Abstract
Female mice generating oocytes lacking complex N- and O-glycans (double mutants (DM)) produce only one small litter before undergoing premature ovarian failure (POF) by 3 months. Here we investigate the basis of the small litter by evaluating ovulation rate and embryo development in DM (Mgat1F/FC1galt1F/F:ZP3Cre) and Control (Mgat1F/FC1galt1F/F) females. Surprisingly, DM ovulation rate was normal at 6 weeks, but declined dramatically by 9 weeks. In vitro development of zygotes to blastocysts was equivalent to Controls although all embryos from DM females lacked a normal zona pellucida (ZP) and ∼30% lacked a ZP entirely. In contrast, in vivo preimplantation development resulted in less embryos recovered from DM females compared with Controls at 3.5 days post coitum (dpc) (3.2±1.3 vs 7.0±0.6). Furthermore, only 45% of mated DM females contained embryos at 3.5 dpc. Of the preimplantation embryos collected from DM females, approximately half were morulae unlike Controls where the majority were blastocysts, indicating delayed embryo development in DM females. Post-implantation development in DM females was analysed to determine whether delayed preimplantation development affected subsequent development. In DM females at 5.5 dpc, only ∼40% of embryos found at 3.5 dpc had implanted. However, at 6.5 dpc, implantation sites in DM females corresponded to embryo numbers at 3.5 dpc indicating delayed implantation. At 9.5 dpc, the number of decidua corresponded to embryo numbers 6 days earlier indicating that all implanted embryos progress to midgestation. Therefore, a lack of complex N- and O-glycans in oocytes during development impairs early embryo development and viability in vivo leading to delayed implantation and a small litter.
Introduction
The fertility of an individual female is a reflection of the number of eggs ovulated and their competence. The development of each oocyte occurs within a follicle which can be considered as the functional unit of the ovary. The development and growth of follicles requires complex endocrine, paracrine and autocrine regulations, as well as bidirectional signals between the oocyte and follicular cells. Each follicle consists of a central oocyte that is surrounded by layers of granulosa cells that are separated from the outer surrounding theca cells by a basal lamina. Upon antrum formation, granulosa cells differentiate into mural granulosa cells that line the follicle wall and cumulus cells that surround the oocyte. Since 1996 when the critical role of the oocyte in follicle development was revealed, elucidating the various functions of the oocyte has been the focus of much research (Dong et al. 1996, Matzuk et al. 2002, Hutt & Albertini 2007, Gilchrist et al. 2008, Kidder & Vanderhyden 2010). Indeed, differentiation of the cumulus cells and their subsequent expansion before ovulation requires oocyte-secreted factors (Diaz et al. 2007, Sugiura et al. 2010).
Essential cell-to-cell signalling between the oocyte and the surrounding granulosa cells occurs via transzonal processes (TZPs). TZPs penetrate from the granulosa cells through the extracellular matrix coat that surrounds the oocyte. This extracellular matrix coat known as the zona pellucida (ZP) is composed of three glycoproteins, ZP1, ZP2 and ZP3 (Bleil & Wassarman 1980b, Wassarman 2005) in the mouse, all of which are expressed exclusively by the oocyte from the primary stage of follicle development onwards (Philpott et al. 1987). Glycans on ZP proteins had been postulated as sperm receptors for many years (Bleil & Wassarman 1980a, van Duin et al. 1994, Litscher et al. 2009, Han et al. 2010). However, mutant mice generating oocytes lacking complex N- and/or O-glycans are fertile demonstrating that these glycans are not required for zona–sperm binding in mice although the ZP is thinner and more fragile if it lacks O-glycans, N-glycans or both (Shi et al. 2004, Williams et al. 2007, Williams & Stanley 2008).
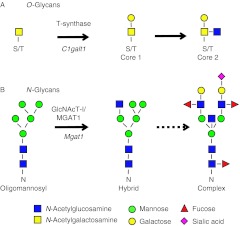
The generation of both core 1 and 2 O-glycans (also referred to as complex O-glycans) and complex and hybrid N-glycans occurs on glycoproteins at specific motifs in a highly regulated manner (Fig. 1). The generation of core 1 and 2 O-glycans requires the glycosyltransferase T-synthase encoded by the C1galt1 gene (previously known as T-syn). Core 1 O-glycans are synthesized by the addition of galactose to the N-acetylgalactosamine (GalNAc) attached via O-linkage to serine or threonine residues. Core 2 O-glycans are generated by the attachment of N-acetylglucosamine (GlcNAc) to the core 1 structure. Mice lacking C1galt1 die during gestation at ∼E14 due to defective angiogenesis (Xia et al. 2004, Williams et al. 2007). Mice lacking complex and hybrid N-glycans also die in utero at E9.5 (Ioffe & Stanley 1994, Metzler et al. 1994). Complex and hybrid N-glycans are generated by modification and extension of oligomannosyl structures which is initiated by the enzyme N-acetylglucosaminyltransferase 1 (GlcNAcT-1, encoded by the Mgat1 gene). Therefore, to study biological functions of these glycans in the oocyte Cre-loxP technology has been used to eliminate the enzymes required for their generation. Cre-loxP technology allows a gene flanked by loxP sites to be deleted upon exposure to the enzyme Cre recombinase. Therefore, by flanking the coding exons of C1galt1 and/or Mgat1 with loxP sites, and using a Cre recombinase transgene under the control of the oocyte-specific ZP3 promoter, mice can be generated which lack these enzymes specifically in the oocyte (Shi et al. 2004, Williams & Stanley 2008, 2011).
Figure 1.
The generation of complex O- and N-glycans. (A) T-synthase (encoded by C1galt1) is required to initiate the synthesis of core 1 O-glycans by transferring galactose (Gal) to N-acetylgalactosamine (GalNAc) on serine/threonine residues (S/T). Core 2 O-glycans are generated by the addition of N-acetylglucosamine (GlcNAc) to the GalNAc of core 1 structure. (B) N-Acetyl-glucosaminyltransferase 1 (GlcNAcT-1, encoded by Mgat1) is required to generate complex and hybrid N-glycans from the oligomannosyl structure.
