Abstract
Research has shown poor performance on verbal memory tasks in patients with major depressive disorder relative to healthy controls, as well as structural abnormalities in the subcortical structures that form the limbic-cortical-striatal-pallidal-thalamic circuitry. Few studies, however, have attempted to link the impairments in learning and memory in depression with these structural abnormalities, and of those which have done so, most have included patients medicated with psychotropic agents likely to influence cognitive performance. This study thus examines the relationship between subcortical structural abnormalities and verbal memory using the California Verbal Learning Test (CVLT) in unmedicated depressed patients. A T1 weighted Magnetic Resonance Imaging scan and the CVLT were obtained on 45 subjects with major depressive disorder and 44 healthy controls. Using the FMRIB’s Integrated Registration and Segmentation Tool (FIRST) volumes of selected subcortical structures were segmented and correlated with CVLT performance. Depressed participants showed significantly smaller right thalamus and right hippocampus volumes than healthy controls. Depressed participants also showed impaired performance on global verbal learning ability, and appeared to depend upon an inferior memory strategy (serial clustering). Measures of serial clustering were correlated significantly with right hippocampal volumes in depressed participants. Our findings indicate that depressed participants and healthy controls differ in the memory strategies they employ, and that while depressed participants had a smaller hippocampal volume, there was a positive correlation between volume and use of an inferior memory strategy. This suggests that larger hippocampal volume is related to better memory recall in depression, but specifically with regard to utilizing an inferior memory strategy.
Keywords: Depression, Verbal Memory, California Verbal Learning Test, Subcortical Volumes, Hippocampus, Serial Clustering
1. Introduction
A diagnosis of major depressive disorder includes clinical features of cognitive dysfunction such as “diminished ability to think or concentrate, or indecisiveness,” (APA 2000). Neuropsychological studies that evaluate the cognitive features of depression have identified deficits in information processing, attention, memory, and executive functioning (Reppermund, Ising et al. 2009). Memory dysfunction is apparent in declarative memory (Campbell and MacQueen 2006), with the largest effects in the domains of encoding and retrieval of episodic memory (Zakzanis, Leach et al. 1998). Studies of episodic memory deficits in depression have examined both autobiographical memory and explicit memory. With regard to autobiographical memory, depressed participants tend to be over-general in their recall of events triggered by a cue word (Williams et al. 2007; Young et al., 2011). Most examinations of explicit memory in depression have focused on verbal memory. For example, in studies comparing depressives and controls for their performance on the California Verbal Learning Test (CVLT; (Delis 2000), an assessment of verbal memory, retention, and retrieval, the depressed participants were slower to acquire new information, and recalled fewer target words. In contrast, memory retention and recognition memory appear relatively preserved in major depression (Otto, Bruder et al. 1994).
Verbal memory deficits observed in patients with depression conceivably may be mediated by the abnormalities in brain structure and function that have been identified in neuroimaging and neuropathological studies of major depression (Sheline, Wang et al. 1996; Gold, Drevets et al. 2002). Such abnormalities commonly have been reported to involve subcortical structures such as the amygdala, hippocampus, striatum and thalamus (Bremner 2005; Amico, Meisenzahl et al. 2011). These structures, along with the prefrontal and temporal cortices, form the limbic-cortical-striatal-pallidal-thalamic circuit that plays a role in supporting affective states, episodic memory, and other types of cognitive processing (Sheline 2000; Bielau, Trubner et al. 2005; Kim, Hamilton et al. 2008).
The hippocampus is critically involved in learning and memory, particularly in consolidation of short-term into long-term explicit memory (Squire, 1992; Schacter, et al., 1996; Eichenbaum, 1997; Nadel et al., 2003). The hippocampus has been linked with performance on tasks of delayed recall (Geuze, Vermetten et al. 2005) and damage to the hippocampus gives rise to explicit memory deficits (Sapolsky 2000). In addition, lesion (Serra-Grabulosa, Junque et al. 2005) and resection (Hermann, Wyler et al. 1994) studies have shown that hippocampal damage impairs performance on recall measures of the CVLT (Hermann, Wyler et al. 1994; Serra-Grabulosa, Junque et al. 2005).
