Abstract
Within the group I family of metabotropic glutamate receptors (mGluRs), substantial evidence points to a role for mGluR5 mechanisms in cocaine's abuse-related behavioral effects, but less is understood about the contribution of mGluR1, which also belongs to the group I mGluR family. The selective mGluR1 antagonist JNJ16259685 [(3,4-dihydro-2H-pyrano-[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone] was used to investigate the role of mGluR1 in the behavioral effects of cocaine and methamphetamine. In drug discrimination experiments, squirrel monkeys were trained to discriminate cocaine from saline by using a two-lever, food-reinforced operant procedure. JNJ16259685 (0.56 mg/kg) pretreatments significantly attenuated cocaine's discriminative stimulus effects and the cocaine-like discriminative stimulus effects of methamphetamine. In monkeys trained to self-administer cocaine or methamphetamine under a second-order schedule of intravenous drug injection, JNJ16259685 (0.56 mg/kg) significantly reduced drug-reinforced responding, resulting in a downward displacement of dose-response functions. In reinstatement studies, intravenous priming with cocaine accompanied by restoration of a cocaine-paired stimulus reinstated extinguished cocaine-seeking behavior, which was significantly attenuated by JNJ16259685 (0.56 mg/kg). Finally, in experiments involving food rather than drug self-administration, cocaine and methamphetamine increased the rate of responding, and the rate-increasing effects of both psychostimulants were significantly attenuated by JNJ16259685 (0.3 mg/kg). At the doses tested, JNJ16259685 did not significantly suppress food-reinforced behavior (drug discrimination or fixed-interval schedule of food delivery), but did significantly reduce species-typical locomotor activity in observational studies. To the extent that the psychostimulant-antagonist effects of JNJ16259685 are independent of motor function suppression, further research is warranted to investigate other mGluR1 antagonists for potential therapeutic value in psychostimulant abuse.
Introduction
Robust glutamatergic innervation of mesocorticolimbic dopamine regions provides an anatomical basis for interactions between glutamate and dopamine that contribute to the addictive effects of drugs of abuse (Kenny and Markou, 2004). Of the three main groups of G protein-coupled metabotropic glutamate receptors (mGluRs), the group I family (comprising mGluR1 and mGluR5 subtypes) has been implicated frequently in abuse-related behavioral effects of cocaine (Bird and Lawrence, 2009). Furthermore, group I mGluRs are expressed in brain regions implicated in the pathology of drug addiction, including the ventral tegmental area, nucleus accumbens, basolateral and central nuclei of the amygdala, hippocampus, and prefrontal cortex (Kenny and Markou, 2004; Mitrano and Smith, 2007) and have therefore been proposed as potentially important in mediating the reinforcing effects of substances of abuse (Kenny and Markou, 2004; Bird and Lawrence, 2009; Olive, 2009).
Of the group I family, the mGluR5 subtype has been extensively investigated and consistently found to play a role in substance abuse (see Bird and Lawrence, 2009 for review), whereas comparatively less is known about the influence of the mGluR1 subtype. The available preclinical evidence suggests, however, that mGluR1 activity can modulate some behavioral effects of drugs of abuse in rodents. For example, the pharmacological inhibition of mGluR1 can attenuate ethanol-induced place preference and ethanol self-administration (Lominac et al., 2006; Besheer et al., 2008). Regarding psychostimulants, the inhibition of mGluR1 activity blocked cocaine-induced psychomotor sensitization (Dravolina et al., 2006; Kotlinska and Bochenski, 2011) and methamphetamine-induced hyperlocomotion (Satow et al., 2008), and amphetamine-induced locomotor activity was greater in mGluR1 knockout mice compared with their wild-type littermates (Mao et al., 2001). In reinstatement studies, cue-induced reinstatement of cocaine seeking (Xie et al., 2010, 2012) and nicotine-induced reinstatement of nicotine seeking (Dravolina et al., 2007) were also attenuated by mGluR1 antagonists. In addition, emerging evidence at the molecular level implicates mGluR1 in the regulation of cocaine's long-term effects because it has been shown that the pharmacological manipulation of mGluR1 activity can alter cocaine-induced trafficking of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (McCutcheon et al., 2011), which are ligand-gated ionotropic glutamatergic receptors critical for cocaine-induced synaptic plasticity (Tzschentke and Schmidt, 2003).
The purpose of the present study was to further characterize the role of the group I family of glutamate receptors in the abuse-related behavioral effects of cocaine and methamphetamine. We investigated the contribution of the mGluR1 subtype by using an antagonist-based strategy in relevant nonhuman primate behavioral models. Pharmacological blockade of mGluR1 was achieved by administration of (3,4-dihydro-2H pyrano-[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl) methanone (JNJ16259685), a selective mGluR1 antagonist that exhibits ∼1000-fold selectivity over mGluR5 (Lavreysen et al., 2004). Specifically, this study investigated the impact of JNJ16259685 on: 1) cocaine's DS effects and the cocaine-like DS effects of methamphetamine, 2) the reinforcing effects of cocaine and methamphetamine, 3) the priming effects of cocaine and a cocaine-paired stimulus in the reinstatement of drug-seeking behavior, and 4) the behavioral stimulant (response rate-increasing) effects of cocaine and methamphetamine on food-reinforced operant behavior. Quantitative observational studies also determined the effects of JNJ16259685 on a range of species-typical unconditioned behaviors.
Materials and Methods
Subjects.
Adult male squirrel monkeys (Saimiri sciureus) weighing 0.8 to 1.2 kg were housed in a climate-controlled vivarium, where they had unlimited access to water and received a nutritionally balanced diet of monkey chow (Harlan Teklad, Madison, WI) supplemented with fresh fruit. A total of 25 monkeys was studied, with groups of four to six monkeys serving as subjects in each experiment. Animals in cocaine discrimination and food reinforcement experiments were maintained at 90 to 95% of their free-feeding body weights by adjusting access to food in the home cages. All monkeys were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011) and the guidelines of the Committee on Animals of Harvard Medical School, and research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Surgery.
In experiments involving drug self-administration, indwelling venous catheters were implanted by using aseptic surgical procedures as described previously (Platt et al., 2011). In brief, monkeys were anesthetized with isoflurane, and the catheter was passed via a femoral or jugular vein to the level of the right atrium. The distal end of the catheter was then passed subcutaneously to the midscapular exit point. Catheters were flushed daily with 0.9% saline solution and sealed with stainless-steel obturators when not in use. Monkeys wore nylon-mesh jackets (Lomir Biomedical, Quebec, Canada) at all times to protect the catheter.
Apparatus.
With the exception of observational studies, all experiments were conducted in ventilated sound-attenuated chambers (MED Associates, St. Albans, VT), which were equipped with white noise to mask external sounds. Within the chambers, the monkeys were seated in chairs (MED Associates) facing a panel that was equipped with response levers and stimulus lights above the levers. In experiments with catheterized subjects, catheters were connected to motor-driven syringe pumps (MED Associates) located outside the chamber. Each operation of the pump delivered a 1-s infusion of 0.18 ml of saline, cocaine, or methamphetamine solution into the catheter. In drug discrimination and food reinforcement experiments, 190-mg sucrose pellets (Bio-Serv, Frenchtown, NJ) could be delivered to a receptacle in the front panel of the chair. Experiments were controlled, and data were recorded via interfaces (MED Associates) and computers located in an adjacent room. Observational studies were conducted in a ventilated, transparent Plexiglas arena (114 × 122 × 213 cm) situated in a lighted room, separate from other animals (Platt et al., 2000). The arena was equipped with perches, suspended plastic chains, manipulable devices, and a wood-chip foraging substrate to permit a range of species-typical behaviors. A video camera was positioned 1 m in front of the chamber to provide an archival record of each subject's behavior during the session.
Cocaine Discrimination.
