Abstract
Cadmium is an important industrial agent and environmental pollutant that is a major cause of kidney disease. With chronic exposure, cadmium accumulates in the epithelial cells of the proximal tubule, resulting in a generalized reabsorptive dysfunction characterized by polyuria and low-molecular-weight proteinuria. The traditional view has been that as cadmium accumulates in proximal tubule cells, it produces a variety of relatively nonspecific toxic effects that result in the death of renal epithelial cells through necrotic or apoptotic mechanisms. However, a growing volume of evidence suggests that rather than merely being a consequence of cell death, the early stages of cadmium-induced proximal tubule injury may involve much more specific changes in cell-cell adhesion, cellular signaling pathways, and autophagic responses that occur well before the onset of necrosis or apoptosis. In this commentary, we summarize these recent findings, and we offer our own perspectives as to how they relate to the toxic actions of cadmium in the kidney. In addition, we highlight recent findings, suggesting that it may be possible to detect the early stages of cadmium toxicity through the use of improved biomarkers. Finally, some of the therapeutic implications of these findings will be considered. Because cadmium is, in many respects, a model cumulative nephrotoxicant, these insights may have broader implications regarding the general mechanisms through which a variety of drugs and toxic chemicals damage the kidney.
Cadmium as an Environmental Health Problem
Cadmium (Cd2+) is a widespread environmental pollutant that is a major cause of kidney disease in many regions of the world. Cadmium is normally found at low concentrations throughout the lithosphere but has become increasingly concentrated in the biosphere through mining, smelting, and agricultural and industrial activities of humans. As a stable, divalent cation, cadmium is not biodegradable and persists in the environment. Despite efforts by many countries and international agencies to reduce the usage of cadmium, it continues to be a major public health problem, especially in emerging industrial nations where environmental controls are still being developed (Satarug et al., 2003; Nordberg, 2004; Teeyakasem et al., 2007; Järup and Akesson, 2009).
Humans are typically exposed to cadmium either in the workplace or through the ingestion of cadmium-contaminated food or water (Agency for Toxic Substances & Disease Registry, Toxicological Profile for Cadmium, 2008, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15; Järup and Akesson, 2009; Satarug et al., 2010). Tobacco contains significant amounts of cadmium, and smoking is a major source of exposure among the general population (Satarug and Moore, 2004; Agency for Toxic Substances & Disease Registry, Toxicological Profile for Cadmium, 2008, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15; Menke et al., 2009). Depending on the dose, route, and duration of exposure, cadmium can damage various organs including the lung, liver, kidney, and bone. Cadmium can also act as an endocrine disruptor, and it is carcinogenic. (Järup et al., 1998; Waalkes, 2003; for reviews, see Agency for Toxic Substances & Disease Registry, Toxicological Profile for Cadmium, 2008, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48 &tid=15; Byrne et al., 2009; Joseph, 2009).
With the chronic, low-level patterns of cadmium exposure that are commonly seen in human populations, the primary target organ of cadmium toxicity is the kidney, in which cadmium causes a generalized dysfunction of the proximal tubule characterized by polyuria and increases in the urinary excretion of glucose, amino acids, electrolytes (particularly Na+, K+, and Ca2+) and low-molecular-weight proteins (Järup, 2002; Agency for Toxic Substances & Disease Registry, Toxicological Profile for Cadmium, 2008, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15). A growing volume of evidence indicates that the adverse renal effects of cadmium can result from even low levels of cadmium exposure and that women, children, and individuals with confounding health conditions, such as diabetes, may be especially susceptible (Järup, 2002; Satarug et al., 2003; Akesson et al., 2005; Nawrot et al., 2008; Agency for Toxic Substances & Disease Registry, Toxicological Profile for Cadmium, 2008, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15; Navas-Acien et al., 2009; Thomas et al., 2009; Suwazono et al., 2010).
Evolving View of the Mechanisms of Cadmium Nephrotoxicity
Whereas the general effects of cadmium on proximal tubule function have been well documented, the specific molecular mechanisms that underlie these effects are not yet fully understood. It should be emphasized that the current uncertainties are not merely due to a lack of attention or information. Even though it is fashionable for authors of these types of reviews (including these authors) to state that “little is known” about the mechanisms of cadmium toxicity, such statements are not completely true. In reality, a great deal is actually known regarding the basic molecular mechanisms by which cadmium can alter renal epithelial cell function (for reviews see Prozialeck, 2000; Järup, 2002; Thévenod, 2009). The problem is that much of this information has been generated from studies on renal epithelial cells in culture, and the relevance of many of these findings to the nephrotoxic effects of cadmium in vivo remain unclear. This issue has been complicated by the unusual toxicokinetics of cadmium in the body and by the ability of cadmium to interact with a vast array of biological molecules. When using in vitro models, it is difficult, if not impossible, to mimic the conditions under which renal epithelial cells are exposed in vivo and to sort out the relevant biological effects from the irrelevant. However, although the problems of identifying the mechanisms of cadmium toxicity have certainly been formidable, they have not been insurmountable. By applying modern techniques of cellular and molecular biology to the study of in vivo model systems, investigators have, in fact, managed to obtain new insights into the molecular basis of cadmium-induced proximal tubular injury.
The purpose of this commentary is to highlight some of the most significant findings in this evolving field of research. This will not be a comprehensive review, but we will focus on recent in vivo findings showing that the early stages of cadmium nephrotoxicity involve specific changes in proximal tubule cell-cell adhesion, cellular signaling cascades, and autophagic responses that occur before the onset of necrosis or apoptosis of proximal tubule cells. We will also highlight recent findings suggesting that it may be possible to detect these early stages of cadmium toxicity through the use of improved biomarkers, such as kidney injury molecule-1 (Kim-1). In discussing these topics, we will consider aspects of the toxicokinetics of cadmium in vivo and some of the key pathological events that are associated with the onset of proximal tubule injury. Finally, some of the potential therapeutic and mechanistic implications of these findings will be considered. In preparing the manuscript, we have tried to integrate and synthesize information from diverse sources to provide a concise overview that will be of use to investigators in the cadmium field. We have also tried to incorporate our own insights and perspectives that we have developed from working in the cadmium field for many years. Because cadmium is, in many respects a model cumulative nephrotoxin, these observations may have broader implications beyond the field of cadmium toxicology.
Toxicokinetics of Cadmium In Vivo
Any discussion of the actions of cadmium in the kidney must begin with consideration of the forms of cadmium that exist under physiological conditions and the distribution of these forms of cadmium within the body. These topics have been the subject of several excellent reviews (Jin et al., 1998; Bridges and Zalups, 2005; He et al., 2009). However, they have often been overlooked in many of the in vitro studies on the cytotoxic actions of cadmium, a fact that has greatly complicated the extrapolation of in vitro mechanistic findings to the actions of cadmium in the whole kidney.
With respiratory exposure, cadmium is efficiently absorbed from the lung; up to 40 to 60% of inhaled cadmium reaches the systemic circulation. With oral exposure, the absorption of cadmium from the gastrointestinal tract is considerably lower (only 5–10%). However, with long-term exposure, even this low level of absorption from the gastrointestinal tract can lead to systemic accumulation of cadmium and subsequent toxicities. The gastrointestinal absorption of cadmium may be substantially higher in individuals with low body stores of iron, which is a factor that could contribute to individual variations in sensitivity to cadmium exposure.
