Abstract
Modeling the binding sites for spermine and ifenprodil on the regulatory (R) domains of the N-methyl-d-aspartate receptor GluN1 and GluN2B subunits was carried out after measuring spermine stimulation and ifenprodil inhibition at receptors containing GluN1 and GluN2B R domain mutants. Models were constructed based on the published crystal structure of the GluN1 and GluN2B R domains, which form a heterodimer (Nature 475:249–253, 2011). The experimental results and modeling suggest that a binding site for spermine was formed by the residues near the cleft between the R1 and R2 lobes of the GluN1 R domain (GluN1R) together with residues on the surface of the R2 (C-terminal side) lobe of the GluN2B R domain (GluN2BR). The ifenprodil binding site included residues on the surface of the R1 lobe (N-terminal side) of GluN1R together with residues near the cleft between the R1 and R2 lobes of GluN2BR. It was confirmed using a Western blot analysis that GluN1R and GluN2BR formed a heterodimer. Models of spermine and ifenprodil binding to the heterodimer were constructed. The modeling suggests that an open space between the two R1 lobes of GluN1R and GluN2BR is promoted through spermine binding and that the R1 lobes of GluN1R and GluN2BR approach each other through ifenprodil binding—an effect opposite to that seen with the binding of spermine.
Introduction
N-Methyl-d-aspartate (NMDA) receptors are tetramers composed of combinations of GluN1, GluN2, and GluN3 subunits (Traynelis et al., 2010). Each subunit contains a distal amino terminal domain (Perin-Dureau et al., 2002), also referred to as the regulatory (R) domain (Masuko et al., 1999), which has structural similarity to bacterial periplasmic binding proteins, in particular, the leucine/isoleucine/valine binding protein, and contains two lobes, R1 and R2. The R domain is linked to the proximal part of the extracellular N-terminal region—the L1 domain—which, together with part of the extracellular M3-M4 loop (the L2 domain), forms the ligand binding site—the glycine binding site in GluN1 and the glutamate binding site in GluN2 (Kuryatov et al., 1994; Laube et al., 1997). The structures of the ligand binding domains (LBDs) of several glutamate receptor subunits, including GluA2, GluK1, GluK2, GluN1, and GluN2A, have been determined by X-ray crystallography, and these domains form bilobed clamshell structures (Armstrong et al., 1998; Mayer et al., 2001; Furukawa and Gouaux, 2003; Furukawa et al., 2005; Mayer, 2005). The crystal structures of the R domains from GluN2B (Karakas et al., 2009), GluA2 (Jin et al., 2009), and GluK2 (Kumar et al., 2009) also have been reported.
Polyamines (putrescine, spermidine, and spermine) are present at millimolar concentrations in cells and play important roles in cell proliferation and differentiation (Igarashi and Kashiwagi, 2010). In the nervous system, spermine may be released from neurons and have effects on NMDA receptors (Masuko et al., 2003). Spermine has multiple effects on NMDA receptors, including stimulation and a weak voltage-dependent channel block that involves the binding of spermine to at least two distinct sites (Benveniste and Mayer, 1993; Williams, 1997; Masuko et al., 1999; Kashiwagi et al., 2002). We have suggested that a spermine binding site for the stimulation of NMDA receptors is located in or near the cleft of the GluN1 R domain (Masuko et al., 1999; Han et al., 2008).
Ifenprodil is an atypical NMDA antagonist that selectively inhibits NMDA receptors containing the GluN2B subunit (Williams, 1993). Mutations at several residues in GluN1R greatly influenced ifenprodil inhibition, and we have suggested that this region may form part of the ifenprodil binding site (Masuko et al., 1999). It also has been reported that mutations at residues in GluN2BR strongly affect ifenprodil inhibition (Perin-Dureau et al., 2002). Indeed, it has been reported recently that GluN1R and GluN2BR form a heterodimer and that ifenprodil binds at the interface between GluN1R and GluN2BR (Karakas et al., 2011).
Using soluble, purified GluN1R, GluN2AR, and GluN2BR, we found that spermine and ifenprodil bound to these domains with affinities in the orders GluN1R > GluN2BR ≫ GluN2AR for spermine and GluN1R ≈ GluN2BR ⋙ GluN2AR for ifenprodil (Han et al., 2008). In this article, we have studied the effects of various mutations in the R domains of GluN1 and GluN2B on spermine stimulation and ifenprodil inhibition at intact GluN1/GluN2B receptors and have modeled the spermine and ifenprodil binding sites on GluN1R, GluN2BR, and the GluN1R/GluN2BR heterodimer based on the reported structures of GluN1R and GluN2BR (Karakas et al., 2011) and on our experimental results.
