Abstract
Norbuprenorphine is a major metabolite of buprenorphine and potent agonist of μ, δ, and κ opioid receptors. Compared with buprenorphine, norbuprenorphine causes minimal antinociception but greater respiratory depression. It is unknown whether the limited antinociception is caused by low efficacy or limited brain exposure. Norbuprenorphine is an in vitro substrate of the efflux transporter P-glycoprotein (Mdr1), but the role of P-glycoprotein in norbuprenorphine transport in vivo is unknown. This investigation tested the hypothesis that limited norbuprenorphine antinociception results from P-glycoprotein-mediated efflux and limited brain access. Human P-glycoprotein-mediated transport in vitro of buprenorphine, norbuprenorphine, and their respective glucuronide conjugates was assessed by using transfected cells. P-glycoprotein-mediated norbuprenorphine transport and consequences in vivo were assessed by using mdr1a(+/+) and mdr1a(−/−) mice. Antinociception was determined by hot-water tail-flick assay, and respiratory effects were determined by unrestrained whole-body plethysmography. Brain and plasma norbuprenorphine and norbuprenorphine-3-glucuronide were quantified by mass spectrometry. In vitro, the net P-glycoprotein-mediated efflux ratio for norbuprenorphine was nine, indicating significant efflux. In contrast, the efflux ratio for buprenorphine and the two glucuronide conjugates was unity, indicating absent transport. The norbuprenorphine brain/plasma concentration ratio was significantly greater in mdr1a(−/−) than mdr1a(+/+) mice. The magnitude and duration of norbuprenorphine antinociception were significantly increased in mdr1a(−/−) compared with mdr1a(+/+) mice, whereas the reduction in respiratory rate was similar. Results show that norbuprenorphine is an in vitro and in vivo substrate of P-glycoprotein. P-glycoprotein-mediated efflux influences brain access and antinociceptive, but not the respiratory, effects of norbuprenorphine.
Introduction
The long-duration opioid buprenorphine has been used for several decades for treating acute and chronic pain and is now also marketed for opioid addiction and withdrawal therapy. Buprenorphine is a partial agonist at the μ opioid receptor and an antagonist at the κ opioid receptor. Like other μ agonists, it causes analgesia, respiratory depression, miosis, and mood changes, but unlike typical μ agonists there is a ceiling effect at higher doses (Cowan et al., 1977; Walsh et al., 1994). Buprenorphine is extensively metabolized in humans, primarily to norbuprenorphine, and both undergo subsequent glucuronidation to buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide (Zacny et al., 1997; Moody et al., 2009). In humans, peak plasma concentrations of norbuprenorphine are similar to or exceed those of buprenorphine. The relative norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide exposures, based on molar area under the plasma concentration versus time curves, are 200, 100, and 600% those of buprenorphine, respectively (Moody et al., 2002).
Norbuprenorphine is pharmacologically active, and norbuprenorphine formation may be a bioactivation pathway, rather than an inactivation pathway, as originally considered (Ohtani et al., 1995). Norbuprenorphine has high affinity for the μ, δ, and κ opioid receptors (Huang et al., 2001; Brown et al., 2011). Norbuprenorphine causes marked respiratory depression in animal models (Ohtani et al., 1997; Brown et al., 2011) but has comparatively less antinociceptive effect (Ohtani et al., 1995). In contrast, buprenorphine causes much greater antinociception but has no respiratory effects in animal models (Ohtani et al., 1995). In rats, norbuprenorphine had 1/50th the analgesic potency of buprenorphine but 10-fold greater respiratory depressant potency (Ohtani et al., 1997). Norbuprenorphine-3-glucuronide (κ, but not μ or δ) and buprenorphine-3-glucuronide (μ and δ, but not κ) also have affinity for opioid receptors and are biologically active, causing mild respiratory and antinociceptive effects, respectively (Brown et al., 2011). The role of norbuprenorphine-3-glucuronide in mediating the effects of norbuprenorphine, norbuprenorphine-3-glucuronide analgesic efficacy, and central nervous system access are unknown. The mechanism of limited norbuprenorphine antinociception, as well as that underlying the differences between norbuprenorphine and buprenorphine, is unknown. These mechanisms have been attributed to either low norbuprenorphine intrinsic analgesic activity or limited brain access of norbuprenorphine (Ohtani et al., 1995).
With the exception of essential nutrients, the movement of endogenous and exogenous substances from the blood to the brain is highly restricted by the blood-brain barrier (BBB). The BBB is formed by a unique suite of tight junction proteins, receptors, and transporters expressed by the endothelial cells that make up the microvasculature of the brain (Abbott, 2005; Engelhardt, 2011). Because of the restrictive nature of the BBB, the vast majority of molecules that bind with high affinity to one or more receptors in the brain have little biological activity in vivo (Pardridge, 1997). P-glycoprotein [P-gp; multidrug resistance protein 1 (MDR1), ABCB1] is one of several transmembrane efflux transporters at the BBB that use ATP hydrolysis to protect the brain from a wide variety of exogenous and endogenous compounds. P-glycoprotein has a broad substrate profile, has been shown to regulate brain access of numerous drugs via active efflux, and determines the pharmacologic effect of several drugs acting in the central nervous system (Kim et al., 1998; Jolliet-Riant and Tillement, 1999; Boulton et al., 2002; Uhr et al., 2003; Dagenais et al., 2004; Wang et al., 2004; Sasongko et al., 2005).
Several opioids have been reported previously in animals and/or humans to be in vitro or in vivo substrates for P-glycoprotein, including loperamide (Wandel et al., 2002; Skarke et al., 2003), morphine (Xie et al., 1999; Wandel et al., 2002; Dagenais et al., 2004), methadone (Dagenais et al., 2004; Kharasch et al., 2004), fentanyl (Wandel et al., 2002; Dagenais et al., 2004), alfentanil (Wandel et al., 2002; Kalvass et al., 2007), and sufentanil (Wandel et al., 2002). Norbuprenorphine has been identified as a substrate for P-gp-mediated efflux by in vitro screening using transfected Madin-Darby canine kidney (MDCK) cells (Tournier et al., 2010).
Mice lacking Mdr1a and/or Mdr1b have been shown to be useful tools for investigating the in vivo role of P-gp in pharmacology (Doran et al., 2005; van Waterschoot and Schinkel, 2011). Genetic P-gp knockouts have been compared with chemical P-gp knockouts in several studies, and the results have shown that genetic disruption of the P-gp gene product has the same effect as chemically inhibiting the transporter (Polli et al., 1999; Zong and Pollack, 2000). Mdr1a(−/−) mice have been used to determine that P-gp limits opioid-induced analgesia of morphine, methadone, and fentanyl (Thompson et al., 2000; Hassan et al., 2009).