Six monkeys were trained to discriminate cocaine from vehicle (0.9% saline solution) injections by using procedures similar to those described previously (Spealman et al., 1991). In brief, 10 consecutive responses [fixed ratio (FR) 10] on one lever (counterbalanced across subjects) produced a sucrose pellet if cocaine was injected, whereas 10 consecutive responses on the other lever produced a sucrose pellet if vehicle was injected. Each response on the inappropriate lever reset the FR10 requirement. Delivery of each sucrose pellet was followed by a 10-s timeout (TO) during which the chamber was dark and lever pressing had no programmed consequences.
During training sessions, this procedure was repeated 10 times per component, and the number of components per session (n = 1–4) was varied daily in an irregular order. Components were terminated after delivery of the 10th reinforcer or 5 min, whichever occurred first. Each component was preceded by an extended (10-min) TO, during which vehicle was administered 5 min before the beginning of the n-1 component(s) of the session, and cocaine was administered 5 min before the nth component. Occasionally saline was administered before all components to avoid invariant association with drug and the last component. The training dose of cocaine was 0.30 mg/kg in four subjects but was increased by ∼1/8 of a log unit to 0.42 mg/kg in two subjects to achieve the performance criteria (see below) for testing. These training doses remained constant for the duration of the study.
Drug testing began when the monkeys made ≥90% of responses on the injection-appropriate lever for at least three consecutive sessions, permitting test sessions to be conducted at approximately weekly intervals. Test sessions consisted of four components, each preceded by an extended TO (10 min). In each component, completion of FR10 on either lever resulted in delivery of a sucrose pellet. Dose-response functions for cocaine and methamphetamine were determined by using a cumulative-dosing procedure in which incremental doses of cocaine or methamphetamine (1/4–1/2 log unit increments) were injected at the 5-min point of the TO periods that preceded sequential components of a test session. To achieve a five-point cumulative dose-response function, which included saline injections, there were two rounds of test sessions in which an overlapping range of doses was tested. In experiments involving drug pretreatments, JNJ16259685 (0.18 and 0.56 mg/kg) or vehicle was administered 25 min before the start of the test session. The pretreatment interval was chosen based on experiments in which JNJ16259685's effects were observed in rhesus monkeys between 10 and 90 min post-treatment (Yamasaki et al., 2012).
Drug Self-Administration.
Monkeys were trained to self-administer cocaine (0.1 mg/kg/injection; n = 6) or methamphetamine (0.03 mg/kg/injection; n = 6) by pressing a lever under a second-order fixed interval (FI)/FR schedule of intravenous drug injection similar to the schedule described previously (Achat-Mendes et al., 2010). In brief, in the presence of a white light, completion of every 10th response during a 5-min FI resulted in a 2-s change in illumination from white to red. Completion of the first FR after expiration of the FI resulted in an intravenous injection of cocaine or methamphetamine simultaneous with the onset of a 2-s red light (the drug-paired stimulus). A 60-s TO period, during which all lights were off and responses had no scheduled consequences, followed each drug injection. If the FR requirement was not completed within 8 min after the expiration of the FI (limited hold), the component ended automatically without an injection. Daily sessions ended after completion of 10 cycles of the second-order schedule or a maximum of 90 min, whichever occurred first.
To investigate the effects of JNJ16259685 on the reinforcing effects of cocaine and methamphetamine, doses of self-administered drug that varied over a 10-fold range for both cocaine (0.03, 0.1, and 0.3 mg/kg/injection) and methamphetamine (0.01, 0.03, and 0.10 mg/kg/injection) were first evaluated. Before a test session, stable baseline response rates were maintained in which no systematic increase or decrease in the rate of responding was observed for a minimum of three consecutive sessions. During test sessions, JNJ16259685 (0.10–0.56 mg/kg) or its vehicle was administered as an intramuscular treatment 25 min before the start of the self-administration session. Each dose of JNJ16259685 was studied once in each monkey, and different doses of JNJ16259685 and self-administered drug were studied in a counterbalanced order among subjects. After a test session, subjects underwent approximately 2 to 3 weeks of self-administration without drug pretreatment to reacquire baseline rates of responding.
Reinstatement of Drug Seeking.
Reinstatement studies were conducted in four monkeys trained to self-administer cocaine by using second-order schedule parameters and procedures identical to those described above. Once stable response rates were established (criteria as described previously) extinction of cocaine-seeking behavior took place during daily sessions in which lever pressing produced saline instead of cocaine injections, and the cocaine-paired stimulus was omitted; all other conditions remained unchanged. Extinction sessions were conducted daily until responding declined and stabilized at ≤10% of the response rate maintained by cocaine self-administration (at least three consecutive sessions), at which time reinstatement testing began. During reinstatement test sessions, only saline was available for self-administration, and the response-contingent presentations of the cocaine-paired stimulus were restored after a noncontingent priming injection of cocaine or saline. Earlier studies showed that robust reinstatement of cocaine-seeking behavior in monkeys is achieved by intravenous priming with cocaine accompanied by restoration of the cocaine-paired stimulus (Spealman et al., 1999).
In addition to a saline prime, a range of priming doses of cocaine was evaluated for their ability to reinstate extinguished drug-seeking behavior. Cocaine and saline priming injections were administered intravenously immediately before the session followed by a saline flush to clear the catheter of residual drug solution. During testing, JNJ16259685 (0.56 mg/kg) or vehicle pretreatments were administered 25 min before an intravenous priming injection of cocaine (0.1–1.0 mg/kg). Because priming with saline did not induce significant reinstatement of cocaine seeking, JNJ16259685 pretreatments were administered only before priming injections of cocaine. The effects of different doses of cocaine and JNJ16259685 were tested on different days, with each test session separated by three or more extinction sessions as described above.
Food-Reinforced Behavior.
To investigate the effects of JNJ16259685 on the behavioral stimulant (rate-increasing) effects of cocaine and methamphetamine on schedule-controlled behavior, four monkeys were trained to respond under a second-order schedule of food presentation with schedule parameters identical to the second-order schedule of drug self-administration described above except completion of the first FR after expiration of the FI resulted in delivery of three sucrose pellets, instead of an injection, coinciding with the 2-s change in stimulus light. Test sessions were conducted when response rates were stable (criteria as described above). Before the start of the test session JNJ16259685 (0.30 mg/kg) or vehicle was administered as a pretreatment (25 min) followed by an injection of cocaine (0.1–1.0 mg/kg), methamphetamine (0.03–0.56 mg/kg), or vehicle immediately before the session.
Observational Studies.
Observation studies were conducted with four monkeys by using procedures similar to those described previously (Lee et al., 2005). After habituation to the observation arena, handling, and injection procedures, 30-min observation sessions were conducted daily, during which the animal's behavior was recorded continuously. Scoring of video recordings was conducted by using the behavioral scoring system described previously (Platt et al., 2000) in which a range of species-typical behaviors (Table 1) was scored as either present or absent in 15-s intervals during three 5-min observation periods across the session (0–5, 12–17, and 24–29 min). Frequency scores were calculated from these data as the number of 15-s intervals in which a particular behavior was observed. In addition, during the 6th, 18th, and 30th min of each session, the monkey was removed from the observational arena by a trained handler and evaluated for ataxia [defined as the inability to balance on and/or grasp a stainless-steel pole (56.0 cm in length; 1.0 cm in diameter) held in a horizontal plane] and muscle resistance (defined as resistance to hind limb extension). For ataxia, a score of 0 indicated that the monkey was able to balance normally on the pole, a score of 1 indicated an inability to balance effectively, and a score of 2 indicated that the monkey could neither balance on nor grasp the pole. For muscle resistance, a score of 0 indicated no change in resistance to hind-limb extension, a score of +1 indicated increased resistance to extension and/or clinging to the grid floor, and a score of −1 indicated decreased resistance to extension and/or flaccidity. Drug test sessions were conducted twice a week with saline control sessions on intervening days. JNJ16259685 (0.18–0.56 mg/kg) was administered intramuscularly 25 min before placing the subject in the observation arena.