Materials and Methods
NMDA Clones and Site-Directed Mutagenesis.
The rat GluN1 clone used in these studies is the GluN1A variant (Moriyoshi et al., 1991), which lacks the 21-amino-acid insert encoded by exon 5. This clone was supplied by Dr. S. Nakanishi (Osaka Bioscience Institute, Osaka, Japan). The rat GluN2A and GluN2B clones (Monyer et al., 1992) were gifts from Dr. P. H. Seeburg (Center for Molecular Biology, University of Heidelberg, Heidelberg, Germany), and the mouse GluN2B clone (Kutsuwada et al., 1992) was a gift from Dr. M. Mishina (Graduate School of Medicine, University of Tokyo, Tokyo, Japan). The preparation of several GluN1 and GluN2B mutants has been described previously (Masuko et al., 1999). Site-directed mutagenesis to construct additional GluN1 and GluN2B mutants was carried out according to the method of Ho et al. (1989) using the polymerase chain reaction. For mutations in GluN2B, the rat clone was mainly used. In some cases, the mouse GluN2B clone containing a 1.7-kb HindIII-SphI fragment of the rat GluN2B clone was used to obtain the mutants more easily (Williams et al., 1998). Amino acids are numbered from the initiator methionine in each subunit. Chimeric constructs of GluN2B and GluN2A—GluN2B(198)2A, GluN2B(357)2A, GluN2B(413)2A, GluN2B(489)2A, and GluN2B(858)2A, in which the number indicates the final amino acid of GluN2B included in the construct—were generated using XhoI, ApaLI, NarI, HpaI, and XbaI for fusion sites, respectively, and EcoRI for the C-terminal side. The GluN2A fragments prepared using the above restriction enzymes were inserted into the same restriction sites of GluN2B. In some cases, the restriction sites were created in either the GluN2A or GluN2B clone by site-directed mutagenesis using the M13 phage system (Kunkel et al., 1987). Mutations and correct fusion of GluN2A and GluN2B were confirmed by DNA sequencing.
Preparation of Oocytes and Voltage Clamp Recording.
Adult female Xenopus laevis (Saitama Experimental Animals Supply Co. Ltd., Saitama, Japan, or Nasco, Fort Atkinson, WI) were chilled on ice or anesthetized with tricaine, and the preparation and maintenance of oocytes were carried out using methods similar to those described previously (Williams et al., 1994). Capped complementary RNAs were prepared from linearized cDNA templates using mMESSAGE mMACHINE kits (Ambion, Austin, TX). Oocytes were injected with GluN1 and GluN2 cRNAs in a ratio of 1:5 (0.2–4 ng of GluN1 plus 1–20 ng of GluN2). Macroscopic currents were recorded with a two-electrode voltage clamp using a GeneClamp 500 amplifier (Molecular Devices, Sunnyvale, CA). Electrodes were filled with 3 M KCl. Oocytes were superfused continuously (approximately 5 ml/min) with a Mg2+-free saline solution [96 mM NaCl, 2 mM KCl, 1.8 mM BaCl2, and 10 mM HEPES (pH 7.5)]. This solution contained BaCl2 rather than CaCl2, and oocytes were injected with K+-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (100 nl, 40 mM, pH 7.0) on the day of recording to eliminate Ca2+-activated Cl− currents (Williams, 1993). Oocytes were voltage-clamped at a holding potential of −20 mV. Data were recorded by using a Digidata 1332A interface with pCLAMP8 software for Windows (Molecular Devices) or using a BIOPAC Systems MP100 interface with AcqKnowledge software (BIOPAC Systems, Camino Goleta, CA).
Molecular Modeling of GluN1R and GluN2BR and Docking Simulation.
Three-dimensional structures of GluN1R and GluN2BR were constructed with Molecular Operating Environment (MOE) (version 2009; CCG Inc., Montreal, QC, Canada) based on the information for NMDA receptors in the Brookhaven Protein Databank (code 3QEL). Spermine and ifenprodil were docked into the appropriate sites of GluN1R or GluN2BR by using the docking module in MOE. The GluN1R and GluN2BR heterodimer in complex with spermine was constructed by taking into consideration the appropriate binding sites of spermine on GluN1R and GluN2BR with MOE. The GluN1R and GluN2BR heterodimer in complex with ifenprodil was constructed by using the homology modeling module in MOE. The molecular mechanics calculations and the calculation of the interaction energy of these complexes were performed by using the Amber99 force field by MOE. The three-dimensional structures of these complexes were displayed using MolFeat (version 4; FiatLux, Tokyo, Japan). Secondary structure alignment was performed based on the structures of GluN1R and GluN2BR (Karakas et al., 2011) using the SWISS-MODEL Workspace (Arnold et al., 2006).