Once absorbed into the bloodstream, cadmium is initially transported to the liver where it is taken up by hepatocytes and induces the synthesis of metallothionein, which binds cadmium, and buffers its toxic effects in the cell. However, as the hepatocytes die off, either through normal turnover or as a result of cadmium injury, the cadmium-metallothionein complex can be released into the bloodstream (Jin et al., 1998; Klaassen et al., 2009). Even though the cadmium-metallothionein complex is nontoxic to most organs, it can be filtered at the glomerulus and taken up by the epithelial cells of the proximal tubule. In this situation, cadmium-metallothionein can have the paradoxical effect of facilitating the delivery of cadmium from the liver to the kidney, and it has been suggested that cadmium-metallothionein may actually mediate some of the toxic effects of cadmium in the proximal tubule (Klaassen and Liu, 1997). However, a great deal of evidence indicates that it is actually ionic cadmium (Cd2+), not cadmium-metallothionein, that injures proximal tubule epithelial cells (Goyer et al., 1989; Klaassen et al., 2009). The fact that metallothionein-null animals are sensitive to cadmium-induced proximal tubule injury provides compelling evidence that cadmium-metallothionein does not play a critical role in directly mediating the nephrotoxic effects of cadmium (Liu et al., 1998).
One especially important aspect of cadmium disposition that has been overlooked frequently is that essentially all cadmium in the plasma is bound to proteins or other molecules. The circulating cadmium may either be tightly bound to specific metal-binding proteins such as metallothionein (Klaassen and Liu, 1997; Klaassen et al., 2009) or may be loosely associated with molecules, such as albumin, amino acids, or low-molecular-weight sulfhydryl compounds such as glutathione or cysteine (Bridges and Zalups, 2005; He et al., 2009). These interactions of cadmium with proteins and low-molecular-weight compounds in plasma have greatly complicated efforts to identify the molecular mechanisms by which cadmium is taken up by proximal tubule epithelial cells in vivo. Various studies to address this issue have shown that cadmium can enter proximal tubule cells through a variety of mechanisms (He et al., 2009). As noted previously, circulating cadmium-metallothionein can be filtered at the glomerulus and taken up by the epithelium of the proximal tubule in a process that involves megalin-mediated transport at the brush border (Squibb and Fowler, 1984; Klaassen et al., 2009). In addition, there is evidence for the uptake of lower-molecular-weight cadmium-thiol conjugates (cysteine and glutathione) by proximal tubule cells (Bridges and Zalups, 2005). However, it is also important to note that the interaction between cadmium and low-molecular-weight thiols is of a low enough affinity that cadmium could dissociate from the thiol and bind to molecules on the cell surface and, in some cases, enter the cell. Indeed, there is evidence that cadmium can enter renal tubular cells through a variety of channels and transporters for ions such as Ca2+, Fe2+, and Zn2+ (Bridges and Zalups, 2005; He et al., 2009). Nebert and coworkers (He et al., 2009) have recently provided compelling evidence that the ZIP8 family of metal ion transporters plays an especially important role in the cellular uptake of cadmium in the kidney. Taken together, these findings suggest that with typical patterns of exposure, multiple mechanisms probably contribute to the uptake of cadmium in the proximal tubule in vivo.
Another important consideration relates to the concentrations of cadmium that are typically achieved in vivo (for review, see Prozialeck and Edwards, 2010). The blood levels of cadmium in nonexposed populations are typically less than 0.5 μg/l. Blood levels higher than 1.0 μg/l are generally indicative of cadmium exposure; levels higher than 5 μg/l are considered hazardous. Urinary levels of cadmium in nonexposed populations are normally less than 0.5 μg/g creatinine; values higher than 1 to 2 μg/g are indicative of exposure or elevated body burden. The critical urinary cadmium concentration that is associated with the onset of renal injury is usually approximately 2 to 10 μg/g creatinine, which corresponds to a renal cortical cadmium concentration of approximately 150 to 200 μg/g tissue (Roels et al., 1979; Järup, 2002). These levels of exposure need to be kept in mind when the possible relevance of in vitro studies to the action of cadmium in vivo is considered. Most in vitro studies typically have involved the exposure of cultured cells to low micromolar concentrations of cadmium for less than 24 h. Although these concentrations are much higher than the concentrations of cadmium in blood (5–10 nM), they are well below the millimolar concentrations of cadmium that are achieved in renal cortical tissue in vivo. It is also important to recognize that individual cells and target molecules in the proximal tubule could actually be exposed to relatively high concentrations of cadmium. For example, consider the situation in which a cadmium-intoxicated proximal tubule cell lyses and releases its cytosolic contents, including cadmium, into the local cellular environment. The localized concentrations of cadmium in the immediate vicinity could easily exceed 1 mM. Even though all of this cadmium would be bound to proteins or low-molecular-weight thiols, it could still undergo equilibrium interactions (dissociation and binding) with potential molecular targets in or on the adjacent cells. From a practical standpoint, it would be almost impossible to replicate these types of exposure conditions in vitro, a fact that further highlights the importance of in vivo models.
In considering the use of in vivo models to study cadmium nephrotoxicity, investigators must balance the need to be able to do the studies in a reasonably short time frame with the need to replicate the toxicokinetics of the long-term, low-level patterns of exposure that are common in humans. For example, even though humans are typically exposed to dietary cadmium over many years or decades, it is simply not possible or practical to replicate this type of exposure in species such as rats or mice. Because these species have shorter life spans and for many practical reasons, exposure levels used in animal studies are usually higher, but shorter in duration, than those seen in humans.
Cadmium is a classic cumulative nephrotoxicant. With higher levels of exposure, nephrotoxic effects occur more quickly than with lower levels of exposure. In commonly used animal models, there is a linear inverse relationship between the dose of cadmium, and the time of exposure causes onset of proximal tubule injury (i.e., doubling the dose produces effects in one-half the time) (Prozialeck et al., 2007). However, higher doses of cadmium can cause injury to organs other than the kidney, in particular, the liver and gonads. For nephrotoxicity studies, one of the most useful approaches has involved the subcutaneous administration of moderate doses of cadmium (0.4–0.8 mg/kg per day) for periods ranging from 4 to 12 weeks. However, even with this approach, slight differences in treatment protocols and methodologies can complicate comparisons of results from different laboratories. To address some of the key issues, our own research groups have been using a treatment protocol that involves the subcutaneous administration of cadmium to rats (0.6 mg/kg, 5 days/week for up to 12 weeks) (Prozialeck et al., 2007, 2009a,b; Prozialeck and Edwards, 2010). This has been a widely used protocol in cadmium research and accurately reflects key pathophysiological features of longer-term exposure in humans. Many of the major conclusions that we will be emphasizing are derived from studies using this protocol. This treatment protocol was approved by the Institutional Animal Care and Use Committee of Midwestern University, and the studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Role of Necrosis, Apoptosis, and Autophagy in Cadmium Nephrotoxicity
Regardless of the uptake mechanisms that are involved, it is clear that over time cadmium can accumulate in the epithelial cells of the proximal tubule. The traditional view has been that when the tissue levels of cadmium exceed a critical concentration of approximately 150 μg/g tissue, intracellular defenses such as metallothionein and glutathione are overwhelmed, and the cells undergo injury and begin to die (Gobe and Crane, 2010; Prozialeck and Edwards, 2010). A very fundamental question that has only been addressed over the past 10 to 15 years concerns the relative roles of apoptotic, necrotic, and autophagic mechanisms in cadmium-induced proximal tubular cell death. This is an important issue because even though all three pathways can result in cell death, each of the pathways involves its own unique sequence of pathophysiological events (for review, see Galluzzi et al., 2007). Apoptosis (or type I cell death) is characterized by mitochondrial depolarization, caspase activation, DNA fragmentation, and cell shrinkage, followed by fragmentation of the cell into small, membrane-coated apoptotic bodies. Necrosis (type III cell death) is characterized by swelling of mitochondria and other organelles, breakdown of the cell membrane and the leakage of cytosolic contents into the surrounding environment. Autophagy is the least understood of these so called “cell death” pathways. Autophagy is a programmed process that involves the internal phagocytosis of damaged proteins and cytosolic elements into double membrane-coated vesicles known as autophagosomes, which in turn are broken down by lysosomes. Whereas low levels of autophagy may actually represent a repair/survival mechanism to preserve cell function, persistent or high levels of autophagy may trigger cell death. The biochemical pathways leading to autophagic cell death may overlap, to a certain extent, with those leading to apoptotic death.
