Abstract
Adult stem cells have shown great promise toward repairing infarcted heart and restoring cardiac function. Mesenchymal stem cells (MSCs), because of their inherent multipotent nature and their ability to secrete a multitude of growth factors and cytokines, have been used for cardiac repair with encouraging results. Preclinical studies showed that MSCs injected into infarcted hearts improve cardiac function and attenuate fibrosis. Although stem cell transplantation is a promising therapeutic option to repair the infarcted heart, it is faced with a number of challenges, including the survival of the transplanted cells in the ischemic region, due to excessive oxidative stress present in the ischemic region. The objective of this study was to determine the effect of Carvedilol (Carv), a nonselective β-blocker with antioxidant properties, on the survival and engraftment of MSCs in the infarcted heart. MSCs were subjected to a simulated host-tissue environment, similar to the one present in the infarcted myocardium, by culturing them in the presence of hydrogen peroxide (H2O2) to induce oxidative stress. MSCs were treated with 2.5 μM Carv for 1 h in serum-free medium, followed by treatment with H2O2 for 2 h. The treated cells exhibited significant protection against H2O2-induced cell death versus untreated controls as determined by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assays. Likewise, transplantation of MSCs after permanent left coronary artery ligation and treatment of animals after myocardial infarction (MI) with Carv (5 mg/kg b.wt.) led to significant improvement in cardiac function, decreased fibrosis, and caspase-3 expression compared with the MI or MSC-alone groups.
Introduction
Cardiovascular disease morbidity and mortality rates have shown a dramatic decrease over the past 50 years because of advances in research and clinical care (Cutler et al., 2006; Yeh et al., 2010b; Lauer, 2012). Nevertheless, heart disease remains the leading cause of death worldwide (Mathers and Loncar, 2006). Cellular cardiomyoplasty has emerged as one of the most promising therapeutic approaches for treating ischemic heart disease since the last decade. Cellular transplantation has rapidly evolved from the benchside to clinical trials (Menasche, 2011). In particular, mesenchymal stem cells (MSCs) have shown promising results in improving cardiac function and attenuation of fibrosis (Tomita et al., 1999; Fazel et al., 2005). However, the survival of the transplanted stem cells is limited not only because of the hypoxic environment of the myocardium, but also because of the abundance of oxidative stress and reactive oxygen species (ROS) in the infarcted heart (Zhang et al., 2001; Wei et al., 2010). Oxidative stress has been implicated in the prognosis after an acute myocardial infarction (MI), particularly during the initiation of reperfusion, because of its direct cytotoxicity (Ferrari et al., 1985; Hill and Singal, 1996), negative inotropism (Hill and Singal, 1996; Flesch et al., 1999), and proapoptotic effect (Krown et al., 1996; Ferrari et al., 2004) contributing to ventricular remodeling (Kinugawa et al., 2000).
β-Blockers are commonly prescribed for patients with acute MI (AMI), and they produce their effect by decreasing heart rate, myocardial contractility, myocardial oxygen consumption, and the overall workload of the heart, hence improving ischemic heart symptoms (Monteiro et al., 2003; Kopecky, 2006). Carvedilol (Carv) is a nonselective β-blocker with β1, β2, and α1 adrenergic receptor blocking properties (Packer, 1998), whereas Atenolol is a selective β1 receptor antagonist. Carv exerts a potential beneficial effect compared with other β-blockers in ventricular remodeling, because of the α-adrenergic blockage properties and its unique antioxidant properties for scavenging superoxide anions and hydroxyl radicals (Yue et al., 1992; Feuerstein et al., 1998). This dual property of Carv makes it substantially unique from drugs with only antioxidant properties. Furthermore, Carv attenuates cardiomyocyte apoptosis and the expression of proapoptotic proteins after ischemia reperfusion injury (Zeng et al., 2003). The antiapoptotic cardioprotective role of Carv is independent of its antiadrenergic properties (Schwarz et al., 2003). Studies have shown that the antioxidant properties of Carv provide it a unique ability to maintain the viability and inhibit apoptosis in the ischemic myocardium (Cargnoni et al., 2000; Carreira et al., 2006). In addition, experimental studies have shown that Carv treatment alleviates left ventricular remodeling, fibrosis, and cardiac dysfunction in rats (Barone et al., 2007; Yoshikawa et al., 2010).
On the basis of the widespread use of Carv in hypertensive, post-MI, and heart failure patients, the focus of this study was to see the combined effect of Carv in rats receiving MSC transplantation. Because the morbidity and mortality rates after AMI have increased; it is clear that combination therapy using complementary approaches would act synergistically to decrease further myocardial damage after MI. In the present study, we have investigated the in vitro protective effect of Carv against H2O2-induced oxidative stress in MSCs. We have also evaluated the in vivo effect of treating of MSCs with Carv before transplantation and the beneficial effect of further continuing treatment of animals with Carv after MI. The in vitro results demonstrated that Carv pretreatment attenuates apoptosis of MSCs, when subjected to H2O2-induced oxidative stress. Likewise, in vivo data showed improved recovery of cardiac function, decreased terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive cells, and caspase-3 expression in the hearts treated with combined Carv and MSC.
Materials and Methods
Chemicals.
Carv (Sigma-Aldrich, St. Louis, MO) was freshly prepared in dimethyl sulfoxide and diluted with phosphate-buffered saline (PBS) before administration. Xanthine and xanthine oxidase were obtained from Sigma-Aldrich. 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEPMPO) was obtained from Radical Vision (Jerome, Marseille, France). All other reagents were analytical grade or higher purchased from Sigma-Aldrich, unless otherwise mentioned.
Measurement of Superoxide Radicals by EPR Spectroscopy.
The superoxide radical-scavenging ability of Carv in vitro was determined by EPR spectroscopy (Khan et al., 2007a). A mixture of xanthine (0.2 mM) and xanthine oxidase (0.02 U/ml) in PBS, pH 7.4, was used to generate superoxide radicals. EPR measurements were performed in PBS, pH 7.4, containing DEPMPO (1 mM) and in the presence or absence of different concentrations of Carv. The superoxide radicals were detected as DEPMPO-OOH adducts. The attenuation of DEPMPO adduct generation was quantified by double integration and expressed as a percentage of untreated (without Carv) levels.