Trypsin Sensitivity of the Complex of the Heterodimer of GluN1R/GluN2BR with Spermine or Ifenprodil.
GluN1R and GluN2BR proteins were purified as described previously (Han et al., 2008). The reaction mixture (0.3 ml) containing 15 μg of GluN1R plus 10 μg of GluN2BR, either 100 μM spermine or 1 μM ifenprodil, 10 mM Tris-HCl (pH 7.5), and 50 mM KCl was incubated in the presence and absence of 1 μg of trypsin at 30°C for 2 h, and 7 μg of anti-GluN1 (sc-9058; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was added to the reaction mixture. Then, antibody-coupled protein was precipitated by the addition of 2 μl of PANSORBIN cells (Calbiochem, San Diego, CA) and incubated at 4°C for 2 h. The precipitate was collected by centrifugation at 30,000g for 5 min, washed three times with a buffer containing 0.5 M Na-phosphate buffer (pH 7.5), 100 mM NaCl, 1% Triton X-100, and 0.1% SDS, and boiled for 5 min in SDS-polyacrylamide gel electrophoresis sample buffer. Western blot analysis was performed by the method of Nielsen et al. (1982) using 3 μg of protein and enhanced chemiluminescence Western blot reagents (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). GluN1R and GluN2BR were detected with anti-GluN1 (sc-9058; Santa Cruz Biotechnology, Inc.) and anti-GluN2B (sc-9057; Santa Cruz Biotechnology, Inc.). These commercially available antibodies were raised against epitopes in the first 70 to 300 amino acids of the N terminus (i.e., the R domain) of each subunit.
Results
Effects of Spermine and Ifenprodil at GluN1 and GluN2B R Domain Mutants and Modeling of Spermine and Ifenprodil Binding Sites.
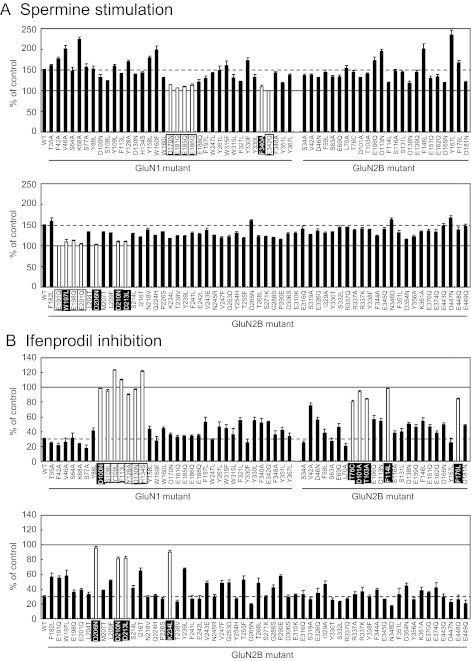
We have reported that several amino acid residues in the R domains of GluN1 and GluN2B strongly influence spermine stimulation and ifenprodil inhibition and are likely to contribute to the binding sites for these modulators (Williams et al., 1995; Masuko et al., 1999; Han et al., 2008). To characterize in more detail the binding sites for spermine and ifenprodil on the R domains of GluN1 and GluN2B, we examined the effects of these modulators using many additional GluN1R and GluN2BR mutants. For the identification of mutants having effects, we selected arbitrary thresholds of <115% of control for spermine (approximately 70% decrease in spermine stimulation compared with wild type) and >80% of control for ifenprodil (approximately 70% decrease in ifenprodil inhibition compared with wild type). As shown in Fig. 1, in addition to 14 amino acid residues shown previously to influence spermine stimulation and ifenprodil inhibition (shown by open columns and with mutants labeled using black letters), 15 amino acid residues were newly identified as being involved in spermine stimulation and ifenprodil inhibition (shown by open columns and with mutants labeled using white letters). A number of additional mutants had effects close to these thresholds—for example, V42A, I216T, and Y239L on ifenprodil inhibition—but are not explicitly discussed further in the modeling studies below. Those mutants lie near the proposed binding sites and may cause local disruptions in structure.
Fig. 1.
