41±0.151 vs 2.44±0.533 h; P=0.87).
Figure 3.
Influence of Oatp1b2-knockout on docetaxel pharmacokinetics. (A) Plasma concentration-time profile of docetaxel in wildtype and Oatp1b2(−/−) mice (i.v. dose, 10 mg/kg). Data represent the mean of at least 6 observations per time point, and error bars represent the standard error. (B) Comparative expression of 839 probe sets for 463 genes, including 49 ATP-binding cassette transporters, 78 cytochrome P450 enzymes, and 336 solute carriers, at baseline in livers of wildtype mice and Oatp1b2(−/−) mice (n=5 per group). Each symbol represents a single probe set, the solid line is the line of identity, and the dotted lines are the 95% confidence intervals. (C) Influence of Oatp1b2 knockout on the ex vivo liver microsomal metabolism of docetaxel (concentration, 2 μM; 15-min incubations) in the absence or presence of ketoconazole (20 μM). Under these conditions, formation of the main murine metabolite of docetaxel (M2) was not different between liver microsomes from wildtype or Oatp1b2(−/−) mice (P=0.29). Data represent the mean of 8 independent observations per group and are expressed as the percent of drug added to the microsomes at time zero. Error bars represent the standard error. The star (*) denotes a significant difference from time zero (P<0.05).
As anticipated, the liver/plasma AUC ratio was significantly reduced in Oatp1b2(−/−) mice (1.32±0.088 vs 8.14±2.39; P=0.0079). The kidney/plasma AUC ratio was also reduced in Oatp1b2(−/−) mice (5.14±0.363 vs 43.0±12.5; P=0.0063), although there was limited shunting of docetaxel in the knockout mice to urine (urinary excretion, 1.68±0.758 vs 1.03±0.676 %dose; P=0.15). To rule out potentially altered, compensatory expression of enzymes and transporters in the liver of Oatp1b2(−/−) mice at baseline, microarrays were used to evaluate differential expression profiles of 839 probe sets for 463 genes, including 49 ATP-binding cassette transporters, 78 cytochrome P450 enzymes, and 336 solute carriers. Compared to levels in liver of wildtype mice, besides probe sets for the Oatp1b2 gene Slco1b2, only transcripts of the enzyme Cyp2b10 were decreased in the Oatp1b2(−/−) mice (Fig. 3B). Since taxanes are not known to be metabolized by Cyp2b10, this genetic alteration is unlikely to directly or indirectly influence docetaxel handling by the liver. Furthermore, there were no potentially compensatory changes in hepatic Cyp3a activity, the main metabolic route for docetaxel, since Oatp1b2-knockout had no influence on the hepatic microsomal metabolism of docetaxel ex vivo in the presence or absence of the Cyp3a inhibitor ketoconazole (Fig. 3C).
Docetaxel pharmacokinetics in patients with different transporter genotypes
To provide preliminary evidence for a possible role of OATP1B1 and OATP1B3 in the clinical pharmacology of docetaxel, an exploratory pharmacogenetic-association analysis was performed in human subjects with cancer undergoing docetaxel-based chemotherapy. To this end, pharmacokinetic and pharmacogenetic data was obtained from 141 predominantly white patients (87 females and 54 males) with a median age of 55 years (Supplementary Table S2). The average clearance of docetaxel in the study population was 41.8±12.3 L/h, with a 6.3-fold difference between the lowest and highest values.
