Abstract
Background
Staphylococcus aureus bacteremia (SAB) is a common, severe infectious disease with accepted standards of care.
Methods
A retrospective cohort study of all 233 SAB cases at the Minneapolis Veterans Affairs Medical Center (MVAMC) between October 2004 and February 2008 was performed to measure the impact of Infectious Disease (ID) consultation on conformance to standards and patient outcomes. Outcomes were classified as survived without relapse, relapsed, or died without relapse. ID involvement was classified as consultation, curbside, or no involvement.
Results
ID involvement occurred in 179/233 cases (77%). Management conformed to accepted standards in 162/197 cases (82%) evaluable for conformance. ID involvement was associated with increased conformance in univariable analysis and multivariable analysis adjusted for propensity for ID consultation (OR 5.9, 95% CI 2.5 - 13.8). Relapse occurred in 14/156 cases (9%) in which therapy conformed to standards compared with 8/35 cases (23%) in which therapy did not conform to standards (p=0.045). Relapse was more common in older patients (OR 1.05, CI 1.01-1.09) and in cases without ID involvement (OR 3.02, CI 1.003-9.1). Death was associated with greater Charlson Index scores (OR 1.89, CI 1.4-2.5). Of 111 cases with definitely or possibly infected devices, relapse occurred in 9/92 cases (9.8%) in which the device was wholly or partially removed compared with 6/19 cases (32%) in which the device was left in place (p=0.02).
Conclusions
ID involvement in SAB cases was associated with increased adherence to accepted standards and fewer relapses. ID consultation should be performed for all SAB cases.
Keywords: Staphylococcus aureus, bacteremia, Referral and Consultation
Introduction
S. aureus is the second most common cause of bacteremia in the developed world with an incidence of approximately 32/100,000 population.1 S. aureus bacteremia (SAB) mortality ranges from 6-20% of cases.2-4 Substantial clinical evidence has demonstrated that three elements of care are associated with better outcomes, and these have become widely accepted standards. First, intravenously administered β-lactam antibiotics are the best drugs for cases of methicillin susceptible S. aureus (MSSA).5-9 Second, intravenous therapy must be given for minimum durations for specific types of infection.3, 10-17 Third, devices known to be or suspected of being infected with S. aureus must be removed whenever possible.5, 15, 18-21
Six previous studies have suggested that infectious diseases (ID) consultation is associated with improved conformance to SAB management standards.2, 4, 22-25 The impact on outcomes has been less clear. Studies have differed substantially in design, institutional setting, and outcome measures.2-4, 22-25 For this study, we hypothesized that ID consultation would be associated with management that conformed to these widely accepted standards and would be associated with lower risk of relapse and of 12-week all-cause mortality. Here, we report our results and review the evidence of direct and indirect benefits from ID consultation for S. aureus bacteremia.
Materials and Methods
Samples
We identified all S. aureus isolates from blood cultures at the Minneapolis Veterans Affairs Medical Center (MVAMC) from October 1, 2004 to February 29, 2008. MVAMC has 279 inpatient beds including 80 in a Community Living Center (CLC) where patients receive acute rehabilitation and low-intensity medical care. CLC patients are managed by staff internists available in the hospital at all times, and access to consultative care is the same as for other inpatient locations. S. aureus identification and oxacillin susceptibility were performed with standard methods.26 The MVAMC IRB considered analysis of the data for publication devoid of patient identifiers exempt under Title 38 Code of Federal Regulations 16.101 (b).
Medical Record Review
MVAMC medical records are entirely computerized. We systematically reviewed records for all patients with SAB. For each case, we extracted patient identification, age, underlying illnesses, location at onset, service making treatment decisions, acquisition, type of infection, infectious disease involvement, results of echocardiography, management, and relapse or death within 12 weeks of onset. Acquisition was classified as hospital-associated if symptoms and signs began after admission to hospital; healthcare-associated if the patient had been hospitalized, had lived in a long-term medical care facility, or had a medical procedure (such as dialysis, surgery, intravenous catheter insertion) in the preceding year;27-29 or community-associated. SAB cases with focal infection sites were categorized as osteomyelitis; joint infection; endocarditis; intravenous catheter infection; skin, soft tissue; septic thrombophlebitis; pneumonia; UTI; wound; or other. Cases without apparent focal infection were categorized as “bacteremia.” Infections were categorized as “definite device-related” infections if the device was suspected of being infected and found to be culture-positive upon removal. Infections were categorized “possible device-related” infections if the device was suspected of being infected but was either not removed or was culture-negative upon removal. All other infections were considered not device-related.
