Abstract
Identifying predictors of antidepressant response will facilitate the successful treatment of patients suffering from depression. Scopolamine produces robust antidepressant responses in unipolar and bipolar depression. Here we evaluate the potential for baseline self-ratings to predict treatment response to scopolamine. Fifty-one unipolar and bipolar patients participated in a double-blind, placebo-controlled crossover trial. Following a single-blind placebo session, participants randomly received P/S or S/P (P=3 placebo; S=3 scopolamine (4ug/kg) sessions). Mood-state self-ratings (POMS and VAS) and depression severity (MADRS) were obtained before each infusion. Day 1 (baseline/placebo) self-ratings were used in a discriminant function analysis to identify linear combinations of individual items that predict response. The discriminant analysis separated responders from non-responders in both the unipolar (Chi-square=22.6, p=0.002) and bipolar (Chi-square=11.2, p=0.025) diagnostic subgroups. The discriminant functions accurately classified over 85% of patients as responders/non-responders. The POMS depression subscale correlated with clinical response (r=-.47; p<0.001), as did the VAS restlessness (r=-.49; p<0.001), sad (r=-.49, p<0.0001) and irritated (r=-.40, p=0.004) scales. These results indicate that self-report mood-ratings obtained prior to treatment can predict response outcome to scopolamine, and suggest that a constellation of mood-state features may be related to clinical response.
Keywords: Mood-disorders, response prediction, scopolamine, Major Depressive Disorder, Bipolar Disorder
1. Introduction
Patient response to antidepressant treatment is varied and currently difficult to predict. While one medication works well for one patient, others will require a different agent or multiple agents to achieve the same clinical response (Simon and Perlis, 2010). Often the first trial is unsuccessful at treating symptoms and as a result, uncovering the correct drug or the correct combination of drugs commonly requires multiple attempts. Moreover, an adequate trial traditionally requires a several week period to sufficiently assess the efficacy of a particular regimen. As a result patients often go many months before experiencing relief from their symptoms, leading to disruption in personal, social, and occupational aspects of their life, and possibly could increase risk of suicide (Insel and Wang, 2009).
A focus on improving upon current approaches to the treatment of patients with mood disorders is critical. In addition to identifying agents that produce faster clinical response (Machado-Vieira et al., 2009), an essential aspect of the development of better treatment methods will be determining how to predict treatment response in individual patients to specific therapeutic agents. The goal would be to determine prior to or early in treatment, if a particular medication will be effective so that patients can avoid unnecessary drugs and attain clinical response more quickly.
Efforts have been made to identify biomarkers that can be used to predict antidepressant treatment response (Leuchter et al., 2010). Measures associated with brain structure (MacQueen et al., 2008), brain function (Salvadore et al., 2009; Leuchter et al., 2010; Pizzagalli, 2011), and genetic factors (Laje and McMahon, 2007; Garriock and Hamilton, 2009) have been evaluated as potential markers of treatment response and may prove to be clinically useful. Evidence suggests that diagnostic subtypes may preferentially respond to medications with a particular mechanism of action (for review see (Leuchter et al., 2010)). Generally the effect sizes are quite small and as a result sample sizes have to be relatively large to achieve significance, and while these findings may reflect significant effects, the ability of these markers to predict response in individual patients has remained limited. The identification of predictors of antidepressant response to specific therapeutic agents, especially predictor tools that are readily available to clinicians, is critical to successful treatment for individual patients.
Recently, we reported that the anticholinergic agent, scopolamine, produces rapid and robust antidepressant response in unipolar and bipolar depression (Furey and Drevets, 2006; Furey et al., 2010). Approximately 50% of patients experienced remission and 65% achieved response following a short treatment period of scopolamine. The remaining 35% showed modest or no improvement in symptoms following scopolamine infusions.
The purpose of this study was to determine whether self-report mood ratings predict antidepressant response in depressed patients treated with scopolamine (Furey and Drevets, 2006; Drevets and Furey, 2009).