Cell Survival and Proliferation Assay.
MSCs (20,000 per well) were cultured in a 96-well plate. After 24 h, the cells were treated with Carv (2.5 μM) or atenolol (2.5 μM) for 1 h before the addition of 800 μM H2O2 in a serum-free medium for 2 h. Controls were used in parallel for each treatment group. A total of 0.5 mg/ml 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) in complete medium was then applied to the cells for 2 h. The yellow tetrazolium MTT was reduced by metabolically active cells to a purple formazan that was solubilized and quantified by spectrophotometry at 570 nm and referenced higher than 650 nm. Likewise, an anti-5-bromo-2′-deoxyuridine (BrdU) proliferation assay was performed. MSCs were incubated with 10 μM BrdU for 2 h. The pyrimidine analog BrdU gets incorporated in place of thymidine into the DNA of an active proliferating cell. The amount of BrdU used by the cells was assayed by an ELISA technique using anti-BrdU antibody and its substrate.
Preparation of Carv Suspension for Oral Administration.
On the basis of the studies in the literature (Abdulla et al., 2011; Kumar et al., 2011), we have selected 5 mg/kg b.wt. as the optimal dose to be administered daily to the rats after MI for 4 weeks. Coreg tablets were crushed to fine powder using a mortar and pestle and mixed with 20 ml of Ora-plus suspending agent to prepare a final concentration of 7.25 mg/ml Carv suspension. The Carv suspension was prepared freshly every day before administration.
Induction of Myocardial Infarction In Vivo.
Fisher-344 rats (200–250 g) were used as an in vivo acute model for MI [permanent left anterior descending coronary artery (LAD) ligation]. Rats were randomly divided into five groups of six animals each: 1) sham (untreated); 2) MI (LAD ligation only); 3) Carv [MI+Carv (5 mg/kg b.wt.)]; 4) MSC (MI+MSC-treated); and 5) MSC+Carv (MI+MSC+Carv treated). Rats were anesthetized with ketamine (50 mg/kg i.p.) and xylazine (5 mg/kg i.p.) and maintained under anesthesia using isoflurane (1.5–2.0%) mixed with air. MI was created by permanently ligating the LAD, as described previously (Khan et al., 2009). After permanent ligation of LAD, successful infarction was confirmed by an ST elevation on electrocardiograms that was recognized in all surgical groups of animals. MSCs or MSCs treated with Carv (2.5 μM) were transplanted in the ischemic heart at 30 min after permanent LAD ligation, and the chest was closed. Shams (untreated animals) were anesthetized as described previously, and the chest cavity was opened and closed without LAD ligation. After reinitiation of spontaneous respiration, animals were extubated and allowed to recover from the anesthesia. The following day after surgery, rats were administered orally with Carv suspension (5 mg/kg b.wt.) daily for 4 weeks using a clean oral gavage. All the procedures were performed with the approval of the Institutional Animal Care and Use Committee of The Ohio State University and conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 86-23; Institute of Laboratory Animal Resources, 1996).
Echocardiography (M-Mode) for Cardiac Functional Analysis.
Assessment of cardiac function was performed at the baseline and at 4 weeks after MI or stem cell transplantation. Rats were anesthetized using 1.5-2% isoflurane, and M-mode ultrasound images were acquired using a Vevo 2100 (VisualSonics, Toronto, ON, Canada) ultrasonic rodent imaging system as described previously (Khan et al., 2012).
Measurement of Fibrosis.
Rats were anesthetized after 4 weeks of MI/stem cell transplantation, and their hearts were excised and washed with ice-cold PBS. The hearts were then frozen for 10 min at −20°C and sliced into 2-mm sections using a heart matrix. The sections were then incubated in formalin overnight for Masson's trichrome staining for collagen/fibrosis. To determine the fibrosis, images were acquired by a dissecting microscope (Nikon, Tokyo, Japan). The fibrosis area was quantified by computerized planimetry using MetaMorph image analysis software (Molecular Devices, Sunnyvale, CA) as described previously (Khan et al., 2009).
Assessment of Apoptosis by TUNEL Assay.
To assess cell death or apoptosis, cultured MSCs/cryopreserved heart sections (5-μm thickness) were fixed in freshly prepared 4% paraformaldehyde for 20 min at room temperature. The cells/slides were incubated for 30 min in PBS and washed twice with fresh PBS. The samples were then incubated with permeabilization solution (0.1% Triton X-100 and 0.1% sodium citrate, freshly prepared) for 2 min on ice. DNA strand breaks were detected using the TUNEL assay kit procured from Roche Diagnostics (Indianapolis, IN). In brief, sections were covered with 50 μl of TUNEL reaction mixture and incubated in this solution for 60 min at 37°C in a humidified chamber. After rinsing with PBS, the sections were mounted with Vectashield HardSet Mounting Medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and visualized under a fluorescence microscope. The apoptotic index (the percentage of TUNEL-positive cardiomyocytes relative to total nuclei) was then calculated from at least three slides, five fields each in the peri-infarct area using MetaMorph image analysis software (Molecular Devices) as published previously (Khan et al., 2010).
Immunostaining for α-Smooth Muscle Actin and von Willebrand Factor.
Immunostaining was performed in formalin-fixed paraffin-embedded heart sections (5-μm). The fixed tissue sections were serially rehydrated as described previously (Khan et al., 2012). The tissue sections were then incubated with 2% goat serum and 5% bovine serum albumin in PBS to reduce nonspecific binding. The sections were then incubated for 2 h with mouse anti-α-smooth muscle actin (α-SMA) and anti-von Willebrand factor (vWF) monoclonal antibodies (Cell Signaling Technology, Danvers, MA). The sections were then incubated with the appropriate antimouse secondary antibodies (1:200) conjugated to Texas Red (α-SMA) and FITC (vWF). The nuclei were counterstained with the hardest DAPI (Vector Laboratories). The tissue sections were visualized by inverted Nikon fluorescence microscope (TE 2000). Blood vessels and capillaries stained positive for α-SMA and vWF in peri-infarct regions of the heart were acquired. Separate sections were also stained without primary antibodies to indentify nonspecific binding.