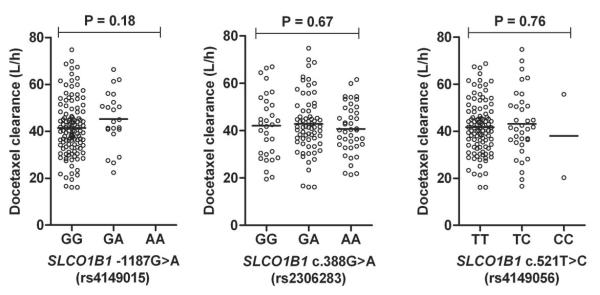
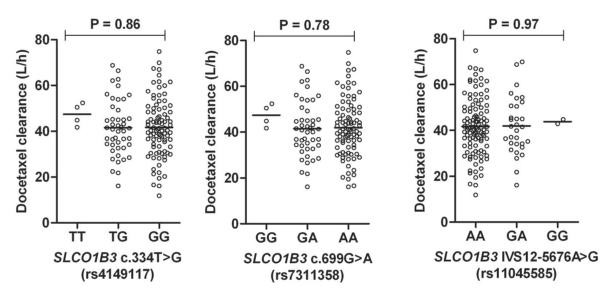
The relative frequencies of the variant alleles in our patient population were comparable with previously reported estimates (16), the distributions of all polymorphisms were in Hardy-Weinberg equilibrium, and demographic characteristics at baseline were similar for individuals carrying 0, 1, or 2 variant alleles at the loci of interest. Despite the observed functional impact of the OATP1B1 c.521C substitution in vitro, none of the individual polymorphisms in OATP1B1 were found to be associated with the clearance of docetaxel in vivo (Fig. 4). Significant associations were also not observed between docetaxel clearance and the studied variants in OATP1B3 (Fig 5), and associations did not improve when individuals were clustered on the basis of observed diplotypes (Supplementary Fig. S4).
Figure 4.
Docetaxel clearance as a function of observed OATP1B1 (SLCO1B1) genotypes. Data were obtained in 141 predominantly white cancer patients receiving docetaxel-based chemotherapy. Each symbol represents an individual patient, and horizontal lines indicate median values. The P-value denotes a statistical comparison of the clearance of docetaxel in the different genotype group.
Figure 5.
Docetaxel clearance as a function of observed OATP1B3 (SLCO1B3) genotypes. Data were obtained in 141 predominantly white cancer patients receiving docetaxel-based chemotherapy. Each symbol represents an individual patient, and horizontal lines indicate median values. The P-value denotes a statistical comparison of the clearance of docetaxel in the different genotype group.
Discussion
The current study provides support for a growing body of knowledge that solute carriers belonging to the family of organic anion transporting polypeptides can have a dramatic impact on the hepatic accumulation and systemic clearance of CYP3A4 substrates. Employing an array of in vitro transport assays, including intracellular accumulation studies in multiple transfected model systems, docetaxel was identified as a high-affinity substrate for human OATP1B1 and OATP1B3. We found that the interaction of docetaxel with OATP1B1 and OATP1B3 was strongly dependent on cell context and culture medium composition, and this has obvious implications for future screening strategies aimed at identifying novel substrates for these transporters.
Our in vitro studies also suggest that docetaxel is a transported substrate of mouse Oatp1b2 and rat Oatp1b2. The rodent Oatp1b2 transporters share more than 60% amino acid sequence homology to human OATP1B1 and OATP1B3, and on the basis of their shared basolateral localization in hepatocytes and overlapping substrate specificity (24), it is possible that in rodents Oatp1b2 fulfills the same function in the liver as OATP1B1 and OATP1B3 in humans. Based on this premise, we evaluated the pharmacokinetic properties of docetaxel in a mouse model with a genetic deletion of Oatp1b2. One possible limitation of this model is that fact that, unlike in humans, mouse hepatocytes express multiple members of Oatp1a, a related subfamily of transporters that can potentially provide compensatory restoration of function when Oatp1b2 is lost (25). However, compared to wildtype mice, the systemic exposure to docetaxel in the Oatp1b2(−/−) mice was remarkably increased by more than 26 fold. Gene expression profiling and Cyp3a activity measurements in liver samples excluded alterations in alternate transport mechanisms or metabolic pathways as a possible cause of the delayed clearance phenotype in Oatp1b2(−/−) mice. These findings suggest that Oatp1b2-mediated transport of docetaxel is likely a critically important rate-limiting process in the elimination of this drug in mice. This supposition is consistent with the notion that the change in clearance of docetaxel observed here in Oatp1b2(−/−) animals is at least as dramatic as compared with phenotypic changes associated with complete deficiency of metabolic Cyp3a activity in mice (20).
