Abstract
There is no proven therapy or prevention for vascular Ehlers-Danlos syndrome (vEDS), a genetic disorder associated with the mutation of procollagen type III and characterized by increased fragility of vascular and hollow organ walls. Heterozygous COL3A1-deficient (HT) mice recapitulate a mild presentation of one of the variants of vEDS: haploinsufficiency for collagen III. Adult HT mice are characterized by increased metalloproteinase (MMP) activity, reduced collagen content in the arterial walls, and spontaneous development of various severity lesions in aorta. We hypothesized that chronic treatment with a MMP inhibitor would increase collagen content and prevent the development of spontaneous aortic lesions. HT mice were treated since weaning with the broad-spectrum MMP inhibitor doxycycline added to food. At the age of 9 months MMP-9 expression was twice as high in the tunica media of aorta in untreated HT mice, whereas total collagen content was 30% lower (p < 0.01) and the cumulative score of aortic lesions was eight times higher than in wild-type (WT) mice (p < 0.01). After 9 months of doxycycline treatment, MMP-9 activity, collagen content, and lesions in the aortas of HT mice were at the level of those of WT mice (p > 0.05). In the mouse model of collagen III haploinsufficiency treatment with broad-spectrum MMP inhibitor that was started early in life normalized increased MMP activity, reduced aortic collagen content in adults, and prevented the development of spontaneous aortic lesions. Our findings provide experimental justification for the clinical evaluation of the benefit of doxycycline at least in the haploinsufficient variety of vEDS.
Introduction
Ehlers-Danlos syndrome (EDS) is a group of connective tissue disorders caused by collagen mutation. According to the Villefranche nosology, the disease is subdivided into six clinical types (Beighton et al., 1997). Among them, EDS type IV or vascular EDS (vEDS; aka Sack-Barabas syndrome, OMIM130050) is the most serious type associated with mutation of procollagen type III (COL3A1) (Pepin et al., 2000; Germain, 2002, 2007). Type III collagen is a homotrimeric fibrillar collagen found abundantly in the wall of arteries, gastrointestinal tract, uterus, and skin. Within arteries, type III collagen is integrated into the elastin lamellae of the media and the collagenous network of the adventitia (Baxter, 2005). As a result of the collagen III mutation, vEDS is associated with increased fragility of the vascular and hollow organ walls. Arterial ruptures, the most serious complication of vEDS, are potentially deadly and usually occur without warning (Arteaga-Solis et al., 2000, Barabas, 2000); As a result, life expectancy for patients with vEDS is reduced to <50 years (Pepin et al., 2000; Watanabe and Shimada, 2008). Currently, there is no established prevention or treatment for vEDS except “common sense” lifestyle modification and genetic counseling (Lum et al., 2011). Results of a completed clinical trial (Ong et al., 2010) suggested serious protective benefits of celiprolol (a combination of β1 adrenergic receptor antagonist with a partial β2 adrenergic receptor agonist); however, that study had some significant limitations, because in 40% of participants the mutation was not confirmed through genetic testing.
The absence of a reliable animal model for vEDS hampers the search for effective treatment. A COL3A1 knockout mouse (COL3A1tm1Jae) had been developed previously (Liu et al., 1997) via targeted replacement of the promoter and first exon of the COL3A1 gene with a phosphoglycerate kinase neo cassette. The resulting total absence of collagen III product from the mutated allele in homozygous knockouts produced a severe, unsustainable phenotype with >90% perinatal mortality, whereas heterozygotes, which represent a haploinsufficiency for collagen III, one of the many representations of vEDS, were reported to have no phenotype. We, on the other hand, have shown through careful histological evaluation that haploinsufficiency for COL3A1 in mice is characterized by a significant number of spontaneous lesions in the aortic wall of adult mice (started at 9 months of age) and thus recapitulates a mild presentation of vEDS in humans and can serve as an experimental model (Cooper et al., 2010).