Females with oocyte-specific deletion of C1galt1 generate oocytes lacking core 1 and 2 O-glycans and exhibit an increase in fertility, revealing a role for these glycans in the regulation of ovulation rate (Williams & Stanley 2008). Oocyte-specific deletion of Mgat1 results in the generation of oocytes lacking complex and hybrid N-glycans. These glycans have a role in generating developmentally competent oocytes, and once fertilised, in embryo implantation and development (Shi et al. 2004). Mgat1F/F:ZP3Cre females have reduced fertility producing smaller litters (Shi et al. 2004). Follicle development is also modified in these mice with considerable abnormalities evident in preovulatory follicles (Williams & Stanley 2009). Moreover, oocyte-specific deletion of both Mgat1 and C1galt1 in double mutant (DM) females results in ovarian dysfunction leading to an altered endocrine profile and premature ovarian failure (POF) by 3 months of age (Williams & Stanley 2011). However, embryonic development and fertility are normal for mice heterozygous for either Mgat1 or C1galt1 generated by crossing Mgat1del/+ or C1galt1del/+ mice (Ioffe & Stanley 1994, Xia et al. 2004). Furthermore, fertility is also normal in mice generating oocytes heterozygous for either Mgat1 or C1galt1 using oocyte-specific deletion (Williams et al. 2007). In addition, females generating oocytes heterozygous for both Mgat1 and C1galt1 have normal fertility (Williams et al. 2007).
The aim of this study was to determine the stage(s) at which the decrease in fertility occurs in DM females. We reveal that the decrease in fertility is not due to a reduced ovulation rate but due to modified embryo development.
Results
Ovulation rate
We have previously demonstrated that DM females mated at 6 weeks have decreased fertility producing only a single small litter of ∼4 pups (Williams et al. 2007, Williams & Stanley 2011). To determine whether the smaller litter size was due to a decrease in the number of eggs ovulated, ovulation rate was assessed by analysis of the number of eggs and/or presumptive zygotes recovered at 0.5 days post coitum (dpc) in females joined with males, at either 6 weeks or 9 weeks of age (termed the ‘6-week group’ and ‘9-week group’ respectively) (Fig. 2).
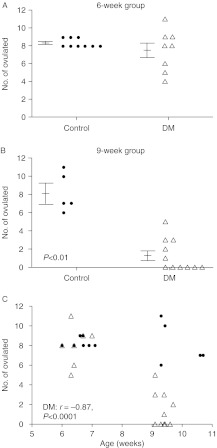
Figure 2.
Ovulation rate determined as the number of presumptive zygotes collected from the oviducts 0.5 dpc from Control and DM females joined with males at (A) 6 weeks old (6-week group) and (B) 9 weeks old (9-week group). (C) Correlation between age and number of eggs collected from (closed circle) Control and (open triangle) DM females. Note: some data points overlap and therefore not all are visible. Results are expressed as mean±s.e.m.
Most surprisingly, the ovulation rate did not differ between Control and DM females in the 6-week group (Control: 8.3±0.2, n=9; DM: 7.5±0.8, n=8), although ovulation rate of DM females was considerably more variable than that of Controls (Fig. 2A). Importantly, the age at which Control and DM females ovulated also did not differ (Control: 6.5±0.1 week; DM: 6.3±0.2 week). However, 3 weeks later in the 9-week group DM females exhibited a marked decrease in ovulation rate (Control: 8.2±0.9, n=5; DM: 1.3±0.5, n=11; P<0.01), revealing a rapid loss of function by DM ovaries (Fig. 2B). Analysis of all ovulation rate data reveals a negative correlation in DM females between age and the number of eggs ovulated (Fig. 2C; r=−0.87, P<0.0001), in contrast to Control females whose ovulation rate remained constant. The sharp drop in DM ovulation rate from the 6- to 9-week group is most likely the main reason DM females do not produce a second litter (Williams & Stanley 2011).
Fertilisation rate and in vitro embryo development
Since, unlike litter size, the ovulation rate of DM females was not decreased in the 6-week group, fertilisation rate was investigated to determine whether the thin fragile ZP of DM eggs (Williams et al. 2007) was affecting fertilisation in vivo. Eggs collected from 6-week group DM and Control females at 0.5 dpc were assumed to have been fertilised and were termed ‘presumptive zygotes’ (Fig. 3A and B, Control: 7.0±0.3 week, DM: 6.1±0.2 week). When collected, the ZP of presumptive zygotes from DM females was either thin or absent compared with the thick ZP around zygotes from Control females (Fig. 3A and B). Thirty percent of DM presumptive zygotes lacked a ZP (Table 1). However, despite the lack of a normal ZP on all presumptive zygotes collected from DM females the percentage that developed to two-cell embryos after culture was not decreased compared with those from Controls (Fig. 3E; Controls: 95.3±2.9%, DM: 96.4±2.3%).
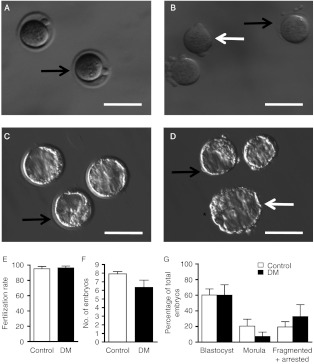
Figure 3.
In vitro development of preimplantation embryos. Images of embryos collected and cultured in vitro from Control (A and C) and DM (B and D) 6-week group females. (A and B) Presumptive zygotes collected at 0.5 dpc. (C and D) Blastocyst after 4 days in culture. Black arrow indicates ZP; white arrows indicate DM embryos without ZP; asterisk indicates a bigger blastocyst that seems to have resulted from the fusion of two embryos because the number of embryos present in this dish was reduced by one. (E) Fertilisation rate of Control (n=12) and DM (n=6) females. (F) Total number of embryos at the end of the culture period from Control and DM females. (G) Percentage of blastocysts, morulae, fragmented and arrested embryos in Control and DM females at 4.5 dpc. Results are expressed as mean±s.e.m. Bar scale=100 μm.
Table 1.