TABLE 1.
Behavioral categories adapted from Platt et al. (2000)
| Behavioral Categories | Description |
|---|---|
| Environment-directed: | |
| Locomotion | Two or more directed steps in the horizontal or vertical plane |
| Object exploration | Tactile or oral manipulation of features of the observational arena |
| Foraging | Sweeping and/or picking through wood-chip substrate |
| Self-directed: | |
| Self-grooming | Picking, scraping, spreading, or licking of fur |
| Scratching | Rapid movement of digits through fur in a rhythmic motion |
| Visual scanning | Directed eye and/or head movements, usually from a sitting position. |
| Vocalization | Any utterance including chirps, twitters, peeps, etc. |
| Resting posture | Species-typical posture associated with sleep: eyes closed, crouched on hind legs, hunched back, tail wrapped around upper body |
| Static posture procumbent posture | Maintenance of a rigid, atypical posture. Lying on cage floor or perch; loose-limbed |
Drugs.
Cocaine hydrochloride and (+)-methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline solution. JNJ16259685 (Tocris Bioscience, Ellisville, MO) was dissolved in 45% (w/v) 2-hydroxypropyl-β-cyclodextrin. Injection volumes did not exceed 0.3 ml per intramuscular injection or 0.18 ml per intravenous injection.
Data Analysis.
In drug discrimination studies, the percentage of drug-lever responding was computed for individual subjects in each component of a test session, with the restriction that response rate was ≥0.1 responses/s during the component. The mean percentage of drug-lever responding and S.E.M. values were then calculated for the group at each dose. The dose of a drug needed to engender 50% cocaine-appropriate responding (ED50) was estimated for individual subjects by linear regression analysis in cases where the linear ascending portion of the log dose-response function was defined by at least three data points or linear interpolation in cases where the log dose-response function was defined best by two points. The mean ± S.E.M. ED50 value for each drug was determined by averaging ED50 values for individual subjects. Response rates in each component for individual subjects were calculated by dividing the total number of responses (regardless of lever) by the total component duration exclusive of timeout periods. Individual data were then averaged across subjects.
In studies involving cocaine- and food-reinforced behavior under the second-order schedule, rates of responding were calculated for each session by dividing the total number of responses by the total elapsed time (excluding timeout periods) for individual subjects across the 10 sequential components of the session as well as for the entire session. In observational experiments, individual scores for each behavior were averaged across the three 5-min observation periods. In each experiment, data from individual subjects were averaged to obtain group means. Data were analyzed by using one-way or two-way repeated-measures ANOVAs and further analyzed by using a priori Bonferroni t tests for planned comparisons. Criterion for significance was p < 0.05 for all analyses.
Results
Effect of JNJ16259685 on the Discriminative Stimulus Effects of Cocaine and the Cocaine-Like Effects of Methamphetamine.
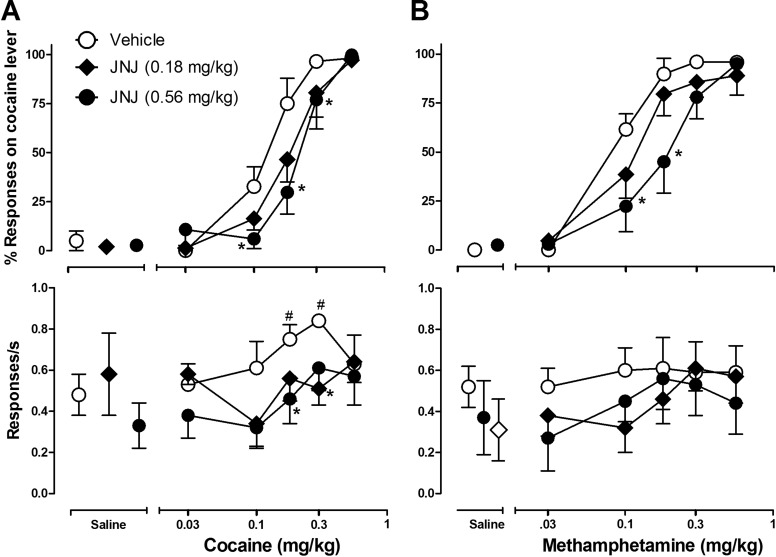
During baseline sessions on the days preceding drug test sessions, the training dose of cocaine (0.3 or 0.42 mg/kg depending on the monkey) engendered 96 to 98% responses on the cocaine-associated lever, whereas administration of saline engendered an average of only 1 to 6% responses on the cocaine-associated lever among individual monkeys. During test sessions, cocaine (cumulative doses: 0.03–0.56 mg/kg) preceded by an injection of vehicle (45% 2-hydroxypropyl-β-cyclodextrin) engendered dose-related increases in cocaine-lever responding, with cumulative doses ≥0.3 mg/kg engendering nearly exclusive cocaine-lever responding and a dose of 0.03 mg/kg engendering nearly exclusive saline-lever responding (Fig. 1A, top, ○).
Fig. 1.
Effects of pretreatment with JNJ16259685 and vehicle on the discriminative stimulus effects of cocaine (A) and the cocaine-like discriminative stimulus effects of methamphetamine (B) in monkeys trained to discriminate cocaine from saline. Top, percentage of responses on the cocaine-associated lever. Bottom, rate of responding. Data are mean ± S.E.M. Points above the Saline column show the effects of pretreatment with vehicle or JNJ16259685 before saline. #, p < 0.05, effects of cocaine or methamphetamine compared with saline. *, p < 0.05, effects of pretreatment with JNJ16259685 compared with its vehicle; Bonferroni t test.
Pretreatment with JNJ16259685 (0.18 or 0.56 mg/kg) produced overall rightward shifts in the cumulative dose-response curve (Fig. 1A, top, ●, ♦). Statistical analysis of the effects of JNJ16259685 by a two-way RM ANOVA revealed a main effect of JNJ16259685 (F1,15 = 6; p < 0.05) and cocaine (F4,15 = 38; p < 0.001), and planned comparisons showed that pretreatment with 0.56 mg/kg JNJ16259685 significantly reduced the percentage of cocaine-lever responses engendered by cocaine doses of 0.1 to 0.3 mg/kg (p < 0.05; Bonferroni t test). Comparison of the average ED50 values for cocaine after pretreatment with vehicle or JNJ16259685 showed that the dose of cocaine required to engender 50% cocaine-lever responding was increased ∼1.5-fold (from 0.18 ± 0.03 to 0.28 ± 0.03 mg/kg) as a result of pretreatment with 0.56 mg/kg JNJ16259685, but not as a result of pretreatment with 0.18 mg/kg JNJ16259685 (0.18 ± 0.03 versus 0.19 ± 0.03 mg/kg). One-way RM ANOVA revealed a significant effect of JNJ16259685 on the ED50 for cocaine (F2,5 = 9; p < 0.01), and planned comparisons using Bonferroni t tests revealed that pretreatment with 0.56 mg/kg JNJ16259685 significantly increased the ED50 for cocaine.
The rate of lever pressing after vehicle pretreatment was characterized by an inverted U-shaped dose-response function (Fig. 1A, bottom, ○), with doses of 0.18 and 0.30 mg/kg cocaine producing maximum rates of responding. Pretreatment with JNJ16259685 shifted this dose-response function downward but did not significantly alter response rates engendered by saline injections and (Fig. 1A, bottom, leftmost symbols). Two-way RM ANOVA revealed significant effects of cocaine (F4,15 = 4; p < 0.05) and JNJ16259685 (F1,15 = 11; p < 0.05) on the rate of responding. Bonferroni t tests showed that response rates were significantly increased by cumulative doses of 0.18 and 0.3 mg/kg cocaine after vehicle pretreatment compared with saline after vehicle pretreatment (p < 0.05) and the rate-increasing effects 0.18 and 0.30 mg/kg cocaine were significantly attenuated after pretreatment with 0.56 mg/kg JNJ16259685 (p < 0.05).