MMPs are important players in numerous biological and pathological processes. The ability to degrade extracellular matrix is an important component in the formation of aortic aneurysms (Sternlicht and Werb, 2001). MMPs have a variety of subtypes. Gelatinases MMP-2 and MMP-9 are involved mainly in the degradation of denatured collagen; thus, their elevation might induce collagen deficiency in the aorta (Collier et al., 1988, Wilhelm et al., 1989, Trocmé et al.,1998, Visse and Nagase, 2003). Therefore, normalizing MMP-2 and MMP-9 with a broad-spectrum MMP inhibitor seemed to be a logical approach for shifting the balance of collagen turnover in a model of collagen haploinsufficiency.
We have reported previously that 3-month pretreatment with the broad-spectrum MMP inhibitor doxycycline (Doxy) protected the aortas of 9-month-old heterozygous COL3A1 KO mice from lesions induced by physical manipulations (Briest et al., 2011). In the present study, we tested a hypothesis that treatment with Doxy starting from weaning would protect the aortas of heterozygous COL3A1 KO mice from the development of spontaneous lesions in adulthood.
Materials and Methods
Subjects.
Heterozygous COL3A1-deficient (HT) mice [strain C.129S4(B6)-COL3A1tm1Jae/J] (Liu et al., 1997) were rederived (The Jackson Laboratory, Bar Harbor, ME) and bred in the vivarium of the National Institute on Aging. The COL3A1 genotype was determined by polymerase chain reaction (5′-CTTCTCACCCTTCTTCATCCC-3′, 5′-AGCCTGTTCAAATCGGTACC-3′, and neo 5′-GCTATCAGGACATAGCGTTGG-3′) after weaning. Animals were housed and studied in conformance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), with institutional Animal Care and Use Committee approval. Mice were maintained on ad libitum food (NIH-07 mouse/rat diet; National Institutes of Health, Bethesda, MD) with permanent access to filtered water.
Experimental Protocol.
Two groups of COL3A1-deficient C.129S4(B6) mice, [HT and wild type (WT)] were treated for 9 months with Doxy starting at 3 weeks of age, i.e., from weaning (HT + Doxy, n = 20; WT + Doxy, n = 20). Treatment was provided with food (compound ID 5281011) containing 800 mg/kg of Doxy (Doxy Diet pellets; BioServ, Frenchtown, NJ). Preliminary measured food intake for this strain was averaged at 3.5 g/day, and the average body weight of animals was 25 g (Cooper et al., 2010), thus the average drug dose for the experiment was 100 mg/kg per day. Two other groups of WT (n = 15) and HT (n = 12) mice remained untreated, were maintained on a regular diet (NIH-07 mouse/rat diet), and served as controls. After 9 months, mice were euthanized by an overdose of isoflurane. Hearts, lungs, livers, spleens, kidneys, and testis were harvested, weighed, and examined with respect to gross histological pathology.
Tissue Collection.
The aorta was dissected free from the surrounding connective tissue and pinned onto a wax block before fixation in 10% formalin for 2 days. Cross-sections of the aorta (2 mm in thickness) were placed in 8% agar to create a block with an average of 20 sections of the aorta. The block was stored in 70% ethanol until it was processed and embedded in paraffin (AML Laboratories, Baltimore, MD).
Histological Analysis.
Sections (5 μm) from each block of aortic sections were stained with hematoxylin and eosin and Masson's trichrome. We counted the number of lesions present in aortas and rated the severity of each lesion on a subjective scale of 1 to 4 according to previously reported criteria (Cooper et al., 2010; see Results). Because of their mild nature, as well as a high frequency in WT mice, grade 1 lesions were excluded from statistical analysis. The sum of the scores of lesions ≥ grade 2 was added for each animal to produce a cumulative score. Radius of lumen and thickness of each layer in aorta were measured with a digital image analyzer (MCID; InterFocus Imaging Ltd., Cambridge, UK). Pathologists assessing histological samples for lesions, collagen content, or MMP expression were blinded with respect to group origin of the samples.