2. Methods
2.1. Participants
Fifty-one patients (Female=31, mean age ± SD = 32.5 ± 8.3) who were in a major depressive episode were enrolled into the study after meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)(Diagnostic and Statistical Manual of Mental Disorders, IV, 2000) criteria for recurrent major depressive disorder (MDD; n= 37) or bipolar disorder (BD; n=14), based upon an unstructured interview conducted by a psychiatrist and the Structured Clinical Interview for DSM-IV (First et al., 2002). Table 1 provides basic demographic information for the patients who participated in the study separated by diagnosis. Participants were evaluated at the National Institutes of Mental Health (NIMH) outpatient clinic. Exclusion criteria included exposure to psychotropic or other medications likely to affect central nervous system or cholinergic function within 3 weeks (8 weeks for fluoxetine), clinically significant suicidal intent, rapid-cycling bipolar illness, delusions or hallucinations, lifetime history of substance dependence or substance abuse within one year, medical or neurological disorders, abnormal electrocardiogram or blood pressure, narrow angle glaucoma, hypersensitivity to anticholinergic agents, hepatic dysfunction, electrolyte disturbance, HIV or hepatitis viral infection, nicotine use, or weight >125 kg. Pregnant or nursing females also were excluded.
Table 1.
Patient Groups
| MDD (N= 37) | BD (N= 14) | |
|---|---|---|
| Mean Age ± SD | 33.3 ± 8.2 | 30.4 ± 8.5 |
| Baseline MADRS ± SD | 30.5 ± 4.6 | 31.5 ± 5.9 |
| Gender (N Female) | 22 | 9 |
| N (%) Treatment Response | 22 (60%) | 8 (57%) |
| N (%) Co-morbid Anxiety | 13 (35%) | 7 (50%) |
| N (%) Chronic Illness (> 2 yrs) | 19 (51%) | 9 (64%) |
The study was approved by the Combined Neuroscience Institutional Review Board (IRB) of the National Institutes of Health (NIH). All subjects provided written informed consent before entry into the study.
2.2. Study Treatment Design
During each of seven sessions, subjects received a 15-minute intravenous infusion of either a placebo (P) saline solution, or 4.0 μg/kg of scopolamine (S). A single-blind, lead-in session was used in which all subjects received a placebo infusion. The remaining 6 sessions were separated into two phases. Individuals were randomized into either a P/S or S/P double-blind, placebo-controlled, cross-over design whereby P constituted a phase with 3 sessions during which participants received placebo and S comprised a phase with 3 sessions during which participants received scopolamine. Follow-up interviews were obtained 3 to 5 days after the final infusion to provide the final clinical assessment. Sessions were scheduled 3-5 days apart. Non-pregnancy was established prior to each session. Randomization sequences were determined by the NIH outpatient pharmacy and assigned by subject number at time of consent. All staff involved in the administration of the infusion and the session assessments remained blind to allocation during the study.
2.3. Assessment
Self-assessments of acute mood-state were performed with Visual analog scales (VAS) and the Profile of Mood State (POMS)(McNair, 1971) at baseline and 20, 60, 120 (VAS only) and 150 minutes relative to infusion start time. The VAS uses a 10-point scale for participants to indicate the extent to which each of the seven items is consistent with how they feel in the moment; the items included happy, restless, sad, anxious, angry, drowsy and alert. The POMS contains 65 adjectives rated by participants on a 5-point scale. Six factors are derived that include tension, depression, anger, fatigue, vigor and confusion.
Prior to each infusion, psychiatric interviews were completed using the Montgomery - Asberg Depression Rating Scale (MADRS) (Khan et al., 2002), Hamilton Anxiety Rating Scale (HAM-A)(Hamilton, 1959), Young Mania Rating Scale (YMRS)(Young et al., 1978), and Clinical Global Impressions- Improvement (CGI-I)(Khan et al., 2002) scales.
2.4. Outcome measures
Baseline mood-state was evaluated for each of the VAS and POMS measures by averaging across time points obtained during the single-blind lead-in placebo session. Thus, a single score reflecting the session mean was calculated for each VAS item and each POMS factor to be used in all analyses. The antidepressant response to scopolamine was evaluated by assessing change in MADRS from baseline to study end.
Treatment response magnitude was calculated as a percent change at study end, relative to baseline measures. Baseline measures included MADRS scores from session 1 (single-blind) and session 2 (as obtained prior to infusion). Patients also were categorized as achieving: 1) response (≥ 50% reduction in MADRS score from baseline); or 2) non-response (< 50% reduction) (Nierenberg and DeCecco, 2001).