The purpose of the current investigation was to test the hypothesis that limited norbuprenorphine antinociception is the result of P-gp-mediated efflux and limited brain access, using in vitro and in vivo methods. It evaluated P-gp-mediated transport of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide in vitro and the antinoceptive and respiratory effects of norbuprenorphine in vivo, using mdr1a-deficient and wild-type mice. This research is significant because it identifies norbuprenorphine as an avid substrate of P-glycoprotein, which influences norbuprenorphine brain access, and it provides evidence for blood-brain barrier transport differentially influencing the classic opioid effects of analgesia and respiratory depression.
Materials and Methods
Materials.
Buprenorphine, norbuprenorphine, and buprenorphine-3-β-d-glucuronide were provided by the National Institute on Drug Abuse (Bethesda, MD). Norbuprenorphine-3-β-d-glucuronide was synthesized as described previously (Fan et al., 2011). Buprenorphine, norbuprenorphine, buprenorphine-3-β-d-glucuronide, and norbuprenorphine-3-glucuronide used as analytical standards were from Cerrilliant (Round Rock, TX). Dulbecco's modified Eagle's media, heat-inactive fetal bovine serum, and Hanks' buffered salt solution (HBSS) were obtained from Mediatech (Herndon, VA). Bac-off antibiotic was obtained from Cell Systems, Inc. (Kirkland, WA). Cell transport assays were conducted by using the Corning HTS Transwell system (Corning Life Sciences, Lowell, MA). All other reagents were from Sigma-Aldrich (St. Louis, MO).
In Vitro Transport Assay.
MDCK cells transfected with the gene encoding human MDR1 (P-gp) were obtained from P. Borst (Netherlands Cancer Institute, Amsterdam, The Netherlands). Both the untransfected and transfected cell lines were cultured in Dulbecco's modified Eagle's media containing 10% fetal bovine serum and 1× Bac-off antibiotic. Cells were seeded onto the Transwell supports at a density of 49,000 cells/well and incubated for 4 days at 37°C/95% humidity/5% CO2.
On the day of the assay the media were aspirated and replaced with HBSS supplemented with 1 mM CaCl2, 0.1 mM MgSO4, and 0.1 mM MgCl2 (HBSSCM) and allowed to equilibrate for 30 min at 37°C. The buffer was then aspirated and replaced with fresh HBSSCM in either the apical (upper) or basolateral (lower) chamber. HBSSCM that contained 2 μM of the test drug was added to the opposite chamber, and the cells were incubated for 2 h at 37°C/95% humidity/5% CO2. The donor and receiver chambers for each well were sampled and analyzed by LC/MS/MS as described below. Monolayer integrity was checked by using lucifer yellow. In brief, 200 μl of a 100 μg/ml lucifer yellow solution in HBSSCM was added to the basolateral compartment of each well, and 100 μl of HBSSCM (without lucifer yellow) was added to the apical chamber. After incubating the cells for 1 h at 37°C/95% humidity/5% CO2 the receiver (apical) chamber and the donor chamber (basolateral) were sampled, and the lucifer yellow-related fluorescence was determined by using a BioTEK SynergyMX plate reader (BioTek Instruments, Winooski, VT). Wells were rejected if the permeability of the lucifer yellow was above 2%.
Apparent permeability (Papp) for both the MDCK wild-type and MDR1-transfected MDCK cells were calculated by using eq. 1, where Vr is the volume of buffer in the receiver, A is the surface area of the Transwell membrane (0.14 cm2), t is the incubation time in seconds, and [Drug]r and [Drug]d are the drug concentrations of the receiver and donor wells, respectively at time t.
The directional ratio for each cell line was calculated by dividing the apparent permeability value for the basolateral-to-apical direction by the apparent permeability value for the apical-to-basolateral direction as in eq. 2.
The net efflux ratio was calculated by dividing the directional ratio value for the MDR1-transfected cell line by the directional ratio of the untransfected MDCK cells by using eq. 3. A net efflux ratio above 2.0 was the criterion used to define significant substrate-transporter interaction (Polli et al., 2001).
The mean and S.D. were calculated from three wells for each direction within the same experiment.
Animals.
Male CF-1 mdr1a(+/+) and mdr1a(−/−) mice (29–35 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA). All mice were group-housed on a 12-h light/dark schedule with ad libitum access to food and water. Experiments were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and approved by the Animal Care and Use Committee of Washington University School of Medicine (St. Louis, MO).
Previous studies in our laboratory showed that a 1 mg/kg dose of norbuprenorphine in wild-type mice elicited a significant decrease in respiratory rate (Brown et al., 2011). Based on the in vitro indication that norbuprenorphine is a P-gp substrate and the hypothesis that decreased efflux from the brain in a P-gp-deficient mouse may result in increased norbuprenorphine effects, a pilot study was performed to determine the norbuprenorphine dose that would elicit the same respiratory effect in the mdr1a(−/−) mice as the 1 mg/kg dose administered to the mdr1a(+/+) mice. mdr1a(+/+) and mdr1a(−/−) mice (n = 4 each) were used in the pilot study. A 0.25 mg/kg dose resulted in a 60% decrease in respiratory rate in the mdr1a(−/−) mice, which was the same response seen in the mdr1a(+/+) mice administered 1 mg/kg. For all further experiments, these equieffective doses of norbuprenorphine were used.
Collection of Tissue Samples.
P-gp-competent mdr1a(+/+) and P-gp-deficient mdr1a(−/−) mice (n = 22 each) were administered norbuprenorphine subcutaneously [1 mg/kg for mdr1a(+/+) and 0.25 mg/kg for mdr1a(−/−) mice]. At 5, 10, 20, 30, 45, and 60 min postdose respiratory rate and antinociception were assessed. Mice were anesthetized with sevoflurane, and blood was collected by cardiac puncture into heparinized Microtainers (BD Biosciences, San Jose, CA). Blood was centrifuged at 14,000 rpm for 1 min to separate plasma. After exsanguination, whole brains were harvested and flash-frozen. Plasma and brain were stored at −20°C until analysis.
Quantitation of Norbuprenorphine, Norbuprenorphine-3-Glucuronide, Buprenorphine, and Buprenorphine-3-Glucuronide.