Effects of spermine and ifenprodil at GluN1/GluN2B receptors containing GluN1 and GluN2B mutants. The effects of spermine (100 μM) and ifenprodil (1 μM) were determined in oocytes expressing GluN1/GluN2B receptors with wild-type (WT) and mutant GluN1 or GluN2B subunits, voltage-clamped at −20 mV and activated by 10 μM glutamate and 10 μM glycine. Values are mean ± S.E.M. from 20 oocytes for wild-type and four oocytes for each mutant. A, mutations that show stimulation <115% (approximately 70% decrease in spermine stimulation compared with wild type) in the presence of spermine are highlighted by open columns. The solid line (100%) indicates the activity in the absence of spermine, and the dotted line indicates the activity in the presence of spermine in wild-type GluN1/GluN2B receptors. B, mutations in which currents are >80% of control in the presence of ifenprodil (approximately 70% decrease in ifenprodil inhibition compared with the wild type) are highlighted by open columns. The solid line (100%) indicates the activity in the absence of ifenprodil, and the dotted line indicates the activity in the presence of ifenprodil in wild-type GluN1/GluN2B receptors. Mutants shown with black letters were identified previously, and mutants shown with white letters in a black box were newly identified.
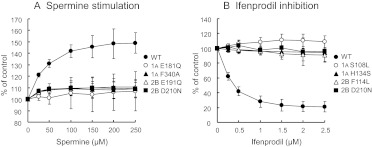
The results shown in Fig. 1 were obtained using 100 μM spermine and 1 μM ifenprodil. In addition, the effects of spermine and ifenprodil on wild-type receptors and receptors containing several key mutants were examined over a range of concentrations of spermine and ifenprodil. As shown in Fig. 2, the degree of spermine stimulation and ifenprodil inhibition at wild-type receptors was concentration dependent, but neither modulator had significant effects at the mutant receptors over the concentration range used. At wild-type receptors, the EC50 values for spermine and ifenprodil were 38 and 0.27 μM, respectively, and the Hill coefficients for spermine and ifenprodil were close to 1.
Fig. 2.
Effects of various concentrations of spermine (A) and ifenprodil (B) at GluN1/GluN2B receptors containing GluN1 and GluN2B mutants. Experiments were carried out as described in the legend to Fig. 1 by changing the concentrations of spermine (25–250 μM) and ifenprodil (0.25–2.5 μM). Values are mean ± S.E.M. from at least five oocytes for each mutant.
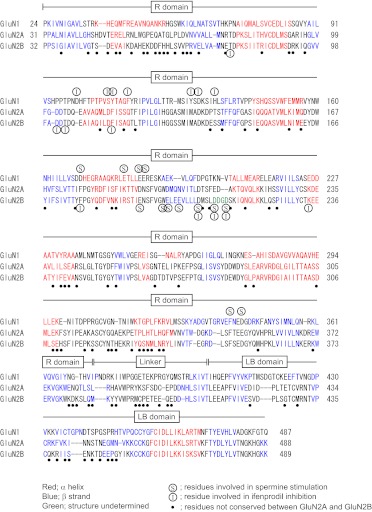
Spermine stimulation and ifenprodil inhibition are observed in receptors containing GluN1 (without the exon 5 insert) and GluN2B (Durand et al., 1993; Williams, 1993; Williams et al., 1994). Through the use of purified, soluble recombinant R domains, the Kd values for the binding of spermine to the R domains were 19 μM (GluN1R), 140 μM (GluN2AR), and 33 μM (GluN2BR), and the Kd values for the binding of ifenprodil were 0.18 μM (GluN1R) and 0.21 μM (GluN2BR); the binding of ifenprodil to GluN2AR was negligible (Han et al., 2008). In Fig. 3, the amino acid sequences of the R domains and immediate distal linker/ LBDs of GluN1, GluN2A, and GluN2B are shown together with amino acid residues that influence spermine stimulation and ifenprodil inhibition as determined at functional, intact receptors (Fig. 1). Although the amino acid sequence of the GluN2AR is similar to that of GluN2BR (57% identical amino acid residues and 72% homologous amino acid residues in 355 amino acid residues), the effects of spermine and ifenprodil on GluN1/GluN2A and GluN1/GluN2B receptors are drastically different. There are three residues (Asp206, Asp210, and Asp213) in GluN2BR that influence both spermine stimulation and ifenprodil inhibition, and the amino acid sequence of this region of GluN2BR differs from that of GluN2AR. It may be that a structural difference of this region strongly influences the effects of spermine and ifenprodil at GluN2A and GluN2B, thus accounting for their selectivity at receptors containing GluN2B. With regard to GluN1R, the residues that influence ifenprodil inhibition were clustered (from Asp100 to His134), whereas those that affect spermine were separated into two regions (from Asp170 to Glu186 and from Glu340 to Glu342). This contrasts with the effects of mutations in GluN2BR, where residues affecting spermine stimulation were clustered (from Glu191 to Asp213), whereas those affecting ifenprodil were separated into two regions (from Thr76 to Phe114 and from Phe176 to Lys234).