It has long been recognized that high nephrotoxic doses of cadmium can cause proximal tubule necrosis. However, it has also been apparent that early manifestations of cadmium-induced proximal tubule dysfunction occur well before the onset of necrosis. In the 1990s, several investigators (Hamada et al., 1991; Tanimoto et al., 1993, 1999; Yan et al., 1997) published results showing that the early stages of cadmium nephrotoxicity were associated with an increase in the number of apoptotic cells in the proximal tubule. In each of these studies, there was no significant evidence of necrotic injury at the time that apoptosis was observed. The studies by Tanimoto et al. were especially noteworthy because the authors also identified proximal tubule cells that appeared to be proliferating as part of the response to apoptotic injury. In another study, Aoyagi et al. (2003) noted an increase in the number of apoptotic cells in the renal cortex of cadmium-treated rats after 4 and 5 weeks of exposure, but that the level of apoptotic labeling was much less pronounced after 6 and 8 weeks of exposure. In more recent studies from our laboratory (Prozialeck et al., 2009a), we also found that cadmium caused a low level of apoptosis in the proximal tubules of subchronically exposed rats. However, the onset of apoptosis appeared to occur after Kim-1-dependent tissue repair processes had already been activated, suggesting that cadmium can produce significant changes in the cells before the onset of apoptosis. In a recent study using an acute (only 5 days), intraperitoneal model of cadmium exposure in the rat, Chargui et al. (2011) identified the activation of a variety of autophagic processes in the proximal tubule that occurred at a time when there was no evidence of apoptosis or general proximal tubule dysfunction. These studies, too, suggest that cadmium is producing early toxic effects within the cells that leads to activation of a repair process, in this case autophagy.
There are several aspects of these studies that merit special attention. First, in all of the studies in which apoptotic cells were identified, the onset of apoptotic cell death appeared to coincide with the onset of proximal tubule dysfunction, as evidenced by polyuria and/or proteinuria. However, it is also noteworthy that in each of these studies, the numbers of proximal tubule cells that were actually undergoing apoptosis were quite low (i.e., well below 5%). The vast majority of proximal tubular cells were largely unaffected by cadmium and/or appeared to dedifferentiate and proliferate as part of the repair process. The fact that only a small percentage of renal cells are being affected by cadmium could greatly complicate efforts to identify the biochemical mechanisms by which the effects are occurring because it can be technically difficult to identify any possible cadmium-induced biochemical changes in a few cells that are located in a sea of cells that are not being affected by cadmium. However, it is also apparent that cadmium causes some sort of injury that triggers this low level of apoptosis. In this context, the studies by Prozialeck at al. (2009a) and Chargui et al. (2011) are especially significant in that they clearly show that cadmium is producing detectable effects, such as up-regulation of Kim-1 and induction of autophagy in proximal tubule cells before there is evidence of apoptosis or proximal tubule dysfunction.
The key question that has yet to be resolved is, How is cadmium causing the initial injury to proximal tubule cells? Studies over the past 10 years have yielded some insights. In general, these studies have implicated three possible early response mechanisms in the proximal tubule. These are disruption of cadherin-mediated cell-cell adhesion, modulation of intracellular signaling cascades, and induction of oxidative stress.
Cadherin Cell Adhesion Molecules as Potential Targets of Cadmium Toxicity
One of the earliest toxic effects of cadmium that is evident in proximal tubule cells, both in vitro and in vivo, involves the disruption of cadherin-mediated cell-cell adhesion (for reviews, see Prozialeck, 2000; Prozialeck et al., 2003; Prozialeck and Edwards, 2007). The cadherins represent a family of calcium-dependent cell adhesion molecules that are usually localized at adherens junctions in epithelial cells (Prozialeck and Edwards, 2007). The extracellular domain of the cadherin contains Ca2+-binding sites and the adhesive regions. The intracellular domain is bound to β-catenin, which is bound to α-catenin, which links the entire complex to the actin cytoskeleton. β-Catenin also functions as a regulator of gene expression through the wingless/Wnt nuclear signaling pathway (Prozialeck and Edwards, 2007; Thévenod, 2009). When β-catenin is released from the junctional complex into the cytosol, it may either be targeted for proteosomal degradation in a process that involves the adenomatous polyposis coli gene product and the serine/threonine kinase glycogen synthase kinase-3β, or it can enter the nucleus, where it can bind to T-cell factor-lymphocyte enhancer factor-1 transcription factors and alter the expression of genes involved in apoptosis and cell-cycle control (Prozialeck and Edwards, 2007; Thévenod et al., 2007; Thévenod, 2009).
The finding that the cadherins are targets of cadmium toxicity stemmed from observations by Prozialeck and Niewenhuis (1991), who found that exposing cultured renal epithelial cells to 5 to 20 μM cadmium for 1 to 4 h caused the cells to separate from each other and change from epithelioid to rounded, an effect that coincided with the loss of E-cadherin from the cell-cell contacts. Subsequent studies showed that cadmium had similar effects on E- and N-cadherin junctions in many types of epithelial cells and on VE-cadherin junctions in vascular endothelial cells (Prozialeck, 2000). The ability of cadmium to disrupt cadherin-dependent cell-cell junctions has been confirmed by many other laboratories and is now generally accepted as one of the primary actions of cadmium on epithelial cells (for review, see Prozialeck and Edwards, 2007). It is also noteworthy that even though cadmium was the first nephrotoxic agent that was found to disrupt cadherin-mediated cell-cell adhesion, it is now recognized that this is a primary effect of many nephrotoxic substances including mercury, bismuth and aminoglycoside antibiotics (for reviews, see Prozialeck and Edwards, 2007; Parrish and Prozialeck, 2010). This fact further illustrates how results of studies on an environmental pollutant such as cadmium can have implications that are relevant to the nephrotoxic actions of drugs and therapeutic agents.