Brain and plasma were prepared for analysis by LC/MS/MS as described previously (Brown et al., 2011). In vitro samples were prepared by the addition of d5-norfentanyl (100 ng/ml) as an internal standard. LC/MS/MS was used to quantify norbuprenorphine and norbuprenorphine-3-glucuronide in brain and plasma and all four compounds in the in vitro assay samples. LC/MS/MS as analysis was performed as described previously (Brown et al., 2011). Brain concentrations were corrected for drug present in brain vasculature, assuming 1% of total brain weight caused by brain vasculature. No correction was applied to plasma drug concentrations.
Tail-Flick Antinociception Assay.
A tail-flick assay (Montana et al., 2009) was used to test the antinociceptive effect of norbuprenorphine in mdr1(−/−) and mdr1(+/+) mice. Tail-flick latency, defined by the time in seconds for tail withdrawal from a warm-water bath (52°C), was measured as described previously (Brown et al., 2011), using an IITC 500 warm-water tail-immersion test analgesia meter (IITC Life Science, Woodland Hills, CA). Baseline tail-flick latency was determined for each mouse before drug dosing. A cutoff of 10 s was used to prevent tissue damage. Animals not responding within 3 s were excluded from the assay. Maximum possible effect (MPE) was calculated as: [(T1 − T0)/(T2 − T0)] × 100, where T0 and T1 represent latencies before and after drug administration, and T2 is the cutoff time. Tail-flick latency was obtained for each animal after removal from a plethysmograph chamber and record of its respiratory rate.
Unrestrained Whole-Body Plethysmography.
Measurements of respiratory rate were obtained by using unrestrained whole-body plethysmography (Buxco Research Systems, Wilmington, NC) as described previously (Brown et al., 2011). The plethysmograph consisted of eight animal chambers with orifices for entry and exit of breathing air and a 1-ml syringe permitting calibrations, connected to a differential pressure transducer. The air entry orifice was connected to a source of compressed breathing air. Immediately before each experiment, each chamber was calibrated with 1 ml of room air. Each awake mouse was placed in a chamber immediately after administration of norbuprenorphine. Respiratory parameters were recorded for 5, 10, 20, 30, 45, and 60 min. Respiratory values were converted by Biosystems XA software (Buxco Research Systems).
Statistical Analysis.
Results are expressed as the mean ± S.D. Two-way repeated-measures analysis of variance (time versus group), followed by the Student-Newman-Keuls test, was used to test for significant differences between groups in mouse experiments (Sigma Plot 12.2; Systat Software, Inc., San Jose, CA).
Norbuprenorphine concentration-effect data in mdr1a(−/−) and mdr1a(+/+) mice were analyzed by nonlinear regression fitting (Sigma Plot 12.2) of the sigmoid Emax model (eq. 4) to antinociception versus plasma or brain concentrations, where Emax was defined as 100%, and γ was constrained to be the same for mdr1a(−/−) and mdr1a(+/+) mice as described previously (Kalvass et al., 2007).
Results
In Vitro Transport Results.
The well established Transwell assay using MDCK cells transfected with human MDR1 was used to determine whether buprenorphine, norbuprenorphine, or their glucuronide metabolites were substrates for P-gp. Transport activity for each drug was measured in the basolateral-to-apical (B → A) and the apical-to-basolateral (A → B) direction across MDCK parental and MDR1-transfected cell lines (Table 1). In the MDR1-transfected MDCK cells the B → A transport of norbuprenorphine was approximately 20-fold greater than that observed for apical-to-basolateral transport (Table 1). When the ratio was corrected for the influence of the endogenous transporters (by comparison against nontransfected cells) the net efflux ratio was nine. Buprenorphine, buprenorphine-3-β-d-glucuronide, and norbuprenorphine-3-β-d-glucuronide all had net efflux ratios less than the cutoff of 2.0 used to define a transporter substrate (Table 1). Loperamide, a known P-gp substrate (Polli et al., 2001), was used as a positive control. The net efflux ratio for loperamide was 13.
TABLE 1.
Bidirectional transport across untransfected and MDR1-transfected MDCK cells
Data are presented as the Papp. The efflux ratio for each cell line was determined as Papp (B→A)/Papp (A→B). The net efflux ratio was calculated as efflux ratioMDR1/efflux ratioMDCK. Compounds are considered P-gp substrates if the net efflux ratio is more than 2.0, as defined under Materials and Methods. Results are the mean ± S.D. for triplicate wells performed on the same day.
|
Papp |
B → A/A → B |
Net Efflux | |||
|---|---|---|---|---|---|
| MDCK | MDR1 | MDCK | MDR1 | ||
| nm/s | |||||
| Buprenorphine | |||||
| B → A | 793 ± 26 | 821 ± 153 | 81.7 | 45.5 | 0.6 |
| A → B | 10 ± 3 | 18 ± 4 | |||
| Norbuprenorphine | |||||
| B → A | 211 ± 5 | 170 ± 6 | 2.2 | 19.8 | 9 |
| A → B | 91 ± 5 | 8 ± 1 | |||
| Buprenorphine 3-glucuronide | |||||
| B → A | 166 ± 1 | 156 ± 19 | 3.8 | 3.8 | 1 |
| A → B | 44 ± 10 | 41 ± 11 | |||
| Norbuprenorphine 3-glucuronide | |||||
| B → A | 159 ± 37 | 222 ± 49 | 1.1 | 0.9 | 0.9 |
| A → B | 197 ± 12 | 205 ± 19 | |||
| Loperamide | |||||
| B → A | 144 ± 17 | 117 ± 43 | 1.8 | 23.3 | 13 |
| A → B | 80 ± 13 | 3 ± 1 | |||
Disposition of Norbuprenorphine in mdr1a(−/−) and mdr1a(+/+) Mice.
The brain-to-plasma ratio of norbuprenorphine in the mdr1a(+/+) mice was low (<0.1) at the 5-min time point and remained essentially unchanged throughout the experiment (Fig. 1). In contrast, the brain-to-plasma ratio in the mdr1a(−/−) mice was >0.1 at the 5-min time point and continued to increase over the time course of the experiment, approaching 1 by the 60-min time point (Fig. 1). For norbuprenorphine 3-glucuronide there was no significant difference in the brain-to-plasma ratios in the mdr1a(−/−) and mdr1a(+/+) mice, and the ratio was less than 0.1 throughout the experiment.
Fig. 1.
Disposition of norbuprenorphine and norbuprenorphine glucuronide. Concentrations of norbuprenorphine (A and B) and norbuprenorphine-3-glucuronide (C and D) were determined in brain homogenate (circles) and plasma (triangles) by LC/MS/MS. Results are shown for mdr1a(+/+) mice (open symbols) and mdr1a(−/−) mice (closed symbols). Measured concentrations are shown in A and C; norbuprenorphine and norbuprenorphine glucuronide brain/plasma ratios are shown in B and D. Each data point is the mean ± S.D. (n = 4). *, significantly different from mdr1a(+/+) controls (p < 0.05) by two-way repeated-measures analysis of variance.