Fig. 3.
Amino acid sequences of the R domains and immediately distal regions in GluN1, GluN2A, and GluN2B. Secondary structure alignment was performed as described under Materials and Methods. The positions of α helices and β strands are shown in red and blue, respectively. Amino acid residues shown in (S) and (I) are residues that influence spermine stimulation and ifenprodil inhibition, respectively. Amino acid residues shown in ● are not conserved between GluN2A and GluN2B.
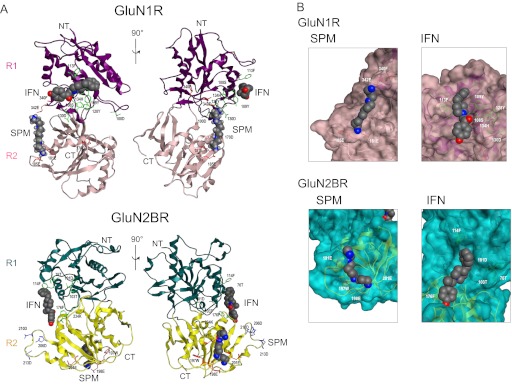
Although spermine and ifenprodil bind to GluN1/GluN2B heterodimers (Karakas et al., 2011; Mony et al., 2011), they also can bind to GluN1 and GluN2B individually, possibly as monomers (Han et al., 2008). Thus, modeling of spermine and ifenprodil binding sites on GluN1R and GluN2BR monomers was carried out first. As shown in Fig. 4A, spermine bound near the cleft of GluN1R, and ifenprodil bound on the surface of the R1 (N-terminal) lobe of GluN1R. In contrast, spermine bound to the surface of the R2 (C-terminal) lobe of GluN2BR, and ifenprodil bound near the cleft of GluN2BR (Fig. 4A). It is noted that the relative position of the spermine binding site near the cleft of GluN1R was different from the position of the ifenprodil binding site near the cleft of GluN2BR. Other views of the sites for spermine and ifenprodil are shown in Fig. 4B, in which more detailed interactions of amino acid residues with spermine and ifenprodil are illustrated. The interactions of spermine and ifenprodil with residues in both GluN1 and GluN2B, of course, do not imply that there are separate functional binding sites for each modulator on GluN1 and GluN2B. Indeed, as discussed below, each subunit R domain (GluN1R and GluN2BR) may contribute to a single functional binding site for each modulator in an intact heterodimer.
Fig. 4.
Modeling of spermine and ifenprodil binding to GluN1R and GluN2BR monomers. Modeling was done as described under Materials and Methods. A, spermine and ifenprodil binding to GluN1R and GluN2BR monomers is shown from two different angles, rotated by 90°. B, expanded spermine and ifenprodil binding sites on GluN1R and GluN2BR monomers. SPM, spermine; IFN, ifenprodil; NT, N terminus; CT, C terminus. Amino acid residues that influence spermine stimulation, ifenprodil inhibition, and both spermine stimulation and ifenprodil inhibition are depicted with a stick model in red, green, and blue, respectively.
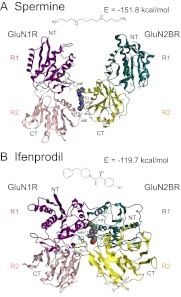
Model structures of a heterodimer of GluN1R and GluN2BR with spermine or ifenprodil were constructed, because there is good evidence that R domains exist and function as a heterodimer (Karakas et al., 2011; Mony et al., 2011). In these models, additional amino acid residues are involved in the interactions with spermine and ifenprodil. Thus, it is expected that spermine and ifenprodil bind to the heterodimer more tightly than to the GluN1R and GluN2BR monomers. As shown in Fig. 5, the relative positions of GluN1R and GluN2BR are altered through the binding of spermine and ifenprodil—the R1 lobes of GluN1R and GluN2BR are separated as a consequence of spermine binding but are brought together through the binding of ifenprodil. This difference may lead ultimately to the stimulation (spermine) or inhibition (ifenprodil) of NMDA receptor activity through the interaction of the R domains with the LBDs.
Fig. 5.
Modeling of spermine (A) and ifenprodil (B) binding sites on GluN1R and GluN2BR heterodimers. Modeling was done as described under Materials and Methods. NT, N terminus; CT, C terminus; R1 and R2, R1 and R2 lobes, respectively. The models show the approximate orientation of GluN1R and GluN2BR. The interaction energy of these complexes also is shown.