Western Blot Analysis for Molecular Signaling.
Western blots for phosphorylated Akt, phosphorylated extracellular signal-regulated kinase (ERK)1/2, Bcl-2, and caspase-3 signaling were performed with the tissue homogenates as described previously (Khan et al., 2012). The anterior walls of the left ventricles were collected from sham, MI, Carv, MSC, and MSC+Carv groups. After the treatment periods in all the groups, rats were anesthetized using pentobarbital sodium, and the hearts were harvested and immediately rinsed in ice-cold PBS, pH 7.4. The protein was separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and probed with primary antibodies for phosphorylated ERK1/2, phosphorylated Akt (ser-473), Bcl-2, and caspase-3 (Cell Signaling Technology). The membranes were incubated overnight at 4°C with the primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) for 1 h. The membranes were then washed and developed using an enhanced chemiluminescence detection system (ECL Advanced kit; GE Healthcare). The same membranes were then reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The protein intensities were quantified by an image-scanning densitometer (Un-Scan It, Orem, UT). To quantify the phospho-specific signal in activated proteins, we first subtracted the background and then normalized the signal to the amount of GAPDH or total target protein in the tissue homogenate (Khan et al., 2012).
Data Analysis.
The statistical significance of the results was evaluated by one-way analysis of variance, and all pairwise multiple comparison procedures were done by Tukey's post hoc test. The values were expressed as mean ± S.D. A p value of <0.05 was considered significant.
Results
Carv Attenuates Superoxide Generation In Vitro.
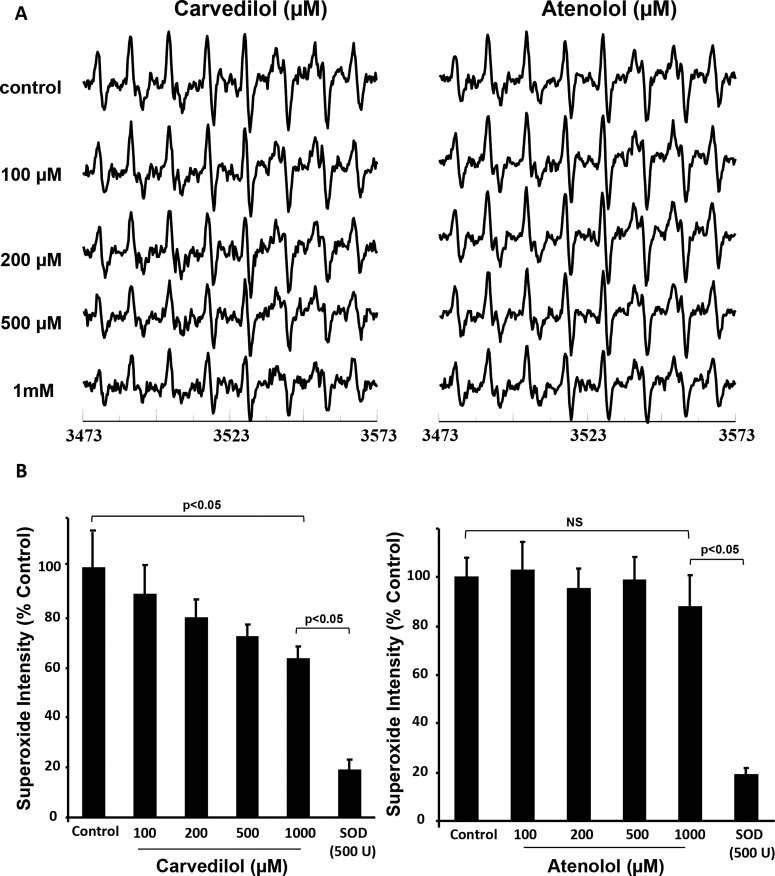
To evaluate the superoxide scavenging property of Carv in vitro, we performed EPR spectroscopy. Detection of superoxide was performed by DEPMPO spin trap (1 mM) as DEPMPO-OO adduct. Carv significantly (p < 0.05) reduced superoxide radical generation in vitro. Figure 1A shows representative spectra obtained by EPR spectroscopy. Figure 1B shows the scavenging effect of Carv against superoxide radicals. Carv (up to 1 mM), challenged against DEPMPO (1 mM), showed a decrease in the intensity of the DEPMPO-OOH spectrum by more than 35% (Fig. 1, A and B). On the other hand, Atenolol (1 mM), which is also a β-blocker, did not have any scavenging effect on superoxide radicals compared with Carv (1 mM). The in vitro data clearly established that Carv is capable of scavenging superoxide radicals in a dose-dependent manner compared with Atenolol.
Fig. 1.
Carv attenuates superoxide radicals. The superoxide-scavenging ability of Carv was determined by EPR spectroscopy using DEPMPO spin trapping. A, representative EPR spectra of DEPMPO spin trap (1 mM) were used to detect superoxide radicals as a DEPMPO-OOH adduct. B, a concentration of 1 mM Carv, challenged against 1 mM DEPMPO, decreased the intensity of the DEPMPO-OOH spectrum by more than 35%, which was not seen with Atenolol. Overall, the EPR data clearly established that the Carv is capable of scavenging superoxide in a dose-dependent manner. NS, not significant. Values are expressed as mean ± S.D.; n = 3.
Carv Pretreatment Improves Cell Survival and Decreases Apoptosis of MSCs under Ischemic Stress.
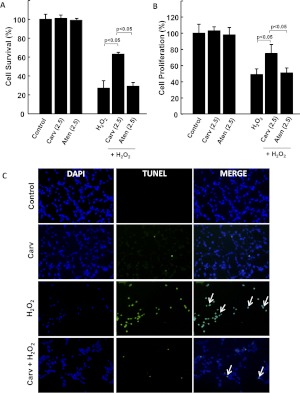
Treatment of MSCs with H2O2 led to significant reduction in cell survival and proliferation. On the other hand, pretreatment of MSCs with Carv showed a significant (p < 0.05) increase in cell survival and proliferation compared with the H2O2-treated group (Fig. 2, A and B). Correspondingly, apoptosis of MSCs assessed by TUNEL assay showed decreased cell death in the Carv+H2O2 group compared with the group treated with H2O2 alone (Fig. 2C).