All distinct SAB episodes in a single patient separated by at least one month without antimicrobial therapy or symptoms of infection were included. We reviewed records for 12 weeks after onset and classified patients into three categories: survived without relapse, relapsed, or died without relapse. Relapse was defined as either recurrent bacteremia or local (tissue) recurrence of infection. After initial analysis, patients who relapsed and subsequently died during the study period were included in the “relapsed” category. This did not materially affect the results or conclusions. Underlying illnesses were used to calculate Charlson co-morbidity scores.30 The study was done in a Veterans Affairs hospital where ID consultation was not mandated and there were no direct financial incentives for ID consultation, but where the majority of patients with SAB had infectious disease involvement.
We considered that management conformed to widely accepted standards when three criteria were met. First, parenteral β-lactams were used for MSSA infections.5-9 Vancomycin was considered appropriate when used for empirical therapy before susceptibilities were known, for cases with methicillin-resistant isolates, or when the case patient could not receive β-lactams because of allergy. Second, for cases of SAB associated with deep-seated infection or SAB cases of unknown source in which echocardiography was not done, parenteral antibiotic therapy was given for at least 28 days.3, 10, 12, 15 Deep-seated infection included S. aureus infections of bone, joint, deep soft tissues, heart valves or other structures that are not easily excised or drained.4, 29, 31, 32 Third, any infected prosthetic device (e.g., catheter, prosthetic heart valve, bone or joint prosthesis) was removed.5, 15, 18-21 For cases with an easily removed or drained focus of infection (e.g., intravenous catheter, pneumonia, cystitis) or for SAB cases of unknown source in which echocardiography was done and did not reveal evidence of endocarditis, we considered 10 to 14 days of parenteral therapy adequate.3, 10-13, 15-17 We did not require that echocardiography be performed.
ID involvement was classified as “consultation” if there was an ID consultation note or if a board certified ID physician was the attending physician. If there was no regular consultation, but primary team notes indicated that they discussed management with a named ID attending, the case was classified as “curbside consultation.” The remainder were classified as “no ID involvement.”
Exclusion Criteria
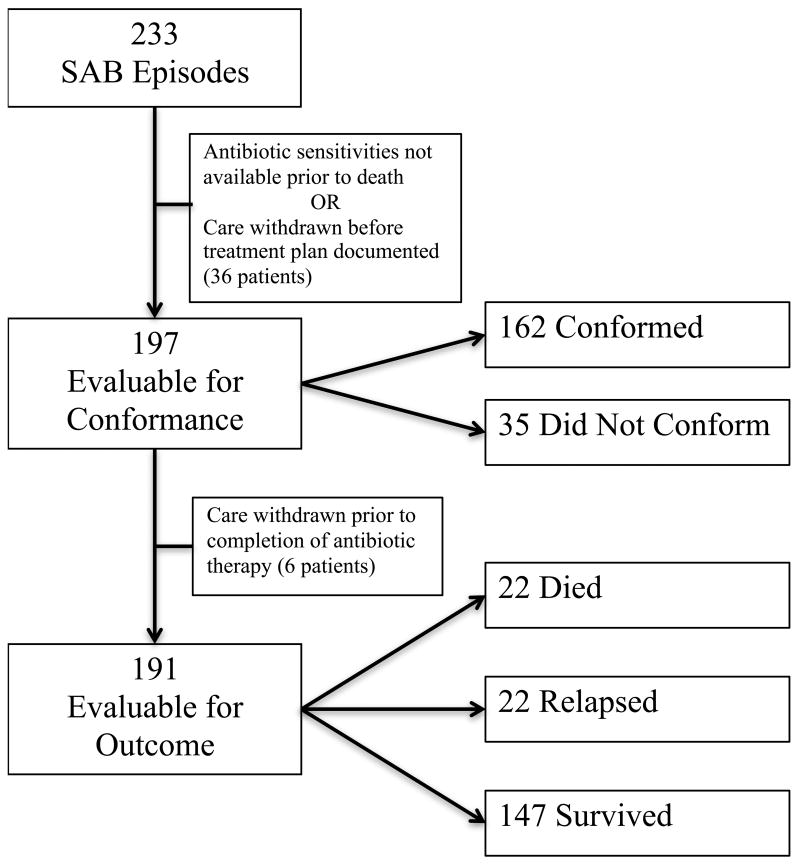
Cases were excluded from the conformance analysis if identification or antibiotic susceptibilities for the isolate were not available prior to death, or if care was withdrawn before a course of treatment was selected. Cases were excluded from the outcome analysis if care was withdrawn prior to the completion of the planned antibiotic therapy (Figure 1).