Number of presumptive zygotes and embryos lacking ZP in both in vitro and in vivo conditions.
| Collected (0.5 dpc) | In vitro (4.5 dpc) | In vivo (3.5 dpc) | ||||
|---|---|---|---|---|---|---|
| No. of presumptive zygotes | No. of ZP-free presumptive zygotes (%) | No. of embryos | No. of ZP-free embryos (%) | No. of embryos | No. of ZP-free embryos (%) | |
| Control | 8.3±0.2 | 0.2±0.2b | 7.9±0.3 | 0.2±0.2a | 7.0±0.6 | 0 |
| (n=12) | (1.9±1.9#) | (n=12) | (3.3±3.3*) | (n=5) | (n=5) | |
| (n=12) | (n=12) | |||||
| DM | 7.0±1.3 | 1.7±0.7b | 6.3±0.8 | 1.3±0.5a | 3.2±1.3 | 1.2±0.5 |
| (n=6) | (30.0±12.0#) | (n=6) | (29.0±10.3*) | (n=5) | (65.0±19.0) | |
| (n=6) | (n=6) | (n=5) | ||||
Mean±s.e.m. Values with the same superscript are significantly different. P=0.05 (a, *); P<0.05 (b, #).
Since a decrease in fertilisation efficiency was not contributing to the decrease in DM litter size (Fig. 3E and F), in vitro embryo development was analysed. Embryos from DM females developed to the blastocyst stage in similar proportions to Controls despite lacking a normal ZP (Table 1 and Fig. 3C, D and G), although they appeared slightly smaller, consistent with other studies of ZP-free blastocysts in mice (Ribas et al. 2006). A unique phenomenon occurred during the culture of embryos from one DM female: two ZP-free morulae fused overnight giving rise to one abnormally large blastocyst (Fig. 3D; asterisk).
In vivo preimplantation embryo development
As mentioned above, development of embryos from DM females in vitro did not differ from Controls and therefore the reason for the decrease in litter size in 6-week females remained undefined. However, the in vitro environment may be more beneficial to development of embryos from DM females, which have a thin fragile ZP, as development occurs in a dish with no movement. In contrast, in vivo development requires the developing embryos to pass through the oviduct. Therefore, we next evaluated preimplantation development in vivo using embryos collected from plugged females at 3.5 dpc (Fig. 4A, B, C, D, E and F). In stark contrast to plugged Control and DM females dissected at 0.5 dpc, all of which contained embryos (Fig. 2A), less than half of all DM females contained embryos at 3.5 dpc (Fig. 4G). The very high proportion of mated DM females that lack embryos at 3.5 dpc is most likely contributing significantly to the 70% of DM females that fail to produce a litter (Williams et al. 2007).
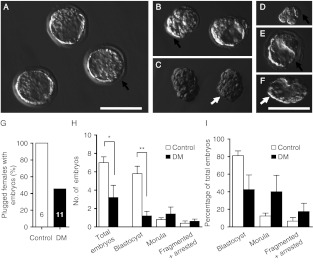
Figure 4.
In vivo developed embryos at 3.5 dpc. Images of embryos from Control (A) and DM (B, C, D, E and F) females in the 6-week group. (A) Blastocysts, (B) blastocyst and morula, (C) morula, (D) arrested embryo at four-cell stage, (E) blastocyst with abnormal ZP and (F) blastocyst without ZP. Black arrow indicates ZP; white arrow indicates a DM morula without ZP. (G) Percentage of mated females, confirmed by presence of vaginal plug, with embryos recovered after flushing. (H) Number of embryos and distribution of embryonic developmental stages in Control (n=5) and DM (n=5) females containing embryos. (I) Proportions of blastocysts, morulae and fragmented and arrested embryos as a percentage of the total number of embryos recovered at 3.5 dpc from Control and DM females. Results are expressed as mean±s.e.m. Bar scale=100 μm. *P=0.05; **P<0.01.
Of the DM females containing embryos, the number of embryos recovered was lower than Controls (Control: 7.0±0.6, DM: 3.2±1.3, P=0.05; Fig. 4H) and only 35% still possessed a ZP (Table 1). Moreover, DM females contained fewer blastocysts than Control females (Control: 5.8±0.8, DM: 1.2±0.5, P<0.01). When expressed as a proportion of total embryos, blastocysts and morulae were equally represented in the embryos from DM females whereas embryos from Control females were 80% blastocysts (Fig. 4I).
In light of these observations, in vivo and in vitro embryonic developments was compared revealing unexpected and striking differences in embryos collected from DM females (Table 2). The total number of in vivo developed embryos from DM females collected at 3.5 dpc was half of that obtained after culture (P=0.08). Furthermore, the number of blastocysts recovered in vivo from DM females was lower than the number obtained in vitro (P=0.05). Conversely, the number of morulae collected from DM females at 3.5 dpc (1.4±0.7) was almost three times higher than the number obtained in culture (0.5±0.3) although this did not reach significance. In stark contrast to embryos developed in vivo from DM females, embryos developed in vivo from Control females collected at 3.5 dpc did not differ from those cultured. However, although the in vitro conditions were better for development of embryos from DM females, the number of fragmented and/or arrested embryos obtained from in vitro culture was three times higher than in vivo for embryos collected from both Control and DM females (although this did not reach significance; Table 2).
Table 2.
In vitro (4.5 dpc) and in vivo (3.5 dpc) development of embryos from 6-week group Control and DM females.
| Control | DM | |||||
|---|---|---|---|---|---|---|
| Total number | In vitro | In vivo | In vivo:in vitro | In vitro | In vivo | In vivo:in vitro |
| Females with embryos | 12 | 5 | – | 6a | 5b | – |
| Embryos per female | 7.9±0.3 | 7.0±0.6 | 0.9 | 6.3±0.8 | 3.2±1.3 | 0.5 |
| Blastocysts per female | 4.8±0.7 | 5.8±0.8 | 1.2 | 3.8±0.9* | 1.2±0.5* | 0.3 |
| Morulae per female | 1.7±0.7 | 0.8±0.2 | 0.5 | 0.5±0.3 | 1.4±0.7 | 2.8 |
| Fragmented+arrested embryos per female | 1.5±0.5 | 0.4±0.2 | 0.3 | 2.0±0.8 | 0.6±0.2 | 0.3 |
| Blastocysts+morulae per female | 6.4±0.6 | 6.6±0.8 | 1.0 | 4.3±1.1 | 2.6±1.0 | 0.6 |
Mean±s.e.m. Values with the same superscript are significantly different, *P=0.05.