The plasma concentrations of norbuprenorphine were 4-fold less in the mdr1a(−/−) mice than the mdr1a(+/+) mice, consistent with the mdr1a(−/−) mice receiving one-fourth the dose of that in the mdr1a(+/+) mice (Fig. 1). Plasma concentrations of norbuprenorphine-3-glucuronide were also 4-fold less in the mdr1a(−/−) mice than the mdr1a(+/+) mice, again consistent with the norbuprenorphine dose difference. Plasma norbuprenorphine-3-glucuronide/norbuprenorphine concentration ratios were similar in the mdr1a(−/−) and mdr1a(+/+) mice.
Antinociceptive Effect of Norbuprenorphine in mdr1a(−/−) and mdr1a(+/+) Mice.
In the mdr1a(+/+) mice, norbuprenorphine peak effect was 30% of MPE (Fig. 2). Maximal antinociception occurred 20 min postdosing, and effects returned to baseline at 45 min. In contrast, the mdr1a(−/−) mice had markedly increased magnitude and duration of norbuprenorphine antinociception. The effect peaked at 100% MPE at 20 min and only declined to 80% by 60 min (Fig. 2).
Fig. 2.
Antinociceptive effects of norbuprenorphine in mdr1a(−/−) and wild-type mice. Time to withdrawal of the tail from a hot-water (52°C) bath, or tail-flick latency was measured after subcutaneous injection of vehicle or norbuprenorphine [1 mg/kg in the mdr1a(+/+) mice; 0.25 mg/kg in the mdr1a(−/−) mice]. The percentage of MPE of the mdr1a(+/+) mice (A) and the mdr1a(−/−) mice (B) was calculated every 15 min for 60 min. Each data point is the mean ± S.D. (10/group). *, significantly different from vehicle control (p < 0.05) by two-way repeated-measures analysis of variance.
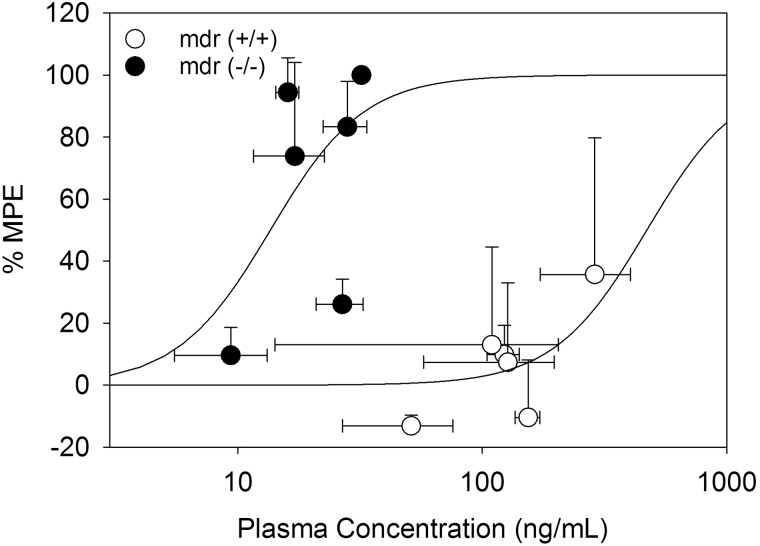
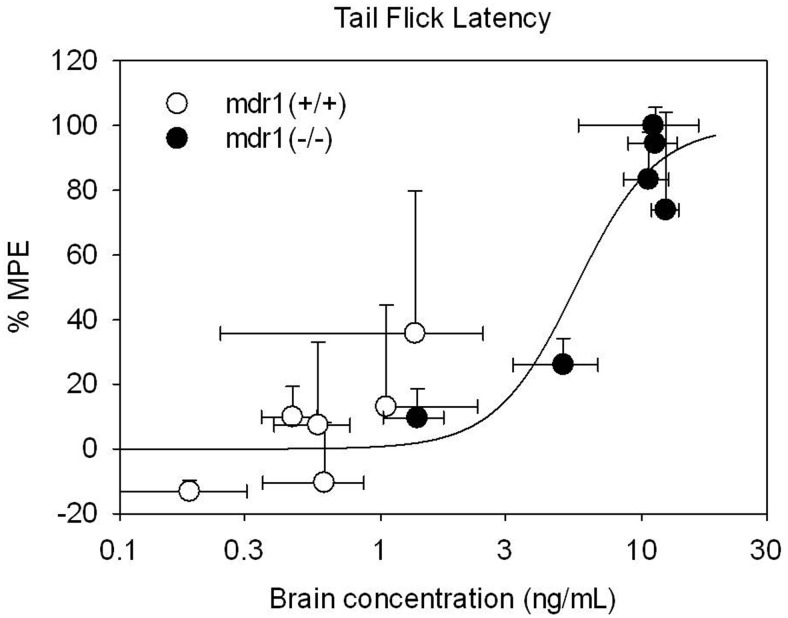
Norbuprenorphine concentration-effect curves were constructed for both plasma and brain concentrations. There was a substantial leftward shift of the plasma concentration-effect curve in the mdr(−/−) compared with the wild-type mice (Fig. 3). The norbuprenorphine plasma EC50 was 14 ± 5 in the mdr1a(−/−) mice compared with 471 ± 255 in the mdr1(+/+) mice. In contrast, there was no apparent difference between the two mouse strains in the brain concentration-effect relationship for buprenorphine, which could be described by a common exponential, with the brain EC50 of 6 ± 1 (Fig. 4).
Fig. 3.
Relationship between antinociception and plasma norbuprenorphine concentration. The relationship shown is after subcutaneous injections of 0.25 mg/kg norbuprenorphine in mdr1a(−/−) mice (●) and 1.0 mg/kg norbuprenorphine in mdr1(+/+) mice (○). Data are mean ± S.D. (n = 4). Lines represent the fit of a sigmoidal Emax model to the effect data.
Fig. 4.
Relationship between antinociception and brain norbuprenorphine concentration. The relationship shown is after subcutaneous injections of 0.25 mg/kg norbuprenorphine in mdr1a(−/−) mice (●) and 1.0 mg/kg norbuprenorphine in mdr1(+/+) mice (○). Data are mean ± S.D. (n = 4). Lines represent the fit of a sigmoidal Emax model to the effect data.