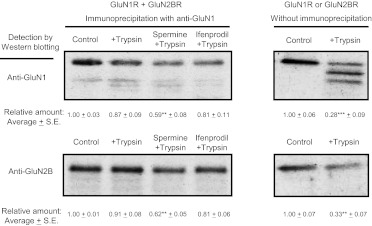
To look for further evidence of a difference in the structure of the GluN1R/GluN2BR heterodimer when spermine or ifenprodil is bound, the trypsin sensitivity of GluN1R and GluN2BR, which were purified as described previously (Han et al., 2008), was examined. As shown in Fig. 6, the GluN1R and GluN2BR monomers were digested readily by trypsin (right panels in Fig. 6), but the heterodimer complex of GluN1R and GluN2BR (studied in the absence of spermine or ifenprodil) was nearly insensitive to trypsin. The recovery of GluN1R and GluN2BR by anti-GluN1 was nearly equal (control in left panels in Fig. 6), confirming that GluN1R and GluN2BR form a heterodimer. Sensitivity of the heterodimer to trypsin was increased in the presence of spermine to a greater extent than in the presence of ifenprodil. This is consistent with the idea that the spermine-GluN1R/GluN2BR complex is more relaxed than the ifenprodil-GluN1R/GluN2BR complex.
Fig. 6.
Trypsin sensitivity of the heterodimer GluN1R/GluN2BR with spermine or ifenprodil and of GluN1R or GluN2BR monomer. The heterodimer GluN1R/GluN2BR with and without spermine (100 μM) or ifenprodil (1 μM) was treated with trypsin, and the level of GluN1R and GluN2BR was measured as described under Materials and Methods. Complexes were immunoprecipitated with anti-GluN1 antibody and detected by Western blot analysis with anti-GluN1 or anti-GluN2B antibodies. In the right panel, GluN1R or GluN2BR monomer was incubated with trypsin and detected by Western blot analysis with anti-GluN1 or anti-GluN2B antibodies. The level of protein was quantified with a LAS-3000 luminescent image analyzer (Fuji Film, Tokyo, Japan). Relative amounts are shown as mean ± S.E. of triplicate determinations. Student's t test was performed for the control values versus the values obtained with trypsin treatment. **, p < 0.01; ***, p < 0.001.
Necessity of GluN2B LBD for Spermine Stimulation and Ifenprodil Inhibition.
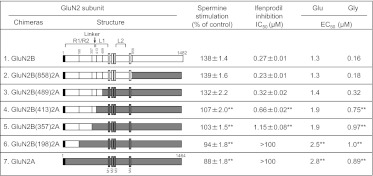
We determined whether an interaction between the R domain and the LBD of GluN2B is necessary for spermine stimulation and ifenprodil inhibition by constructing various fusion genes of GluN2A and GluN2B, because there are differences in the amino acid sequences in the linker region between the R domain and the N-terminal region (L1 lobe) of the LBDs (Fig. 3). It was found that not only the linker region but also part of the L1 lobe of GluN2B is necessary for spermine stimulation and ifenprodil inhibition [Fig. 7, construct 3, GluN2B(489)2A]. If only the R domain and linker region of GluN2B was fused to the residual part of GluN2A [Fig. 7, construct 4, GluN2B(413)2A], then spermine stimulation and ifenprodil inhibition were smaller than those seen in GluN2B or GluN2B(489)2A. The results suggest that an interaction between the R domain and the L1 lobe of the LBD of GluN2B is necessary for spermine stimulation and ifenprodil inhibition in GluN1/GluN2B receptors. In addition, the EC50 for glutamic acid and glycine was influenced strongly by the R domains. When GluN2BR and the L1 lobe of GluN2B were present instead of GluN2AR and the L1 lobe of GluN2A, the EC50 values for glutamic acid and glycine were reduced. The results again support the idea that the R domain influences the function of the LBD allosterically. These results also support the idea that the functional unit of NMDA receptors consists of a heterodimer of GluN1 and GluN2, because a structural change of GluN2 influences the affinity for glycine, which binds to the LBD of GluN1.
Fig. 7.
Effects of spermine and ifenprodil at GluN1/GluN2 receptors containing wild-type or chimeric GluN2B and GluN2A subunits. Schematics of the chimeric GluN2B and GluN2A constructs are shown in the figure. R1, R2, L1, and L2 indicate lobes of the R domains and LBDs, respectively. Linker (↓) indicates the linker region between the R domains and the LBDs. Spermine stimulation and ifenprodil inhibition were measured at GluN1/GluN2 receptors containing wild-type or chimeric GluN2 subunits using 100 μM spermine and various concentrations of ifenprodil in oocytes voltage-clamped at −20 mV. The EC50 values for glutamic acid and glycine were determined from currents measured over a range of concentrations of glutamic acid and glycine. Values are means from at least five oocytes. Statistical analysis was performed using one-way analysis of variance with a Tukey-Kramer post hoc test. **, p < 0.01 versus values obtained from GluN1/GluN2B receptors.