Like cocaine, methamphetamine, after pretreatment with vehicle, engendered dose-related increases in drug-lever responding, with cumulative doses ≥0.18 mg/kg methamphetamine engendering ≥90% drug-lever responding, and a dose of 0.03 mg/kg methamphetamine engendering nearly exclusive saline-lever responding (Fig. 1B, top, ○). Pretreatment with 0.18 mg/kg had minimal effects on the methamphetamine cumulative dose-response curve, whereas 0.56 mg/kg JNJ16259685 resulted in a rightward shift (Fig. 1B, top, ●, ♦). Two-way RM ANOVA revealed a main effect of methamphetamine (F4,11 = 60; p < 0.001), but not of JNJ16259685 (F1,11 = 3; p > 0.05). Planned comparisons using a priori Bonferroni t tests showed that the percentage of drug-lever responses engendered by 0.10 and 0.18 mg/kg methamphetamine was significantly reduced after pretreatment with JNJ16259685 (0.56 mg/kg) compared with pretreatment with vehicle (p < 0.05). Furthermore, the average dose of methamphetamine required to engender 50% drug-lever responding was increased approximately ∼1.9-fold [from 0.12 (± 0.01) to 0.23 (± 0.04) mg/kg] after pretreatment with JNJ16259685 (0.56 mg/kg) compared with pretreatment with vehicle. One-way RM ANOVA revealed an effect of JNJ16259685 (0.56 mg/kg) on the ED50 for methamphetamine that approached significance (F1,11 = 6; p = 0.05).
Cumulative dosing with methamphetamine after vehicle pretreatment yielded a narrow range of response rates that was similar to the rate of responding after saline and vehicle treatment (Fig. 1B, bottom, ○, ♢). Pretreatment with JNJ16259685 (0.18 and 0.56 mg/kg) did not alter response rates engendered by any dose of methamphetamine (Fig. 1B, bottom, ●, ♦). Two-way RM ANOVA revealed significant effects of methamphetamine (F4,16 = 5; p < 0.05), but not JNJ16259685 (F1,16 = 0.6; p > 0.05) on the rate of responding. Although response rates after administration of saline and the lowest dose of methamphetamine (0.03 mg/kg) seemed to be reduced by JNJ16259685 pretreatment, these observations were not confirmed statistically (p > 0.05; Bonferroni t test).
Effect of JNJ16259685 on Cocaine and Methamphetamine Self-Administration.
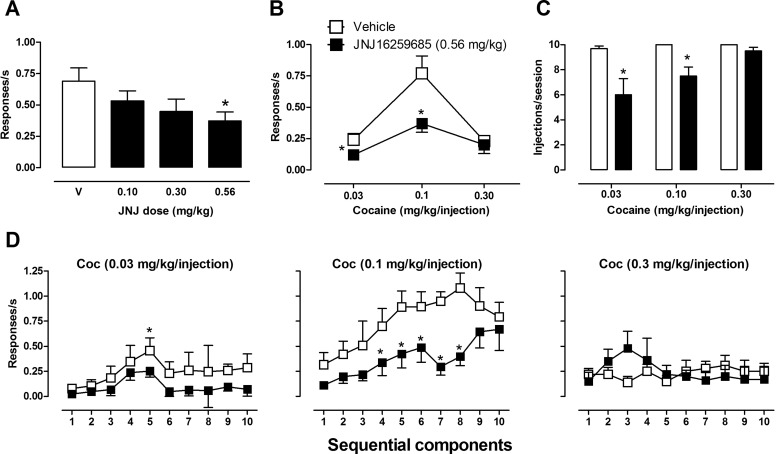
Cocaine maintained consistent self-administration under the second-order schedule throughout the study in all monkeys. Baseline response rates after vehicle pretreatment averaged 0.69 (± 0.10) responses/s for the group of six monkeys (Fig. 2A, empty bar). With rare exceptions, all subjects self-administered the maximum possible number of injections per session (10) under baseline conditions. Initial studies evaluating the effects of a range of doses of JNJ16259685 (0.10–0.56 mg/kg) while keeping the dose of self-administered cocaine constant at 0.1 mg/kg/injection revealed a dose-dependent decrease in the rate of cocaine-maintained responding (Fig. 2A, filled bars). RM ANOVA showed a significant effect of JNJ16259685 (F3,15 = 6; p < 0.05), and Bonferroni t tests showed that the rate of cocaine-maintained responding was significantly reduced after pretreatment with 0.56 mg/kg JNJ16259685 compared with vehicle (p < 0.05). Doses of JNJ16259685 lower than 0.56 mg/kg resulted in an average of 8 to 10 injections/session, whereas pretreatment with 0.56 mg/kg significantly reduced the number of self-administered cocaine injections (F1,11 = 11; p < 0.05; data not shown).
Fig. 2.
Effects of pretreatment with JNJ16259685 and vehicle on cocaine self-administration under a second-order schedule of intravenous drug delivery. A, JNJ16259685 dose-response curve; the empty bar shows the effects of the JNJ16259685 vehicle. B, cocaine dose-response function after pretreatment with JNJ16259685 or vehicle. C, number of injections/session self-administered during cocaine availability. D, within-session analyses showing the effects of pretreatment with JNJ16259685 or vehicle on the rate of responding during sequential components of cocaine self-administration sessions. *, p < 0.05, effects of pretreatment with JNJ16259685 compared with vehicle, Bonferroni t test.
The effects of JNJ16259685 were further evaluated by testing a range of self-administered doses of cocaine (0.03–0.30 mg/kg/injection) after pretreatment with either vehicle or 0.56 mg/kg JNJ16259685. After vehicle pretreatment, increasing cocaine doses produced a typical biphasic dose-response curve (Fig. 2B), with maximum response rates maintained by an intermediate dose of cocaine (0.1 mg/kg/injection). Compared with vehicle, JNJ16259685 pretreatment resulted in a downward shift in the ascending limb of the cocaine dose-response curve. RM ANOVAs revealed a significant effect of JNJ16259685 dose (F1,9 = 26; p < 0.05), cocaine dose (F2,9 = 8; p < 0.05), and JNJ16259685 × cocaine interaction (F2,9 = 7; p < 0.05) on response rates. Planned comparisons showed significant attenuation by JNJ16259685 of responding maintained by the 0.03 and 0.10 mg/kg/injection doses of self-administered cocaine (p < 0.05; Bonferroni t tests). After vehicle pretreatment, the number of self-administered cocaine injections averaged 10 injections/session across the cocaine doses tested (Fig. 2C). RM ANOVAs revealed a significant effect of JNJ16259685 on the number of injections/session during self-administration of 0.03 mg/kg cocaine (F1,11 = 9; p < 0.05) and 0.10 mg/kg cocaine (F1,11 = 12; p < 0.05), but not when 0.3 mg/kg cocaine was available (F1,11 = 2; p > 0.05).
Within-session analysis of the effects of JNJ16259685 across sequential components of the self-administration sessions revealed that, compared with vehicle, pretreatment with JNJ16259685 resulted in response rates that were consistently lower when either the lower or intermediate cocaine dose was available (Fig. 2D). Two-way RM ANOVAs revealed a significant effect of JNJ16259685 (F1,36 = 8; p < 0.05) and session component (F9,36 = 2; p < 0.05) for a 0.03 mg/kg/injection of cocaine and a significant effect of JNJ16259685 (F1,45 = 19; p < 0.05), session component (F9,45 = 7; p < 0.001), and JNJ16259685 × component interaction (F9,45 = 4; p < 0.05) for a 0.10 mg/kg/injection of cocaine. For the highest cocaine dose tested, RM ANOVA revealed no main effect of JNJ16259685 on response rates (F1,45 = 1; p > 0.05). Planned comparisons showed that, compared with vehicle, JNJ16259685 pretreatment significantly reduced the rate of responding during the fifth component of the cocaine session (0.03 mg/kg/injection) and during the fourth through eighth components of the cocaine session (0.10 mg/kg/injection) (p < 0.05, Bonferroni t tests).