Placebo response was calculated as percent change in MADRS during phase I, from baseline to the session five assessment, thus placebo response was evaluated based on the phase I of the study where one group received placebo and one group received scopolamine. Placebo response was assessed only in those participants who were randomized into P/S and therefore received four placebo infusions before receiving scopolamine. To compare directly study phase I placebo response to phase I treatment response, phase I treatment response also was calculated as percent change in MADRS from baseline to the session five assessment for patients randomized into S/P and therefore received scopolamine in study phase I. These two correlations were compared directly using the Fisher R to Z transformation. All placebo analyses were based on percent change; no categorical “responders vs non-responders” analysis was conducted as so few participants achieved response during the placebo period.
2.5 Data Analysis
Session mean POMS factor scores and the seven items included on the 10-point VAS scales were evaluated as potential predictors of treatment response. Discriminant analyses were performed to determine if a linear combination of items from pre-infusion rating scales predicted antidepressant response to scopolamine. Behavioral ratings to be included in the discriminant analysis were chosen using an automated stepwise process. Initially, no variable was included in the model. At each step, the Wilks’ Lambda was calculated for the next variable considered and for each variable already included in the model. The Wilks’ Lambda significance threshold for retention in the model was 0.25, which was derived as a general recommendation from Monte Carlo simulation studies (Costanza and Afifi, 1979). If any variable met criteria to enter the model, it was entered, and if any variable in the model failed to meet the criteria, it was removed. When no more variables could be added to or removed from the model, the procedure ended. This procedure resulted in the identification of the variables that had the greatest power to discriminate between responders (50% reduction in MADRS) and non-responders (less than 50% reduction in MADRS).
A canonical discriminant analysis subsequently was conducted, in which linear combinations of differentially weighted variables (as identified above) were constructed which best summarized the differences between responders and non-responders. A cross-validation also was performed, where the discriminant function was derived leaving out each observation in turn, and then classifying that observation to determine the accuracy of the derived discriminant function. Canonical correlations between the discriminant function and the response status are provided, and the significance of the function is determined using chi-square tests. Differences in the mean value of the discriminant function for responders versus non-responders were also evaluated using two-sample t-tests. Discriminant function analyses were conducted on MDD and BD patient groups separately. All statistical analyses were conducted using SPSS.
The specificity of the predictive ability of the discriminant function to the scopolamine response was evaluated by correlating the phase I placebo and treatment response magnitude with the discriminant function scores. The correlations reflect the extend to which the change in MADRS in phase I correlates with the calculated discriminant function scores for the placebo group (the P/S group) and the treatment group (S/P group). Pearson correlations were conducted for the MDD groups, and no correlations were conducted in the BP phase I placebo and treatment groups as the sample sizes are small. These correlations then were compared directly to address the specificity of the discriminant function to predict treatment response to scopolamine as compared to response in general (i.e. placebo response).
To evaluate the relation between individual baseline mood-state measures on POMS and VAS scales and treatment response, univariate Pearson correlation analyses were used. Statistical significance was taken at 0.004 (p of 0.05/13) to control for multiple comparisons.
3. Results
In the discriminant function analysis with MDD subjects, seven behavioral measures were identified in the model to best discriminate responders from non-responders including the depression, anger, vigor and fatigue scales from the POMS, and the alert, drowsy, and happy scales from the VAS. The canonical correlation between the response groups and the selected behavioral variables was 0.716, and the derived discriminant function was significant (Chi-Square = 22.6, df=7, p=0.002). The discriminant function is plotted for each subject, grouped by response versus non-response, in Figure 1A. Of the non-responders, 85.7 were correctly classified, while 87% of responders were correctly classified. In the leave-one-out cross-validation, 71.4% of the non-responders and 69.6% of the responders were correctly classified, well above chance. The mean of the discriminant function was -1.28 in the non-responders and 0.78 in the responders; this difference was significant (t= 6.06, p<0.001).
Figure 1.