Figure 1. Study Design.
Of the 233 cases, 197 were evaluable for conformance. Cases were not included in the conformance analysis if the patient died before culture results and sensitivities were available, or if care was withdrawn before a treatment plan was documented in the medical record. Six additional patients were excluded from the outcome analysis as care was withdrawn prior to the completion of the planned course of antibiotic therapy.
Analysis
We used Student's t test, chi-square analyses, and Fisher's exact test to determine significance of differences in baseline characteristics, conformance, and outcomes. We used binomial logistic regression to determine variables associated with improved conformance and multinomial logistic regression for patient outcome (Statistical Program for the Social Sciences [SPSS] for Windows, version 19, Chicago, Ill). Propensity scores were calculated to adjust for covariate imbalances between cases with and without ID involvement.33, 34
Results
During the 40-month study period, there were 233 separate episodes of SAB. Most patients were male, on the inpatient ward, and cared for by the internal medicine service (Table 1). The mean Charlson Index was 3.8. ID involvement ranged from 83 percent of patients with onset in the CLC to 56 percent of patients in an intensive care unit (p=0.003). ID involvement occurred in 78-100% of patients with bone and joint infections, endocarditis, IV catheter related bacteremia, and skin and soft tissue infections, but only 56% of patients with primary bacteremia (p < 0.001). Baseline differences in percentages of ID involvement in various categories were similar, and the few differences between groups disappeared after propensity score adjustment (Table 1).
Table 1. Characteristics of Patients with SAB.
| Infectious Diseases Involvement | Total | ||
|---|---|---|---|
|
| |||
| Yes, n=179 (77%) | No, n=54 (23%) | ||
| Age [years, mean ± SD, (median)] | 66 ± 12 (65) | 68 ± 12 (68) | |
| Male Gender [n] | 178 | 52 | |
| Charlson index (mean ± SD, median) | 3.7 ± 2.5, 3 | 4.3 ± 2.8, 4 | |
| Location at onset [n, (%)]* | |||
| Community | 15 (79) | 4 (21) | 19 |
| Community Living Center | 30 (83) | 6 (17) | 36 |
| Inpatient ward | 111 (81) | 26 (19) | 137 |
| Intensive care | 23 (56) | 18 (44) | 39 |
| Management by [n, (%)] | |||
| Internal medicine | 124 (78) | 36 (23) | 160 |
| Community Living Center | 26 (84) | 5 (16) | 31 |
| Other disciplines | 29 (69) | 13 (31) | 42 |
| MRSA [no (%)] | 102 (75) | 34 (25) | 136 |
| Acquisition [n, (%)] | |||
| Hospital-associated | 89 (73) | 33 (27) | 122 |
| Healthcare-associated | 72 (80) | 18 (20) | 90 |
| Community-associated | 18 (86) | 3 (14) | 21 |
| Type of Infection [n, (%)] | |||
| Bacteremia | 27 (56) | 21 (44) | 48 |
| Bone/Joint | 34 (90) | 4 (11) | 38 |
| Endocarditis | 15 (100) | 0 (0) | 15 |
| IV Catheter | 40 (78) | 11 (22) | 51 |
| Skin/Soft Tissue/Wound | 37 (80) | 9 (20) | 46 |
| Other | 26 (74) | 9 (26) | 35 |
| Device-Related Infections: | |||
| Definitely Device Related | 63 (80) | 16 (20) | 79 |
| Possibly Device Related | 38 (70) | 16 (30) | 54 |
Number, percentages of each row with or without ID involvement.
Conformance
Conformance was evaluable for 197 cases (Figure 1). Management conformed to accepted standards in 162 cases (82%). Reasons for non-conformance in the other 35 cases were that an infected device was not removed in 14 (40%); treatment duration was too short in 6 (17%); vancomycin was used to treat MSSA infections in 6 (17%); the patient received oral therapy for a portion of the treatment instead of parenteral therapy in 5 (14%), and multiple or miscellaneous reasons in four (11%).