Data are presented for females whose zygotes were cultured.
Eleven DM females were evaluated; however, only five contained embryos.
Implantation analysis
The high proportion of morulae collected from DM females at 3.5 dpc compared with Controls (Fig. 4I and Table 2 although significance was not reached) may indicate a delay in early development of embryos from DM females that could affect implantation. To evaluate whether these morulae are able to progress further and implant, the 6-week group of Control and DM females was mated and embryo development was analysed at 5.5 dpc. The number of implantation sites was unsurprisingly lower in DM females at 5.5 dpc (1.3±0.4) than in Control females (7.3±0.6, P<0.01; Table 3). However, the number of implantation sites at 5.5 dpc in DM females was around half the number of embryos recovered at 3.5 dpc (3.5 dpc: 3.2±1.3, 5.5 dpc: 1.3±0.4, Tables 2 and 3). Furthermore, unfertilised eggs and non-implanted embryos were recovered in DM females in contrast to Control females, indicating that this process is aberrant in DM females.
Table 3.
Implantation sites, CL, eggs and non-implanted embryos in Control and DM females at 5.5 dpc.
| No. of females (age plugged; weeks) | Implantation sites | Eggs | Non-implanted embryos | CL | |||
|---|---|---|---|---|---|---|---|
| Total | Normal | Abnormal | |||||
| Control | 8 (6.5±0.1) | 7.3±0.6b | 0 | 0 | 8.3±0.3c | 8.1±0.3 | 0.1±0.1a |
| DM | 4 (6.4±0.0) | 1.3±0.4b,#,*,§ | 1.3±0.7 | 1.3±0.9 | 11.8±1.0c,# | 7.8±1.1* | 4.0±0.9a,§ |
Mean±s.e.m. Values with the same superscript are significantly different: P<0.05 (a, #, * and §); P<0.01 (b and c).
To determine whether there was a delay in implantation in DM females thus explaining the lower number of implantation sites at 5.5 dpc compared with the mean litter size of 4.0±2.7 (Williams et al. 2007), we analysed implantation 24 h later at 6.5 dpc (Table 4). Interestingly, the number of implantation sites in DM females at 6.5 dpc (Table 4) was more than double that at 5.5 dpc (Table 3) and is consistent with the number of embryos at 3.5 dpc (Table 2).
Table 4.
Implantation sites and CL in control and DM females at 6.5 dpc.
| No. of females (age plugged; weeks) | Implantation sites | CL | |||
|---|---|---|---|---|---|
| Total | Normal | Abnormal | |||
| Control | 4 (6.7±0.2) | 7.8±0.3a | 8.3±0.6 | 8.0±0.4 | 0.3±0.3 |
| DM | 5 (6.3±0.1) | 3.2±1.0a,#,* | 11.5±1.6# | 9.3±0.7* | 2.3±0.9 |
Mean±s.e.m. Values with the same superscript are significantly different: P<0.05 (# and *); P<0.01 (a).
Postimplantation embryo development
Analysis of embryos implanted at 5.5 and 6.5 dpc revealed a delay in implantation. To determine whether development of the implanted embryos was altered due to a difference in the timing of implantation, we analysed embryos at 9.5 dpc in females of the 6-week group. Uterine horns of DM females contained less deciduae and healthy embryos than those of Control females (P<0.05 and P<0.001 respectively; Table 5). The number of decidua in DM females (3.0±0.6) did not differ from the number of embryos recovered at 3.5 dpc (3.2±1.3), nor the number of implantations at 6.5 dpc (3.2±1.0), and is consistent with a litter size of 4.0±2.7 when DM females are mated at 6 weeks of age (Williams et al. 2007). However, not all deciduae in both, Control and DM females contained normal healthy embryos (Table 5), demonstrating that not all implanted embryos generated from either normal or mutant eggs are able to develop to term. Consistent with this, the number of healthy embryos in DM females at 9.5 dpc (2.0±0.3) was similar to the number of morphologically normal embryos (blastocyst+morula) recovered before implantation (2.6±1.0; Table 2).
Table 5.
Number of decidua, embryos and CL in Control and DM females at 9.5 dpc.
| No. of females (age plugged; weeks) | Decidua | Healthy embryos | Unhealthy embryos and reabsorptions | CL | |||
|---|---|---|---|---|---|---|---|
| Total | Normal | Abnormal | |||||
| Control | 5 (6.6±0.3) | 7.6±0.5a | 7.2±0.4d | 0.6±0.2 | 8.2±0.6c | 7.6±0.5 | 0.6±0.4b |
| DM | 6 (6.3±0.1) | 3.0±0.6a,#,* | 2.0±0.3d | 1.0±0.5 | 11.3±0.6c,# | 8.3±0.5* | 3.0±0.7b |
Mean±s.e.m. Values with same superscript are significantly different: P<0.05 (a and b); P<0.01 (c, # and *); P<0.001 (d).
Embryo and implantation site comparison at the five developmental stages
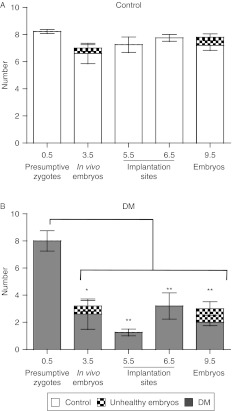
The number of preimplantation embryos, implanted embryos and deciduae at the five different developmental stages are compared in Fig. 5. At each stage of development, the number of implantations in DM females was markedly lower than that of Controls which was consistently between seven and eight. However, the number of presumptive zygotes recovered in DM at 0.5 dpc was significantly higher than the number of embryos found at any other stage. Therefore, the number of deciduae in DM females at 5.5 dpc is markedly lower than at any of the other time points analysed.