Collagen Detection by Picrosirius Red Staining.
To examine collagen content in the vessel wall, 5-μm sections of aorta were stained with picrosirius red. Digital images of stained sections were obtained from light microscopy by using polarized filters and analyzed by using a MCID digital imaging analysis system (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The total collagen content in the aortic wall and the collagen content of tunicae adventitia and media separately were calculated as a percentage of the total area of the wall or its respective components (Seeland et al., 2007).
Immunohistochemical Staining.
For MMP-2 and MMP-9 immunohistochemical analysis aorta sections (5 μm thick) were cut and placed onto SuperFrost glass slides (Thermo Fisher Scientific, Waltham, MA). After deparaffinization, sections were incubated in 3% H2O2 for 5 min to inactivate endogenous peroxidase. Deparaffinized and rehydrated specimens were boiled in 10 mM citrate buffer, pH 6.0, for 5 min. The samples were cooled to room temperature and incubated with bovine serum albumin (Sigma, St. Louis, MO) for 30 min at room temperature followed by incubation with antibodies overnight at 4°C with MMP-2 (1:100; Calbiochem, San Diego, CA) and MMP-9 (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibodies. The sections were washed with phosphate-buffered saline three times (5 min each) and incubated for 1 h with biotinylated anti-goat and anti-mouse IgG secondary antibodies. After rinsing, immune complexes were visualized by the standard avidin biotin complex method using the avidin biotin complex kit (Vector Laboratories, Burlingame CA). Nuclei were counterstained with hematoxylin, and then examined under a microscope (Carl Zeiss, Jena, Germany).
Statistical Analysis.
Numerical data were analyzed and expressed as means ± S.E.M. A multiple-sample comparison [analysis of variance (ANOVA)] and the multiple range tests as post hoc test using the criterion of the least significant differences) was applied to assess the differences between groups. A value of p < 0.05 was considered to be significant. Aortic collagen content was analyzed by one-way ANOVA followed by Tukey's post hoc comparison.
Results
Organ Anatomy and Histology.
During the study all mice gained weight according to their age, and to the end of 9 months there were no differences in body weight among WT and HT mice untreated or treated with Doxy (Supplemental Table 1). The weights of heart, liver, kidney, spleen, lung, thymus, and testis expressed as a ratio to a body weight also did not differ among groups (Supplemental Table 1). Organs had no unusual microscopy or lesions.
Morphometry and Histopathology of the Aorta.
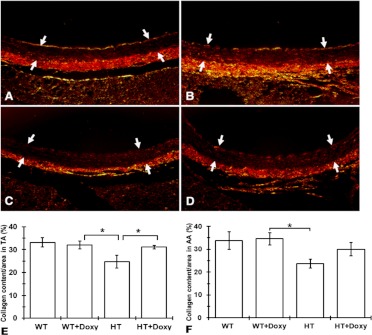
The representative picrosirius red-stained slices of aorta viewed under polarized light are presented in Fig. 1. There was a trend for a reduction of total collagen content in the entire aorta wall and separately in tunica media and adventitia in both thoracic and abdominal segments of aorta among untreated HT mice in comparison with untreated WT mice; there was also a trend for normalization of reduced collagen content in aorta among HT mice treated with Doxy (HT + Doxy) (Supplemental Table 2). However, a statistically significant reduction of collagen in HT mice (25% less than in WT) with its normalization in HT + Doxy mice was observed only in the tunica media of thoracic aorta (Fig. 1E). The lumen diameter of aorta did not differ between WT and HT mice and was not affected by Doxy treatment (Table 1). Thickness of tunica adventitia was not different among groups and was not affected by treatment. However, thickness of tunica media was reduced in HT mice compared with WT (p < 0.05), but increased in Doxy-treated HT mice to the level of WT (Table 1).
Fig. 1.