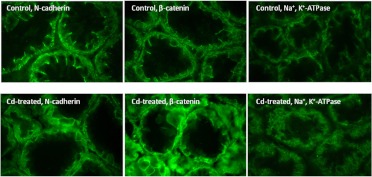
Whereas much of the original work showing that cadherins are targets of cadmium toxicity involved studies on epithelial cells in culture, it was less clear whether cadmium can disrupt cadherin-dependent cell junctions in vivo. This issue was complicated by the unexpected finding that proximal tubule epithelial cells exhibit different patterns of cadherin expression in vivo than they do in vitro. Most proximal tubule-derived cell lines, including the cell lines that were used for our in vitro studies (for review, see Prozialeck, 2000), primarily express E-cadherin, which is the main cadherin expressed in most types of epithelial cells. However, when we tried to visualize E-cadherin in rat kidney, we found that the predominant cadherin in the proximal tubule was not E-cadherin but N-cadherin (Prozialeck et al., 2003, 2004). Once this issue was resolved, we were able to show that cadmium caused pronounced alterations in the pattern of N-cadherin localization in the proximal tubule without affecting E-cadherin in other nephron segments. Photos showing the effects of cadmium on N-cadherin localization in the S-3 segment of the proximal tubule are found in Fig. 1. As may be seen, the N-cadherin labeling in the control sample was highly concentrated along the basolateral cell surface and at the lateral cell-cell contacts. In contrast, the sample from the cadmium-treated animal shows a marked reduction in the labeling at the lateral cell-cell contacts. In addition, the labeling along the basolateral surface was much more diffuse than in the control samples. It is important to emphasize that these changes in N-cadherin localization were widespread and were readily apparent in all of the samples from the cadmium-treated animals that were examined. Other investigators have shown at about this same stage of inquiry significant changes in the microvilli and actin cytoskeleton (Sabolic et al., 2001, 2006). Together, these findings suggest that the cadherins or their associated catenins or cytoskeletal elements may be key early targets of cadmium toxicity.
Fig. 1.
Effects of cadmium on the localization of N-cadherin, β-catenin, and Na+,K+-ATPase in proximal tubule. Adult male Sprague-Dawley rats were treated with cadmium (0.6 mg/kg s.c.) 5 days/week for 6 weeks, whereas control animals received saline vehicle alone. The kidneys were removed and processed for immunofluorescent visualization of the molecules of interest as described previously (Prozialeck et al., 2003). These particular images show the patterns of labeling in the S-3 segment of the proximal tubules in the inner cortex, near the outer stripe of the medulla. Original magnification, 410×. [Images of N-cadherin and β-catenin labeling are reproduced from Prozialeck WC, Lamar PC, and Lynch SM (2003) Cadmium alters the localization of N-cadherin, E-cadherin, and β-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol 189:180–195. Copyright © 2003 Elsevier. Used with permission.]
Because the cadherins play a critical role in establishing and maintaining the epithelial polarity that is essential for the normal functioning of the proximal tubule (Molitoris and Marrs, 1999; Prozialeck and Edwards, 2007), we hypothesized that the cadmium-induced loss of N-cadherin-mediated adhesion in the proximal tubule might lead to changes in epithelial polarity and barrier function. As a first step to address this issue, we examined the effects of cadmium on the localization of Na+,K+-ATPase in the proximal tubule. Under normal circumstances, this transport protein is localized at the basolateral surface of the epithelial cells, where it plays a key role in sodium and fluid reabsorption. It is thought that the cadherin-dependent cell-cell junctions serve as a “fence” to confine Na+,K+-ATPase to the basolateral cell surface. The images at the right of Fig. 1 show that cadmium does, in fact, cause alterations in the localization of Na+,K+-ATPase in the proximal tubule. Note that in the control kidney, Na+,K+-ATPase is localized along the basolateral surface of the epithelial cells of the proximal tubule. In contrast, in the sample from the cadmium-treated animal, the Na+,K+-ATPase labeling is present over the entire cell surface and, in some areas, appears on the apical surface. These changes in Na+,K+-ATPase localization are similar to those described previously by Sabolic et al. (2006). These findingssuggest that cadmium-induced changes in cadherin dependent cell-cell adhesion may result in changes in epithelial polarity that are similar to those that have been described in ischemic kidney injury (for review, see Molitoris and Marrs, 1999).
The finding that the early stages of cadmium-induced proximal tubule injury are associated with changes in N-cadherin localization raises important questions regarding the mechanisms by which cadmium is producing these effects. Is cadmium altering the genetic expression of N-cadherin or is altering the function of the molecule, either directly or indirectly?
These findings also raise the important question as to whether the disruption of N-cadherin-mediated adhesion results in the activation of β-catenin-regulated gene expression. This is potentially very significant because β-catenin is a key regulator of a variety of genes that are involved in cell cycle control, cell differentiation, and apoptosis (for reviews, see Thévenod, 2009; Thévenod and Chakraborty, 2010).
Previous studies from our laboratories and more recent studies by Thévenod and coworkers (Prozialeck et al., 2002; Thévenod et al., 2007; Thévenod, 2009; Chakraborty et al., 2010; Thévenod and Chakraborty, 2010) have provided evidence that the disruption of cadherin-mediated adhesion by cadmium results in the nuclear accumulation of β-catenin and activation of β-catenin-regulated gene expression. The study by Chakraborty et al. (2010) is particularly significant. Using an in vivo mouse model of long-term cadmium ingestion, the investigators showed that cadmium caused the up-regulation in the expression of specific Wnt ligands and receptors that coincided with increases in the expression of several β-catenin-regulated genes including c-myc, cyclin D1, and Abcb1.
To further address these issues we have used real-time RT-PCR techniques to analyze the effects of cadmium on the patterns of gene expression in the renal cortex. Specific genes that were examined included N-cadherin, E-cadherin, VE-cadherin, and β-catenin along with a panel of β-catenin-regulated genes (cyclin DI, matrilysin, fibronectin, c-myc, and c-Jun), the cadmium-binding protein metallothionein, and a panel of stress response genes (super oxide dismutase, glutathione transferase, heme oxygenase, and NADPH oxidase). Results of these studies are summarized in Table 1. Note that cadmium differentially affected the expression of E-cadherin, VE-cadherin, and N-cadherin. There were no effects on the expression of E-cadherin or VE-cadherin at either 6 or 12 weeks or on expression of N-cadherin at 6 weeks. However, at 12 weeks, the expression of N-cadherin mRNA decreased significantly. The fact that the expression of N-cadherin changes in response to cadmium exposure, whereas the expression of E-cadherin and VE-cadherin do not, is consistent with our findings that N-cadherin is a target of cadmium toxicity. Moreover, the fact that there is no change in N-cadherin mRNA or protein levels (protein data not shown) at 6 weeks, a time at which changes in the localization of N-cadherin in the proximal tubule are readily apparent, suggests that the initial effects of cadmium involve either direct effects on N-cadherin or its associated molecules, or actions on one of the signaling pathways that regulate the function of N-cadherin.
TABLE 1.