Norbuprenorphine Respiratory Effects in mdr1a(−/−) and mdr1a(+/+) Mice.
By experimental design (see Materials and Methods) a dose of 0.25 mg/kg norbuprenorphine elicited the same effect in mdr1a(−/−) mice as that seen in the mdr1a(+/+) mice that received 1 mg/kg (Fig. 5). Respiratory rates decreased approximately 60% from baseline in both groups. There was no change in tidal volume after administration of norbuprenorphine in either mdr1a(−/−) or mdr1a(+/+) mice.
Fig. 5.
Respiratory rate in mdr1a(−/−) and wild-type mice administered norbuprenorphine. Respiratory rate and tidal volume in mdr1a(+/+) mice (A) and mdr1a(−/−) mice (B) after subcutaneous injection of norbuprenorphine or saline vehicle were measured by unrestrained whole-body plethysmography. Results are mean ± S.D. (four per group). *, significantly different from vehicle control (p < 0.05) by two-way repeated-measures analysis of variance. There was no effect of norbuprenorphine on tidal volume.
Behavioral Observations.
During the course of the experiment, several behaviors were observed in the P-gp-deficient mdr1a(−/−) mice that received norbuprenorphine. At 15 min after administration of norbuprenorphine, the mdr1a(−/−) mice exhibited Straub tails (Bilbey et al., 1960). The mdr1a(−/−) mice exhibited loss of the righting reflex (Oka et al., 1992; Kamei et al., 1996), with the mice unable to right themselves upon being released after the tail-flick procedure. In comparison, the mdr1a(+/+) mice did not show Straub tails or loss of righting reflex. Both groups of mdr1a(+/+) and mdr1a(−/−) mice seemed equally sedated after norbuprenorphine, with minimal movements approximately 15 min after norbuprenorphine dosing.
Discussion
The purpose of this investigation was to evaluate P-gp-mediated transport of buprenorphine, norbuprenorphine, norbuprenorphine-3-glucuronide, and buprenorphine-3-glucuronide in vitro and test the hypothesis that P-gp-mediated efflux limits brain access of norbuprenorphine (and possibly norbuprenorphine-3-glucuronide) and influences norbuprenorphine antinociception. P-gp-transfected MDCK cells were used to screen for P-gp substrates specifically, rather than as a model for blood-brain transport more generally. The major result was that norbuprenorphine was a P-gp substrate both in vitro and in vivo. As measured by the net efflux ratio in transfected cells, P-gp-mediated norbuprenorphine efflux was substantial and approximated that of the unambiguous P-gp substrate loperamide (Polli et al., 2001) (9 versus 13, respectively). P-gp-mediated norbuprenorphine brain efflux was also observed in vivo. In mdr1a(+/+) animals, the norbuprenorphine brain/plasma ratio was negligible and constant. In contrast, in mdr1a(−/−) animals the norbuprenorphine brain/plasma ratio increased steadily, approaching unity. This demonstrates that P-gp represents a major barrier to norbuprenorphine entering the brain and elimination of transporter function resulted in increased norbuprenorphine brain concentrations, which essentially equaled plasma concentrations. Thus, absent P-gp-mediated efflux, norbuprenorphine accumulates in the brain until the drug is equally distributed between brain and plasma. The low norbuprenorphine brain concentration in mdr1a(+/+) mice probably is caused by a combination of low passive permeability and substantial P-gp-mediated efflux (Ohtani et al., 1995).
P-gp-mediated brain access significantly influenced the pharmacologic effect of norbuprenorphine. Antinociception was greater in both magnitude and duration in mdr1a(−/−) compared with mdr1a(+/+) mice despite the mdr1a(−/−) mice receiving one-fourth the norbuprenorphine dose of the mdr1a(+/+) mice. Moreover, the duration of norbuprenorphine antinociception in mdr1a(−/−) mice was even greater than that observed after a maximally antinociceptive buprenorphine dose in mdr1a(+/+) mice (Brown et al., 2011). There was a substantial shift of the norbuprenorphine plasma concentration-effect curve for antinociception and more than an order of magnitude difference in the plasma EC50 between mdr1a(−/−) and mdr1a(+/+) mice, yet there was no difference in the brain concentration-effect curves. In addition, some characteristic opioid-induced behaviors were observed after norbuprenorphine in mdr1a(−/−) but not mdr1a(+/+) mice. The mdr1a(−/−) mice administered norbuprenorphine exhibited Straub tails, the tail held erect because of opioid-induced contraction of the sacrococcygenus muscle (Bilbey et al., 1960; Aceto et al., 1969). This has been shown to be mediated through the central μ2 opioid receptor subtype (Nath et al., 1994). The mdr1a(−/−) mice also exhibited loss of righting reflex, another μ2 opioid receptor-mediated effect (Oka et al., 1992; Kamei et al., 1996). Together, these findings strongly support the hypothesis that P-gp regulates brain access of norbuprenorphine, and greater norbuprenorphine brain access in P-gp-deficient mice caused greater antinociceptive and behavioral effects.
Norbuprenorphine is a μ, δ, and κ receptor agonist, as shown in vitro (Huang et al., 2001; Brown et al., 2011). Thus, the poor antinociceptive effects of norbuprenorphine in wild-type mice could be caused by limited brain exposure rather than low intrinsic analgesic efficacy of norbuprenorphine. Although norbuprenorphine was shown to have an analgesic effect one-fourth that of buprenorphine after an intraventricular dose in rats (Ohtani et al., 1995), intraventricular dosing in a wild-type animal may still be accompanied by substantial P-gp-mediated efflux and therefore less overall brain exposure.
Both buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide are abundant buprenorphine metabolites in humans in vivo (McCance-Katz et al., 2006, 2007) and were found to be biologically active in mice (Brown et al., 2011). The potential contribution of norbuprenorphine-3-glucuronide to norbuprenorphine effects and influence of P-gp deficiency on norbuprenorphine effects were unknown. Therefore, P-gp-mediated norbuprenorphine-3-glucuronide transport was evaluated, along with that of buprenorphine and buprenorphine-3-glucuronide. In vitro, neither buprenorphine, buprenorphine-3-glucuronide, nor norbuprenorphine-3-glucuronide were P-gp substrates. The net efflux ratio was approximately one for each. In vivo norbuprenorphine-3-glucuronide brain/plasma concentration ratios after norbuprenorphine were very small and unaffected by P-gp deficiency, indicating that norbuprenorphine-3-glucuronide has limited brain access, and P-gp does not influence norbuprenorphine-3-glucuronide efflux or brain concentrations in vivo.