Collagen content of aorta in 9-month-old mice. A to D, representative picrosirius red-stained slides of aortas of 9-month-old mice viewed under polarized light (×400). Arrows indicate tunica media. Shown are WT untreated mice (A), WT doxycycline-treated mice (WT + Doxy) (B), HT untreated mice (HT) (C), and HT doxycycline-treated mice (HT + Doxy) (D). E and F, average content of collagen in thoracic aorta (TA) (E) and abdominal aorta (AA) (F). Values are means ± S.E.M. ANOVA with Duncan post hoc test. *, p < 0.05.
TABLE 1.
Average thickness of aortic wall in 9-month-old WT and HT mice untreated or treated with doxycycline since weaning
Values are means ± S.E.M.
| WT | WT + Doxy | HT | HT + Doxy | ANOVA | |
|---|---|---|---|---|---|
| Diameter of lumen, μm | 664.05 ± 44.37 | 619.64 ± 21.05 | 667.65 ± 44.45 | 606.57 ± 20.17 | |
| Thickness of adventitia, μm | 23.12 ± 2.97 | 19.84 ± 1.11 | 20.79 ± 3.82 | 16.69 ± 1.18 | |
| Thickness of media, μm | 28.57 ± 1.14AB | 30.74 ± 0.92A | 25.56 ± 1.29B | 29.51 ± 1.27A | * |
, p < 0.05, ANOVA with post-hoc Tukey test. Different letters indicate significant difference from other groups.
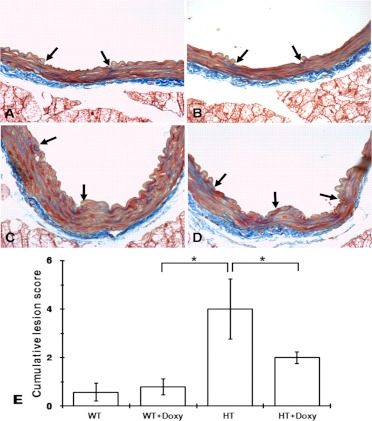
The spectrum of lesions in aortic wall among HT mice had been characterized previously (Cooper et al., 2010; Briest et al., 2011) and could be divided into four grades according to severity. All grades of lesions included fragmentation of the internal elastic lamina (IEL). Grade 1 lesions appeared as a small break in the IEL with no significant spindle cell proliferation (data not shown). The broken ends of the lamina frequently curled under into the media. In grade 2 lesions, the distance between the fragmented ends of the IEL was wider than in grade 1 (Fig. 2, A and B). Grade 3 lesions were larger, with florid medial spindle cell proliferation and often fragmentation of one to two medial elastic laminae (Fig. 2C). In grade 4 lesions (Fig. 2D), there was marked and abrupt attenuation of the wall thickness with abundant fibrosis and more severe fragmentation of several elastic laminae (Ramot et al., 2009; Briest et al., 2011). Cooper et al. (2010) reported that grade 1 lesions were seen in 55% of studied mice and distributed equally among WT and HT mice of both sexes. For this reason, grade 1 lesions were excluded from our statistical analysis. Cumulative lesion scores (lesions ≥ grade 2) for each group are shown in Fig. 2E. Lesion score was significant, 8-fold higher in HT mice compared with WT mice. Doxy treatment did not affect cumulative lesion score in WT mice. The lesion score in HT + Doxy mice was significantly smaller than in HT mice and did not differ from both WT groups.
Fig. 2.
Spontaneously developed lesions in aortas of 9-month-old mice. A to D, typical representations of spontaneous lesions. Sections were stained with Masson's trichrome. A, lesion grade 2 in WT mouse. B, section of aorta from lesion grade 2 in HT mouse. C, lesion grade 3 lesion; a large defect in the internal elastic lamina and significant subintimal spindle cell proliferation with deposition of collagen. D, lesion grade 3, similar to grade 4 but with a defect area much larger. E, cumulative lesion score (lesions ≥ grade 2) in each group. Values are means ± S.E.M. *, p < 0.05, ANOVA with Tukey post hoc test.