It is interesting to note that a previous study demonstrated that mice deficient for all Oatp1a and Oatp1b genes display only a rather modest 2-fold increase in concentrations of paclitaxel in plasma, presumably due to decreased uptake of the drug into the liver compared to wildtype mice (26). The reasons underlying the apparent differences in outcome of the study with paclitaxel and our current results for docetaxel are not entirely clear. It is possible that the background strains onto which these respective knockout mice were developed (FVB vs DBA1/lacJ, respectively) differentially impact any resulting phenotypes for structurally similar xenobiotics. Regardless of the exact mechanism, the observations made in the mice provide further evidence that hepatic OATP transporters can affect the pharmacokinetic properties of a remarkably broad range of substrates that include charged organic anions (eg, methotrexate), charged organic cations (eg, imatinib), polar zwitterions (eg, fexofenadine), and uncharged hydrophobic agents (eg, taxanes).
Based on in vitro uptake studies, multiple functionally different haplotypes, including OATP1B1*5 and OATP1B1*15, were found to have a detrimental impact on docetaxel transport. This finding is consistent with previously studies showing substantially diminished transport activity of several OATP1B1 substrates by these particular variants when transfected into mammalian cells (27). In vivo, these variants have been associated with altered systemic exposure and toxicity in response to multiple substrate drugs (28).
Interestingly, the relevance of these genetic variants in OATP1B1 could not be confirmed in our prospectively conducted pharmacogenetic-association study done in a group of predominantly white cancer patients receiving treatment with docetaxel. It is possible that additional rare genetic variants or haplotypes in OATP1B1 of importance to the transport docetaxel in this population are yet to be discovered, and that much larger numbers of patients are then needed to more precisely quantify genotype-phenotype associations.
We also considered the possibility that the interaction of docetaxel with OATP1B1 may be masked by the pharmaceutical vehicle polysorbate 80 (Tween 80), which is used to solubilize docetaxel in clinical preparations. Indeed, the presence of polysorbate 80, even in relatively low amounts, completely nullified the genotype-dependent transport of docetaxel by OATP1B1 observed in the absence of polysorbate 80. Although further investigation is required to confirm direct involvement of polysorbate 80-mediated inhibition of OATP1B1 as the primary mechanistic basis for the observed in vivo effects, it is of note that similarly altered hepatic uptake has been described for colchicine in the presence of Solutol HS15 (29) and for paclitaxel in the presence of Cremophor (12). If confirmed, these observations suggest that the impact of reduced function variants of OATP1B1 on the clearance of docetaxel may be much more pronounced for polysorbate 80-free formulations of the drug, such as nab-docetaxel (ABI-008).
In our study, several genetic variants in OATP1B3 were also not significantly associated with the pharmacokinetics of docetaxel. This is in line with previously published data that we collected in another predominantly white, independent cohort of patients (4). It should be pointed out that this finding is at odds with several other investigations performed in patients of Asian descent. For example, homozygosity (GG) for rs11045585 was associated with reduced clearance of docetaxel, compared with patients carrying the AA or AG genotypes (5). In another study, a particular OATP1B3 genotype combination comprising the reference allele at IVS4+76G>A (rs4149118) and variant alleles at 699G>A (rs7311358), IVS12+5676A>G (rs11045585), and *347_*348insA (rs3834935)indel was also linked with reduced clearance of docetaxel (30). It is possible that differences in outcome with our study are associated with the fact that such variants may occur at different frequencies between Asians and Caucasians, and/or on different, ethnicity-dependent haplotype structures.
Regardless of any potential ethnic considerations, the existence of at least two potentially redundant uptake transporters in the human liver with similar affinity for docetaxel supports the possibility that functional defects in both of these proteins may be required to confer substantially altered disposition phenotypes such as those seen in the Oatp1b2(−/−) mice. While complete functional deficiency of either OATP1B1 or OATP1B3 has been recorded to occur (31), deficiency of both transporters is very rare, with an estimated frequency in the human population of about 1 in a million (32). It can thus be postulated that intrinsic physiologic and environmental variables influencing OATP1B1- or OATP1B3-mediated uptake of docetaxel into hepatocytes may have a more profound influence on the clearance of docetaxel in the general population than do defective genetic variants. This recognition is particularly relevant in the context of the recent guidelines offered by The International Transporter Consortium regarding preclinical criteria needed to trigger the conduct of clinical studies to evaluate drug-transporter interactions (33). Indeed, it is conceivable that instances of idiosyncratic hypersensitivity to docetaxel are the result of currently unrecognized drug-drug interactions at the level of hepatocellular uptake mechanisms (see Supplementary Table S3 for examples).