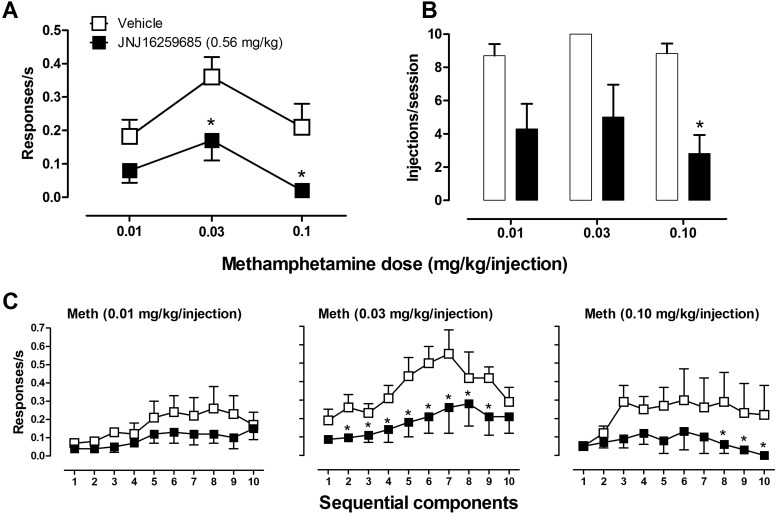
In a separate group of monkeys, methamphetamine, like cocaine, maintained consistent self-administration under the second-order schedule throughout the study. Methamphetamine (0.03 mg/kg/injection) engendered an average baseline response rate of 0.36 (± 0.06) responses/s after vehicle pretreatment for the group of six monkeys. Subjects typically self-administered 8 to 10 injections/session, depending on the individual monkey. The effects of JNJ16259685 on methamphetamine self-administration were evaluated by testing a range of doses of methamphetamine (0.01–0.10 mg/kg/injection) after pretreatment with either vehicle or the dose of JNJ16259685 (0.56 mg/kg) that significantly attenuated the reinforcing effects of cocaine (above).
After pretreatment with vehicle, methamphetamine self-administration was characterized by a biphasic dose-response curve similar to that engendered by cocaine (Fig. 3A, □), with maximum response rates maintained by the intermediate dose of methamphetamine (0.03 mg/kg/injection). Compared with vehicle pretreatment, JNJ16259685 (0.56 mg/kg) produced an overall downward shift in the methamphetamine dose-response curve (Fig. 3A, ■). Two-way RM ANOVA revealed a significant effect of JNJ16259685 (F1,6 = 12; p < 0.05) but not methamphetamine (F2,6 = 4; p > 0.05), and additional planned comparisons using Bonferroni t tests showed that JNJ16259685 significantly attenuated the rate of responding maintained by 0.03 and 0.10 mg/kg injections of methamphetamine. For each dose of methamphetamine, JNJ16259685 pretreatment decreased the number of self-administered injections/session compared with vehicle (Fig. 3B). RM ANOVAs revealed a significant effect of JNJ16259685 on injections/session during self-administration of methamphetamine at 0.10 mg/kg/injection (F1,11 = 49; p < 0.05) and an effect of JNJ16259685 on the number of injections/session that approached significance during self-administration of methamphetamine at 0.03 mg/kg/injection (F1,11 = 7; p = 0.05) and 0.01 mg/kg/injection (F1,11 = 6; p = 0.05).
Fig. 3.
Effects of pretreatment with JNJ16259685 and vehicle on methamphetamine self-administration under a second-order schedule of intravenous drug delivery. A, methamphetamine dose-response curve after pretreatment with JNJ16259685 or vehicle. B, number of injections/session self-administered during methamphetamine availability. C, within-session analyses showing effects of pretreatment with JNJ16259685 or vehicle on the rate of responding during sequential components of methamphetamine self-administration sessions. *, p < 0.05, effects of pretreatment with JNJ16259685 compared with vehicle, Bonferroni t test.
Within-session analyses of response rates revealed that, compared with vehicle, JNJ16259685 produced downward shifts in the component-response curves during self-administration of methamphetamine at 0.01, 0.03, and 0.10 mg/kg/injection (Fig. 3C). Two-way RM ANOVAs revealed significant effects of JNJ16259685 on responding maintained by 0.03 mg/kg/injection (F1,45 = 60; p < 0.05) and 0.10 mg/kg/injection (F1,45 = 7; p < 0.05) and an effect of JNJ16259685 that approached significance (F1,45 = 6; p = 0.05) on responding maintained by 0.01 mg/kg. Planned comparisons by Bonferroni t tests showed that, compared with vehicle, pretreatment with JNJ16259685 significantly decreased response rates during the second through ninth components of the 0.03 mg/kg/injection session (p < 0.05) and the eighth through 10th components of the 0.1 mg/kg/injection session (p < 0.05).
Effect of JNJ16259685 on the Reinstatement of Cocaine Seeking.
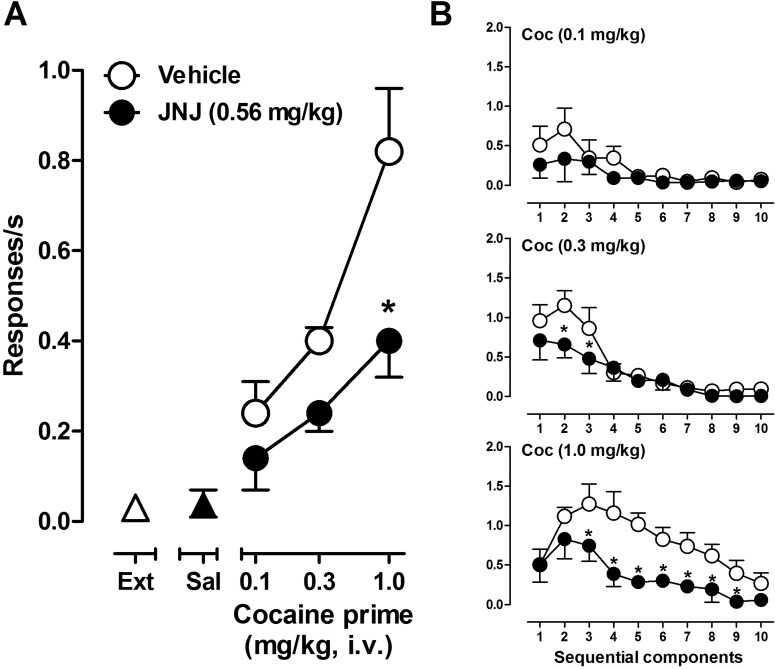
Reinstatement studies were conducted in four monkeys that were trained to self-administer cocaine under the second-order schedule described above and then underwent extinction training before subsequent testing. During the self-administration phase of the study, cocaine (0.18 mg/kg/injection) maintained consistent responding with an average response rate of 0.96 (±0.09) responses/s, and subjects typically self-administered the maximum possible number of injections per session. During extinction training, in which saline was substituted for cocaine and the cocaine-paired stimulus was omitted, responding declined and stabilized at a low rate of 0.03 ± 0.02 responses/s (Fig. 4A, ▵). Priming with saline resulted in average response rates that were not significantly different from response rates yielded during extinction (Fig. 4A, ▴), and RM ANOVA of response rates revealed no significant difference between extinction and saline prime conditions (F1,7 = 2; p > 0.05).