Regular consultation occurred in 150 cases, a board certified ID physician was the attending physician in 3 cases, and curbside consultation was documented in 6 cases. In the remaining 38 cases, there was no ID involvement (Table 2). To simplify most analyses, we combined cases with regular consultation, an ID attending, and curbside consultation into the “ID involvement” category, which did not materially affect the results. Management conformed to standards in 141 of 159 cases (89%) with ID involvement, compared with 21 of 38 cases (55%) without ID involvement (p <0.001). Fewer cases with definite device-related infections conformed to accepted standards (58/76, 76%) compared with cases without infected devices (71/80, 89%, p=0.04).
Table 2. Conformance and Outcomes in Cases with Regular Consultations, Curbside Consultations, and No ID Involvement.
| No ID Involvement | Curbside Consultation | Regular Consultation or ID Attending | |
|---|---|---|---|
| Conformance with Accepted Standards* | 21/38 (55%) | 5/6 (83%) | 136/153 (89%) |
| Outcomes† | |||
| Survived, no relapse | 25/36 (69%) | 6/6 (100%) | 116/149 (78%) |
| Relapse | 7/36 (19%) | 0/6 (0%) | 15/149 (10%) |
| Died within 12 weeks of onset | 4/36 (11%) | 0/6 (0%) | 18/149 (12%) |
197 evaluable cases
191 evaluable cases
In multivariable analysis, ID involvement was strongly associated with increased conformance to standards (OR adjusted with propensity scores 5.9, 95% CI 2.5 - 13.8). When curbside consultations were removed from the analysis, the association was nearly identical (OR 5.9, 95% CI 2.5 – 13.9). Age, Charlson Index, location at onset, service when blood culture turned positive, methicillin susceptibility, and whether the infection was device related were not significantly associated with conformance (data not shown).
Outcomes
Outcomes were evaluable for 191 cases (Figure 1). For the 12-week period after onset, S. aureus infection relapsed in 22 patients, and 22 patients died. In univariable analysis, relapse occurred in 14 (9%) of 156 cases in which therapy conformed to standards compared with eight (23%) of 35 cases in which therapy did not conform to standards (p=0.045). Relapse occurred in 15 (9.7%) of 155 cases with ID involvement compared with seven (19%) of 36 cases without ID involvement, but this difference was not significant (Table 2). Of 111 cases with definitely or possibly infected devices, relapse occurred in nine (9.8%) of 92 cases in which the device was wholly or partially removed compared with six (32%) of 19 cases in which the device was left in place (p = 0.02). Relapse rates were similar in cases with or without echocardiography (data not shown). In multivariable analysis, in which death and relapse were compared with the reference condition “survived with no relapse, ” significant associations were observed between relapse and both increasing age and lack of ID involvement (Table 3). Charlson Index was not associated with relapse. When the six curbside consultation cases were removed from multivariable analysis, the association between lack of ID involvement and relapse was weaker (OR 2.8, 95% CI 0.94 to 8.5). The other associations were slightly but not meaningfully different.
Table 3. Multinomial Analysis of Outcomes*.
| Variable | Coefficient | SE | Wald X2 | p | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Relapse: | ||||||
| Age | 0.05 | 0.02 | 4.81 | 0.03 | 1.05 | 1.01-1.1 |
| Charlson Index | 0.08 | 0.11 | 0.45 | 0.5 | 1.08 | 0.9-1.3 |
| CLC | 1.7 | 1.0 | 3.0 | 0.09 | 5.4 | 0.8-38 |
| Endocarditis and Bacteremia | 1.3 | 1.0 | 1.7 | 0.2 | 0.3 | 0.04-1.9 |
| No ID involvement | 1.1 | 0.6 | 3.9 | 0.049 | 3.02 | 1.003-9.1 |
| Death: | ||||||
| Age | 0.04 | 0.03 | 2.5 | 0.12 | 1.04 | 0.99-1.1 |
| Charlson Index | 0.6 | 0.15 | 17 | <0.001 | 1.89 | 1.4-2.5 |
| CLC | -2.4 | 1.0 | 5.9 | 0.02 | 0.09 | 0.01-0.6 |
| Endocarditis and Bacteremia | 2.2 | 1.1 | 4.3 | 0.04 | 8.8 | 1.1-69 |
| No ID involvement | 0.4 | 0.8 | 0.2 | 0.6 | 1.5 | 0.3-7.3 |
Covariates included in the multinomial logistic regression model were age (continuous variable), Charlson Index (continuous variable), service (CLC, medicine, surgery), origin, type of infection, ID involvement (regular consultation, ID attending, and curbside consultation vs. no involvement), and propensity score for ID involvement. Dependent variable was relapse (top half) or death within 12 weeks (bottom half). The baseline outcome state was “survived without relapse.”