Structural Magnetic Resonance Imaging (MRI) studies show smaller hippocampal volumes in major depression (Videbech and Ravnkilde 2004; Clark, Chamberlain et al. 2009; Ystad, Lundervold et al. 2009), although this finding remains inconsistent in the literature (Ashtari, Greenwald et al. 1999; Vythilingam, Vermetten et al. 2004). Although in some cases differences in the results across studies may be due to methodological issues such as the use of lower resolution MRI techniques and differences in segmentation procedures, in other cases they may reflect the biological heterogeneity extant within the population encompassed by the major depressive disorder criteria (Sapolsky 2000; Sheline 2000). For example, theories exist regarding the relationship between cortisol hypersecretion, hippocampal abnormalities and verbal memory deficits and the neurobiology of depression. The prevailing hypothesis is that hippocampal neuronal damage may be caused by excess secretion of cortisol, particularly in the context of prolonged stress, and/or glutamatergic excitotoxicity (for review see Gold, Drevets, & Charney, 2002), although the relationship between these pathological constructs has been only partly elucidated.
Other subcortical structures implicated in the pathophysiology of major depressive disorder, such as the basal ganglia, play roles in implicit learning and memory, and in working memory (Packard and Knowlton 2002; Ring and Serra-Mestres 2002; Graybiel 2005). Also, the basal ganglia have also been implicated in mediating certain memory strategies, such as route recognition, via their limbic-cortical-striatal-pallidal-thalamic circuit interactions (Voermans et. al., 2004). The basal ganglia have been implicated in depression due to the comorbidity of depression with neurodegenerative disorders that involve the basal ganglia, such as Parkinson’s disease and Huntington’s disease (Husain, McDonald et al. 1991; Krishnan, McDonald et al. 1992; Ring and Serra-Mestres 2002). Neuroimaging studies have shown smaller putamen and caudate volumes (Husain, McDonald et al. 1991; Krishnan, McDonald et al. 1992), while postmortem studies have shown smaller putamen, pallidum, and accumbens volumes in depressed participants compared to healthy controls (Baumann, Danos et al. 1999). In addition, lesion studies link the caudate nucleus and the putamen with depression, and patients with lesions in the caudate and putamen tend to have both higher frequency and higher severity of depression(Starkstein, Robinson et al. 1988).
The thalamus also has been implicated in the pathophysiology of major depression in neuroimaging studies (e.g., (Drevets 1998; Kim, Hamilton et al. 2008). Volumetric MRI (Kim, Hamilton et al. 2008) and post-mortem (Bielau, Trubner et al. 2005) studies identified smaller thalamic volumes in depressed participants compared to healthy controls. In addition, patients with thalamic lesions tend to have memory difficulties that include retrieval and encoding deficits (Van der Werf, Witter et al. 2000).
In summary, while both verbal memory deficits and subcortical abnormalities have been demonstrated in patients suffering from major depression, the relationship between subcortical volume and verbal memory remains unclear. Further insight into this relationship could facilitate our understanding of the pathophysiology underlying mood disorders, and potentially may contribute to improved diagnosis and treatment for major depressive disorder. We predicted that volumes of subcortical regions involved in the limbic-cortical-striatal-pallidal-thalamic circuit that support verbal memory processing would be reduced in depressed participants as compared to healthy controls. Moreover, we expected that volumes in areas showing group differences would correlate with indices of verbal memory dysfunction.
2. Methods and Materials
2.1 Participants
Forty-five currently unmedicated (for at least 2 weeks, and at least 4 weeks for those treated with fluoxetine) patients were enrolled in this study after meeting Diagnostic and Statistical Manual for Mental Disorders (4th ed., text rev.; DSM-IV-TR; APA, 2000) criteria for Major Depressive Disorder without psychotic features (18 males, 27 females; mean age = 36 ± 10; range 19-56 years). Mental health status was determined by the Structured Clinical Interview for the DSM-IV-TR (SCID) (First 1995) administered by trained research nurses with at least .80 interrater reliability and confirmed via an unstructured interview with a psychiatrist. Healthy controls (20 males, 24 females; age = 33 ± 10; range 20-57 years) had no current lifetime or history of a psychiatric disorder (also ascertained via the SCID and an unstructured interview with a psychiatrist), and did not have a first-degree relative with a mood or anxiety disorder. Exclusion criteria for all participants as determined by medical history, physical examination and laboratory testing included significant medical or neurological disorders, head injury with loss of consciousness, pregnancy, general MRI exclusions, meeting DSM-IV-TR (APA, 2000) criteria for substance abuse within the preceding 90 days, or a positive urine toxicology screen. All participants were evaluated at the National Institute of Mental Health outpatient clinic. Written informed consent was obtained from all participants, and all data were collected as approved by the National Institutes of Health Combined Neuroscience Institutional Review Board.