Collectively, our findings demonstrate the importance of OATP1B-type solute carriers as the initial, rate-limiting step in the elimination of docetaxel. Our results suggest that genetic defects leading to impaired function of both OATP1B1 and OATP1B3 may be required to confer substantially reduced clearance of this drug in humans. In view of the established exposure-toxicity relationships for docetaxel, we suggest that extreme caution is warranted if docetaxel has to be administered together with agents that potently inhibit both of these transporters.
Supplementary Material
Translational Relevance.
Docetaxel is widely used for the treatment of multiple solid tumors, including cancers of the breast, lung, head and neck, stomach, and prostate. The interindividual pharmacokinetic variability seen with docetaxel treatment remains high, and this phenomenon may have important ramification for the agent’s clinical activity and toxicity. We speculated that differential expression of polymorphic transporters involved in the hepatic elimination of docetaxel plays a crucial role in explaining this pharmacologic variability. Here, we investigated the contribution of organic anion transporting polypeptides to the disposition of docetaxel using an array of in vitro and in vivo model systems. Our results indicate the existence of at least two potentially redundant uptake transporters in the human liver with similar affinity for docetaxel (OATP1B1 and OATP1B3) that regulate the initial, rate-limiting step in the elimination of docetaxel. In view of the established exposure-toxicity relationships for docetaxel, our results suggest that extreme caution is warranted if docetaxel has to be administered together with agents that potently inhibit both these transporters.
Acknowledgments
We would like to thank Richard Kim and Jeffrey Stock for providing the Oatp1b2(−/−) mice.
Acknowledgment of research support: This study was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), Dutch Cancer Society Grant EMCR 2010-4664 (to RHNvS and RHJM), USPHS Cancer Center Support Grant 3P30CA021765 (to SDB), and NCI Grant 5R01CA151633-01 (to AS).
Footnotes
Disclosure of Potential Conflict of Interest The authors declared no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflicts of interest: the authors indicated no potential conflicts of interest.
References
- 1.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel : recent developments. Clin Pharmacokinet. 2006;45:235–52. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. Journal of Clinical Oncology. 1998;16:187–96. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 3.Bruno R, Vivier N, Veyrat-Follet C, Montay G, Rhodes GR. Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Invest New Drugs. 2001;19:163–9. doi: 10.1023/a:1010687017717. [DOI] [PubMed] [Google Scholar]
- 4.Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, et al. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–63. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew SC, Singh O, Chen X, Ramasamy RD, Kulkarni T, Lee EJ, et al. The effects of CYP3A4, CYP3A5, ABCB1, ABCC2, ABCG2 and SLCO1B3 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of docetaxel in nasopharyngeal carcinoma patients. Cancer Chemother Pharmacol. 2011;67:1471–8. doi: 10.1007/s00280-011-1625-9. [DOI] [PubMed] [Google Scholar]
- 6.Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. Journal of Clinical Oncology. 2002;20:3683–90. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Kim KP, Ahn JH, Kim SB, Jung KH, Yoon DH, Lee JS, et al. Prospective evaluation of the drug-metabolizing enzyme polymorphisms and toxicity profile of docetaxel in Korean patients with operable lymph node-positive breast cancer receiving adjuvant chemotherapy. Cancer chemotherapy and pharmacology. 2012 doi: 10.1007/s00280-011-1816-4. [DOI] [PubMed] [Google Scholar]
- 8.Lewis LD, Miller AA, Rosner GL, Dowell JE, Valdivieso M, Relling MV, et al. A comparison of the pharmacokinetics and pharmacodynamics of docetaxel between African-American and Caucasian cancer patients: CALGB 9871. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:3302–11. doi: 10.1158/1078-0432.CCR-06-2345. [DOI] [PubMed] [Google Scholar]