Variables with p<0.1 on univariable analysis or in preliminary models of multivariable analysis were included in the final multivariable analysis model.
Five of the eleven patients with endocarditis died within 12 weeks, a rate which was significantly greater than for other categories of S. aureus infection (p=0.001). In multivariable analysis, death was associated with greater Charlson Index scores and having bacteremia or endocarditis (Table 3). Death was less likely for patients on CLC than on other inpatient wards. Age and lack of ID involvement were not associated with death. Location at onset of infection, hospital service, origin of infection, methicillin resistance, and whether infection was device related or not were not associated with death or relapse.
Discussion
ID involvement was strongly associated with management that conformed to accepted standards, while lack of ID involvement was associated with relapse. This association was significant in multivariable analysis with or without adjustment by propensity scores. The effect of ID involvement appeared to be mediated by conformance with standards, so conformance was not included in the final model. In patients with infected devices, relapse was less likely if the infected device was removed. This confirms previous observations that devices infected with S. aureus must be removed whenever possible.5, 15, 18-21
Criteria for appropriate standards of SAB management have differed in all previous studies on the impact of ID consultation for SAB. To be conservative, we used criteria for “accepted standards” that were based on evidence that they are associated with improved outcomes and are common clinical practice. The first criterion was that intravenously administered β-lactam antibiotics were used to treat cases of MSSA.5-9 In a recent study that supported the use of β-lactams for MSSA bacteremia, the adjusted odds ratio for treatment failure was 3.53 for those receiving vancomycin compared with those receiving cefazolin.8 Second was that intravenous therapy was given for adequate durations. Evidence supports that deep-seated infections should be treated for 4 to 6 weeks and that bacteremia associated with minor infections or intravenous catheters should be treated for 10 to 14 days.3, 10-13, 15-17 Third was that devices known to be or suspected of being infected were removed.5, 15, 18-21 For example, in one recent study, relapse and death were 6.5 times more likely among patients who had an infected intravascular device left in place than among patients who had an infected device removed or those without an infected device.3 Our study provided additional evidence that removal of an infected device decreases likelihood of relapse. Several other criteria have been proposed or used by investigators in just one or two studies, but they have not gained wide acceptance. We did not require that patients receive follow up blood cultures at defined intervals. Patients with persistently positive blood cultures often have other clinical signs or symptoms suggestive of progressive or disseminated infection.29 No study has demonstrated that results of follow-up blood cultures affect outcomes independently of clinical signs or symptoms of infection. We did not require that echocardiography be performed. Echocardiography is often done and reveals unsuspected evidence of endocarditis or complications of endocarditis in a minority of cases, but no well-controlled study has demonstrated better outcomes for SAB cases with echocardiography compared with those without echocardiography.
Several themes have emerged from this and previous studies on the impact of ID consultation in SAB. ID consultation was associated with conformance to management standards in several studies (Table 4).4, 22-25 Our study is the only one to demonstrate an association between ID involvement and a decreased risk of relapse. In one study, cure was more likely and relapse less likely in cases in which all ID consultation recommendations were followed.3 In three studies, mortality was significantly lower in cases with ID consultation than in cases without ID consultation.22, 24, 25 In two studies,2, 35 mortality appeared lower in cases with ID consultation in univariable but not multivariable analysis. In one study, 30-day mortality decreased as the numbers of ID consultation performed increased, but no direct relationship between ID consultation and mortality in individual cases was reported.23 Different mortality definitions were used, and in one,22 the SAB cases were derived from cases selected for a different study. Interestingly, the studies that showed statistically significant improvements in mortality with ID consultation were in hospitals with ID consultation in 33% to 67% of cases.22, 24, 25 In studies (including ours) that did not observe a significant survival advantage with ID consultation, the proportion of cases with ID consultation involvement tended to be greater.2, 4 As ID involvement increases above 50%, any statistically significant mortality benefit would be more difficult to detect. The data are also consistent with the hypothesis that frequent ID involvement led to greater awareness of appropriate SAB management among providers, which tended to improve outcomes regardless of whether ID had been consulted. While SAB is severe and common enough to be well studied, there is a growing body of literature from many countries documenting the value of ID consultation in management of other complicated infections. ID consultation has been associated with improved diagnostic workups,36 improved antimicrobial selection,36-40 improved patient outcomes,37, 39 and lower costs.38
Table 4. Summary of literature on impact of ID consultation on SAB.