Discussion
In this study, we tried to identify the binding sites for spermine and ifenprodil on the heterodimer of rat GluN1R and GluN2BR subunits, based on the results of studies measuring spermine stimulation and ifenprodil inhibition at intact receptors containing GluN1R and GluN2BR mutants. This approach was driven in part by the report that GluN1R and GluN2BR exist as a heterodimer (Karakas et al., 2011). We confirmed that a heterodimer complex is formed in experiments using recombinant R domain proteins, in which an antibody against GluN1R precipitated both GluN1R and GluN2BR. In modeling studies, it was found that the structure of the complex of spermine-GluN1R/GluN2BR was different from that of the complex of ifenprodil-GluN1R/GluN2BR. Although an open space between the R1 lobes of GluN1R and GluN2BR was promoted by the binding of spermine, the R1 lobes of GluN1R and GluN2BR became closer after the binding of ifenprodil. Because the relative positions of the C termini of GluN1R and GluN2BR in a spermine-GluN1R/GluN2BR complex were different from those in an ifenprodil-GluN1R/GluN2BR complex (Fig. 5), the relative positions or spacing of the R domain and LBD may be changed through the binding of spermine and ifenprodil. In this way, an R domain heterodimer may have different effects on the LBDs of GluN1 and GluN2B, depending on whether spermine or ifenprodil is bound to the R domain. Indeed, different conformational states of R domains have been observed in native α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor complexes (Nakagawa et al., 2005).
With regard to the complex of spermine-GluN1R/GluN2BR, a similar model has been proposed based on the measurement of NMDA receptor activity using mutants at a number of cysteine and acidic amino acid residues (Mony et al., 2011). We have taken a similar approach, but our model allows for more detail because many amino acid residues involved in spermine stimulation were identified (Fig. 1). In our model, not only the R2 lobes of both GluN1R and GluN2BR but also the R1 lobe of GluN1R are involved in the formation of the complex with spermine. Spermine bound at the interface between GluN1R and GluN2BR rather than within the cleft of GluN1R and GluN2BR.
With regard to the ifenprodil-GluN1R/GluN2BR complex, the reported X-ray crystallographic structure was determined using a splice variant of GluN1R from Xenopus laevis that includes exon 5 (Karakas et al., 2011), rather than a GluN1R variant that lacks exon 5 (used in the present work), together with rat GluN2BR. Our model constructed with rat GluN1R (lacking exon 5) and GluN2BR was essentially the same as the published crystal structure (Karakas et al., 2011). The results confirmed that the effects of ifenprodil on GluN1/GluN2B receptors are not affected markedly by the presence or absence of the exon 5 insert (Pahk and Williams, 1997).
It was shown that residues Asp206, Asp210, and Asp213 in GluN2BR are involved in both spermine stimulation and ifenprodil inhibition. The structure of the region containing these three residues may be flexible and not contribute directly to the binding sites for spermine and ifenprodil. However, this region may be important for the formation of a ternary complex containing GluN1R, GluN2BR, and spermine (or ifenprodil). Notably, the structure of this region in GluN2AR is different from the structure in GluN2BR, and spermine stimulation and ifenprodil inhibition are not observed in receptors containing GluN1 plus GluN2A. Thus, this region may be the key determinant of the subunit-specific effects of spermine and ifenprodil.
In conclusion, the structure of the GluN1R/GluN2BR heterodimer was changed significantly through the binding of spermine or ifenprodil. Such a structural change is probably involved in alterations in NMDA receptor channel gating through an interaction with the LBDs.
Acknowledgments
We thank Drs. S. Nakanishi, P. H. Seeburg, and M. Mishina for the supply of rat and mouse GluN1, GluN2A, and GluN2B clones.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS35047]; and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- NMDA
- N-methyl-d-aspartate
- LBD
- ligand binding domain
- MOE
- Molecular Operating Environment
- R
- regulatory.
Authorship Contributions
Participated in research design: Tomitori, Williams, Kashiwagi, and Igarashi.
Conducted experiments: Tomitori, Suganami, Saiki, Mizuno, Yoshizawa, Masuko, Tamura, Nishimura, Toida, and Williams.
Performed data analysis: Tamura, Williams, Kashiwagi, and Igarashi.