Figure 5.
Numbers of eggs ovulated and pre- and post-implantation embryos in Control and DM females at four developmental stages. The graph shows the number of eggs ovulated (0.5 dpc; presumptive zygotes), preimplantation embryos (3.5 dpc) and implanted embryos (5.5, 6.5 and 9.5 dpc) in Control (A) and DM (B) females. *P<0.05; **P<0.01, with regard to the zygotes collected at 0.5 dpc. Results are expressed as mean±s.e.m.
Corpora lutea observations
At all of the developmental stages studied, the number of corpora lutea (CL) was comparable with the number of deciduae in Controls, but in DM ovaries, the number of CL was significantly higher than the number of deciduae (Tables 3, 4 and 5). However, the CL in DM ovaries were not consistent in size or colour compared with Control CL. Some DM CL were smaller than control CL and pale (abnormal CL) compared with the bright red Control CL (normal CL). Despite the low number of implantations, the number of normal CL in DM ovaries was similar to the number of CL in Control females, consistent with a normal ovulation rate of DM females at 6 weeks (Tables 3, 4 and 5). However, at all stages of development, the number of implantation sites or decidua in DM females was lower than the number of normal CL (P<0.05).
Discussion
DM females generating oocytes lacking complex N- and O-glycans produce only one small litter from mating at 6 weeks before undergoing POF by 3 months of age (Williams et al. 2007, Williams & Stanley 2011). To determine the basis of the small litter size we investigated ovulation rate and embryo development. Ovulation rate was unexpectedly normal in the DM females in the 6-week group, but decreased dramatically by 9 weeks, consistent with our previous findings (Williams et al. 2007, Williams & Stanley 2011). However, since ovulation rate was normal in the 6-week group, further analyses were required to reveal the basis for the decrease in litter size. Surprisingly, the number of embryos at 3.5 dpc was around half the ovulation rate 3 days earlier, revealing a considerable loss of embryos surviving during blastogenesis. Furthermore, embryo development in those embryos that did survive in DM females was delayed resulting in delayed implantation.
When mated at 6 weeks of age, DM females produce only a single small litter (Williams et al. 2007) yet, on average, they ovulate normal numbers of eggs, although the number of eggs ovulated per female is highly variable. This is consistent with the presence of morphologically normal follicles in 6-week DM ovaries, although ovaries also contain some follicular abnormalities such as oocytes lacking cumulus cells or empty follicles (Williams & Stanley 2011). Therefore, the most likely explanation for the precipitous decline in ovulation rate in DM females from 6 to 9 weeks is defective follicle development that gets more severe with age, explaining the lack of a second litter in these females (Williams et al. 2007, Williams & Stanley 2011).
Despite the fact that ovulation rate is normal at 6 weeks in DM females, only 45% of mated DM females contained embryos at 3.5 dpc. The very high proportion of mated DM females that lack embryos at 3.5 dpc is most likely contributing significantly to the 70% of DM females that fail to produce a litter (Williams et al. 2007). Furthermore, in those DM females that did contain embryos, the number was significantly lower than Controls. This was in stark contrast to just 3 days earlier when ovulation rate in DM females was normal and eggs were fertilised equivalently to those from Control females. These data indicate that the lack of complex N- and O-glycans during oocyte development results in a substantial decrease in embryo viability in vivo with an associated decrease in female fertility. In addition, around half of the morphologically normal embryos recovered from DM females at 3.5 dpc were morulae compared with ∼11% in Controls, indicating a delay in development. Delayed development of embryos from DM females was not unexpected as embryos generated from oocytes lacking only complex N-glycans are also retarded (Shi et al. 2004). However, the development of embryos from DM females was supported in vitro, with the same proportion of embryos developing to the blastocyst stage as embryos from Controls. This demonstrates that in vivo development of embryos from DM females is affected by factors not present in vitro. Furthermore, these factors can either be intrinsic to the developing embryo or result from a modified environment within DM females as embryo development is affected by both oocyte quality and the environment in which the embryo develops (Mann & Lamming 2001, Van der Auwera & D'Hooghe 2001, Kidder & Vanderhyden 2010).
Focusing first on the embryo, as a consequence of the lack of N- and O-glycans, the ZP of DM oocytes is thinner and more fragile (Williams et al. 2007, Williams & Stanley 2009). During the transit through the oviduct, embryos develop within the protective coat of the ZP undergoing cleavage, compaction and cavitation before developing into a blastocyst. Blastocysts hatch from the ZP and implant in the endometrial lining of the uterus (Nagy et al. 2003). We have observed that eggs and preimplantation embryos from DM females often lack a ZP which is most likely removed either during ovulation or whilst in transit from the oviduct to the uterus. Analysis of ZP-free eggs and embryos that were reintroduced to the oviduct illustrated a critical role for the ZP in preventing adherence of developing embryos to the oviduct (Modlinski 1970). These data indicate that the dramatic reduction in DM embryos flushed from 3.5 dpc as opposed to the number found at 0.5 dpc may be attributed to adherence of naked DM pre-blastocyst embryos to the oviduct. Furthermore, embryos with the ZP removed early in development have reduced developmental potential (Bronson & McLaren 1970). In addition, embryo lethality induced by shear stress (caused by the movement of fluid over the surface of cells) is hastened by ZP removal (Xie et al. 2006) which may also contribute to embryo loss in DM females. The ZP has also a role maintaining embryo morphology including the connections between blastomeres which can influence embryo development in mice (Suzuki et al. 1995, Ribas et al. 2005). Moreover, removal of the ZP in mice results in reduced DNA methylation at the two-cell and four-cell stages (Ribas et al. 2006). In addition, the thickness of the ZP or its variation has been used as a marker of successful implantation of human embryos (Garside et al. 1997, Gabrielsen et al. 2001, Shen et al. 2005). Based on these observations it is likely that the altered thickness or absence of a functional ZP in DM embryos has a role in the aberrant early embryogenesis in embryos from DM females that is not observed in vitro.