Real-time RT-PCR analyses of the effects of cadmium on gene expression in the renal cortex
Adult male Sprague-Dawley rats were treated with cadmium (0.6 mg/kg s.c., 5 days/week for 6 or 12 weeks), whereas control animals received saline vehicle alone. Samples of renal cortex were analyzed for patterns of gene expression using standard real time RT-PCP techniques performed by Jie Liu in the laboratory of Dr. Michael Waalkes at the National Cancer Institute/National Institute of Environmental Health Sciences. Results are expressed as a percentage of control values and represent the mean ± S.E.M. from five to seven replicate samples. Results of these studies were originally presented in 2006 at the 45 Annual Meeting of the Society of Toxicology, and some of the data were included in portions of Prozialeck et al. (2007) Prozialeck et al. (2009a).
| Gene Category | Specific Protein | Cadmium |
|
|---|---|---|---|
| 6 Weeks (n = 7) | 12 Weeks (n = 7) | ||
| % control | |||
| Cell adhesion/scaffolding molecules | N-cadherin | 101 ± 16 | 30 ± 5* |
| E-cadherin | 114 ± 7 | 101 ± 25 | |
| VE-cadherin | 103 ± 18 | 117 ± 29 | |
| β-Catenin | 129 ± 19 | 69 ± 27 | |
| β-Catenin-regulated genes | Cyclin D1 | 80 ± 10 | 73 ± 13 |
| c-Myc | 154 ± 16* | 69 ± 20 | |
| Matrilysin | 210 ± 20* | 534 ± 77* | |
| Fibronectin | 90 ± 20 | 120 ± 20 | |
| c-Jun | 68 ± 18 | 119 ± 35 | |
| Metallothionein | 970 ± 155* | 1512 ± 329* | |
| Metal-binding proteins and stress indicators | Superoxide dismutase | 72 ± 12 | 36 ± 13* |
| Glutathione transferase | 117 ± 15 | 78 ± 8 | |
| Heme oxygenase | 75 ± 11 | 162 ± 29 | |
| α1-Acid glycoprotein | 190 ± 24* | 142 ± 11* | |
| NADPH oxidase | 173 ± 8* | 172 ± 28 | |
| Biomarker | Kim-1 | 611 ± 158* | 2434 ± 193* |
Levels of expression in the cadmium-treated animals were significantly different from control values (P < 0.05) as determined by Student's t tests.
It is also noteworthy that cadmium had no effect on the expression of β-catenin even though immunofluorescence (Fig. 1) and Western blot analyses (data not shown) revealed a pronounced redistribution of β-catenin from the cell borders to the cytosol. Moreover, cadmium had no effect on the mRNA expression of several β-catenin responsive genes (cyclin D1, fibronectin, and c-Jun) but did increase the expression of c-myc and the matrix metalloproteinase matrilysin. This indicates that even though cadmium causes the breakdown of the N-cadherin/β-catenin complex in the proximal tubule, it only results in partial activation of β-catenin-regulated gene expression.
Another interesting finding from the mRNA analyses is that even though cadmium caused a marked increase in the expression of metallothionein, it had little or no effect on the expression of various stress response elements such as heme oxygenase, glutathione transferase, and superoxide dismutase. This finding suggests that at the time cadmium-induced changes in N-cadherin localization are occurring, the renal epithelial cells are not undergoing a generalized stress response and, at most, may only be undergoing a very mild level of oxidative stress. Again, this finding suggests that cadmium is probably acting on specific molecular targets within the epithelial cells although the targets have yet to be identified.
Cadmium and Cellular Signaling Cascades
These findings strongly suggest that alterations in N-cadherin function and epithelial polarity represent very early events in the pathophysiology of cadmium-induced proximal tubule. However, the specific molecular mechanisms that mediate these effects have yet to be elucidated. Results of studies using renal epithelial cells in culture and polypeptide analogs of E-cadherin have shown that cadmium can interact with the extracellular Ca+2-binding domains on the molecule and alter its adhesive properties (for review, see Prozialeck, 2000). This mechanism appears to account for the ability of cadmium to disrupt cadherin-dependent cell-cell junctions in vitro, when cells are exposed to micromolar concentrations of free cadmium. However, it is less clear whether this mechanism can explain the actions of cadmium on N-cadherin in the proximal tubule, where the epithelial cells would be exposed to unknown concentrations of cadmium in the form of cadmium-protein or cadmium-thiol conjugates (Bridges and Zalups, 2005). Results of our own in vivo mechanistic studies to date have shown that at the time the initial cadmium-induced changes in N-cadherin localization and Kim-1 expression are occurring, there is no evidence of necrosis, and only minimal evidence of oxidative stress or apoptosis in the proximal tubule (Prozialeck et al., 2003, 2009a,b; Prozialeck and Edwards, 2010). Again, these findings strongly suggest that cadmium may exert relatively specific effects on one of the many signaling pathways that regulate cadherin-mediated cell-cell adhesion in the proximal tubule. Indeed, there is a large volume of literature showing that cadmium can affect a variety of cellular signaling pathways in epithelial cells (for review, see Thévenod, 2009). Some of the specific pathways that have been shown to be affected by cadmium include protein kinase C, cAMP, nitric oxide, mitogen-activated protein kinases (extracellular signal-regulated kinase 1/2, p38, c-Jun NH2-terminal kinase, and others), nuclear factor-κB, p53, and wnt/β-catenin. However, with the exception of the recent studies by Chakraborty et al. (2010), essentially all of these studies have involved the use of in vitro models and exposure of the cells to micromolar concentrations of cadmium. Again, the relevance of these reported effects to the actions of cadmium in the intact kidney are not clear. There is currently very little information on the possible mechanisms by which cadmium alters N-cadherin function in the intact kidney. Further studies are needed to resolve this issue.
Cadmium and Oxidative Stress
One final aspect of cadmium nephrotoxicity that merits special discussion is the role of oxidative stress in pathophysiological processes. cadmium is not a Fenton metal, and, as a stable divalent cation, it does not undergo redox cycling. However, cadmium is clearly able to induce oxidative stress, and this mechanism has long been thought to play a role in cadmium-induced kidney injury (for reviews, see Liu et al., 2009; Gobe and Crane, 2010). Rather than directly causing oxidative stress, cadmium appears to act indirectly by binding to intracellular thiols such as glutathione and/or interfering with the actions of various enzymes that protect against oxidative stress. Through these indirect mechanisms, cadmium can greatly amplify the actions of normal oxidative processes within the cell, which results in oxidative stress. Whereas oxidative stress has long been thought of as a relatively nonspecific mechanism of cellular injury, it is now recognized that oxidative stress, particularly at low to moderate levels, may actually trigger the activation of specific oxidative signaling pathways (Liu et al., 2009; Thévenod, 2009). It is noteworthy that many of these so called oxidative signaling pathways have also been shown to be modulated by cadmium exposure (Thévenod, 2009). However, at the present time, the cause-effect relationship between the signaling effects of cadmium and the development of oxidative stress are not clear. Our own studies (Prozialeck et al., 2003; see also data in Table 1) indicate that at the time the initial changes in N-cadherin localization, Kim-1 expression, and even apoptosis begin to occur in rat kidney, there is little evidence of oxidative stress. However, some of those studies involved the analysis of total nonprotein thiols and thiobarbituric acid-reactive substances and the expression of stress response genes, which are all relatively crude and late markers of oxidative stress. Additional in vivo studies using more sensitive and specific indicators of oxidative stress are needed to resolve this issue.