Fig. 2.
Effect of Carv on the survival and proliferation of MSC. MSCs (2 ′ 104 per well) were cultured in a 96-well plate. After 24 h, the cells were pretreated with Carv (2.5 μM) or Atenolol (2.5 μM) for 2 h before the addition of 800 μM H2O2 in a serum-free medium for 2 h. Controls were used in parallel for each treatment group. Carv-treated MSCs showed increased protection against H2O2-induced cell death than the Atenolol-treated group. A and B, MTT (A) and BrdU (B) assays were used to assess cell viability and proliferation, respectively. C, The effect of Carv treatment on apoptosis was assessed by TUNEL assay. Treatment of MSCs with 800 μM H2O2 increased the number of TUNEL-positive cells. However, preconditioning with Carv protected against H2O2-induced cell death and oxidative stress. The arrows (white) indicate TUNEL-positive cells. Values are expressed as mean ± S.D.; n = 3.
Carv Adjuvant Treatment with MSC Improves Cardiac Function.
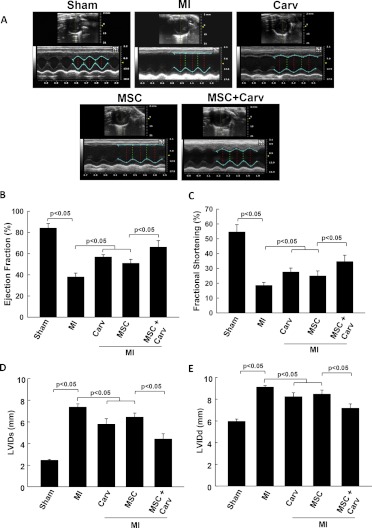
Cardiac function was measured at baseline and 4 weeks after MI by M-mode ultrasound echocardiography. Figure 3A shows representative images of M-mode echocardiography. The recovery of cardiac function was significantly (p < 0.05) improved in all treatment groups compared with the MI group. However, the functional recovery (ejection fraction and fractional shortening) was further improved in rats treated with MSC+Carv compared with the MSC-alone group (p < 0.05; Fig. 3, B and C). In addition, the left ventricular inner diameters at end-systole and end-diastole were significantly (p < 0.05) restored in the MSC+Carv group compared with the MI, Carv, and MSC groups (Fig. 3, D and E).
Fig. 3.
Carv adjuvant treatment with stem cells improves cardiac function. A, representative images of ultrasound echocardiography (M-mode) performed on rats to assess the cardiac function. B–E, The functional parameters ejection fraction (B), fractional shortening (C), left ventricular internal dimensions at end-systole (D), and left ventricular internal dimensions at end-diastole (E) were analyzed at the baseline and at 4 weeks after MI or stem cell transplantation. The MSC+Carv-treated group showed enhanced functional recovery after MI compared with the MSC- or Carv-alone-treated groups. Values are expressed as mean ± S.D.; n = 6 animals per group.
Carv and/or Stem Cell Treatment Ameliorates Cardiac Fibrosis.
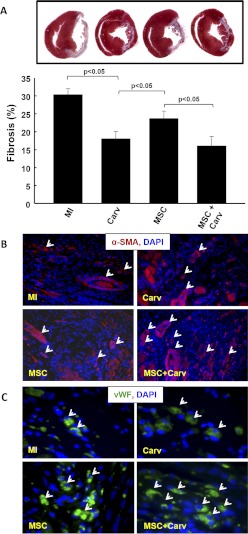
To further understand whether improved cardiac function correlates to cardiac fibrosis, Masson's trichrome staining was performed for the assessment of fibrosis 4 weeks after MI. Data showed a significant (p < 0.05) decrease in fibrosis in the Carv-, MSC-, and MSC+Carv-treated groups compared with the MI group (Fig. 4A). Moreover, the fibrosis was significantly lower in the Carv and MSC+Carv groups compared with the MSC-alone group. The fibrosis data supports our hypothesis that adjuvant treatment of MSCs with Carv attenuates cardiac fibrosis.
Fig. 4.
Effect of Carv and/or stem cell treatment on myocardial fibrosis and angiogenesis. A, Masson's trichrome staining for cardiac fibrosis showed a significant decrease in fibrosis in the MSC+Carv-treated groups compared with the MI or MSC group (p < 0.05). B, α-SMA, a marker for vasculogenesis (white arrows), was increased in the MSC+Carv group compared the MSC group. C, v-WF, a marker for neovascularization or capillary density, was also increased in the MSC+Carv group compared with the MSC group. Values are expressed as mean ± S.D.; n = 6 animals per group.
Carv and /or MSC Treatment Increases Angiogenesis.
To detect neovascularization in the infarcted heart, the cardiac tissue sections were stained with smooth muscle cell marker (α-SMA) and endothelial cell surface marker (vWF). The results demonstrated an increased number of α-SMA- and vWF-positive vessels/capillaries, respectively, in the peri-infarct regions of the Carv+MSC group compared with the MSC-alone group.
Carv and/or MSC Treatment Decreases Myocardial Apoptosis.
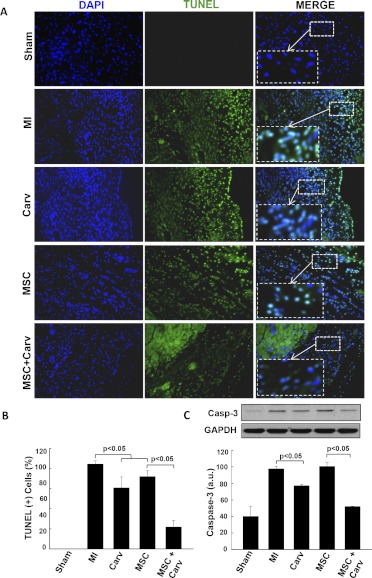
One of the key events after AMI, the apoptosis of cardiomyocytes is due to the activation of caspases that cleave DNA and other components. Apoptosis data in heart sections were evaluated at 4 weeks after MI by TUNEL assay. The data showed decreased TUNEL-positive cells in the Carv- and MSC+Carv-treated groups compared with the MSC-alone group (Fig. 5A), indicating that adjuvant treatment of MSCs along with Carv further decreases apoptosis of cardiomyocytes in the infarcted heart.