Verbal Memory Task
The California Verbal Learning Test (CVLT;(Delis 2000)) was used to assess verbal learning, retention, and retrieval. The CVLT is a retrieval-trial, list-learning test, with two 16-item lists. List A, which consists of four semantic categories, is read to the participants, and is presented so that no two words from the same category occur in sequence. Participants are not informed of the semantic structure. The list is presented for five trials, after each of which the participant is asked to recall as many of the words as possible (immediate free recall). A Total Free Recall score is computed by adding trials one through five. Immediately after trial 5, List B is presented (again this list is comprised of four semantic categories, two of which overlap with List A), and participants were asked to recall these words once. Following this single trial with list B, the participants are asked to recall List A once with no prompt (short delay free recall), and then a second time when prompted with semantic category (short delay cued recall). Following a 20 minute delay, participants are asked to recall as many words from list A as possible, first via free recall (long delay free recall) then via cued recall (long delay cued recall). Finally, a List A recognition trial included the presentation of a word list comprised of List A words, list B words and novel words. The CVLT also includes scores for semantic clustering, which refers to the consecutive recall of words from the same category, and serial clustering, which is the recall of words in the same order they were presented either from the beginning of the list specifically (serial clustering forward), or from the beginning or end of the list (serial clustering bidirectional). For additional details on the CVLT see (Delis 2000).
2.2 Image Acquisition
MRI scans were conducted on two scanners. Seventy-one (35 healthy controls; 36 depressed participants) participants were scanned on a long bore GE 3 Tesla scanner with a single channel coil running an MP-RAGE pulse sequence (echo time = 2.7 ms, repetition time = 7.3 ms, prep time = 725 ms). Eighteen participants (9 healthy controls; 9 depressed participants) were scanned on a short bore GE 3 Tesla MRI scanner acquiring IR-fSPGR images with an 8 channel coil (echo time = 2.6ms, repetition time = 5.9 ms, inversion time = 450ms). All images were acquired axially at a resolution of 224×224×124, resampled to 256×256×124 with a 22cm field of view and final resolution of 0.9×0.9×1.2mm.
2.3 Image Processing
Following acquisition, the MINC (McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, Canada) tool N3 was used to correct the images for intensity non-uniformity. After spatial normalization the FMRIB’s Integrated Registration and Segmentation Tool (FIRST) software was utilized to segment the images (details described in (Patenaude, Smith et al. 2008). Images were processed using the run_first_all routine, distributed as part of the FIRST software. Correction for voxels on the boundary of each segmentation was also included in the run_first_all routine. Regions of interest included right and left thalamus, caudate, putamen, pallidum, and hippocampus. We have previously found the FIRST technique to be reliable across our scanning platforms in these regions; although the FIRST tool can also be used to segment the accumbens and amygdala, we found the reliability of these volumes to be relatively low on our scanners (Nugent 2012).
2.4 Statistical Analysis
All data analysis was performed utilizing the Statistical Package for the Social Sciences (SPSS 2010). For demographic characteristics, a chi square analysis was used to compare the categorical variables and a one-way analysis of variance was used to compare the continuous variables between groups. The CVLT measures and brain regions were divided into primary and secondary analyses. The primary analyses consisted of CVLT measures and regions that were well supported by the literature, while the secondary analyses consisted of CVLT measures and regions with less evidence for abnormality in major depression. Therefore, the primary region of interest was the hippocampus, while the thalamus, caudate, putamen and pallidum were secondary regions; left and right lateralized regions were examined separately. Of the apriori outcome measures of the CVLT, seven were considered primary (total free recall, short delay free recall, short delay cued recall, long delay free recall, long delay cued recall, Yes/No Recognition Total Hits and Total False Positives) and five were considered secondary (Semantic Clustering trials 1-5, Semantic Clustering SDFR, Semantic Clustering LDFR, Serial Clustering Forward, Serial Clustering Bidirectional). An analysis of covariance was used to identify group differences in region volumes and CVLT outcome measures. Cohen’s d is used to show effect sizes for mean comparisons. A Pearson’s correlation was used to determine if volumes of brain areas that differed between groups correlated with CVLT outcome measures that also differed between groups. Gender and scanner were included as covariates in each of the appropriate analyses (Kramer, Yaffe et al. 2003). The Bonferroni correction was applied to control for multiple comparisons, making the significance level for the primary regions of interest .025, and .006 for secondary regions of interest; the significance level for primary CVLT measures of interest is .008, and .01 for secondary CVLT measures of interest. Finally, the significance level for Pearson’s correlations between significant regions and significant measures is .004. Pearson’s correlations found to be significant in one group were compared between groups via Fisher’s r-to-z transformation to evaluate the significance of the difference between the correlation coefficients.