Emerging Model of Cadmium-Induced Proximal Tubule Injury
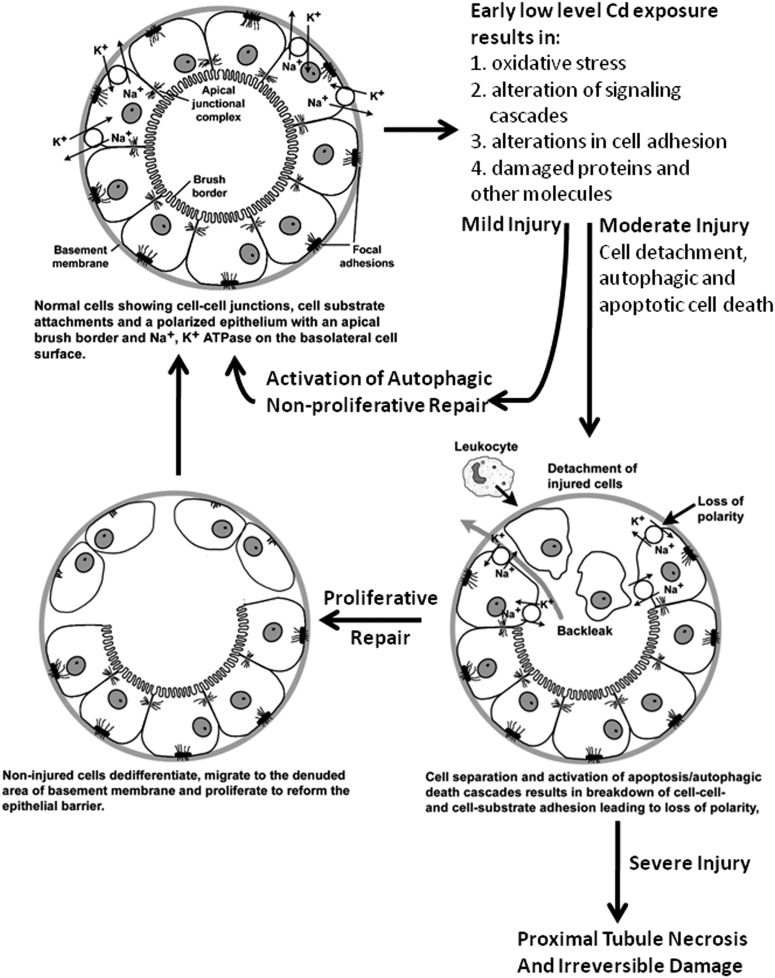
From these observations, we have developed a model of cadmium-induced proximal tubular injury that resembles a model of ischemic kidney injury that was first proposed more than 20 years ago (Molitoris and Marrs, 1999). This emerging cadmium model is summarized in Fig. 2. Under normal circumstances, the tubular epithelial cells are attached to each other and the basement membrane/extracellular matrix through specialized junctional complexes. Over time, the proximal tubule begins to accumulate cadmium that eventually begins to affect epithelial cell function. These early effects appear to involve mild oxidative stress, disruption of cellular signaling cascades, and alterations in cell adhesion. These effects, in turn, trigger autophagic responses in the cells. If the level of injury is mild, the autophagic response may be sufficient to repair damage. However, if the injury is more severe, apoptosis and/or autophagic cell death can occur. This in turn triggers epithelial to mesenchymal transformation of surviving cells and a proliferative repair response. If the injury to cells is widespread and severe, repair processes are inadequate, resulting in necrosis of the proximal tubule cells.
Fig. 2.
Schematic diagram summarizing the toxic effects of cadmium in the proximal tubule.
Cadmium and Glomerular Injury
Whereas the proximal tubule is the primary target of cadmium-induced kidney injury, there is evidence that cadmium, particularly at higher levels of exposure, can also affect the glomeruli (Xiao et al., 2009). Changes in classic markers of glomerular dysfunction such as serum or urinary creatinine are generally not seen during the early or mild stages of cadmium-induced kidney injury (Prozialeck and Edwards, 2007; Prozialeck et al., 2009a). However, several investigators have reported associations between cadmium exposure and alterations (either increased or decreased) creatinine clearance (Mueller et al., 1998; Bernard, 2004; Trzcinka-Ochocka et al., 2004; Navas-Acien et al., 2009). Some studies have also shown increased urinary excretion of albumin during the early stages of cadmium toxicity (Mueller, 1993; Mueller et al., 1998; Haswell-Elkins et al., 2008), which is classically interpreted as a marker of glomerular damage. Navas-Acien et al. (2009) have reported significant alterations in glomerular filtration along with albuminuria in subjects exposed to cadmium and lead. At present, the relative contributions and relationship of glomerular injury and proximal tubule injury to these reported increases in urinary albumin excretion remain unclear.
Implications for Biomonitoring and Therapeutic Interventions
The finding that the early toxic effects of cadmium in the proximal tubule may involve relatively specific changes in cell-cell adhesion and cellular signaling cascades has very important implications for biomonitoring cadmium-exposed populations and for the potential treatment of cadmium nephrotoxicity. The monitoring of at risk populations for early signs of cadmium nephrotoxicity has posed special challenges (for reviews, see Bernard, 2004; Prozialeck and Edwards, 2010). As a result of the tendency of cadmium to accumulate in tissues such as the liver and kidney, monitoring of blood and urinary cadmium often provides an incomplete reflection of the level of exposure and kidney disease. Because of these problems, investigators have used various biomarkers to characterize the severity of cadmium-induced kidney disease. Some of the urinary biomarkers that have been used for this purpose include the cadmium-binding protein metallothionein, low-molecular-weight proteins such as β2-microglobulin, proximal tubule-derived enzymes such as N-acetyl-β-glucosaminidase and even cadmium itself (Bernard, 2004). Although these markers have been used to monitor cadmium toxicity in humans and experimental animals, several problems remain. Most significantly, these current markers only identify relatively late stages of cadmium-induced kidney injury. By the time these markers appear in the urine, the injury to the kidney is generally considered to be irreversible and untreatable. Thus, there is a need for better early biomarkers of cadmium-induced kidney injury.
One novel marker that has shown exceptional promise in preclinical studies is Kim-1. Kim-1 is a transmembrane protein that is not detectable in normal kidney but is expressed at high levels in the proximal tubule after ischemic or toxic injury (Vaidya et al., 2008). Kim-1 acts as a regulator of cell adhesion and endocytosis in regenerating cells of the injured tubule as they reform a functional epithelial barrier. This process is associated with the proteolytic cleavage of the ectodomain of Kim-1 into the urine. The ectodomain is stable in urine and has been shown to be a sensitive marker of renal injury induced by a variety of agents (Vaidya et al., 2008).
In studies using a rat model of cadmium-induced kidney injury, Kim-1 outperformed traditional urinary markers (Prozialeck et al., 2007, 2009a,b). Kim-1 was detected in the urine 4 to 5 weeks before onset of proteinuria and 2 to 5 weeks before the appearance of other markers such a metallothionein and CC16. Other studies showed that the cadmium-induced increase in Kim-1 expression occurred at a time when there was little or no evidence of either necrosis or apoptosis of proximal tubule epithelial cells (Prozialeck et al., 2009a). The fact that Kim-1 can be detected at a time before lethal injury to proximal tubule epithelial cells has occurred may be especially significant. Perhaps, with earlier detection via Kim-1, it may be possible to reverse, or at least more effectively treat, cadmium-induced kidney injury. In light of this possibility, studies on the utility of Kim-1 as a marker of cadmium toxicity in humans are certainly warranted.