Fig. 5.
Carv treatment decreases myocardial apoptosis after MI. Evaluation of cellular apoptosis in heart tissue was performed by TUNEL assay. A, TUNEL (positive) cells (green) and total nuclei (DAPI/blue) were imaged in the peri-infarct regions of the heart tissues at 4 weeks after MI. B, Increased TUNEL-positive nuclei (green) were seen in the MI group, whereas Carv-treated hearts showed a significant decrease (p < 0.05) in TUNEL-positive nuclei compared with the MSC group. C, Western blot analysis of caspase-3 expression in heart tissues showed significant (p < 0.05) reduction in caspase-3 expression in the combined MSC+Carv-treated groups compared with the single treated groups (MI, Carv, and MSC groups). Values are expressed as mean ± S.D.; n = 4.
Carv Adjuvant Treatment with MSC Attenuates Caspase-3 Expression In Vivo.
Caspase-3 expression was found to be significantly (p < 0.05) decreased in rat hearts treated with Carv and MSC+Carv groups compared with the MI or MSC-alone group (Fig. 5C). The results indicate that Carv as an adjuvant treatment plays a crucial role in attenuating apoptosis of surviving myocytes in the infarcted area of the damaged heart. Because MSCs were pretreated with Carv before transplantation and, consecutively, animals were treated with Carv daily for 4 weeks, this combined treatment might be responsible for significant decrease in caspase-3 expression in the MSC and Carv combined group compared with the MSC-alone group.
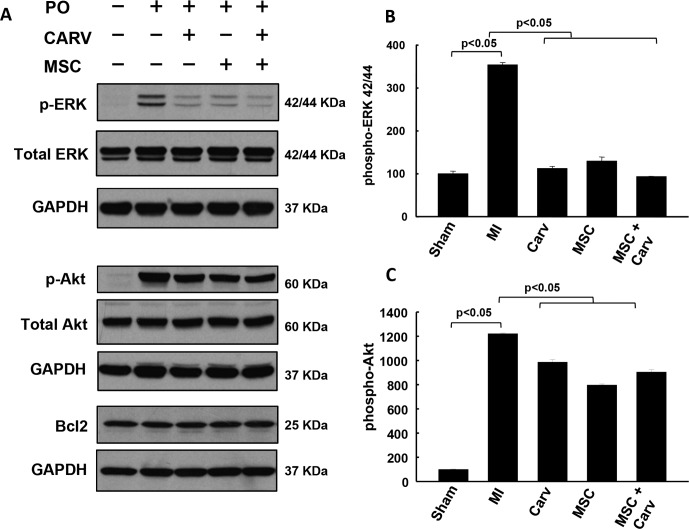
Carv and MSC Treatment Modulates the Phosphorylation of Akt and ERK1/2.
Phosphatidylinositol 3-kinase-Akt signaling and ERK1/2 signaling cascades are activated due to a wide range of receptors and are involved in regulating cell survival, proliferation, and differentiation (Widmann et al., 1999; Cross et al., 2000). The results of our study showed a significant increase in phosphorylation of Akt and ERK1/2 in MI hearts compared with all the treated groups. It is noteworthy that the levels of phospho-Akt and ERK1/2 were significantly down-regulated (p < 0.05) in the Carv, MSC, and MSC+Carv groups compared with the MI group (Fig. 6).
Fig. 6.
Effect of Carv and/or stem cell treatment on modulation of AKT, ERK1/2, and Bcl-2 after 4 weeks of MI. A, representative Western blots for different groups were normalized with GAPDH. B and C, Western blot analysis data showed a significant (p < 0.05) increase in phosphorylation of Akt (B) and ERK1/2 (C) in the MI group. However, the phosphorylation of AKT and ERK1/2 was significantly (p < 0.05) attenuated in all the treated groups, i.e., Carv, MSC, and MSC+Carv. On the other hand, there was no change in the expression of prosurvival Bcl-2 in any of the groups. Values are expressed as mean ± S.D.; n = 3.
Discussion
Overall, the present study showed that the pretreatment of stem cells with Carv decreases H2O2-induced cell death and caspase-3 expression in vitro. Furthermore, in vivo studies showed that transplantation of Carv pretreated stem cells along with daily treatment of Carv 1) enhanced the functional recovery of the heart, 2) decreased cardiac fibrosis, 3) increased angiogenesis, and 4) decreased caspase-3 expression, thereby suppressing apoptosis of surviving myocytes in the infarcted heart. The beneficial effects of Carv in protecting cardiomyocyte apoptosis and survival may seem to be due to its antioxidant properties.
Although, numerous preclinical studies have demonstrated the benefits of MSC therapy for treating the damaged heart after MI (Tomita et al., 1999; Kudo et al., 2003; Fazel et al., 2005), one of the key factors that determine the clinical outcomes of transplanted stem cells is their survival in the ischemic heart. Previous studies from our group have shown that the important determinants that might affect the survival of stem cells in the ischemic heart are the apoptosis of transplanted cells due to low oxygen and oxidative stress (Khan et al., 2007b; Wisel et al., 2009). Therefore, the main goal of this study was to understand whether pretreatment and/or postconditioning of animals with Carv, a nonselective β-blocker, could improve the outcome of stem cell therapy.
Antioxidant Effect of Carv on MSC In Vitro.
The potential sources of ROS in cardiomyocytes include mitochondrial electron transport chain, xanthine oxidase, nonphagocytic NADPH oxidases, dysfunctional NO synthase, lipoxygenase, heme-oxygenase, and the cytochrome P450 mono-oxygenases. During cardiac disease, the major sources of ROS in cardiac disease are the mitochondria, the xanthine oxidase, and the NADPH oxidase (Grieve et al., 2004; Arozal et al., 2010). One of the important observations of our study is the dose-dependent increase in the superoxide-scavenging property of Carv compared with Atenolol. Likewise, MSCs subjected to oxidative stress by H2O2 treatment were protected when they were pretreated with Carv before H2O2 treatment. The decrease in the number of TUNEL-positive cells further indicated the antiapoptotic effect of Carv pretreatment on stem cells. These data showed the potential benefit of Carv pretreatment to decrease the apoptosis of transplanted cells. We speculate that through Carv's dual properties as a β-blocker and antioxidant, it maintains a unique ability to restore cardiac function and scavenge superoxide radicals (Hayashi et al., 2010). These dual properties of Carv distinguish it from drugs with only antioxidant properties.