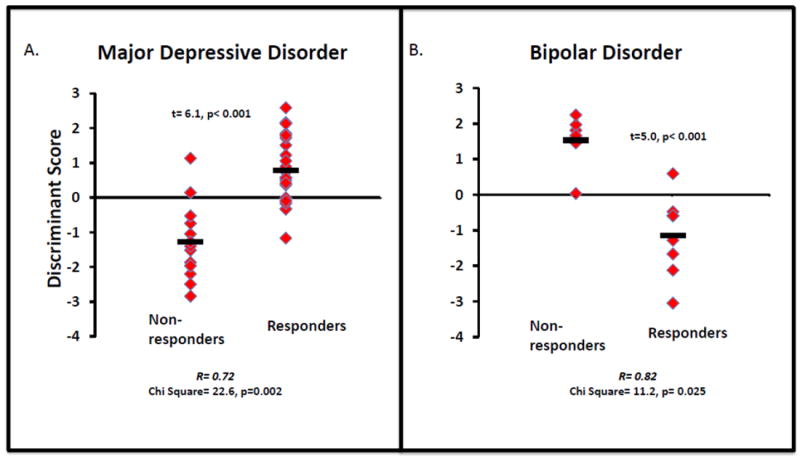
Scatterplot of individual subjects discriminant function scores are shown for responders and non-responders with major depressive disorder (panel A) and with bipolar disorder (panel B) separately. The bars indicate the group means. In major depressive disorder, the stepwise analysis identified seven mood-rating variables that best discriminated responders from non-responders, including depression, anger, fatigue and vigor from the POMS, and altertness, drowsy, and happy from the VAS. The discriminant function significantly separates responders from non-responders (X2= 22.6, p= 0.002) and the mean function scores differs between the two groups (t= 6.1, p<0.001). In bipolar disorder, the stepwise analysis identified seven mood-rating variables that best discriminated responders from non-responders, including depression and anger from the POMS, and the restlessness and irritated scales from the VAS. The discriminant function significantly separates responders from non-responders (X2= 11.2, p= 0.025) and the mean function scores differs between the two groups (t= 5.0, p<0.001).
In the bipolar subjects, four behavioral measures were chosen as discriminating including the depression and anger subscales of the POMS and the restless and irritated scales of the VAS. The canonical correlation between the response groups and the selected behavioral variables was 0.820, and the derived discriminant function was significant (Chi-Square = 11.2, df=4, p=0.025). The discriminant function is plotted for each subject, grouped by response, in Figure 1B. Of the non-responders, 88.3% were correctly classified, while 87.5% of the responders were successfully classified. Results for the leave-one-out cross-validation were identical. Mean of the discriminant function was 1.53 in the non-responders and -1.15 in the responders; this difference was significant (t=4.96, p<0.001).
In order to address specificity, the magnitude of placebo response was correlated with the discriminant function scores from the MDD group of patients who received placebo during the study phase I (n=17). The correlation for the placebo response and the discriminant function score was not significant (r= .22, p= 0.40), suggesting that the discriminant function score does not predict the placebo response. The correlation was significant for the study phase I scopolamine treatment response and the discriminant function score (r= .59, p= 0.006), and the correlations obtained for placebo and treatment differed significantly (z= 3.02, p= 0.003) suggesting that the predictive value of the discriminant function is specific to the scopolamine response in the MDD group. Moreover, in the same P/S group of patients with MDD where no correlation was evident under placebo, there was a significant correlation between the discriminant function score and the percent change at the end of the study (r= 0.70, p= 0.002) demonstrating that the correlation developed following response to scopolamine.
Table 2 provides Pearson correlation coefficients for items in the POMS and VAS as well as percent change in MADRS score. Significant correlations were observed between percent change in the MADRS and individual baseline ratings on the depression-scale from the POMS (r= -.466, p= 0.0006), and the restless (r= -.448, p= 0.001), sad (r= -.493, p= 0.0003), and irritated (r= -.403, p= 0.0037) scales from the VAS. Scatterplots showing each of the significant correlations are presented in Figure 2, A through D. Trends were observed in the confusion (r= -.378, p= 0.007) and tension (r= -.378, p= 0.01) scales from the POMS, and the drowsy (r= -.371, p= 0.008) scale from the VAS. The correlations indicate that lower scores on the POMS depression, confusion, and tension scales, and the VAS sad, restless, irritated, and drowsy scales are associated with larger treatment response. Importantly, baseline MADRS (r= -.07, p> 0.05; Figure 2E) and baseline HAM-A (r= -.023, p> 0.05) scores did not correlate with treatment response to scopolamine.
Table 2.