Although brain norbuprenorphine-3-glucuronide concentrations were low, they were not zero, indicating that despite the highly hydrophilic nature of this glucuronide, it did pass the blood-brain barrier. Limited but detectable brain access of norbuprenorphine-3-glucuronide is consistent with previous results, which found norbuprenorphine-3-glucuronide in brain after administration of either norbuprenorphine or the glucuronide and a small pharmacologic effect of norbuprenorphine-3-glucuronide (Brown et al., 2011). The determinants of norbuprenorphine-3-glucuronide brain access, including passive permeability and/or active uptake, are not known. Organic anion-transporting polypeptides are expressed in both human and murine brain microvessel endothelial cells (Hagenbuch and Meier, 2004). These transporters can transport glucuronide conjugates of drugs (Hagenbuch and Meier, 2004) and may be responsible, in vivo, for the brain exposure of glucuronides. Further testing is required to determine any possible role for uptake transporters in the brain exposure of norbuprenorphine-3-glucuronide (or buprenorphine-3-glucuronide).
The norbuprenorphine-3-glucuronide results provide additional insights into the mechanism of norbuprenorphine effects. Norbuprenorphine-3-glucuronide was shown previously to have high affinity for κ and nociceptin but not μ or δ receptors, and, when administered to mice, substantially decreased tidal volume but not respiratory rate (Brown et al., 2011). In the present experiment, brain norbuprenorphine-3-glucuronide concentrations were very low after norbuprenorphine and did not differ between mdr1a(+/+) and mdr1a(−/−) mice. Respiratory rate but not tidal volume was affected in both mdr1a(+/+) and mdr1a(−/−) mice. This suggests that norbuprenorphine-3-glucuronide did not contribute to the respiratory effect after a norbuprenorphine dose. Sedation is another effect of both norbuprenorphine and norbuprenorphine-3-glucuronide (Brown et al., 2011). Both the mdr1a(−/−) and mdr1a(+/+) mice were equally sedated after norbuprenorphine, despite the large differences in brain concentrations of both norbuprenorphine and norbuprenorphine-3-glucuronide between the two strains. Thus, it cannot be concluded whether the sedation observed in this study was attributable to norbuprenorphine, norbuprenorphine-3-glucuronide, or both.
The role of brain access via passive permeability and/or P-gp-mediated efflux and contribution to the respiratory effect of norbuprenorphine is not yet clear. The dose of norbuprenorphine given to the mdr1a(−/−) mice was selected to match the respiratory effects of norbuprenorphine in the mdr1a(+/+) mice. It is noteworthy that the significant respiratory depression in mdr1a(+/+) mice occurred despite very low brain norbuprenorphine concentrations. In addition, the time course of decreased respiratory rate did not parallel the time course of norbuprenorphine brain concentration. Although there was an increase in brain norbuprenorphine corresponding to the decrease in respiratory rate in the mdr1a(−/−) mice, there was no change in brain norbuprenorphine in the mdr1a(+/+) mice. A plausible hypothesis for the profound norbuprenorphine effect despite such low brain concentrations is high respiratory depressant potency. Although norbuprenorphine potency has been modeled in silico (Yassen et al., 2007), potency of norbuprenorphine respiratory depression remains to be elucidated in vivo. Furthermore, the receptor type involved in mediating norbuprenorphine-induced respiratory depression is still unresolved.
P-gp magnifies the discrepancy between the pharmacologic effects of buprenorphine and norbuprenorphine. Buprenorphine is not a P-gp substrate (Nekhayeva et al., 2006; Hassan et al., 2009; Tournier et al., 2010), and P-gp does not influence the pharmacologic effect of buprenorphine in mice (Hassan et al., 2009). In contrast, norbuprenorphine clearly is a P-gp substrate. Norbuprenorphine has significant respiratory effects in both mdr1a(−/−) and mdr1a(+/+) mice, but only in the absence of P-gp does norbuprenorphine have a substantial antinociceptive effect. This suggests that the sites or mechanism of action for norbuprenorphine analgesia and respiratory depression may be distinct, and P-gp-mediated efflux restricts norbuprenorphine access to the analgesia effect site but not the respiratory effect site. This could be caused by the differential expression of P-gp in various locations of the blood-central nervous system brain barrier. For example, there is lower P-gp expression in murine endothelial cells of the blood-spinal cord barrier compared with the blood-brain barrier (Ge and Pachter, 2006). In addition, P-gp influenced blood-brain but not blood-spinal cord morphine distribution in mdr1a(−/−) compared with mdr1a(+/+) mice (Zong and Pollack, 2000). Thus differences in brain P-gp and norbuprenorphine efflux between mdr1a(+/+) and mdr1a(−/−) mice may be greater than differences in spinal cord P-gp and efflux, potentially influencing analgesia more than respiratory depression, depending on their respective sites of action.
The influence of P-gp on norbuprenorphine effects can be compared with that of other opioids. The increase in maximum antinociception (MPE) in mdr1a(−/−) compared with mdr1a(+/+) mice was 60% for norbuprenorphine, but 20, 40, and 50% for methadone (Thompson et al., 2000; Hassan et al., 2009), morphine (Zong and Pollack, 2000), and fentanyl (Hamabe et al., 2006), respectively. In addition, the 8-fold increase in the brain/plasma norbuprenorphine concentration ratio in the absence of P-gp-mediated efflux is greater than that of other opioids. Brain/plasma concentration ratios of morphine, alfentanil, and methadone increased only 2-, 2.4, and 3.5-fold, respectively, in mdr1(−/−) mice compared with mdr1(+/+) mice (Zong and Pollack, 2000; Kalvass et al., 2007; Hassan et al., 2009). Moreover, the more than order of magnitude decrease in plasma EC50 in the absence of P-gp was much greater for norbuprenorphine than for other opioids, such as alfentanil (Kalvass et al., 2007). Thus the degree to which P-gp impedes brain access and antinociception is greater for norbuprenorphine than these other opioids.