| Author, year | Study period | Study Design | SAB cases | Impact on Management | Impact on Cure, Relapse, or Mortality | Proportion with ID consultation |
|---|---|---|---|---|---|---|
| Fowler, 19983 | 1994-1996 | Prospective cohort | 244 | ID consultation advice followed in 46% of cases and partially or not followed in 54% of cases | Cure more likely and relapse less likely in cases in which ID consultation recommendations were followed. No significant mortality difference | 100% |
| Mylotte, 200035 | 1995-1999 | Retrospective cohort | 293 | Not reported | Mortality benefit in cases with ID consultation in univariable but not multivariable analysis | 36% |
| Kaech, 20062 | 1998-2002 | Retrospective cohort | 308 | Not reported | Mortality benefit in cases with ID consultation in univariable but not multivariable analysis | 82% |
| Lahey, 200922 | 2002-2006 | Retrospective case control, derived from larger study | 240 | Follow-up blood culture, antibiotic selection, duration of therapy, and removal of pus or infected prosthetic devices improved in cases with ID consultation | Reduced in all-cause and SAB-related in hospital mortality in cases with ID consultation | 51% |
| Reig, 200924 | 2002-2007 | Retrospective/prospective cohort | 521 | Echocardiography, bone scan, and ≥14 days of parenteral antimicrobial therapy more likely in cases with ID consultation | Reduced in-hospital and 90 day mortality in cases with ID consultation | Increased from 33% to >80%, 67% overall |
| Nagao, 200923 | 2002-2008 | Retrospective cohort | 346 | Echocardiography, follow-up blood cultures, ≥14 days of antimicrobial therapy, and appropriate drugs for MRSA infection improved as ID consultation numbers increased. | 30-day mortality improved as ID consultation numbers increased | Not reported |
| Jenkins, 20084 | 2004-2006 | Retrospective cohort | 234 | Removal of intravascular foci (devices?), follow-up blood cultures, parenteral beta-lactam therapy, and ≥28 days of therapy more common after ID consultation became “routine” | No significant difference in cure, recurrence, or mortality | Increased from 53% to 90%, 69% overall |
| Honda, 201025 | 2005-2007 | Prospective cohort | 341 | Appropriate antimicrobial agents, transesophageal echocardiography, and appropriate planned duration of antimicrobial therapy more common in cases with ID consultation | Reduced 28-day mortality in cases with ID consultation | 33% |
| Present Study | 2004-2008 | Retrospective cohort | 233 | Beta lactam therapy where possible, adequate duration of intravenous therapy, and drainage of pus or removal of infected devices more likely in cases with ID consultation | Greater risk of relapse in cases without ID consultation | 77% |
Abbreviations: ID consultation = infectious disease consultation
All studies on the value of ID consultation in SAB cases have been observational or quasiexperimental. Patient populations, definitions of what investigators considered appropriate therapy, types of consultation that were available, and outcome measurements differed considerably. Our study adds in several ways to the body of evidence. We evaluated both mortality and relapse, and our study is the first to demonstrate that ID involvement is associated with a lower risk of relapse. This is clinically significant, as relapse of SAB incurs additional risk to the patient and additional medical costs. We excluded patients in whom care was withdrawn during the study period, as treatment of their SAB was no longer a goal of care and this may have influenced whether or not ID was consulted. Furthermore, no changes in the availability of ID consultation or changes in hospital policy regarding ID consultation occurred during the study period.
This is the only study that has included cases with informal (“curbside”) consultation. Curbside consultation typically makes up a substantial percentage of the volume of infectious disease involvement.41-45 If the ID service had less effective involvement in cases with curbside consultation than in cases with regular consultation, the effect of including them would be to weaken associations between ID consultation and outcome. In fact, conformance in cases with curbside consultation was excellent, and relapse did not occur.