In vitro analysis revealed one unexpected occurrence of embryo fusion in embryos from DM female. Observations of in vitro cultured embryos revealed that although five DM zygotes were present at the beginning of the culture, at the end, only four embryos were present including one abnormally large blastocyst (Fig. 3D). Fusion of two of the ZP-free embryos is the only logical conclusion in the absence of a degenerating embryo. The formation of natural chimeras has been described in mammals, including in humans (Strain et al. 1998). Although chimeric embryos can be generated by injection of stem cells into blastocysts, they can also be generated by culture of embryonic stem cells which aggregate with denuded morulae in vitro which develop normally upon transfer to a pseudo-pregnant recipient (Prather et al. 1989, Eakin & Hadjantonakis 2006). Although our data indicate that this phenomena can occur in ZP-free DM embryos in vitro, its occurrence in vivo is unknown. However, it is unlikely to be involved in decreasing the number of embryos and pups as none of the embryos collected at 3.5 dpc were abnormally large.
The delay in in vivo embryo development in DM females, revealed by the high proportion of embryos at the morula stage, was reflected in a delay in implantation. At 5.5 dpc, only around a third of the number of the preimplantation embryos recovered at 3.5 dpc were implanted (Fig. 5). However, 1 day later at 6.5 dpc, the number of embryos implanted was equivalent to the number found at 3.5 dpc (Fig. 5). In addition, the number of deciduae found at 9.5 dpc in DM females was similar to the number of embryos recovered at 3.5 dpc and to the number of implantations at 6.5 dpc. However, not all deciduae in DM females contained normal healthy embryos with a number of healthy embryos equal to the number of morphologically normal embryos recovered before implantation. The difference between the number of healthy embryos found in this study and the litter size previously published (4.2±2.7) (Williams et al. 2007), could be explained by the low sample size (only three of ten mated produced a litter) and the high variability of the fertility data in DM females. Recent studies reveal an active role of the embryo in regulating the expression of uterine genes involved in implantation and decidualization (Kashiwagi et al. 2007, Park & Dufort 2011). It is possible that embryos generated from oocytes lacking complex N- and O-glycans do not induce the appropriate expression of required genes for timely implantation. Implantation is a complex process, ovarian steroid-dependant and only possible during a limited period in which the uterus is receptive to the competent embryo. This process requires complex interactions between the blastocyst and the receptive uterus in many species including mice and humans (Simon et al. 2000, Dey et al. 2004). Interestingly, the delay in embryo development and implantation was not uniformly deleterious to subsequent development of embryos as some embryos that implanted at 6.5 dpc remained healthy at 9.5 dpc (Fig. 5). Transferring embryos at different stages of development (zygotes and eight-cell embryos) into the uterus of pseudo-pregnant mice revealed that the ‘window of implantation’ could be expanded (Ueda et al. 2003). This may explain why all the blastocysts and morulae found at 3.5 dpc in DM females are able to implant over an extended period.
In addition to modified embryo competence, the maternal endocrine environment may also have a role in modifying embryo development. Maternal ovarian steroids including estradiol and progesterone regulate embryo development and uterus receptivity to implantation (Dey et al. 2004, Mohamed et al. 2004, 2005). However, although the endocrine profile in DM females is modified by 3 months of age with mice exhibiting elevated FSH, and decreased Inhibin A and testosterone, this is in association with a severe lack of ovarian follicles and ovarian dysfunction (Williams & Stanley 2011). Most importantly, estradiol and progesterone concentrations were normal in DM females at 3 months (Williams & Stanley 2011) and therefore, it is likely that estradiol and progesterone concentrations are also normal in the 6-week old mice used in these studies. We therefore conclude that modified maternal estradiol and progesterone are unlikely to be involved in modifying embryo development in DM females.
The increased number of CL in DM females was unexpected. We have previously shown in DM females that at 3 weeks follicle development is vastly normal; however, by 6 weeks, ovaries contained fewer follicles and more follicular abnormalities (Williams & Stanley 2011). CL in postpubertal females (6 weeks and 3 months) were not counted or measured in these analyses and therefore the increased number of CL was not revealed. Interestingly, the number of normal CL in DM females corresponds to the ovulation rate of DM (and Control females) at 6 weeks. Therefore, the abnormal CL may be present due to abnormal CL regression as DM females do not exhibit an increased ovulation rate. However, how oocyte-specific deletion of glycosyltransferases results in modified CL regression is intriguing and is under further investigation.
Female mice heterozygous for both Mgat1 and C1galt1 exhibit normal fertility (Williams et al. 2007), demonstrating that aberrant embryo development in DM females results from oocyte glycoproteins lacking complex O- and N-glycans during oocyte development and early embryogenesis before activation of the paternal genome. Furthermore, it is clear that activation of the paternal genome during preimplantation embryo development (De Vries et al. 2004) cannot rescue later development as the proportion of embryos dying from 6.5 to 9.5 dpc is considerably higher in DM females than in Controls.
In summary, our results reveal that a lack of complex N- and O-glycans during oocyte development impairs early embryo development and viability in vivo, which could lead to delayed implantation and reduced final litter size. Moreover, these data demonstrate that oocyte glycoproteins have a role in early embryo development and the interaction of the embryo with the maternal environment before implantation.
Materials and Methods
Mice
Female mice carrying floxed Mgat1 and C1galt1 alleles and a ZP3Cre transgene were used as experimental females whereas females carrying the floxed Mgat1 and C1galt1 alleles but not the ZP3Cre transgene were used as controls because the floxed alleles function as wild-type genes (Shi et al. 2004, Williams et al. 2007). Mice were kept under controlled conditions in 12 h light:12 h darkness cycles. All the experiments involving mice were done with permission from the Home Office and the Local Ethical Review Committee.