Fig. 4.
Effects of pretreatment with JNJ16259685 and vehicle on reinstatement of drug seeking induced by cocaine priming and restoration of the cocaine-paired stimulus. A, cocaine priming dose-response curve after pretreatment with JNJ16259685 or vehicle. ▵ shows the baseline response rate under extinction conditions. ▴ shows the response rate after priming with a saline injection. B, within-session analyses showing the effects of pretreatment with JNJ16259685 or vehicle on the rate of responding during sequential components of reinstatement test sessions. *, p < 0.05, effects of pretreatment with JNJ16259685 compared with vehicle, Bonferroni t test.
In a pilot study with two monkeys, priming with 1.0 mg/kg cocaine accompanied by restoration of the cocaine-paired stimulus induced marked reinstatement of drug seeking, which was reduced by ∼50% after pretreatment with 0.56 mg/kg JNJ16259685 (data not shown). Consequently, this dose was used in a more comprehensive experiment in which JNJ16259685 and its vehicle were tested as a pretreatment before priming with increasing doses (0.1–1.0 mg/kg) of cocaine. Priming with cocaine after vehicle pretreatment produced dose-related increases in the level of reinstated drug seeking (Fig. 4A, ○). Pretreatment with JNJ16259685 (0.56 mg/kg) before cocaine priming attenuated cocaine-induced reinstatement of drug seeking (Fig. 4A, ●). Two-way RM ANOVA revealed a significant effect of JNJ16259685 (F1,6 = 18; p < 0.05), cocaine (F2,6 = 17; p < 0.05), and JNJ16259685 × cocaine interaction (F2,6 = 7; p < 0.05). Planned comparisons using Bonferroni t tests showed that, compared with vehicle, pretreatment with JNJ16259685 significantly attenuated the rate of responding induced by the highest priming dose of cocaine (1.0 mg/kg).
Within-session analyses of the effects of JNJ16259685 across sequential components of the reinstatement session revealed that, compared with vehicle, JNJ16259685 pretreatment produced a component-dependent reduction in response rate (Fig. 4B). Two-way RM ANOVAs revealed significant effects of JNJ16259685 on the rate of responding (F1,27 = 15; p < 0.05 and F1,27 = 28; p < 0.05) generated by 0.3 and 1.0 mg/kg cocaine priming doses, respectively. There was no significant effect of JNJ16259685 pretreatment on cocaine (0.1 mg/kg)-primed reinstatement (F1,27 = 1; p > 0.05). Planned comparisons revealed that, compared with vehicle, pretreatment with 0.56 mg/kg JNJ16259685 significantly reduced the rate of responding during the second and third components of the session after priming with 0.3 mg/kg cocaine and during the third to eighth components of the session after priming with 1.0 mg/kg cocaine (p < 0.05; Bonferroni t test).
Effect of JNJ16259685 on Cocaine- and Methamphetamine-Induced Increases in Food-Maintained Responding.
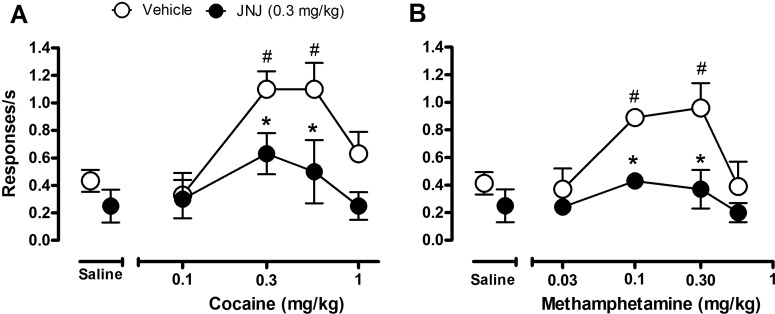
To evaluate the ability of JNJ16259685 to block the behavioral stimulant (response rate-increasing) effects of cocaine and methamphetamine, JNJ16259685 (0.3 mg/kg) or vehicle was administered as a pretreatment before administration of saline or a range of doses of cocaine or methamphetamine in monkeys trained to respond under a second-order schedule of food reinforcement. Under baseline conditions, responding was maintained at an average rate of 0.57 (± 0.09) responses/s. Pretreatment with JNJ16259685 in the absence of cocaine did not significantly affect the rate of responding compared with pretreatment with saline (Fig. 5A, points above Saline column). Pretreatment with JNJ16259685 did, however, attenuate the increases in response rate produced by maximally effective doses of cocaine (Fig. 5A, ●). Two-way RM ANOVA revealed a significant effect of cocaine (F4,16 = 8; p = 0.001) and JNJ16259685 (F1,16 = 9; p < 0.05), and planned comparisons showed that the increases in response rate produced by 0.30 and 0.56 mg/kg cocaine were significantly attenuated after pretreatment with JNJ16259685 compared with pretreatment with vehicle (p < 0.05; Bonferroni t test).
Fig. 5.
Effects of pretreatment with JNJ16259685 and vehicle on the behavioral stimulant effects of cocaine and methamphetamine under a second-order schedule of food delivery. A, cocaine dose-response curve after pretreatment with JNJ16259685 or vehicle. B, methamphetamine dose-response curve after pretreatment with JNJ16259685 or vehicle (right). Points above the Saline column show effects of pretreatment with JNJ16259685 or its vehicle before saline. #, p < 0.05, effects of cocaine or methamphetamine compared with saline. *, p < 0.05, effects of pretreatment with JNJ16259685 compared with pretreatment with vehicle; Bonferroni t test.
Methamphetamine also produced dose-related increases in the rate of responding (Fig. 5B, ○), which were attenuated by pretreatment with JNJ16259685 (Fig. 5B, ●). Two-way RM ANOVA revealed significant effects of methamphetamine (F4,12 = 3; p < 0.05) and JNJ16259685 (F1,12 = 18; p < 0.05), and planned comparisons by Bonferroni t tests showed that, compared with saline control, 0.1 and 0.3 mg/kg methamphetamine significantly increased the rate of responding, whereas pretreatment with JNJ16259685 significantly reduced methamphetamine's rate-increasing effects (p < 0.05).
Behavioral Observations.
JNJ16259685 (0.18–0.56 mg/kg) administered 25 min before the observation session had no significant effect on the majority of behaviors examined, including self-directed behaviors, visual scanning, and vocalization. JNJ16259685 also did not significantly affect resting, static or procumbent postures, ataxia, muscle resistance, or composite categories indicative of sedation (procumbent posture with ataxia) or catalepsy (static posture with muscle rigidity). JNJ16259685 did, however, produce an overall reduction in the environmentally directed category of behavior, which was caused primarily by a significant reduction in locomotion (F3,12 = 23, p < 0.05) and nonsignificant reductions in foraging (F3,12 = 2; p > 0.05) and object exploration (F3,12 = 2; p > 0.05). Planned comparisons revealed significant attenuation in locomotion after pretreatment with 0.18, 0.3, and 0.56 mg/kg JNJ16259685 (p < 0.05; Bonferroni t test), but no significant attenuation in foraging or object exploration (Table 2). In two monkeys JNJ16259685 (0.3 and 0.56 mg/kg) also induced emesis.
TABLE 2.
Effects of JNJ1625985 on environmentally directed behaviors
| Treatment | Locomotion | Foraging | Object Exploration |
|---|---|---|---|
| Vehicle | 8 ± 1.8 | 0.2 ± 0.03 | 3.8 ± 3.5 |
| 0.18 mg/kg JNJ1625985 | 0.4 ± 0.6* | 0.2 ± 0.02 | 0.1 ± 0.1 |
| 0.3 mg/kg JNJ1625985 | 0.9 ± 0.8* | 0.1 ± 0.01 | 0.1 ± 0.3 |
| 0.56 mg/kg JNJ1625985 | 0.5 ± 0.5* | 0.1 ± 0.05 | 0.3 ± 0.8 |
, P < 0.05, effects of pretreatment with JNJ16259685 compared with pretreatment with vehicle; Bonferroni t test.