Effect of Carv and MSC on Cardiac Function and Fibrosis.
Several preclinical studies have shown an improvement in cardiac function after MSC transplantation (Tomita et al., 1999; Kudo et al., 2003; Fazel et al., 2005). However, this is the first study to show that the combination of Carv along with MSC treatment enhances the functional recovery of the heart compared with MSC cardiomyoplasty alone. These data demonstrated the clinical significance of Carv in patients who are on Carv treatment and undergoing stem cell transplantation. Patients might have a better clinical outcome in terms of improvement of cardiac function than patients receiving Carv only. On the other hand, cardiac fibrosis, which is another key determinant in the process of left ventricular remodeling, was attenuated in both the Carv and MSC groups. To an even greater extent, the antifibrotic effect was further enhanced in the Carv+MSC group. The results suggest the crucial role of Carv, either alone as a β-blocker or in conjunction with stem cells, showing phenomenal benefits in attenuation of myocardial fibrosis.
Effect of Carv and MSC on Angiogenesis.
We have previously reported that MSCs transplanted in the ischemic heart induce angiogenesis (Khan et al., 2009, 2012). The immunohistological findings from our current study showed an increased vessel and capillary density in the combined Carv and MSC group (Fig. 4, B and C). The results strongly suggest that the combinatorial treatment enhanced angiogenesis. However, the exact mechanism by which the combined treatment affects angiogenesis needs further investigation.
Effect of Carv and MSC on Cardiomyocyte Apoptosis and Caspase-3 Expression.
One of the potential pathophysiological mechanisms in the progression of heart failure is cardiomyocyte apoptosis (Sabbah, 2000; Abbate et al., 2002), which is responsible for cardiac cell loss after AMI (Olivetti et al., 1996). This leads to changes in the shape, size, and contractility of the left ventricle. Cardiac remodeling is not limited to the first 7 days after AMI; it is a progressive process that continues for weeks and up to several months after AMI (Abbate et al., 2002; Jessup and Brozena, 2003). Studies in human postmortem and animal tissues have demonstrated an increased rate of apoptosis in the peri-infarct and remote myocardium for several months after AMI (Palojoki et al., 2001; Baldi et al., 2002). Apoptosis seemed to be higher in the peri-infarct regions, particularly in cases of recurrent ischemia (Abbate et al., 2002). A recent study by Abbate et al. (2007) showed that permanent occlusion of infarct-related artery is associated with increased apoptosis of cardiomyocytes in the peri-infarct regions of the heart and persisted for up to 8 weeks after MI in an animal model of permanent occlusion of the left coronary artery. In the present study, we have shown that in animals treated with Carv and MSCs, there was a significant reduction in apoptosis as indicated by decreased TUNEL-positive cells (Li et al., 2010). In addition, caspase-3, which is an important mediator in regulating apoptosis, was down-regulated in the combined treatment group (Baldi et al., 2002). The results demonstrated that the decrease in apoptosis might be due to the synergism between the antioxidant properties of Carv and the paracrine signaling effect of MSCs ultimately leading to a decrease in cardiomyocytes apoptosis in the peri-infarct regions of the heart. Overall, the data demonstrate the significance of combined therapy with β-blocker (Carv) and stem cells for ameliorating myocardial apoptosis after AMI.
Effect of Carv and MSCs on Phosphorylation of AKT and ERK.
The increase in phosphatidylinositol-3-kinase/Akt activation and ERK pathways play an important role in the regulation of cardiomyocyte apoptosis and left ventricular function (Li et al., 2009). It has been reported that activation of ERK is associated with inhibition of cardiomyocyte apoptosis both in in vitro and in vivo models of ischemia-reperfusion (Bueno et al., 2000; Foncea et al., 2000). A study by Li et al. (2009) showed an increase in ERK phosphorylation in saline-injected control hearts at 1 week after MI, and this increase was considerably less pronounced in MSC-transplanted hearts. In addition, increased p-ERK1/2 activation was reported in infarcted and remote regions in the post-MI hearts (Yeh et al., 2010a). The study also suggested that the increase in mitogen-activated protein kinase kinase-1/ERK signaling may be predominantly localized in the nonmyocytes in the scar and fibrotic regions. Although, cardiomyocytes contribute to the majority of the adult myocardial mass, they comprise only 30% of the total cells in the heart, and the remaining are poised of nonmyocyte cells (Zak, 1973); these nonmyocyte cells might be responsible for alterations in Akt and ERK1/2 in post-MI hearts. It is noteworthy that the results from our study indicated an increased phosphorylation of Akt and ERK1/2 in post-MI hearts at 4 weeks. In contrast, there was a significant decrease in phosphorylation of Akt and ERK1/2 in Carv, MSC, and MSC+Carv treatment groups. The decreased activation of AKT and ERK1/2 in the treated groups might be due to decreased cardiomyocytes apoptosis. Furthermore, increased activation of Akt and ERK1/2 in cardiac fibroblasts (as seen in MI hearts) might lead to increased fibrosis. Additional studies are needed to understand the mechanism by which Carv and MSC treatment modulates the activation of Akt and ERK1/2.
Conclusions
Our study suggests that Carv treatment along with MSC transplantation improved cardiac function, decreased fibrosis, enhanced cardiomyocyte survival, and decreased caspase-3 expression in the infarcted heart. The combined treatment strategy might be very useful in clinical settings, where infarcted patients are frequently treated with Carv, which could be supplemented with allogenic stem cell transplantation, thereby producing a more promising clinical outcome in recovery of cardiac function.