- 9.Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V, et al. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clinical pharmacology and therapeutics. 2006;79:570–80. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Tsai SM, Lin CY, Wu SH, Hou LA, Ma H, Tsai LY, et al. Side effects after docetaxel treatment in Taiwanese breast cancer patients with CYP3A4, CYP3A5, and ABCB1 gene polymorphisms. Clin Chim Acta. 2009;404:160–5. doi: 10.1016/j.cca.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni WC, Combest AJ, DeLoia JA, Edwards RP, Bredges AS, Zamboni BA, et al. Pharmacologic and phenotypic study of docetaxel in patients with ovarian or primary peritoneal cancer. Cancer chemotherapy and pharmacology. 2011;68:1255–62. doi: 10.1007/s00280-011-1609-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–8. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 13.Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, et al. Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomed Pharmacother. 2011;65:417–26. doi: 10.1016/j.biopha.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey LB, Bruun GH, Yang W, Trevino LR, Vattathil S, Scheet P, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22:1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, et al. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74:320–9. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engels FK, Mathot RA, Loos WJ, van Schaik RH, Verweij J. Influence of high-dose ketoconazole on the pharmacokinetics of docetaxel. Cancer Biol Ther. 2006;5:833–9. doi: 10.4161/cbt.5.7.2839. [DOI] [PubMed] [Google Scholar]
- 19.Kaliss N, Pressman D. Plasma and blood volumes of mouse organs, as determined with radioactive iodoproteins. Proc Soc Exp Biol Med. 1950;75:16–20. doi: 10.3181/00379727-75-18083. [DOI] [PubMed] [Google Scholar]
- 20.van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, et al. Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest. 2007;117:3583–92. doi: 10.1172/JCI33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke RM, Carducci MA, Rudek MA, Baker SD, Sparreboom A. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4562–7. doi: 10.1200/JCO.2010.30.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno R, Vivier N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24:153–72. doi: 10.1007/BF02353487. [DOI] [PubMed] [Google Scholar]
- 23.Ten Tije AJ, Loos WJ, Verweij J, Baker SD, Dinh K, Figg WD, et al. Disposition of polyoxyethylated excipients in humans: implications for drug safety and formulation approaches. Clinical pharmacology and therapeutics. 2003;74:509–10. doi: 10.1016/j.clpt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–87. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iusuf D, van de Steeg E, Schinkel AH. Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci. 2012;33:100–8. doi: 10.1016/j.tips.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 26.van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, et al. High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res. 2011;17:294–301. doi: 10.1158/1078-0432.CCR-10-1980. [DOI] [PubMed] [Google Scholar]
- 27.Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15:513–22. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- 28.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157–81. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 29.Bravo Gonzalez RC, Boess F, Durr E, Schaub N, Bittner B. In vitro investigation on the impact of Solutol HS 15 on the uptake of colchicine into rat hepatocytes. Int J Pharm. 2004;279:27–31. doi: 10.1016/j.ijpharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Chew SC, Sandanaraj E, Singh O, Chen X, Tan EH, Lim WT, et al. Influence of SLCO1B3 Haplotype-tag SNPs on Docetaxel Disposition in Chinese Nasopharyngeal Cancer Patients. Br J Clin Pharmacol. 2011 doi: 10.1111/j.1365-2125.2011.04123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SR, Saito Y, Sai K, Kurose K, Maekawa K, Kaniwa N, et al. Genetic variations and frequencies of major haplotypes in SLCO1B1 encoding the transporter OATP1B1 in Japanese subjects: SLCO1B1*17 is more prevalent than *15. Drug Metab Pharmacokinet. 2007;22:456–61. doi: 10.2133/dmpk.22.456. [DOI] [PubMed] [Google Scholar]
- 32.van de Steeg E, Stranecky V, Hartmannova H, Noskova L, Hrebicek M, Wagenaar E, et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012;122:519–28. doi: 10.1172/JCI59526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.