Discussion
The principal findings in our study show that selective mGluR1 antagonism by JNJ16259685 attenuated: 1) the DS effects of cocaine and the cocaine-like DS effects of methamphetamine, 2) the self-administration of cocaine and methamphetamine, 3) the reinstatement of cocaine-seeking behavior, and 4) the rate-increasing (behavioral stimulant) effects of cocaine and methamphetamine on food-reinforced operant behavior. Antagonism by JNJ16259685, however, was not without side effects, which included reduced locomotion and, in some cases, emesis. These results extend the evidence for the role of group I glutamate receptors in abuse-related behavioral effects of psychostimulants by demonstrating that antagonism of the mGluR1 subtype can attenuate cocaine- and methamphetamine-induced behaviors, similar to the effects produced by antagonism of the mGluR5 subtype under similar testing conditions (Lee et al., 2005; Platt et al., 2008). Furthermore, the findings from drug discrimination, self-administration, and food reinforcement experiments revealed that the behavioral effects of cocaine and methamphetamine were similarly attenuated by JNJ16259685, suggesting that mGlur1 antagonists may serve as functional antagonists of psychostimulant drugs regardless of whether these drugs act primarily by inhibiting dopamine uptake (cocaine) or stimulating dopamine release (methamphetamine).
In drug discrimination experiments, attenuation of cocaine's DS effects by JNJ16259685 was accompanied by attenuation of the rate-increasing effects of cocaine, suggesting that the impact of JNJ16259685 was not the result of perceptual masking (Gauvin and Young, 1989). Rather, the effects of JNJ16259685 seemed to be caused by functional antagonism because both the DS effects of cocaine and the behavioral stimulant effects of cocaine were attenuated. Our finding that antagonism of the mGluR1 subtype by JNJ16259685 can block the DS effects of cocaine has not been reported previously. Along these lines, however, an earlier study using a similar experimental design showed that antagonism of the mGluR5 subtype by 2-methyl-6-(phenylethynyl)-pyridine can similarly attenuate cocaine's DS effects in monkeys (Lee et al., 2005). Together, these findings suggest that both subtypes of group I mGluRs might contribute to the transduction of cocaine's subjective effects. Our results also show antagonism of the cocaine-like DS effects of methamphetamine by JNJ16259685, although comparison of the effects of 2-methyl-6-(phenylethynyl)-pyridine showed no antagonism of methamphetamine's DS effects in rats (Wooters et al., 2011). These apparently disparate observations could be caused by a number of factors, notably the training drug (cocaine versus methamphetamine), species, and subtype selectivity of the mGluR antagonist.
Self-administration of cocaine and methamphetamine also was significantly attenuated after pretreatment with JNJ16259685, suggesting that blocking mGluR1 activity reduced the reinforcing effects of both psychostimulants. There appear to be no other published reports investigating the direct role of the mGluR1subtype in cocaine's reinforcing effects, but mGluR1 signaling has been implicated in the reinforcing effects of other drugs of abuse (Olive, 2009 for review). In a previous study in rats, for example, JNJ16259685 pretreatment significantly decreased the break point at which ethanol was self-administered under a progressive ratio schedule (Besheer et al., 2008).
In our study, within-session analyses of the rate of responding during cocaine and methamphetamine self-administration revealed that the most prominent reductions in response rate by JNJ16259685 usually were observed during the middle and/or late portions of the self-administration session, rather than at the beginning. It is noteworthy that the rate of responding during the first component of the cocaine or methamphetamine self-administration session was not significantly affected by JNJ16259685 pretreatment. A possible explanation of this finding could be that the pretreatment time was too short such that JNJ16259685's onset of action occurred after the first component of the session. However, in monkeys, JNJ16259685's antagonism of mGluR1 was observed 10 min postadministration and was maintained for at least 90 min after pretreatments (Yamasaki et al., 2012). The lack of significant effects of JNJ16259685 during the first component, before the first drug injection, suggests that the significant attenuation in response rates in subsequent components was specific to the reinforcing effects of cocaine or methamphetamine and not caused by a nonspecific impairment of motor function by JNJ16259685.
Reinstatement of extinguished drug-seeking behavior induced by cocaine priming and restoration of the cocaine-paired stimulus also was significantly attenuated by JNJ16259685, implicating the mGluR1 subtype. Within-session analysis during reinstatement sessions revealed that JNJ16259685 pretreatment reduced reinstated drug seeking beginning in components in which cocaine primes elicited the highest rates of responding, further supporting the cocaine antagonist properties of JNJ16259685. Similar to our findings, nicotine-seeking behavior induced by nicotine priming and nicotine-associated cues was attenuated by selective antagonism of mGluR1 in rats (Dravolina et al., 2007). Xie et al. (2010, 2012) also found that blocking mGluR1 activity in the dorsal hippocampus or the nucleus accumbens core inhibited context-induced reinstatement of cocaine seeking. Our results are therefore consistent with recent reports that mGluR1 signaling might play a role in specific neural networks to mediate reactivity to cocaine-associated cues and subsequent relapse to cocaine seeking.
In experiments involving food-reinforced behavior, intermediate doses of cocaine and methamphetamine significantly increased the rate of responding, and these rate-increasing effects were attenuated by JNJ16259685 pretreatment. These results are similar to the effects of JNJ16259685 in our drug discrimination study in which the rate-increasing effects of cocaine also were reduced by JNJ16259685 pretreatment. Consistent with our findings, inhibition of mGluR1 activity has been previously associated with a reduction in the locomotor stimulant effects of cocaine and methamphetamine in rats. For example, administration of the mGluR1 antagonists, EMQMCM [(3-ethyl-2-methyl-quinolin-6-yl)-(4-methoxycyclohexyl)-methanone methanesulfonate] and FTIDC [4-[1-(2-fluoropyridine-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methyl-3,6-dihydropyridine-1(2H)-carboxamide], blocked cocaine-induced psychomotor sensitization (Dravolina et al., 2006; Kotlinska and Bochenski, 2011) and methamphetamine-induced hyperlocomotion (Satow et al., 2008).
When administered alone, JNJ16259685 significantly reduced spontaneous locomotor activity in monkeys without markedly affecting other species-typical behaviors, balance, or muscle resistance. These findings are generally in accord with those reported by Hodgson et al. (2011) in which a variety of motor activity assays were used in rodents. Hodgson et al. found that JNJ16259685 typically had little or no effect on well established motor behavior, although it did affect the acquisition of novel motor skills. The main differences between our results and theirs are that we found a significant effect of JNJ16259685 on locomotion in monkeys, whereas Hodgson et al. did not in rats. Despite attenuation of spontaneous locomotor activity in the observation studies, JNJ16259685 (0.3 mg/kg) did not significantly affect food-reinforced responding under the second-order schedule. Furthermore, JNJ16259685 (0.56 mg/kg) did not significantly affect rates of responding when administered before saline in drug discrimination experiments and during the first component of drug self-administration sessions, before the first drug injection. Collectively, these findings suggest that JNJ16259685 has a relatively restricted profile of effects on motor performance, significantly attenuating spontaneous locomotor activity while having minimal effects on locomotor activity under drug-reinforced operant conditioning parameters.