This work was supported by the National Institutes of Health National Institute of Biomedical Imaging and Bioengineering [Grant R01-EB006153] (to P.K.); and the American Heart Association [Grant SDG 0930181N] (to M.K.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- MSC
- mesenchymal stem cell
- ROS
- reactive oxygen species
- LAD
- left anterior descending
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- DEPMPO
- 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide
- MI
- myocardial infarction
- Carv
- Carvedilol
- PBS
- phosphate-buffered saline
- MTT
- 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
- DAPI
- 4,6-diamidino-2-phenylindole
- α-SMA
- α-smooth muscle actin
- vWF
- von Willebrand factor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- AMI
- acute MI
- BrdU
- 5-bromo-2′-deoxyuridine
- EPR
- electron paramagnetic resonance.
Authorship Contributions
Participated in research design: Fatemat and Khan.
Conducted experiments: Hassan, Meduru, Taguchi, Mostafa, and M. L. Kuppusamy.
Contributed new reagents or analytic tools: P. Kuppusamy and Khan.
Performed data analysis: Hassan, Meduru, Taguchi, and Khan.
Wrote or contributed to the writing of the manuscript: Fatemat Hassan and Khan.
References
- Abbate A, Biondi-Zoccai GG, Baldi A. (2002) Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 193:145–153 [DOI] [PubMed] [Google Scholar]
- Abbate A, Morales C, De Falco M, Fedele V, Biondi Zoccai GG, Santini D, Palleiro J, Vasaturo F, Scarpa S, Liuzzo G, et al. (2007) Ischemia and apoptosis in an animal model of permanent infarct-related artery occlusion. Int J Cardiol 121:109–111 [DOI] [PubMed] [Google Scholar]
- Abdulla MH, Sattar MA, Abdullah NA, Khan MA, Anand Swarup KR, Johns EJ. (2011) The effect of losartan and carvedilol on vasopressor responses to adrenergic agonists and angiotensin II in the systemic circulation of Sprague Dawley rats. Auton Autacoid Pharmacol 31:13–20 [DOI] [PubMed] [Google Scholar]
- Arozal W, Watanabe K, Veeraveedu PT, Ma M, Thandavarayan RA, Sukumaran V, Suzuki K, Kodama M, Aizawa Y. (2010) Protective effect of carvedilol on daunorubicin-induced cardiotoxicity and nephrotoxicity in rats. Toxicology 274:18–26 [DOI] [PubMed] [Google Scholar]
- Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, Dobrina A, Rossiello R, Silvestri F, Baldi F, et al. (2002) Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol 34:165–174 [DOI] [PubMed] [Google Scholar]
- Barone FC, Willette RN, Nelson AH, Ohlstein EH, Brooks DP, Coatney RW. (2007) Carvedilol prevents and reverses hypertrophy-induced cardiac dysfunction. Pharmacology 80:166–176 [DOI] [PubMed] [Google Scholar]
- Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, et al. (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19:6341–6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnoni A, Ceconi C, Bernocchi P, Boraso A, Parrinello G, Curello S, Ferrari R. (2000) Reduction of oxidative stress by carvedilol: role in maintenance of ischaemic myocardium viability. Cardiovasc Res 47:556–566 [DOI] [PubMed] [Google Scholar]
- Carreira RS, Monteiro P, Gon Alves LM, Providência LA. (2006) Carvedilol: just another beta-blocker or a powerful cardioprotector? Cardiovasc Hematol Disord Drug Targets 6:257–266 [DOI] [PubMed] [Google Scholar]
- Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. (2000) Serine/threonine protein kinases and apoptosis. Exp Cell Res 256:34–41 [DOI] [PubMed] [Google Scholar]
- Cutler DM, Rosen AB, Vijan S. (2006) The value of medical spending in the United States, 1960–2000. N Engl J Med 355:920–927 [DOI] [PubMed] [Google Scholar]
- Fazel S, Chen L, Weisel RD, Angoulvant D, Seneviratne C, Fazel A, Cheung P, Lam J, Fedak PW, Yau TM, et al. (2005) Cell transplantation preserves cardiac function after infarction by infarct stabilization: augmentation by stem cell factor. J Thorac Cardiovasc Surg 130:1310. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Ceconi C, Curello S, Guarnieri C, Caldarera CM, Albertini A, Visioli O. (1985) Oxygen-mediated myocardial damage during ischaemia and reperfusion: role of the cellular defences against oxygen toxicity. J Mol Cell Cardiol 17:937–945 [DOI] [PubMed] [Google Scholar]
- Ferrari R, Guardigli G, Mele D, Percoco GF, Ceconi C, Curello S. (2004) Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des 10:1699–1711 [DOI] [PubMed] [Google Scholar]
- Feuerstein G, Yue TL, Ma X, Ruffolo RR. (1998) Novel mechanisms in the treatment of heart failure: inhibition of oxygen radicals and apoptosis by carvedilol. Prog Cardiovasc Dis 41:17–24 [DOI] [PubMed] [Google Scholar]
- Flesch M, Maack C, Cremers B, Bäumer AT, Südkamp M, Böhm M. (1999) Effect of beta-blockers on free radical-induced cardiac contractile dysfunction. Circulation 100:346–353 [DOI] [PubMed] [Google Scholar]
- Foncea R, Galvez A, Perez V, Morales MP, Calixto A, Melendez J, Gonzalez-Jara F, Diaz-Araya G, Sapag-Hagar M, Sugden PH, et al. (2000) Extracellular regulated kinase, but not protein kinase C, is an antiapoptotic signal of insulin-like growth factor-1 on cultured cardiac myocytes. Biochem Biophys Res Commun 273:736–744 [DOI] [PubMed] [Google Scholar]
- Grieve DJ, Byrne JA, Cave AC, Shah AM. (2004) Role of oxidative stress in cardiac remodelling after myocardial infarction. Heart Lung Circ 13:132–138 [DOI] [PubMed] [Google Scholar]
- Hayashi T, De Velasco MA, Saitou Y, Nose K, Nishioka T, Ishii T, Uemura H. (2010) Carvedilol protects tubular epithelial cells from ischemia-reperfusion injury by inhibiting oxidative stress. Int J Urol 17:989–995 [DOI] [PubMed] [Google Scholar]
- Hill MF, Singal PK. (1996) Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol 148:291–300 [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jessup M, Brozena S. (2003) Heart failure. N Engl J Med 348:2007–2018 [DOI] [PubMed] [Google Scholar]