Wrote or contributed to the writing of the manuscript: Williams, Kashiwagi, and Igarashi.
References
- Armstrong N, Sun Y, Chen GQ, Gouaux E. (1998) Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 395:913–917 [DOI] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201 [DOI] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. (1993) Multiple effects of spermine on N-methyl-d-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol 464:131–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Bennett MV, Zukin RS. (1993) Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA 90:6731–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. (2003) Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J 22:2873–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. (2005) Subunit arrangement and function in NMDA receptors. Nature 438:185–192 [DOI] [PubMed] [Google Scholar]
- Han X, Tomitori H, Mizuno S, Higashi K, Füll C, Fukiwake T, Terui Y, Leewanich P, Nishimura K, Toida T, et al. (2008) Binding of spermine and ifenprodil to a purified, soluble regulatory domain of the N-methyl-D-aspartate receptor. J Neurochem 107:1566–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59 [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42:39–51 [DOI] [PubMed] [Google Scholar]
- Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, Jin Y, Gouaux E. (2009) Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J 28:1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. (2009) Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J 28:3910–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. (2011) Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature 475:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K, Masuko T, Nguyen CD, Kuno T, Tanaka I, Igarashi K, Williams K. (2002) Channel blockers acting at N-methyl-D-aspartate receptors: differential effects of mutations in the vestibule and ion channel pore. Mol Pharmacol 61:533–545 [DOI] [PubMed] [Google Scholar]
- Kumar J, Schuck P, Jin R, Mayer ML. (2009) The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat Struct Mol Biol 16:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. (1987) Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol 154:367–382 [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. (1994) Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron 12:1291–1300 [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. (1992) Molecular diversity of the NMDA receptor channel. Nature 358:36–41 [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. (1997) Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron 18:493–503 [DOI] [PubMed] [Google Scholar]
- Masuko T, Kashiwagi K, Kuno T, Nguyen ND, Pahk AJ, Fukuchi J, Igarashi K, Williams K. (1999) A regulatory domain (R1–R2) in the amino terminus of the N-methyl-d-aspartate receptor: effects of spermine, protons, and ifenprodil, and structural similarity to bacterial leucine/isoleucine/valine binding protein. Mol Pharmacol 55:957–969 [DOI] [PubMed] [Google Scholar]
- Masuko T, Kusama-Eguchi K, Sakata K, Kusama T, Chaki S, Okuyama S, Williams K, Kashiwagi K, Igarashi K. (2003) Polyamine transport, accumulation, and release in brain. J Neurochem 84:610–617 [DOI] [PubMed] [Google Scholar]
- Mayer ML. (2005) Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron 45:539–552 [DOI] [PubMed] [Google Scholar]
- Mayer ML, Olson R, Gouaux E. (2001) Mechanisms for ligand binding to GluR0 ion channels: crystal structures of the glutamate and serine complexes and a closed apo state. J Mol Biol 311:815–836 [DOI] [PubMed] [Google Scholar]
- Mony L, Zhu S, Carvalho S, Paoletti P. (2011) Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J 30:3134–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256:1217–1221 [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature 354:31–37 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. (2005) Structure and different conformational states of native AMPA receptor complexes. Nature 433:545–549 [DOI] [PubMed] [Google Scholar]
- Nielsen PJ, Manchester KL, Towbin H, Gordon J, Thomas G. (1982) The phosphorylation of ribosomal protein S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J Biol Chem 257:12316–12321 [PubMed] [Google Scholar]
- Pahk AJ, Williams K. (1997) Influence of extracellular pH on inhibition by ifenprodil at N-methyl-d-aspartate receptors in Xenopus oocytes. Neurosci Lett 225:29–32 [DOI] [PubMed] [Google Scholar]
- Perin-Dureau F, Rachline J, Neyton J, Paoletti P. (2002) Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci 22:5955–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. (1993) Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44:851–859 [PubMed] [Google Scholar]
- Williams K. (1997) Interactions of polyamines with ion channels. Biochem J 325:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Kashiwagi K, Fukuchi J, Igarashi K. (1995) An acidic amino acid in the N-methyl-d-aspartate receptor that is important for spermine stimulation. Mol Pharmacol 48:1087–1098 [PubMed] [Google Scholar]
- Williams K, Pahk AJ, Kashiwagi K, Masuko T, Nguyen ND, Igarashi K. (1998) The selectivity filter of the N-methyl-d-aspartate receptor: a tryptophan residue controls block and permeation of Mg2+. Mol Pharmacol 53:933–941 [PubMed] [Google Scholar]
- Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. (1994) Sensitivity of the N-methyl-d-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol 45:803–809 [PubMed] [Google Scholar]