Immunohistochemistry for MMP-2 and MMP-9.
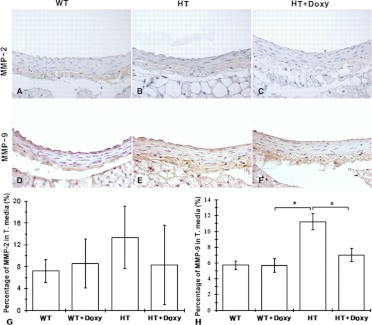
Representative sections of aortas from different groups subjected to immunohistochemical staining for MMP-2 and MMP-9 are shown in Fig. 3, A–F. There was a trend for an increase of MMP-2 in tunica media among untreated HT mice and its normalization in Doxy-treated HT animals (Fig. 3G); however, statistically significant differences were observed only for MMP-9 (Fig. 3H). MMP-9 expression was significantly elevated in HT mice compared with WT mice and normalized in HT + Doxy mice.
Fig. 3.
MMP-2 and MMP-9 in aortas of 9-month-old mice untreated or treated with doxycycline since weaning. A to F, representative immunohistochemical staining of aorta from different groups for MMP-2 (A–C) and MMP-9 (D–F). G and H, quantitative presentation of MMP-2 (G) and MMP-9 (H) in tunica media. Values are means ± S.E.M. *, p < 0.05, ANOVA with Tukey post hoc test.
Discussion
Currently there is no animal model for vEDS associated with missense and splicing mutations representing the majority of patients with vEDS. We have reported a mouse experimental model of heterozygosity for the COL3A1 null mutation (Cooper et al., 2010). This genetic abnormality represents approximately 4% of patients with vEDS and is characterized by a milder presentation and delayed onset of symptoms (Leistritz et al., 2011). Our mouse model does not present a significant mortality from aortic or arterial ruptures; it does exhibit, however, a greatly increased number of histologically identifiable lesions of aorta. The HT mice from this model also have lower collagen content in aorta compared with WT mice (Cooper et al., 2010; Briest et al., 2011) and increased expression of MMPs, particularly MMP-9 (Briest at al., 2011). Thus, this model allows testing of therapeutic interventions to arrest or alleviate the progression and complications of vEDS.
Doxycycline is a tetracycline antibiotic that is also a nonselective and broad-spectrum MMP inhibitor. The effect of Doxy to alleviate the progression of abdominal aortic aneurysm has been proven in animal research (Chung et al., 2008; Turner et al., 2008; Xiong et al., 2008; Tedesco et al., 2009; Sheth et al., 2010; Yang et al., 2010) and demonstrated in some clinical studies (Curci et al., 2000; Mosorin et al., 2001; Baxter et al., 2002; Lindeman et al., 2009). Doxy in a subantimicrobal dose is also the only MMP inhibitor approved by the Food and Drug Administration. It is currently used for the treatment of periodontal disease (Wennström et al., 2001) and rosacea (Del Rosso et al., 2008).
HT mice used in this study demonstrated previously described characteristics of haploinsufficiency for collagen III: reduced collagen content in aorta, particularly tunica media and increased MMP activity, especially MMP-9 (Cooper et al., 2010; Briest et al., 2011). Two gelatinases, MMP-2 and MMP-9, had been considered functionally similar and important in vascular remodeling, particularly in smooth muscle cell migration and matrix degradation (Yasumitsu et al., 1992). Eventually it became clear that their expression in the vascular wall is differently controlled (Whatling et al., 2004); MMP-9 happened to be more important for the organization of collagen by smooth muscle cells than MMP-2, and its expression was more apparent in the presence of vascular injuries (Johnson and Galis, 2004). In light of these findings, it came to no surprise than we observed predominantly elevated expression of MMP-9 in the vascular wall of 9-month-old mice haploinsufficient for collagen III, at the age when aortic lesions in these KO mice became apparent, whereas MMP-2 expression in aortic wall remained at the level of wild-type mice. In our previous study (Briest et al., 2011) we also observed elevated expression on MMP-9 in the aortic wall associated with lesions, but predominant elevation of MMP-2 expression in the skin of COL3A1 heterozygous mice.