- Khan M, Kutala VK, Vikram DS, Wisel S, Chacko SM, Kuppusamy ML, Mohan IK, Zweier JL, Kwiatkowski P, Kuppusamy P. (2007a) Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. Am J Physiol Heart Circ Physiol 293:H2129–H2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Mohan IK, Kutala VK, Kumbala D, Kuppusamy P. (2007b) Cardioprotection by sulfaphenazole, a cytochrome p450 inhibitor: mitigation of ischemia-reperfusion injury by scavenging of reactive oxygen species. J Pharmacol Exp Ther 323:813–821 [DOI] [PubMed] [Google Scholar]
- Khan M, Meduru S, Mohan IK, Kuppusamy ML, Wisel S, Kulkarni A, Rivera BK, Hamlin RL, Kuppusamy P. (2009) Hyperbaric oxygenation enhances transplanted cell graft and functional recovery in the infarct heart. J Mol Cell Cardiol 47:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Meduru S, Mostafa M, Khan S, Hideg K, Kuppusamy P. (2010) Trimetazidine, administered at the onset of reperfusion, ameliorates myocardial dysfunction and injury by activation of p38 mitogen-activated protein kinase and Akt signaling. J Pharmacol Exp Ther 333:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Meduru S, Gogna R, Madan E, Citro L, Kuppusamy ML, Sayyid M, Mostafa M, Hamlin RL, Kuppusamy P. (2012) Oxygen cycling in conjunction with stem cell transplantation induces NOS3 expression leading to attenuation of fibrosis and improved cardiac function. Cardiovasc Res 93:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. (2000) Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87:392–398 [DOI] [PubMed] [Google Scholar]
- Kopecky SL. (2006) Effect of beta blockers, particularly carvedilol, on reducing the risk of events after acute myocardial infarction. Am J Cardiol 98:1115–1119 [DOI] [PubMed] [Google Scholar]
- Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA. (1996) Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest 98:2854–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. (2003) Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol 35:1113–1119 [DOI] [PubMed] [Google Scholar]
- Kumar A, Prakash A, Dogra S. (2011) Neuroprotective effect of carvedilol against aluminium induced toxicity: possible behavioral and biochemical alterations in rats. Pharmacol Rep 63:915–923 [DOI] [PubMed] [Google Scholar]
- Lauer MS. (2012) Advancing cardiovascular research. Chest 141:500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Malhotra D, Yeh CC, Tu R, Zhu BQ, Birger N, Wisneski A, Cha J, Karliner JS, Mann MJ. (2009) Myocardial survival signaling in response to stem cell transplantation. J Am Coll Surg 208:607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Turdi S, Thomas DP, Zhou T, Ren J. (2010) Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction. Toxicol Lett 195:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P. (2011) Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol 50:258–265 [DOI] [PubMed] [Google Scholar]
- Monteiro P, Duarte AI, Moreno A, Gonçalves LM, Providência LA. (2003) Carvedilol improves energy production during acute global myocardial ischaemia. Eur J Pharmacol 482:245–253 [DOI] [PubMed] [Google Scholar]
- Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. (1996) Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol 28:2005–2016 [DOI] [PubMed] [Google Scholar]
- Packer M. (1998) Beta-adrenergic blockade in chronic heart failure: principles, progress, and practice. Prog Cardiovasc Dis 41:39–52 [DOI] [PubMed] [Google Scholar]
- Palojoki E, Saraste A, Eriksson A, Pulkki K, Kallajoki M, Voipio-Pulkki LM, Tikkanen I. (2001) Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 280:H2726–H2731 [DOI] [PubMed] [Google Scholar]
- Sabbah HN. (2000) Apoptotic cell death in heart failure. Cardiovasc Res 45:704–712 [DOI] [PubMed] [Google Scholar]
- Schwarz ER, Kersting PH, Reffelmann T, Meven DA, Al-Dashti R, Skobel EC, Klosterhalfen B, Hanrath P. (2003) Cardioprotection by Carvedilol: antiapoptosis is independent of beta-adrenoceptor blockage in the rat heart. J Cardiovasc Pharmacol Ther 8:207–215 [DOI] [PubMed] [Google Scholar]
- Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. (1999) Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100:II247–II256 [DOI] [PubMed] [Google Scholar]
- Wei H, Li Z, Hu S, Chen X, Cong X. (2010) Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem 111:967–978 [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79:143–180 [DOI] [PubMed] [Google Scholar]
- Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. (2009) Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther 329:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CC, Li H, Malhotra D, Turcato S, Nicholas S, Tu R, Zhu BQ, Cha J, Swigart PM, Myagmar BE, et al. (2010a) Distinctive ERK and p38 signaling in remote and infarcted myocardium during post-MI remodeling in the mouse. J Cell Biochem 109:1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. (2010b) Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 362:2155–2165 [DOI] [PubMed] [Google Scholar]
- Yoshikawa E, Marui A, Tsukashita M, Nishina T, Wang J, Muranaka H, Ikeda T, Komeda M. (2010) Carvedilol may alleviate late cardiac remodelling following surgical ventricular restoration. Eur J Cardiothorac Surg 37:362–367 [DOI] [PubMed] [Google Scholar]
- Yue TL, Cheng HY, Lysko PG, McKenna PJ, Feuerstein R, Gu JL, Lysko KA, Davis LL, Feuerstein G. (1992) Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther 263:92–98 [PubMed] [Google Scholar]
- Zak R. (1973) Cell proliferation during cardiac growth. Am J Cardiol 31:211–219 [DOI] [PubMed] [Google Scholar]
- Zeng H, Liu X, Zhao H. (2003) Effects of carvedilol on cardiomyocyte apoptosis and gene expression in vivo after ischemia-reperfusion in rats. J Huazhong Univ Sci Technolog Med Sci 23:127–130 [DOI] [PubMed] [Google Scholar]
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. (2001) Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 33:907–921 [DOI] [PubMed] [Google Scholar]