3. Results
Demographic and clinical characteristics of the healthy controls and participants with major depression are presented in Table 1. No significant difference was observed between the two groups on age, education, IQ, or gender distribution, however, the depressed participants scored significantly higher on the Montgomery-Asberg Depression Rating Scale (Montgomery and Asberg 1979) than the healthy controls
Table 1.
Sample Characteristics Mean(SD)
| HC | MDD | |
|---|---|---|
| N | 44 | 45 |
| Age | 34 (10) | 36 (10) |
| Age Range | 20-57 | 19-56 |
| Gender (M:F) | 20:24 | 18:27 |
| *Education Years (n=88) | 16 (2) | 16 (2) |
| *Education Range (n=88) | 12-22 | 11-21 |
| *IQ (n=87) | 117 (12) | 117 (11) |
| *IQ Range (n=87) | 75-133 | 96-141 |
| *MADRS (n=78) | .23 (.73) | 24.88 (5.67) |
| *MADRS Range(n=78) | 0-3 | 14-37 |
| *Length of Illness (n=79) | N/A | 17.11 (8.5) |
| *Length of Illness Range (n=79) | N/A | 3-37 |
= some participants missing this information, HC=Healthy Control, MDD=participants with depression, N= number of participants in the group, M=male, F=female, MADRS=Montgomery Asberg Depression Rating Scale, Length of Illness is in years
Table 2 lists the mean regional volumes for depressed and control samples. For the primary region of interest, depressed participants showed significantly smaller mean right hippocampal volumes than the healthy controls (F=6.386, p=.013; d=.62), and within secondary regions of interest, depressed participants showed significantly smaller right thalamic volumes than the healthy controls (F=8.625, p=.004; d=.68).
Table 2.
Mean Volume Differences in mm3
| HC | MDD | F | Sig | |
|---|---|---|---|---|
| Primary Analyses | ||||
| Left Hippocampus | 3839.4 | 3672.9 | 2.452 | .121 |
| Right Hippocampus | 3938.4 | 3699.3 | 6.386 | .013* |
| Secondary Analyses | ||||
| Left Thalamus | 8272.4 | 7848.1 | 5.966 | .017 |
| Right Thalamus | 8085.2 | 7587.3 | 8.625 | .004* |
| Left Caudate | 3436.3 | 3397.1 | .009 | .926 |
| Right Caudate | 3652.2 | 3520.8 | .641 | .426 |
| Left Putamen | 5039.4 | 4919.9 | .178 | .674 |
| Right Putamen | 4884.5 | 4757.9 | .172 | .679 |
| Left Pallidum | 1712.5 | 1673.9 | .608 | .438 |
| Right Pallidum | 1763.1 | 1701.9 | 1.517 | .221 |
indicates continued significance when controlling for multiple comparisons
As depicted in Table 3, for the primary measures-of-interest, healthy controls had a significantly higher mean Total Free Recall (TFR; F=7.420, p=.008; d=.58) than the depressed participants. For the secondary measures-of-interest, healthy controls used semantic clustering more often than depressed participants did during Long Delay Free Recall (F=9.154, p=.003; d=.64) while depressed participants showed a nonsignificant trend toward using bidirectional serial clustering more often than healthy controls (F=.698, p=.011; d=.20).
Table 3.