The results of the experiment confirmed our hypothesis: treatment with a broad-spectrum MMP inhibitor, doxycycline, started early in life (immediately after weaning) resulted in the normalization of increased MMP activity and normalization of the reduced aortic collagen content in adult, 9-month-old mice. The treatment also prevented the development of spontaneous aortic lesions in HT mice. These findings are in concert with our previous report that in the same experimental model 3-month treatment with Doxy prevented the development of induced lesions of aorta (Briest et al., 2011).
Taking together, our findings provide experimental justification for the clinical evaluation of doxycycline at least in the haploinsufficient variety of vEDS. The recommendation for testing this therapeutic intervention for the rest of patients with vEDS, whose collagen III is not reduced in volume but has an altered structure, cannot be done on the basis of existing experimental evidences. Development of the adequate mouse model is a crucial next step.
Supplementary Material
This work was fully supported by the Intramural Research Program of the National Institutes of Health National Institute on Aging.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- EDS
- Ehlers-Danlos syndrome
- vEDS
- vascular EDS
- HT
- heterozygous COL3A1-deficient
- Doxy
- doxycycline
- WT
- wild type
- KO
- knockout
- MMP
- metalloproteinase
- IEL
- internal elastic lamina
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Tae, Marshall, Briest, and Talan.
Conducted experiments: Tae, Marshall, and Zhang.
Performed data analysis: Tae, Wang, and Talan.
Wrote or contributed to the writing of the manuscript: Tae, Marshall, and Talan.
References
- Arteaga-Solis E, Gayraud B, Ramirez F. (2000) Elastic and collagenous networks in vascular diseases. Cell Struct Funct 25:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas AP. (2000) Ehlers-Danlos syndrome type IV. N Engl J Med 343:366; author reply 368 [DOI] [PubMed] [Google Scholar]
- Baxter BT. (2005) Heritable diseases of the blood vessels. Cardiovasc Pathol 14:185–188 [DOI] [PubMed] [Google Scholar]
- Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, et al. (2002) Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg 36:1–12 [DOI] [PubMed] [Google Scholar]
- Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. (1998) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 77:31–37 [DOI] [PubMed] [Google Scholar]
- Briest W, Cooper TK, Tae HJ, Krawczyk M, McDonnell NB, Talan MI. (2011) Doxycycline ameliorates the susceptibility to aortic lesions in a mouse model for the vascular type of Ehlers-Danlos syndrome. J Pharmacol Exp Ther 337:621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Yang HH, Radomski MW, van Breemen C. (2008) Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 102:e73–e85 [DOI] [PubMed] [Google Scholar]
- Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He CS, Bauer EA, Goldberg GI. (1988) H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 263:6579–6587 [PubMed] [Google Scholar]
- Cooper TK, Zhong Q, Krawczyk M, Tae HJ, Müller GA, Schubert R, Myers LA, Dietz HC, Talan MI, Briest W. (2010) The haploinsufficientCol3a1 mouse is a model for the vascular Ehlers-Danlos syndrome. Vet Pathol 47:1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, Sicard GA, Thompson RW. (2000) Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg 31:325–342 [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ, Schlessinger J, Werschler P. (2008) Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol 7:573–576 [PubMed] [Google Scholar]
- Germain DP. (2002) Clinical and genetic features of vascular Ehlers-Danlos syndrome. Ann Vasc Surg 16:391–397 [DOI] [PubMed] [Google Scholar]
- Germain DP. (2007) Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Johnson C, Galis ZS. (2004) Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol 24:54–60 [DOI] [PubMed] [Google Scholar]