The role of P-gp in mediating brain access of norbuprenorphine in humans and the pharmacologic contribution of norbuprenorphine to buprenorphine clinical effects are unknown. If, however, these are clinically relevant, then findings described in this article may explain, in part, clinical observations about buprenorphine (Bruce and Altice, 2006; McCance-Katz et al., 2007). Atazanavir and atazanavir/ritonavir increased plasma buprenorphine and norbuprenorphine concentrations 1.3- to 2.5-fold, albeit not into ranges shown previously to cause side effects (McCance-Katz et al., 2007). Nevertheless, the interaction caused some subjects to become sedated. The adverse side effects after buprenorphine/atazanavir were attributed to inhibition of CYP3A4-mediated buprenorphine metabolism by atazanvir. However, atazanavir is also a P-gp inhibitor (Bierman et al., 2010), and, in light of the findings in the present study, it is possible that atazanavir-enhanced buprenorphine effects could be attributable, at least in part, to P-gp inhibition and increased norbuprenorphine brain access. Tipranavir/ritonavir had no effect on plasma buprenorphine concentrations but diminished norbuprenorphine concentrations to one-fifth of control and had no effect on opioid withdrawal (Bruce et al., 2009). Although this was considered mechanistically unclear, tipranavir and ritonavir are both P-gp inhibitors (Storch et al., 2007) and therefore might have increased brain norbuprenorphine exposure and contributed to obviating withdrawal. Rifampin induced buprenorphine clearance and caused significant withdrawal in opioid-dependent patients (McCance-Katz et al., 2011). Although withdrawal was attributed to diminished buprenorphine concentrations, the decrease in plasma norbuprenorphine concentrations was substantially greater than that of buprenorphine (8- versus 3-fold). Because rifampin induces brain P-gp (Bauer et al., 2006), the combined effects of rifampin to decrease plasma norbuprenorphine concentrations and up-regulate norbuprenorphine brain efflux could have markedly decreased brain norbuprenorphine exposure and contributed to the withdrawal. Nevertheless, the role of norbuprenorphine and P-gp-mediated brain access in humans remains unknown.
Two limitations of the present investigation merit mention. Buprenorphine was found to bind readily to the polystyrene used for the in vitro Transwell studies, which potentially confounded the accurate determination of the extent of transcellular movement. Because of differences in the surface areas of the apical and basolateral compartments, correction for nonspecific binding was not possible. Therefore, the buprenorphine data should be interpreted carefully. Nonspecific buprenorphine binding has also been reported previously (Tournier et al., 2010). Because of the unexpectedly large effect of P-gp on brain norbuprenorphine access, there was not sufficient overlap of norbuprenorphine brain concentrations between mdr1a(−/−) and mdr1a(+/+) mice to separately model the brain concentration-antinociception data in the two groups, thus they were modeled together.
In conclusion, the limited antinociceptive effect of norbuprenorphine is caused by P-gp-mediated inhibition of brain access. P-gp does not influence the brain disposition of buprenorphine-3-glucuronide or norbuprenorphine-3-glucuronide. These findings add to the complexity of buprenorphine pharmacology.
Acknowledgments
We thank Rolley (Ed) Johnson and Moo Kwang Park for assistance in obtaining norbuprenorphine and Jeremy Morrissey for assistance in harvesting mouse tissue.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01 DA25931, K24 DA00417] (to E.D.K.); and the National Institutes of Health Neuroscience Blueprint Interdisciplinary Center [Core Grant P30 NS057105] (to Washington University).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- BBB
- blood-brain barrier
- P-gp
- P-glycoprotein
- MDR
- multidrug resistance protein
- MDCK
- Madin-Darby canine kidney
- Papp
- apparent permeability
- MPE
- maximum possible effect
- B → A
- basolateral to apical
- A → B
- apical to basolateral
- HBSS
- Hanks' buffered salt solution
- HBSSCM
- HBSS supplemented with 1 mM CaCl2, 0.1 mM MgSO4, and 0.1 mM MgCl2
- LC/MS/MS
- liquid chromatography/tandem mass spectrometry.
Authorship Contributions
Participated in research design: Brown, Campbell, and Kharasch.
Conducted experiments: Brown, Campbell, Crafford, and Regina.
Contributed new reagents or analytic tools: Holtzman.
Performed data analysis: Brown, Campbell, and Crafford.
Wrote or contributed to the writing of the manuscript: Brown, Campbell, and Kharasch.
References
- Abbott NJ. (2005) Physiology of the blood-brain barrier and its consequences for drug transport to the brain. Int Congress Ser 1277:3–18 [Google Scholar]
- Aceto MD, McKean DB, Pearl J. (1969) Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol 36:225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol 70:1212–1219 [DOI] [PubMed] [Google Scholar]
- Bierman WF, Scheffer GL, Schoonderwoerd A, Jansen G, van Agtmael MA, Danner SA, Scheper RJ. (2010) Protease inhibitors atazanavir, lopinavir and ritonavir are potent blockers, but poor substrates, of ABC transporters in a broad panel of ABC transporter-overexpressing cell lines. J Antimicrob Chemother 65:1672–1680 [DOI] [PubMed] [Google Scholar]
- Bilbey DL, Salem H, Grossman MH. (1960) The anatomical basis of the straub phenomenon. Br J Pharmacol Chemother 15:540–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton DW, DeVane CL, Liston HL, Markowitz JS. (2002) In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci 71:163–169 [DOI] [PubMed] [Google Scholar]
- Brown SM, Holtzman M, Kim T, Kharasch ED. (2011) Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology 115:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Altice FL. (2006) Three case reports of a clinical pharmacokinetic interaction with buprenorphine and atazanavir plus ritonavir. AIDS 20:783–784 [DOI] [PubMed] [Google Scholar]
- Bruce RD, Altice FL, Moody DE, Lin SN, Fang WB, Sabo JP, Wruck JM, Piliero PJ, Conner C, Andrews L, et al. (2009) Pharmacokinetic interactions between buprenorphine/naloxone and tipranavir/ritonavir in HIV-negative subjects chronically receiving buprenorphine/naloxone. Drug Alcohol Depend 105:234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. (1977) Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais C, Graff CL, Pollack GM. (2004) Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol 67:269–276 [DOI] [PubMed] [Google Scholar]
- Doran A, Obach RS, Smith BJ, Hosea NA, Becker S, Callegari E, Chen C, Chen X, Choo E, Cianfrogna J, et al. (2005) The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab Dispos 33:165–174 [DOI] [PubMed] [Google Scholar]
- Engelhardt B. (2011) Neuroscience. Blood-brain barrier differentiation. Science 334:1652–1653 [DOI] [PubMed] [Google Scholar]