Since the study was retrospective, there was no possibility that knowledge of the study could have influenced provider behavior. We chose a 12-week follow up period because it is sufficient to find disease recurrence, but it is unlikely that cases occurring in this interval represent new S. aureus infections. This study was done in a VA hospital where consultants had no financial incentives to provide consultation, and patients were not charged for consultations. This minimized the possibility of financial incentives influencing consultation practices.
This study had limitations. It was a retrospective observational study with 191 cases evaluable for outcome. ID involvement occurred in 77% of cases, and relapse occurred in only 12% of cases. These characteristics made it difficult for the study to detect a relationship between ID involvement and outcomes, but an association between lack of ID involvement and relapse did emerge. Twenty-three patients had more than one episode of bacteremia during the study period, and six of these patients had more than one recurrence. These cases were not completely independent. Curbside consultations may have occurred without documentation. If curbside consultations were missed, the effect would have been to dilute differences between the groups.
A growing body of evidence now strongly suggests that ID involvement increases conformance to widely accepted management standards and improves survival in SAB. In addition, our study demonstrates that ID involvement reduces the risk of SAB relapse following treatment. We conclude that physicians should request infectious disease consultation for SAB treatment and health care organizations should consider policies that encourage or require infectious disease involvement in SAB cases.
Acknowledgments
We thank James R. Johnson, M.D., for helpful ideas and suggestions. AAP thanks the University of Minnesota Infectious Disease Fellowship Program and NIAID (T32-AI55433) for salary support. There are no other sources of financial support.
Sources of Support: AAP was supported by a National Institutes of Health training grant [T32-AI55433].
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 2.Kaech C, Elzi L, Sendi P, et al. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect. 2006;12:345–352. doi: 10.1111/j.1469-0691.2005.01359.x. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27:478–486. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins TC, Price CS, Sabel AL, et al. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:1000–1008. doi: 10.1086/529190. [DOI] [PubMed] [Google Scholar]
- 5.Chang FY, Peacock JE, Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003;82:333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 6.Fortun J, Navas E, Martinez-Beltran J, et al. Short-course therapy for right-side endocarditis due to Staphylococcus aureus in drug abusers: cloxacillin versus glycopeptides in combination with gentamicin. Clin Infect Dis. 2001;33:120–125. doi: 10.1086/320869. [DOI] [PubMed] [Google Scholar]
- 7.Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–1231. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44:190–196. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 9.Gentry CA, Rodvold KA, Novak RM, et al. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy. 1997;17:990–997. [PubMed] [Google Scholar]
- 10.Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 11.Raad II, Sabbagh MF. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin Infect Dis. 1992;14:75–82. doi: 10.1093/clinids/14.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Malanoski GJ, Samore MH, Pefanis A, et al. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med. 1995;155:1161–1166. doi: 10.1001/archinte.155.11.1161. [DOI] [PubMed] [Google Scholar]
- 13.Iannini PB, Crossley K. Therapy of Staphylococcus aureus bacteremia associated with a removable focus of infection. Ann Intern Med. 1976;84:558–560. doi: 10.7326/0003-4819-84-5-558. [DOI] [PubMed] [Google Scholar]
- 14.Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987;9:891–907. doi: 10.1093/clinids/9.5.891. [DOI] [PubMed] [Google Scholar]
- 15.Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med. 1976;60:495–500. doi: 10.1016/0002-9343(76)90715-4. [DOI] [PubMed] [Google Scholar]
- 16.Bayer AS, Tillman DB, Concepcion N, et al. Clinical value of teichoic acid antibody titers in the diagnosis and management of the staphylococcemias. West J Med. 1980;132:294–300. [PMC free article] [PubMed] [Google Scholar]
- 17.Ehni WF, Reller LB. Short-course therapy for catheter-associated Staphylococcus aureus bacteremia. Arch Intern Med. 1989;149:533–536. [PubMed] [Google Scholar]