Correlations between Treatment Response and Baseline Mood-Ratings
| Tension | Depression | Anger | Vigor | Fatigue | Confusion | Happy | Restless | Sad | Anxious | Irritated | Drowsy | Alert | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent Change (MADRS) | Corr Coef | -0.359 | -0.466 | -0.273 | 0.214 | -0.26 | -0.378 | 0.126 | -0.448 | -0.493 | -0.301 | -0.403 | -0.371 | 0.26 |
| Sig (2-t) | 0.01 | <0.001 | 0.055 | 0.135 | 0.069 | 0.007 | 0.383 | <0.001 | <0.001 | 0.033 | 0.004 | 0.008 | 0.068 |
Significant (≤0.004; corrected for multiple comparisons at p<0.05) correlations with treatment response (percent change) shown in BOLD
Trend level correlations with treatment response shown in underline
Figure 2.
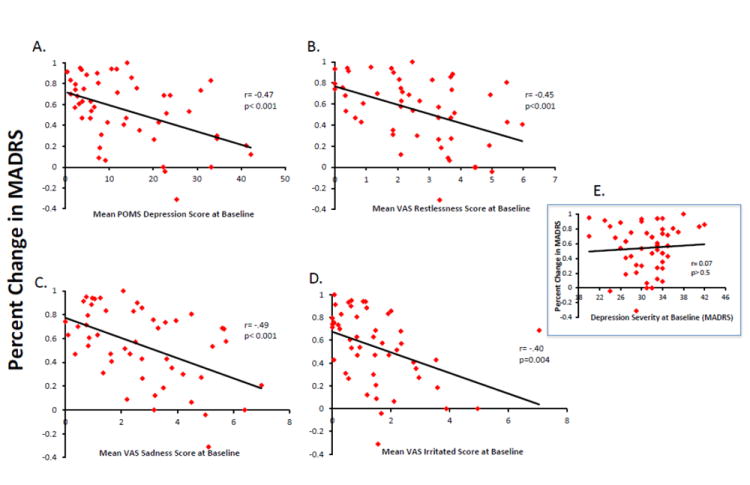
Scatterplots reflect correlations between the magnitude of antidepressant response (percent improvement in MADRS score from baseline to study end) and baseline self-report mood ratings in a patient group that includes major depressive and bipolar disorder patients. The magnitude of antidepressant response correlated significantly with the depression subscale of the POMS (panel A), as well as the restlessness (panel B), sadness (panel C) and irritated (panel D) scales of the VAS. The correlation value and associated probability value are presented for each graph. The absence of a correlation between the magnitude of the antidepressant response and baseline depression severity is reflected in the panel insert (panel E).
4. Discussion
The results of this study indicate that baseline self-report mood-ratings obtained prior to treatment can predict response outcome to scopolamine.
The discriminant analysis identified a combination of the baseline rating scale variables that together predict response or non-response. The discriminant functions obtained for the separate diagnostic subgroups accurately classified over 85 percent of all unipolar and bipolar patients as responders or non-responders. Little overlap in the factor scores exists for responders and non-responders, especially within the bipolar group. Moreover, in the cross-validation analysis when each subject was removed individually and subsequently classified based on the resulting discriminant function, over 70% of unipolar patients and over 85% of bipolar patients were correctly classified. This result suggests that baseline mood ratings may successfully predict treatment response to scopolamine in individual patients.
Importantly, the magnitude of response during placebo did not correlate with the discriminant function scores, suggesting that the predictive value of the discriminant function is specific to treatment response and does not predict improvement associated with placebo. In addition, in the MDD group in study phase I where the sample size was sufficient to estimate the placebo response separately from the treatment response, the placebo and treatment correlations differed, suggesting that the discriminant function selectively predicts scopolamine response and not placebo response. In addition, while the discriminant function did not predict response to placebo in the MDD group receiving placebo first (P/S), the function did predict subsequent response to scopolamine, providing further evidence that the predictive ability of the discriminant function is specific to the treatment response. The sample sizes for the BP group during study phase I became too small when separating the placebo (n=8) and treatment (n=6) groups and therefore we were unable to confirm specificity in the bipolar sub-group.