Timed mating
Control and DM female mice aged either 6 or 9 weeks were joined with fertile males (C1galt1F/FMgat1F/F±ZP3Cre) for mating for up to a maximum of 2 weeks. Embryos generated from C1galt1F/FMgat1F/F females will be C1galt1F/FMgat1F/F±ZP3Cre and embryos generated from C1galt1F/FMgat1F/F:ZP3Cre females will be Mgat1del/FC1galt1del/F±ZP3Cre with the deleted genes (del) originating from oocyte-specific deletion in the mother and the floxed genes from the sperm of the father. Those joined with males at 6 weeks are herein termed the 6-week group, and those joined with males at 9 weeks are herein termed the 9-week group. Females were examined in the morning daily for evidence of mating by the presence of a vaginal plug and if present, removed from the cage. Midday of the day a plug was visible was deemed 0.5 dpc.
Ovulated egg, presumptive zygote and embryo analysis
For collection of eggs and presumptive zygote, females joined with males at 6 and 9 weeks of age were killed at 0.5 dpc and oviducts were collected in warmed M2 medium (Sigma–Aldrich) and examined. Eggs collected from Control and DM females at 0.5 dpc were assumed to have been fertilised and were termed ‘presumptive zygotes’. If they were visible in the oviduct, the oviducts were ripped open to release the presumptive zygotes. Cumulus cells were disaggregated with 0.3 mg/ml of hyaluronidase (Sigma–Aldrich) in M2 media for up to 3 min and the number of presumptive zygotes was recorded. If no presumptive zygotes were visible, the oviducts were flushed with M2 media to collect them and the number was recorded.
In vivo preimplantation embryo development was analysed at 3.5 dpc from female mice joined with males at 6 weeks. Females were killed and the uterus and oviduct collected and placed in warmed M2 media. Embryos were flushed from the oviduct and uterus with warmed M2 media and classified as blastocysts, morulae, and arrested or fragmented embryos, based on morphology.
In vitro embryo development
Presumptive zygotes obtained as described above at 0.5 dpc from the 6-week female group were cultured for 4 days in 50 μl drops of KSOM media (Millipore, Billerica, MA, USA) supplemented with essential and non-essential amino acids (Gibco), covered with mineral oil (Sigma–Aldrich) and incubated at 37 °C in air with 5% CO2. The fertilisation rate was determined after 24 h of culture by analysing the number that had developed into two-cell embryos. Development was analysed and images were collected using a Leica M125 microscope (Leica Microsystems, Germany) and MicroPublisher 5.0 RTV camera (Qimaging, London, UK) every 24 h during the 4-day culture period until 4.5 dpc. In vitro embryo culture was for 1 day longer than in vivo because embryo development in vitro takes longer than in vivo (Van der Auwera & D'Hooghe 2001). Embryos were classified as either blastocysts, morulae, fragmented or arrested based on morphology.
Implantation analysis
Implantation was evaluated in females in the 6-week group at 5.5 and 6.5 dpc. To assist visualisation of implantation sites at 5.5 dpc, females were injected intravenously under anaesthesia with 100 μl of 1% Chicago Sky Blue (Sigma–Aldrich) in ddH2O, and killed 5 min later. The uterine horns were removed and the number of implantation sites visible as blue dots recorded. Uterine horns were also flushed at 5.5 dpc to recover any non-implanted embryos. Implantation sites at 6.5 dpc were easily identified. At both time points, ovaries were dissected to determine the number of CL.
Post-implantation embryo development
Post-implantation embryo development was assessed in the 6-week group. Plugged females were killed at 9.5 dpc and the number of deciduae (each developing around an implanted embryo) determined. To analyse embryonic development, the deciduae were dissected, embryos extracted and morphology evaluated. Embryos classified as healthy had normal gross morphology and were alive determined by the presence of a beating heart. Ovaries were collected and dissected to determine the number of CL.
Statistical analysis
Results are presented as mean±s.e.m. The Mann–Whitney U test was performed for all statistical analyses with the exception of the Pearson test, which was used to assess the correlation between the age and the number of eggs ovulated. All analyses were performed using Prism software version 4.0b (GraphPad Software, Inc., version 4.0b, 2004. La Jolla, CA, USA).
Acknowledgements
We thank Brad Joyce and Dr Shankar Srinivas for their help and advice with the embryo experiments. We also thank members of the animal house facility, in particular, Denise Jelfs and James Ward for their technical assistance with the mice.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by a grant awarded from the Medical Research Council to S A Williams. H Kaune is a recipient of Becas Chile PhD scholarship from the Government of Chile and PhD scholarship for Academics from Diego Portales University, Chile.
References
- Bleil JD, Wassarman PM. Mammalian sperm–egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980a;20:873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Developmental Biology. 1980b;76:185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Bronson RA, McLaren A. Transfer to the mouse oviduct of eggs with and without the zona pellucida. Journal of Reproduction and Fertility. 1970;22:129–137. doi: 10.1530/jrf.0.0220129. [DOI] [PubMed] [Google Scholar]
- De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB. Maternal β-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocrine Reviews. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Developmental Biology. 2007;305:300–311. doi: 10.1016/j.ydbio.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- van Duin M, Polman JE, De Breet IT, van Ginneken K, Bunschoten H, Grootenhuis A, Brindle J, Aitken RJ. Recombinant human zona pellucida protein ZP3 produced by chinese hamster ovary cells induces the human sperm acrosome reaction and promotes sperm–egg fusion. Biology of Reproduction. 1994;51:607–617. doi: 10.1095/biolreprod51.4.607. [DOI] [PubMed] [Google Scholar]
- Eakin GS, Hadjantonakis AK. Production of chimeras by aggregation of embryonic stem cells with diploid or tetraploid mouse embryos. Nature Protocols. 2006;1:1145–1153. doi: 10.1038/nprot.2006.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A, Lindenberg S, Petersen K. The impact of the zona pellucida thickness variation of human embryos on pregnancy outcome in relation to suboptimal embryo development. A prospective randomized controlled study. Human Reproduction. 2001;16:2166–2170. doi: 10.1093/humrep/16.10.2166. [DOI] [PubMed] [Google Scholar]
- Garside WT, Loret de Mola JR, Bucci JA, Tureck RW, Heyner S. Sequential analysis of zona thickness during in vitro culture of human zygotes: correlation with embryo quality, age, and implantation. Molecular Reproduction and Development. 1997;47:99–104. doi: 10.1002/(SICI)1098-2795(199705)47:1<99::AID-MRD13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Human Reproduction Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- Han L, Monne M, Okumura H, Schwend T, Cherry AL, Flot D, Matsuda T, Jovine L. Insights into egg coat assembly and egg–sperm interaction from the X-ray structure of full-length ZP3. Cell. 2010;143:404–415. doi: 10.1016/j.cell.2010.09.041. [DOI] [PubMed] [Google Scholar]
- Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reproductive BioMedicine Online. 2007;14:758–764. doi: 10.1016/S1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. PNAS. 1994;91:728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A, DiGirolamo CM, Kanda Y, Niikura Y, Esmon CT, Hansen TR, Shioda T, Pru JK. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology. 2007;148:4173–4184. doi: 10.1210/en.2007-0268. [DOI] [PubMed] [Google Scholar]
- Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Canadian Journal of Physiology and Pharmacology. 2010;88:399–413. doi: 10.1139/Y10-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litscher ES, Williams Z, Wassarman PM. Zona pellucida glycoprotein ZP3 and fertilization in mammals. Molecular Reproduction and Development. 2009;76:933–941. doi: 10.1002/mrd.21046. [DOI] [PubMed] [Google Scholar]
- Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction. 2001;121:175–180. doi: 10.1530/rep.0.1210175. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO Journal. 1994;13:2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlinski JA. The role of the zona pellucida in the development of mouse eggs in vivo. Journal of Embryology and Experimental Morphology. 1970;23:539–547. [PubMed] [Google Scholar]
- Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biology of Reproduction. 2004;71:417–424. doi: 10.1095/biolreprod.103.025692. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/β-catenin signaling is required for implantation. PNAS. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K & Behringer R 2003 Manipulating the Mouse Embryo. A Laboratory Manual. 3rd Edn. New York, NY, USA: Cold Spring Harbor Laboratory Press.