- Leistritz DF, Pepin MG, Schwarze U, Byers PH. (2011) COL3A1 haploinsufficiency results in a variety of Ehlers-Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet Med 13:717–722 [DOI] [PubMed] [Google Scholar]
- Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. (2009) Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119:2209–2216 [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Krane S, Jaenisch R. (1997) Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A 94:1852–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum YW, Brooke BS, Arnaoutakis GJ, Williams TK, Black JH., 3rd (2011) Endovascular procedures in patients with Ehlers Danlos syndrome: a review of clinical outcomes and iatrogenic complications. Ann Vasc Surg 26: 25–33 [DOI] [PubMed] [Google Scholar]
- Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. (2001) Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 34:606–610 [DOI] [PubMed] [Google Scholar]
- Ong KT, Perdu J, De Backer J, Bozec E, Collignon P, Emmerich J, Fauret AL, Fiessinger JN, Germain DP, Georgesco G, et al. (2010) Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet 376:1476–1484 [DOI] [PubMed] [Google Scholar]
- Pepin M, Schwarze U, Superti-Furga A, Byers PH. (2000) Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 342:673–680 [DOI] [PubMed] [Google Scholar]
- Ramot Y, Manno RA, Okazaki Y, Krakovsky M, Lamensdorf I, Meiron M, Toren A, Zehavi-Goldstein E, Vezzali E, Nyska A. (2009) Spontaneous aortitis in the Balb/c mouse. Toxicol Pathol 37:667–671 [DOI] [PubMed] [Google Scholar]
- Seeland U, Selejan S, Engelhardt S, Müller P, Lohse MJ, Böhm M. (2007) Interstitial remodeling in β1-adrenergic receptor transgenic mice. Basic Res Cardiol 102:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth RA, Maricevich M, Mahmood U. (2010) In vivo optical molecular imaging of matrix metalloproteinase activity in abdominal aortic aneurysms correlates with treatment effects on growth rate. Atherosclerosis 212:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco MM, Terashima M, Blankenberg FG, Levashova Z, Spin JM, Backer MV, Backer JM, Sho M, Sho E, McConnell MV, et al. (2009) Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler Thromb Vasc Biol 29:1452–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocmé C, Gaudin P, Berthier S, Barro C, Zaoui P, Morel F. (1998) Human B lymphocytes synthesize the 92-kDa gelatinase, matrix metalloproteinase-9. J Biol Chem 273:20677–20684 [DOI] [PubMed] [Google Scholar]
- Turner GH, Olzinski AR, Bernard RE, Aravindhan K, Karr HW, Mirabile RC, Willette RN, Gough PJ, Jucker BM. (2008) In vivo serial assessment of aortic aneurysm formation in apolipoprotein E-deficient mice via MRI. Circ Cardiovasc Imaging 1:220–226 [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shimada T. (2008) Vascular type of Ehlers-Danlos syndrome. J Nippon Med School 75:254–261 [DOI] [PubMed] [Google Scholar]
- Wennström JL, Newman HN, MacNeill SR, Killoy WJ, Griffiths GS, Gillam DG, Krok L, Needleman IG, Weiss G, Garrett S. (2001) Utilization of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multi-centre trial of 2 treatment approaches. J Clin Periodontol 28:753–761 [DOI] [PubMed] [Google Scholar]
- Whatling C, McPheat W, Hurt-Camejo E. (2004) Matrix management assigning different roles for MMP-2 and MMP-9 in vascular remodeling. Atheroscler Thromb Vasc Biol 24:10–11 [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant GA, Goldberg GI. (1989) SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem 264:17213–17221 [PubMed] [Google Scholar]
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. (2008) Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg 47:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. (2010) Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J Thorac Cardiovasc Surg 140:305–312.e2 [DOI] [PubMed] [Google Scholar]
- Yasumitsu H, Miyazaki K, Umenishi F, Koshikawa N, Umeda M. (1992) Comparison of extracellular matrix-degrading activities between 64-kDa and 90-kDa gelatinases purified in inhibitor-free forms from human schwannoma cells. J Biochem 111:74–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.