- Fan J, Brown SM, Tu Z, Kharasch ED. (2011) Chemical and enzyme-assisted syntheses of norbuprenorphine-3-β-d-glucuronide. Bioconjug Chem 22:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pachter JS. (2006) Isolation and culture of microvascular endothelial cells from murine spinal cord. J Neuroimmunol 177:209–214 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hamabe W, Maeda T, Fukazawa Y, Kumamoto K, Shang LQ, Yamamoto A, Yamamoto C, Tokuyama S, Kishioka S. (2006) P-glycoprotein ATPase activating effect of opioid analgesics and their P-glycoprotein-dependent antinociception in mice. Pharmacol Biochem Behav 85:629–636 [DOI] [PubMed] [Google Scholar]
- Hassan HE, Myers AL, Coop A, Eddington ND. (2009) Differential involvement of P-glycoprotein (ABCB1) in permeability, tissue distribution, and antinociceptive activity of methadone, buprenorphine, and diprenorphine: in vitro and in vivo evaluation. J Pharm Sci 98:4928–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 297:688–695 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jolliet-Riant P, Tillement JP. (1999) Drug transfer across the blood-brain barrier and improvement of brain delivery. Fundam Clin Pharmacol 13:16–26 [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Pollack GM. (2007) Pharmacokinetics and pharmacodynamics of alfentanil in P-glycoprotein-competent and P-glycoprotein-deficient mice: P-glycoprotein efflux alters alfentanil brain disposition and antinociception. Drug Metab Dispos 35:455–459 [DOI] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M, Nagase H. (1996) Possible involvement of μ2-opioid receptor-mediated mechanisms in morphine-induced enhancement of the pentobarbital-induced loss of the righting reflex in the mouse. Life Sci 59:PL349–PL353 [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Hoffer C, Whittington D. (2004) The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br J Clin Pharmacol 57:600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. (1998) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse GD, Friedland G, Pade P, Baker J, Alvanzo A, Smith P, Ogundele A, Jatlow P, et al. (2006) Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis 43 (Suppl 4):S224–S234 [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse GD, Ma Q, DiFrancesco R, Friedland G, Pade P, Rainey PM. (2007) Interaction between buprenorphine and atazanavir or atazanavir/ritonavir. Drug Alcohol Depend 91:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Prathikanti S, Friedland G, Rainey PM. (2011) Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients. Drug Alcohol Depend 118:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RW., 4th (2009) The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J Pharmacol Exp Ther 330:834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DE, Chang Y, Huang W, McCance-Katz EF. (2009) The in vivo response of novel buprenorphine metabolites, M1 and M3, to antiretroviral inducers and inhibitors of buprenorphine metabolism. Basic Clin Pharmacol Toxicol 105:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. (2002) A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem 306:31–39 [DOI] [PubMed] [Google Scholar]
- Nath C, Gupta MB, Patnaik GK, Dhawan KN. (1994) Morphine-induced straub tail response: mediated by central μ2-opioid receptor. Eur J Pharmacol 263:203–205 [DOI] [PubMed] [Google Scholar]
- Nekhayeva IA, Nanovskaya TN, Hankins GD, Ahmed MS. (2006) Role of human placental efflux transporter P-glycoprotein in the transfer of buprenorphine, levo-α-acetylmethadol, and paclitaxel. Am J Perinatol 23:423–430 [DOI] [PubMed] [Google Scholar]
- Ohtani M, Kotaki H, Nishitateno K, Sawada Y, Iga T. (1997) Kinetics of respiratory depression in rats induced by buprenorphine and its metabolite, norbuprenorphine. J Pharmacol Exp Ther 281:428–433 [PubMed] [Google Scholar]
- Ohtani M, Kotaki H, Sawada Y, Iga T. (1995) Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther 272:505–510 [PubMed] [Google Scholar]
- Oka T, Liu XF, Kajita T, Ohgiya N, Ghoda K, Taniguchi T, Arai Y, Matsumiya T. (1992) Effects of the subcutaneous administration of enkephalins on tail-flick response and righting reflex of developing rats. Brain Res Dev Brain Res 69:271–276 [DOI] [PubMed] [Google Scholar]
- Pardridge WM. (1997) Drug delivery to the brain. J Cereb Blood Flow Metab 17:713–731 [DOI] [PubMed] [Google Scholar]
- Polli JW, Jarrett JL, Studenberg SD, Humphreys JE, Dennis SW, Brouwer KR, Woolley JL. (1999) Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm Res 16:1206–1212 [DOI] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. (2001) Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther 299:620–628 [PubMed] [Google Scholar]
- Sasongko L, Link JM, Muzi M, Mankoff DA, Yang X, Collier AC, Shoner SC, Unadkat JD. (2005) Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin Pharmacol Ther 77:503–514 [DOI] [PubMed] [Google Scholar]
- Skarke C, Jarrar M, Schmidt H, Kauert G, Langer M, Geisslinger G, Lötsch J. (2003) Effects of ABCB1 (multidrug resistance transporter) gene mutations on disposition and central nervous effects of loperamide in healthy volunteers. Pharmacogenetics 13:651–660 [DOI] [PubMed] [Google Scholar]
- Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. (2007) Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol 73:1573–1581 [DOI] [PubMed] [Google Scholar]
- Thompson SJ, Koszdin K, Bernards CM. (2000) Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology 92:1392–1399 [DOI] [PubMed] [Google Scholar]
- Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Declèves X. (2010) Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int J Neuropsychopharmacol 13:905–915 [DOI] [PubMed] [Google Scholar]
- Uhr M, Grauer MT, Holsboer F. (2003) Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol Psychiatry 54:840–846 [DOI] [PubMed] [Google Scholar]
- van Waterschoot RA, Schinkel AH. (2011) A critical analysis of the interplay between cytochrome P450 3A and P-glycoprotein: recent insights from knockout and transgenic mice. Pharmacol Rev 63:390–410 [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. (1994) Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther 55:569–580 [DOI] [PubMed] [Google Scholar]
- Wandel C, Kim R, Wood M, Wood A. (2002) Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology 96:913–920 [DOI] [PubMed] [Google Scholar]
- Wang JS, Ruan Y, Taylor RM, Donovan JL, Markowitz JS, DeVane CL. (2004) Brain penetration of methadone (R)-and (S)-enantiomers is greatly increased by P-glycoprotein deficiency in the blood-brain barrier of Abcb1a gene knockout mice. Psychopharmacology 173:132–138 [DOI] [PubMed] [Google Scholar]
- Xie R, Hammarlund-Udenaes M, de Boer AG, de Lange EC. (1999) The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a(−/−) and mdr1a(+/+) mice. Br J Pharmacol 128:563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M. (2007) Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats. J Pharmacol Exp Ther 321:598–607 [DOI] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. (1997) Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther 282:1187–1197 [PubMed] [Google Scholar]
- Zong J, Pollack GM. (2000) Morphine antinociception is enhanced in mdr1a gene-deficient mice. Pharm Res 17:749–753 [DOI] [PubMed] [Google Scholar]