- Park CB, Dufort D. Nodal expression in the uterus of the mouse is regulated by the embryo and correlates with implantation. Biology of Reproduction. 2011;84:1103–1110. doi: 10.1095/biolreprod.110.087239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott CC, Ringuette MJ, Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Developmental Biology. 1987;121:568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- Prather RS, Hagemann LJ, First NL. Preimplantation mammalian aggregation and injection chimeras. Gamete Research. 1989;22:233–247. doi: 10.1002/mrd.1120220210. [DOI] [PubMed] [Google Scholar]
- Ribas R, Oback B, Ritchie W, Chebotareva T, Ferrier P, Clarke C, Taylor J, Gallagher EJ, Mauricio AC, Sousa M, et al. Development of a zona-free method of nuclear transfer in the mouse. Cloning and Stem Cells. 2005;7:126–138. doi: 10.1089/clo.2005.7.126. [DOI] [PubMed] [Google Scholar]
- Ribas RC, Taylor JE, McCorquodale C, Mauricio AC, Sousa M, Wilmut I. Effect of zona pellucida removal on DNA methylation in early mouse embryos. Biology of Reproduction. 2006;74:307–313. doi: 10.1095/biolreprod.105.046284. [DOI] [PubMed] [Google Scholar]
- Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Human Reproduction. 2005;20:1596–1606. doi: 10.1093/humrep/deh811. [DOI] [PubMed] [Google Scholar]
- Shi S, Williams SA, Seppo A, Kurniawan H, Chen W, Ye Z, Marth JD, Stanley P. Inactivation of the Mgat1 gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Molecular and Cellular Biology. 2004;24:9920–9929. doi: 10.1128/MCB.24.22.9920-9929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Baillière's Best Practice & Research. Clinical Obstetrics & Gynaecology. 2000;14:815–826. doi: 10.1053/beog.2000.0121. [DOI] [PubMed] [Google Scholar]
- Strain L, Dean JC, Hamilton MP, Bonthron DT. A true hermaphrodite chimera resulting from embryo amalgamation after in vitro fertilization. New England Journal of Medicine. 1998;338:166–169. doi: 10.1056/NEJM199801153380305. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Molecular Endocrinology. 2010;24:2303–2314. doi: 10.1210/me.2010-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Togashi M, Adachi J, Toyoda Y. Developmental ability of zona-free mouse embryos is influenced by cell association at the 4-cell stage. Biology of Reproduction. 1995;53:78–83. doi: 10.1095/biolreprod53.1.78. [DOI] [PubMed] [Google Scholar]
- Ueda O, Yorozu K, Kamada N, Jishage K, Kawase Y, Toyoda Y, Suzuki H. Possible expansion of "Window of Implantation" in pseudopregnant mice: time of implantation of embryos at different stages of development transferred into the same recipient. Biology of Reproduction. 2003;69:1085–1090. doi: 10.1095/biolreprod.103.017608. [DOI] [PubMed] [Google Scholar]
- Van der Auwera I, D'Hooghe T. Superovulation of female mice delays embryonic and fetal development. Human Reproduction. 2001;16:1237–1243. doi: 10.1093/humrep/16.6.1237. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Contribution of mouse egg zona pellucida glycoproteins to gamete recognition during fertilization. Journal of Cellular Physiology. 2005;204:388–391. doi: 10.1002/jcp.20389. [DOI] [PubMed] [Google Scholar]
- Williams SA, Stanley P. Mouse fertility is enhanced by oocyte-specific loss of core 1-derived O-glycans. FASEB Journal. 2008;22:2273–2284. doi: 10.1096/fj.07-101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Stanley P. Oocyte-specific deletion of complex and hybrid N-glycans leads to defects in preovulatory follicle and cumulus mass development. Reproduction. 2009;137:321–331. doi: 10.1530/REP-07-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Stanley P. Premature ovarian failure in mice with oocytes lacking core 1-derived O-glycans and complex N-glycans. Endocrinology. 2011;152:1057–1066. doi: 10.1210/en.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. Journal of Cell Science. 2007;120:1341–1349. doi: 10.1242/jcs.004291. [DOI] [PubMed] [Google Scholar]
- Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. Journal of Cell Biology. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biology of Reproduction. 2006;75:45–55. doi: 10.1095/biolreprod.105.049791. [DOI] [PubMed] [Google Scholar]