The measures that were selected as discriminating variables for the unipolar and bipolar patient groups overlapped (both included depression and anger) but unique variables also were selected in the discriminant analyses for the diagnostic groups. This suggests that different baseline mood features better discriminate between responders and non-responders for the MDD and BD subgroups. While the smaller size of the BD group limits the sensitivity and generalizability of the results in this sample, the results suggest that the mood features that best discriminate responders from non-responders to scopolamine differ based on diagnostic subgroup.
The results from the Pearson correlation analyses show that the ability of the discriminant function to predict treatment response is based on more than simple correlations between ratings scales and treatment response. The POMS depression subscale score is the only rating that both correlated with treatment response and contributed to each of the discriminant function scores. The VAS irritated item also correlated with treatment outcome and contributed to the BD group discriminant functions. The POMS anger subscale contributed to both discriminant functions but did not correlate with treatment outcome, while the VAS sadness scale did correlate with treatment outcome but did not contribute to either of the discriminant functions. Thus, while some of the individual items did correlate with treatment response, accounting for 16 to 25 percent of the variance, the predictive ability of the discriminant functions exceeds these individual items by properly classifying over 85% of MDD and BD patients as responders or non-responders.
The relative pattern of scores on these specific mood scales may reflect features of patient subgroups that preferentially respond to scopolamine. Importantly, there is no relation between severity of depressive illness (MADRS) or severity of anxiety symptoms (HAM-A) with response outcome. As a result, the negative correlations that show greater treatment response is associated with lower baseline values on the POMS depression score and the sad VAS scale do not suggest that treatment responders tend to be less severely ill patients. Moreover, the correlations with specific rating scale items were selective to changes in depression severity following treatment with scopolamine, as no such correlations between baseline mood-state rating items and changes in illness severity were evident during placebo.
Researchers have attempted to identify patient sub-groups who preferentially respond to specific agents or classes of antidepressants as a way to improve upon treatment efficacy. Researchers have reported differences in response rates based on diagnostic sub-groups, such as patients with melancholic depression showing better response to TCAs versus SSRIs (Roose et al., 1986) or patients with atypical depression responding better to MAOIs than to TCAs (Quitkin et al., 1993). However these findings are generally inconsistent (Peselow et al., 1992; Nelson et al., 1994) and the effects are small (Fava et al., 1997) indicating that treatment strategies based on diagnostic subgroups are unlikely to improve treatment efficacy. These efforts may prove to be more successful if the relative constellation of symptom features were evaluated as potential predictors of response.
More recently, focus has shifted towards identifying biological markers of treatment response. Brain imaging methods have been used to identify baseline structural features or functional brain responses that discriminate patient responders from non-responders following treatment. For example, hippocampal volumes are smaller in patients with MDD(Campbell et al., 2004; Videbech and Ravnkilde, 2004), and larger volume correlates with subsequent treatment response(MacQueen et al., 2008). Higher pre-treatment activity in the anterior cingulate cortex correlates with antidepressant response to ketamine (Salvadore et al., 2009; Salvadore et al., 2010) thus identifying pre-treatment, brain-based differences among patients that predict treatment response. These findings argue that biological explanations underlie the clinical variability associated with patient response to antidepressant treatment, providing evidence that a more personalized approach to treatment for patients with mood disorders is attainable.
Cognitive and neuropsychological approaches have characterized early changes in the presence of behavioral and neural correlates of emotional processing biases within days of initiating antidepressant treatment (Harmer et al., 2009; Miskowiak et al., 2009). Patients with mood disorders experience biases towards processing negatively valenced emotional stimuli (Bradley et al., 1996; Murphy et al., 1999; Elliott et al., 2000). The shift away from a negative emotional processing bias appears within 3 days after the onset of treatment of erythropoietin (a novel antidepressant agent) in patients (Miskowiak et al., 2009, 2010) but the relation between these changes and subsequent clinical antidepressant responses has not been determined. Emotional processing biases also were shown to differ between patients groups three hours after the administration of placebo or reboxetine (Harmer et al., 2009), but again no assessment to determine if these changes in processing biases predicted response outcome was reported.
Caution is in order when considering the results observed in the bipolar group. While the effect size is large, the sample size is relatively small and thus the generalizability of our findings should be determined following further study.