- 18.Fowler VG, Jr, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;40:695–703. doi: 10.1086/427806. [DOI] [PubMed] [Google Scholar]
- 19.Fowler VG, Jr, Kong LK, Corey GR, et al. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients. J Infect Dis. 1999;179:1157–1161. doi: 10.1086/314712. [DOI] [PubMed] [Google Scholar]
- 20.Hartstein AI, Mulligan ME, Morthland VH, et al. Recurrent Staphylococcus aureus bacteremia. J Clin Microbiol. 1992;30:670–674. doi: 10.1128/jcm.30.3.670-674.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marr KA, Sexton DJ, Conlon PJ, et al. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med. 1997;127:275–280. doi: 10.7326/0003-4819-127-4-199708150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Lahey T, Shah R, Gittzus J, et al. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 2009;88:263–267. doi: 10.1097/MD.0b013e3181b8fccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagao M, Iinuma Y, Saito T, et al. Close cooperation between infectious disease physicians and attending physicians can result in better management and outcome for patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2010;16:1783–1788. doi: 10.1111/j.1469-0691.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 24.Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation--a study of 521 patients in Germany. J Infect. 2009;59:232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Honda H, Krauss MJ, Jones JC, et al. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123:631–637. doi: 10.1016/j.amjmed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, Penn.: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 27.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 28.Buck JM, Como-Sabetti K, Harriman KH, et al. Community-associated methicillin-resistant Staphylococcus aureus, Minnesota, 2000-2003. Emerg Infect Dis. 2005;11:1532–1538. doi: 10.3201/eid1110.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler VG, Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–2072. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 32.Libman H, Arbeit RD. Complications associated with Staphylococcus aureus bacteremia. Arch Intern Med. 1984;144:541–545. [PubMed] [Google Scholar]
- 33.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 35.Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis. 2000;31:1170–1174. doi: 10.1086/317421. [DOI] [PubMed] [Google Scholar]
- 36.Petrak RM, Sexton DJ, Butera ML, et al. The value of an infectious diseases specialist. Clin Infect Dis. 2003;36:1013–1017. doi: 10.1086/374245. [DOI] [PubMed] [Google Scholar]
- 37.Gomez J, Conde Cavero SJ, Hernandez Cardona JL, et al. The influence of the opinion of an infectious disease consultant on the appropriateness of antibiotic treatment in a general hospital. J Antimicrob Chemother. 1996;38:309–314. doi: 10.1093/jac/38.2.309. [DOI] [PubMed] [Google Scholar]
- 38.Nathwani D, Davey P, France AJ, et al. Impact of an infection consultation service for bacteraemia on clinical management and use of resources. QJM. 1996;89:789–797. doi: 10.1093/qjmed/89.10.789. [DOI] [PubMed] [Google Scholar]
- 39.Fluckiger U, Zimmerli W, Sax H, et al. Clinical impact of an infectious disease service on the management of bloodstream infection. Eur J Clin Microbiol Infect Dis. 2000;19:493–500. doi: 10.1007/s100960000306. [DOI] [PubMed] [Google Scholar]
- 40.Byl B, Clevenbergh P, Jacobs F, et al. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis. 1999;29:60–6. doi: 10.1086/520182. discussion 67-68. [DOI] [PubMed] [Google Scholar]
- 41.Grace C, Alston WK, Ramundo M, et al. The complexity, relative value, and financial worth of curbside consultations in an academic infectious diseases unit. Clin Infect Dis. 2010;51:651–655. doi: 10.1086/655829. [DOI] [PubMed] [Google Scholar]
- 42.Leblebicioglu H, Akbulut A, Ulusoy S, et al. Informal consultations in infectious diseases and clinical microbiology practice. Clin Microbiol Infect. 2003;9:724–726. doi: 10.1046/j.1469-0691.2003.00584.x. [DOI] [PubMed] [Google Scholar]
- 43.Manian FA, McKinsey DS. A prospective study of 2,092 “curbside” questions asked of two infectious disease consultants in private practice in the midwest. Clin Infect Dis. 1996;22:303–307. doi: 10.1093/clinids/22.2.303. [DOI] [PubMed] [Google Scholar]
- 44.Myers JP. Curbside consultation in infectious diseases: a prospective study. J Infect Dis. 1984;150:797–802. doi: 10.1093/infdis/150.6.797. [DOI] [PubMed] [Google Scholar]
- 45.Jover-Diaz F, Cuadrado-Pastor JM, Matarranz-del Amo M. Curbside consultation: another healthcare activity of the infectious disease specialist] Enferm Infecc Microbiol Clin. 2010;28:355–357. doi: 10.1016/j.eimc.2009.05.009. [DOI] [PubMed] [Google Scholar]