It should be emphasized that even though Kim-1 shows considerable promise as an early biomarker of cadmium toxicity, there are still several important questions that need to be resolved. For example, How does Kim-1 expression change with higher levels of cadmium exposure and how does it change when cadmium exposure is stopped? Likewise, from a mechanistic perspective, it is unclear how the disruption of cadherin-mediated adhesion might be related to the activation of Kim-1 expression. Ichimura et al. (2008) have shown that Kim-1-expressing cells “phagocytize” debris from necrotic and apoptotic cells through the binding of Kim-1 to phosphatidylserine residues on damaged cells. However, it is not clear how these findings relate to the action of cadmium in the proximal tubule. At the time Kim-1 is expressed in the proximal tubule, there is no evidence of necrosis and only modest evidence of apoptosis (Prozialeck et al., 2009a). At this same time, there are widespread alterations in the localization of N-cadherin (Prozialeck et al., 2003). Perhaps, the loss of N-cadherin-mediated cell-cell adhesion is a key event in triggering the expression of Kim-1. Moreover, it is not clear how this disruption of cell-cell adhesion and the expression of Kim-1 might be related to the onset of oxidative stress and/or the disruption of cellular signaling cascades. Additional studies are needed to resolve these issues.
An important caveat that needs to be considered is that even with the use of very sensitive biomarkers such as Kim-1, cadmium exposure is not evident until some sort of toxic injury has occurred. One of the more intriguing aspects of the studies summarized here is that some of the findings suggest that it may be possible to detect evidence of cadmium exposure before toxic cellular injury occurs. For example, cadmium acts by affecting cellular signaling cascades, it may be possible to detect such alterations in function before the cytopathological cascade of injury starts. One possible avenue of research might be to monitor the effects of cadmium on changes in the phosphorylation status of proteins or the identification of phospho-protein residues in urine. In addition, recent studies suggest that post-translational modifications of protein expression may also play a role in cadmium-induced injury. For example, our own recent studies have revealed that cadmium causes changes in the patterns of microRNA expression in renal epithelial NRK-52E cells (De La Fuente et al., 2011) and in rat kidney (W. C. Prozialeck and M. J. Fay, unpublished observation). Determining how such cadmium-induced alterations in microRNA expression might influence protein expression and kidney function would also seem to be an interesting area for future research.
With respect to treatment, there are currently no proven effective treatments for cadmium-induced kidney disease. Traditional chelating agents that are effective in treating poisoning with other metals, either do not mobilize cadmium from intracellular stores or they have the paradoxical effects of facilitating the delivery of cadmium to the kidney and actually increasing the level of kidney injury. As noted previously, the studies summarized here strongly suggest that cadmium appears to specifically affect cadherin-mediated cell adhesion and cellular signaling pathways well before the onset of apoptosis or necrosis in the proximal tubule. These findings along with early detection with novel biomarkers such as Kim-1 suggest that it may be possible to use pharmacological agents to modulate or even halt these pathophysiological processes before they become irreversible. It is our hope that this review will serve as a framework for future studies in this area.
Acknowledgments
We sincerely thank Drs. Jie Liu and Michael Waalkes of the National Cancer Institute National Institute for Environmental Health Services for performing the real-time RT-PCR analyses; Vicki Sears and Laura Phelps of Midwestern University for help in preparing the manuscript and the figures; and Peter Lamar of Midwestern University for his excellent technical assistance.
This work was supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grant R01-ES006478].
Parts of this work were presented previously at the following conference: Edwards J, Lamar PC, Diamantakos E, Peuler JD, Liu J, Waalkes JP, and Prozialeck WC (2006) Cadmium-induced disruption of proximal tubule cell adhesion is associated with redistribution of cell adhesion molecules and loss of epithelial polarity, 45th Annual Meeting of the Society of Toxicology and ToxExpo; 2006 Mar 6–9; San Diego, CA. Society of Toxicology, Reston, VA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- Kim-1
- kidney injury molecule-1
- RT-PCR
- reverse transcription-polymerase chain reaction
- VE-cadherin
- vascular endothelial cadherin.
Authorship Contributions
Participated in research design: Prozialeck and Edwards.
Performed data analysis: Prozialeck and Edwards.
Wrote or contributed to the writing of the manuscript: Prozialeck and Edwards.
Other: Prozialeck.
References
- Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Strömberg U, Skerfving S. (2005) Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect 113:1627–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T, Hayakawa K, Miyaji K, Ishikawa H, Hata M. (2003) Cadmium nephrotoxicity and evacuation from the body in a rat modeled subchronic intoxication. Int J Urol 10:332–338 [DOI] [PubMed] [Google Scholar]
- Bernard A. (2004) Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals 17:519–523 [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. (2005) Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204:274–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. (2009) Cadmium—a metallohormone? Toxicol Appl Pharmacol 238:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty PK, Scharner B, Jurasovic J, Messner B, Bernhard D, Thévenod F. (2010) Chronic cadmium exposure induces transcriptional activation of the Wnt pathway and upregulation of epithelial-to-mesenchymal transition markers in mouse kidney. Toxicol Lett 198:69–76 [DOI] [PubMed] [Google Scholar]
- Chargui A, Zekri S, Jacquillet G, Rubera I, Ilie M, Belaid A, Duranton C, Tauc M, Hofman P, Poujeol P, et al. (2011) Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure. Toxicol Sci 121:31–42 [DOI] [PubMed] [Google Scholar]
- De La Fuente A, Alt AC, Fay MJ, Prozialeck WC. (2011) Identification of cadmium regulated miRNAs in rat renal proximal tubule epithelial NRK52E cells. In Vitro Cell Dev Biol Anim 47:S31–S32 [Google Scholar]
- Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. (2007) Cell death modalities: classification and pathophysiological implications. Cell Death Differ 14:1237–1243 [DOI] [PubMed] [Google Scholar]
- Gobe G, Crane D. (2010) Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett 198:49–55 [DOI] [PubMed] [Google Scholar]
- Goyer RA, Miller CR, Zhu SY, Victery W. (1989) Non-metallothionein-bound cadmium in the pathogenesis of cadmium nephrotoxicity in the rat. Toxicol Appl Pharmacol 101:232–244 [DOI] [PubMed] [Google Scholar]
- Hamada T, Nakano S, Iwai S, Tanimoto A, Ariyoshi K, Koide O. (1991) Pathological study on beagles after long-term oral administration of cadmium. Toxicol Pathol 19:138–147 [DOI] [PubMed] [Google Scholar]
- Haswell-Elkins M, Satarug S, O'Rourke P, Moore M, Ng J, McGrath V, Walmby M. (2008) Striking association between urinary cadmium level and albuminuria among Torres Strait Islander people with diabetes. Environ Res 106:379–383 [DOI] [PubMed] [Google Scholar]
- He L, Wang B, Hay EB, Nebert DW. (2009) Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol Appl Pharmacol 238:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. (2008) Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118:1657–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Järup L. (2002) Cadmium overload and toxicity. Nephrol Dial Transplant 17 (Suppl 2):35–39 [DOI] [PubMed] [Google Scholar]