The limited amount of research addressing the role of mGluR1, compared with the role of mGlur5, on abuse-related behavioral effects of cocaine and methamphetamine precludes direct comparisons of our findings with other animal models of psychostimulant self-administration, discrimination, and relapse. Nevertheless, our findings extend the modulatory role of the group I mGluRs in substance abuse by revealing that the effects of JNJ16259685 in the present study are similar to the effects of mGluR5 antagonism on psychostimulant-induced behaviors. As in the case of mGluR1 antagonism, antagonism of the mGluR5 subtype significantly reduces cocaine self-administration behavior in monkeys (Lee et al., 2005; Platt et al., 2008) and rodents (Kenny et al., 2005; Martin-Fardon et al., 2009) and attenuates reinstatement of drug seeking induced by cocaine (Lee et al., 2005; Kumaresan et al., 2009) or methamphetamine (Gass et al., 2009).
The precise mechanisms by which group I mGluRs regulate the abuse-related behavioral effects of cocaine and methamphetamine remain to be determined. It has been shown, however, that group I mGluR activity can influence striatal dopamine release (Bruton et al., 1999; Tokunaga et al., 2009). Moreover, under conditions of chronic cocaine exposure, group I receptors can regulate the expression and function of the ionotropic class of glutamate receptors (Bellone and Lüscher, 2006; McCutcheon et al., 2011), which have been consistently linked to addiction (Tzschentke and Schmidt, 2003). It has also been proposed that molecular interactions between the mGluR1 and mGluR5, for example, at Homer proteins where their distinct signaling pathways converge (Swanson et al., 2001), play an integral role in drug-induced synaptic plasticity and the subsequent addiction-related behaviors (see Grueter et al., 2007 for review). Understanding the extent to which these related receptors alter psychostimulant-induced behaviors could contribute to resolving neurobiological mechanisms underlying persistent drug use and help determine whether pharmacological agents acting at group I mGluR subtypes have therapeutic potential for psychostimulant addiction. Although JNJ16259685 itself is probably not a viable candidate medication because of side effects such as those observed in the present study (hypolocomotion, occasional emesis), other mGluR1 agents with more favorable side effect profiles are currently under development (Krystal et al., 2010; Ribeiro et al., 2010) and may be better suited to this purpose.
Acknowledgments
We thank Laura Teixeira, Rebecca Smith, and Shana Langer for technical expertise and Donna Reed for assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA017700; DA011054]; the National Institutes of Health National Center for Research Resources [Grant RR00168]; and the National Institutes of Health Office of Research Infrastructure Programs/Office of the Director [Grant 5P51 OD011103].
Portions of this work were presented previously: Achat-Mendes C, Platt DM, and Spealman RD (2009) Attenuation of the discriminative stimulus effects of cocaine by the mGluR1 antagonist JNJ16259685 in squirrel monkeys, at the Society for Neuroscience Meeting; 2009 Oct 17–21; Chicago, IL. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- mGluR
- metabotropic glutamate receptor
- DS
- discriminative stimulus
- FR
- fixed ratio
- FI
- fixed interval
- JNJ16259685
- (3,4-dihydro-2H pyrano-[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl) methanone
- RM
- repeated measures
- ANOVA
- analysis of variance
- TO
- timeout
- JNJ
- JNJ16259685
- Coc
- cocaine
- Meth
- methamphetamine.
Authorship Contributions
Participated in research design: Achat-Mendes, Platt, and Spealman.
Conducted experiments: Achat-Mendes and Platt.
Performed data analysis: Achat-Mendes, Platt, and Spealman.
Wrote or contributed to the writing of the manuscript: Achat-Mendes, Platt, and Spealman.
References
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. (2010) Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther 334:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9:636–641 [DOI] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. (2008) Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol 42:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. (2009) Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol 2: 83–94 [DOI] [PubMed] [Google Scholar]
- Bruton RK, Ge J, Barnes NM. (1999) Group I mGlu receptor modulation of dopamine release in the rat striatum in vivo. Eur J Pharmacol 369:175–181 [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. (2006) Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 187:397–404 [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. (2007) mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology 52:263–269 [DOI] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. (2009) mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 34:820–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Young AM. (1989) Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology (Berl) 98:212–221 [DOI] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. (2007) Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol 36:232–244 [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Hyde LA, Guthrie DH, Cohen-Williams ME, Leach PT, Kazdoba TM, Bleickardt CJ, Lu SX, Parker EM, Varty GB. (2011) Characterization of the selective mGluR1 antagonist, JNJ16259685, in rodent models of movement and coordination. Pharmacol Biochem Behav 98:181–187 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (2011) Guide for the Care and Use of Laboratory Animals 8th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. (2005) Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 179:247–254 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. (2004) The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci 25:265–272 [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M. (2011) Pretreatment with group I metabotropic glutamate receptors antagonists attenuates lethality induced by acute cocaine overdose and expression of sensitization to hyperlocomotor effect of cocaine in mice. Neurotox Res 19:23–30 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. (2010) Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24:669–693 [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. (2009) Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nóbrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. (2004) JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology 47:961–972 [DOI] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. (2005) Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther 312:1232–1240 [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. (2006) Behavioral and neurochemical interactions between group I mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend 85:142–156 [DOI] [PubMed] [Google Scholar]
- Mao L, Conquet F, Wang JQ. (2001) Augmented motor activity and reduced striatal preprodynorphin mRNA induction in response to acute amphetamine administration in metabotropic glutamate receptor 1 knockout mice. Neuroscience 106:303–312 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. (2009) Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther 329:1084–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. (2011) Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci 31:14536–14541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. (2007) Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol 500:788–806 [DOI] [PubMed] [Google Scholar]
- Olive MF. (2009) Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev 2:83–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. (2011) Models of neurological disease (substance abuse): self-administration in monkeys, in Current Protocols in Pharmacology (Enna S, Williams M, eds), Chapter 10, Unit 10.5 Wiley, New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2000) Dissociation of cocaine-antagonist properties and motoric effects of the D1 receptor partial agonists SKF 83959 and SKF 77434. J Pharmacol Exp Ther 293:1017–1026 [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2008) Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 200:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. (2010) Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets 9:574–595 [DOI] [PubMed] [Google Scholar]
- Satow A, Maehara S, Ise S, Hikichi H, Fukushima M, Suzuki G, Kimura T, Tanak T, Ito S, Kawamoto H, et al. (2008) Pharmacological effects of the metabotropic glutamate receptor 1 antagonist compared with those of the metabotropic glutamate receptor 5 antagonist and metabotropic glutamate receptor 2/3 agonist in rodents: detailed investigations with a selective allosteric metabotropic glutamate receptor 1 antagonist, FTIDC [4-[1-(2-fluoropyridine-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methyl-3,6-dihydropyridine-1(2H)-carboxamide]. J Pharmacol Exp Ther 326:577–586 [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. (1999) Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav 64:327–336 [DOI] [PubMed] [Google Scholar]
- Spealman RD, Bergman J, Madras BK, Melia KF. (1991) Discriminative stimulus effects of cocaine in squirrel monkeys: involvement of dopamine receptor subtypes. J Pharmacol Exp Ther 258:945–953 [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. (2001) Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci 21:9043–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M, Seneca N, Shin RM, Maeda J, Obayashi S, Okauchi T, Nagai Y, Zhang MR, Nakao R, Ito H, et al. (2009) Neuroimaging and physiological evidence for involvement of glutamatergic transmission in regulation of the striatal dopaminergic system. J Neurosci 29:1887–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. (2003) Glutamatergic mechanisms in addiction. Mol Psychiatry 8:373–382 [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. (2011) Discriminative stimulus effects of NMDA, AMPA, and mGluR5 glutamate receptor ligands in methamphetamine-trained rats. Behav Pharmacol 22:516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. (2012) Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol 17:287–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. (2010) Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 208:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Fujinaga M, Maeda J, Kawamura K, Yui J, Hatori A, Yoshida Y, Nagai Y, Tokunaga M, Higuchi M, et al. (2012) Imaging for metabotropic glutamate receptor subtype 1 in rat and monkey brains using PET with [18F]FITM. Eur J Nucl Med Mol Imaging 39:632–641 [DOI] [PubMed] [Google Scholar]