The search for markers that predict treatment response has produced little that translates into clinically useful pre-treatment assessment tools. The current findings suggest that the POMS and VAS mood-scales together characterize a behavioral phenotype of depression that preferentially responds to scopolamine treatment. The potential for acute mood scales such as the POMS and VAS to discriminate responders from non-responders following other antidepressant treatments remains empirical, but the ease of administration argues that the effort is worthwhile. Moreover, the development of additional tools designed specifically to characterize baseline mood features with the goal of better distinguishing the expression of relative symptomatology may improve prediction beyond that reported here.
Acknowledgments
We thank Ashish Khanna, Mark Opal and Summer Peck for technical support, Michele Drevets and Joan Williams for patient recruitment and evaluation, Paul Carlson, Alan Mallinger, and the 5SW Day Hospital nursing staff for medical support. This research was supported by the NIH NIMH-DIRP.
Footnotes
Disclosure/Conflict of Interest
The NIMH has filed a use-patent for the use of scopolamine in the treatment of depression, and Dr.’s Furey and Drevets are identified as co-inventors on this pending patent application in the US and an existing patent in Europe.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bradley BP, Mogg K, Millar N. Implicit memory bias in clinical and non-clinical depression. Behav Res Ther. 1996;34:865–879. doi: 10.1016/s0005-7967(96)00074-5. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. American Journal of Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Costanza MC, Afifi AA. Comparison of Stopping Rules in Forward Stepwise Discriminant Analysis. Journal of the American Statistical Association. 1979;74:777–785. [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders, IV. American Psychiatric Association; 2000. [Google Scholar]
- Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biological Psychiatry. 2009;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum JF. Major depressive subtypes and treatment response. Biological Psychiatry. 1997;42:568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient edition (SCID-I/P, 11/2002 Revision) New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Archives of General Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35:2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock HA, Hamilton SP. Genetic studies of drug response and side effects in the STAR*D study, part 1. Journal of Clinical Psychiatry. 2009;70:1186–1187. doi: 10.4088/JCP.09ac05519. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. American Journal of Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatric Services. 2009;60:1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- Khan A, Khan SR, Shankles EB, Polissar NL. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression rating scale and the Clinical Global Impressions rating scale in antidepressant clinical trials. Int Clin Psychopharmacol. 2002;17:281–285. doi: 10.1097/00004850-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Laje G, McMahon FJ. The pharmacogenetics of major depression: past, present, and future. Biological Psychiatry. 2007;62:1205–1207. doi: 10.1016/j.biopsych.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, Faull K, Whitelegge J, Andrews AM, Loo J, Way B, Nelson SF, Horvath S, Lebowitz BD. Biomarkers to predict antidepressant response. Current Psychiatry Report. 2010;12:553–562. doi: 10.1007/s11920-010-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA., Jr Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. Journal of Clinical Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ, Harmer CJ. Effects of erythropoietin on emotional processing biases in patients with major depression: an exploratory fMRI study. Psychopharmacology (Berl) 2009;207:133–142. doi: 10.1007/s00213-009-1641-1. [DOI] [PubMed] [Google Scholar]
- Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ, Harmer CJ. Erythropoietin modulates neural and cognitive processing of emotional information in biomarker models of antidepressant drug action in depressed patients. Psychopharmacology (Berl) 2010;210:419–428. doi: 10.1007/s00213-010-1842-7. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Mazure CM, Jatlow PI. Characteristics of desipramine-refractory depression. Journal of Clinical Psychiatry. 1994;55:12–19. [PubMed] [Google Scholar]
- Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):5–9. [PubMed] [Google Scholar]
- Peselow ED, Sanfilipo MP, Difiglia C, Fieve RR. Melancholic/endogenous depression and response to somatic treatment and placebo. American Journal of Psychiatry. 1992;149:1324–1334. doi: 10.1176/ajp.149.10.1324. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitkin FM, Stewart JW, McGrath PJ, Tricamo E, Rabkin JG, Ocepek-Welikson K, Nunes E, Harrison W, Klein DF. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. British Journal of Psychiatry Supplement. 1993:30–34. [PubMed] [Google Scholar]
- Roose SP, Glassman AH, Walsh BT, Woodring S. Tricyclic nonresponders: phenomenology and treatment. Amrican Journal of Psychiatry. 1986;143:345–348. doi: 10.1176/ajp.143.3.345. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biological Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments? American Journal of Psychiatry. 2010;167:1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Amrican Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]