- Järup L, Akesson A. (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208 [DOI] [PubMed] [Google Scholar]
- Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. (1998) Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health 24 (Suppl 1):1–51 [PubMed] [Google Scholar]
- Jin T, Lu J, Nordberg M. (1998) Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology 19:529–535 [PubMed] [Google Scholar]
- Joseph P. (2009) Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol 238:272–279 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J. (1997) Role of metallothionein in cadmium-induced hepatotoxicity and nephrotoxicity. Drug Metab Rev 29:79–102 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Diwan BA. (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. (1998) Susceptibility of MT-null mice to chronic CdCl2-induced nephrotoxicity indicates that renal injury is not mediated by the CdMT complex. Toxicol Sci 46:197–203 [DOI] [PubMed] [Google Scholar]
- Liu J, Qu W, Kadiiska MB. (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. (2009) Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect 117:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Marrs J. (1999) The role of cell adhesion molecules in ischemic acute renal failure. Am J Med 106:583–592 [DOI] [PubMed] [Google Scholar]
- Mueller PW. (1993) Detecting the renal effects of cadmium toxicity. Clin Chem 39:743–745 [PubMed] [Google Scholar]
- Mueller PW, Price RG, Finn WF. (1998) New approaches for detecting thresholds of human nephrotoxicity using cadmium as an example. Environ Health Perspect 106:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. (2009) Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 170:1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Van Hecke E, Thijs L, Richart T, Kuznetsova T, Jin Y, Vangronsveld J, Roels HA, Staessen JA. (2008) Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ Health Perspect 116:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg GF. (2004) Cadmium and health in the 21st century—historical remarks and trends for the future. Biometals 17:485–489 [DOI] [PubMed] [Google Scholar]
- Parrish AR, Prozialeck WC. (2010) Metals and cell adhesion molecules, in Cellular and Molecular Biology of Metals (Koropatkick J., Zalups R.K. eds) pp 327–350, Taylor and Francis, Oxford, UK [Google Scholar]
- Prozialeck WC. (2000) Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol 164:231–249 [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR. (2007) Cell adhesion molecules in chemically-induced renal injury. Pharmacol Ther 114:74–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR. (2010) Early biomarkers of cadmium exposure and nephrotoxicity. Biometals 23:793–809 [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. (2009a) Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 238:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. (2009b) Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol Appl Pharmacol 238:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Grunwald GB, Dey PM, Reuhl KR, Parrish AR. (2002) Cadherins and NCAM as potential targets in metal toxicity. Toxicol Appl Pharmacol 182:255–265 [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Appelt DM. (2004) Differential expression of E-cadherin, N-cadherin and β-catenin in proximal and distal segments of the rat nephron. BMC Physiol 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Lynch SM. (2003) Cadmium alters the localization of N-cadherin, E-cadherin, and β-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol 189:180–195 [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Niewenhuis RJ. (1991) Cadmium (Cd2+) disrupts Ca2+-dependent cell-cell junctions and alters the pattern of E-cadherin immunofluorescence in LLC-PK1 cells. Biochem Biophys Res Commun 181:1118–1124 [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. (2007) Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int 72:985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H, Bernard A, Buchet JP, Goret A, Lauwerys R, Chettle DR, Harvey TC, Haddad IA. (1979) Critical concentration of cadmium in renal cortex and urine. Lancet 1:221. [DOI] [PubMed] [Google Scholar]
- Sabolic I, Herak-Kramberger CM, Antolovic R, Breton S, Brown D. (2006) Loss of basolateral invaginations in proximal tubules of cadmium-intoxicated rats is independent of microtubules and clathrin. Toxicology 218:149–163 [DOI] [PubMed] [Google Scholar]
- Sabolić I, Herak-Kramberger CM, Brown D. (2001) Subchronic cadmium treatment affects the abundance and arrangement of cytoskeletal proteins in rat renal proximal tubule cells. Toxicology 165:205–216 [DOI] [PubMed] [Google Scholar]
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR. (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83 [DOI] [PubMed] [Google Scholar]
- Satarug S, Garrett SH, Sens MA, Sens DA. (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Moore MR. (2004) Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 112:1099–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squibb KS, Fowler BA. (1984) Intracellular metabolism and effects of circulating cadmium-metallothionein in the kidney. Environ Health Perspect 54:31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwazono Y, Uetani M, Akesson A, Vahter M. (2010) Recent applications of benchmark dose method for estimation of reference cadmium exposure for renal effects in man. Toxicol Lett 198:40–43 [DOI] [PubMed] [Google Scholar]
- Tanimoto A, Hamada T, Higashi K, Sasaguri Y. (1999) Distribution of cadmium and metallothionein in CdCl2-exposed rat kidney: relationship with apoptosis and regeneration. Pathol Int 49:125–132 [DOI] [PubMed] [Google Scholar]
- Tanimoto A, Hamada T, Koide O. (1993) Cell death and regeneration of renal proximal tubular cells in rats with subchronic cadmium intoxication. Toxicol Pathol 21:341–352 [DOI] [PubMed] [Google Scholar]
- Teeyakasem W, Nishijo M, Honda R, Satarug S, Swaddiwudhipong W, Ruangyuttikarn W. (2007) Monitoring of cadmium toxicity in a Thai population with high-level environmental exposure. Toxicol Lett 169:185–195 [DOI] [PubMed] [Google Scholar]
- Thévenod F. (2009) Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol 238:221–239 [DOI] [PubMed] [Google Scholar]
- Thévenod F, Chakraborty PK. (2010) The role of Wnt/β-catenin signaling in renal carcinogenesis: lessons from cadmium toxicity studies. Curr Mol Med 10:387–404 [DOI] [PubMed] [Google Scholar]
- Thévenod F, Wolff NA, Bork U, Lee WK, Abouhamed M. (2007) Cadmium induces nuclear translocation of β-catenin and increases expression of c-myc and Abcb1a in kidney proximal tubule cells. Biometals 20:807–820 [DOI] [PubMed] [Google Scholar]
- Thomas LD, Hodgson S, Nieuwenhuijsen M, Jarup L. (2009) Early kidney damage in a population exposed to cadmium and other heavy metals. Environ Health Perspect 117:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzcinka-Ochocka M, Jakubowski M, Razniewska G, Halatek T, Gazewski A. (2004) The effects of environmental cadmium exposure on kidney function: the possible influence of age. Environ Res 95:143–150 [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. (2008) Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48:463–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP. (2003) Cadmium carcinogenesis. Mutat Res 533:107–120 [DOI] [PubMed] [Google Scholar]
- Xiao W, Liu Y, Templeton DM. (2009) Pleiotropic effects of cadmium in mesangial cells. Toxicol Appl Pharmacol 238:315–326 [DOI] [PubMed] [Google Scholar]
- Yan H, Carter CE, Xu C, Singh PK, Jones MM, Johnson JE, Dietrich MS. (1997) Cadmium-induced apoptosis in the urogenital organs of the male rat and its suppression by chelation. J Toxicol Environ Health 52:149–168 [DOI] [PubMed] [Google Scholar]