Mean Score Differences on the CVLT
| HC | MDD | F | Sig | |
|---|---|---|---|---|
| Primary Analyses | ||||
| Total Free Recall | 58.59 | 51.36 | 7.420 | .008* |
| Short Delay Free Recall | 12.30 | 11.53 | 1.262 | .264 |
| Short Delay Cued Recall | 13.32 | 11.93 | 4.840 | .030 |
| Long Delay Free Recall | 13.00 | 11.82 | 3.476 | .066 |
| Long Delay Cued Recall | 13.57 | 12.47 | 3.197 | .077 |
| Total Hits Y/N Recognition | 15.39 | 14.18 | 4.211 | .043 |
| False Positives Y/N Recognition | 1.57 | .89 | 2.196 | .142 |
| Secondary Analyses | ||||
| Semantic Clustering Trials 1-5 | 2.01 | .905 | 4.357 | .040 |
| Semantic Clustering SDFR | 3.37 | 2.34 | 2.217 | .140 |
| Semantic Clustering LDFR | 4.99 | 2.97 | 9.154 | .003* |
| Serial Clustering Forward | .53 | 1.06 | 3.761 | .056 |
| Serial Clustering Bidirectional | .56 | 1.34 | 0.698 | .011* |
SDFR = Short Delay Free Recall, LDFR = Long Delay Free Recall, Y/N = Yes/No,
indicates continued significance when controlling for multiple comparisons
Healthy controls did not show significant Pearson’s correlations between volume and CVLT performance in any region where group differences in volume were evident. In contrast, depressed participants showed a significant correlation between serial clustering forward and the right hippocampus volume (r=.439, p=.004), and bidirectional serial clustering and the right hippocampus volume (r=.447, p=.003) (Tables 4 and 5; Figures 1 and 2).
Table 4.
Correlations between Volume in mm3 and Task Performance in HCs
| L Thalamus | R Thalamus | R Hippocampus | L Hippocampus | |
|---|---|---|---|---|
| Total Free Recall | r=.001, p=.994 | r=.036, p=.882 | r=.140, p=.383 | r=.313, p=.046 |
| Short Delay Cued Recall | r=-.030, p=.854 | r=.005,p=.975 | r=.135, p=.400 | r=.314, p=.046 |
| Semantic Clustering Trials 1-5 | r=.114, p=.480 | r=.138,p=.389 | r=.089, p=.580 | r=.351, p=.025 |
| Semantic Clustering LDFR | r=.207, p=.193 | r=.263,p=.096 | r=.192, p=.229 | r=.490, p=.001* |
| Serial Clustering Forward | r=.153, p=.339 | r=.183,p=.252 | r=.177, p=.270 | r=-.060, p=.709 |
| Serial Clustering Bidirectional | r=.014, p=.931 | r=.002,p=.988 | r=.064, p=.689 | r=-.202, p=.205 |
| Total Hits Y/N Recognition | r=-.098,p=.542 | r=-.062,p=.701 | r=.038, p=.812 | r=-.003, p=.984 |
SDFR = Short Delay Free Recall, LDFR = Long Delay Free Recall, Y/N = Yes/No,
indicates continued significance when controlling for multiple comparisons
Table 5.
Correlations between Volume in mm3 and Task Performance in MDDs
| L Thalamus | R Thalamus | R Hippocampus | L Hippocampus | |
|---|---|---|---|---|
| Total Free Recall | r=.050, p=.757 | r=-.072, p=.657 | r=.312, p=.047 | r=.268, p=.090 |
| Short Delay Cued Recall | r=-.148, p=.357 | r=-.250, p=.115 | r=.112, p=.484 | r=.030, p=.853 |
| Semantic Clustering Trials 1-5 | r=-.058, p=.718 | r=-.131, p=.414 | r=-.090, p=.575 | r=.021, p=.897 |
| Semantic Clustering LDFR | r=-.238, p=.134 | r=-.272, p=.085 | r=-.191, p=.231 | r=-.115, p=.475 |
| Serial Clustering Forward | r=.371, p=.017 | r=.369, p=.018 | r=.439, p=.004* | r=.312, p=.047 |
| Serial Clustering Bidirectional | r=.196, p=.219 | r=.222, p=.163 | r=.447, p=.003* | r=.208, p=.191 |
| Total Hits Y/N Recognition | r=-.047, p=.771 | r=-.062, p=.701 | r=-.028, p=.861 | r=-.036, p=.825 |
SDFR = Short Delay Free Recall, LDFR = Long Delay Free Recall, Y/N = Yes/No,
indicates continued significance when controlling for multiple comparisons
Figure 1.
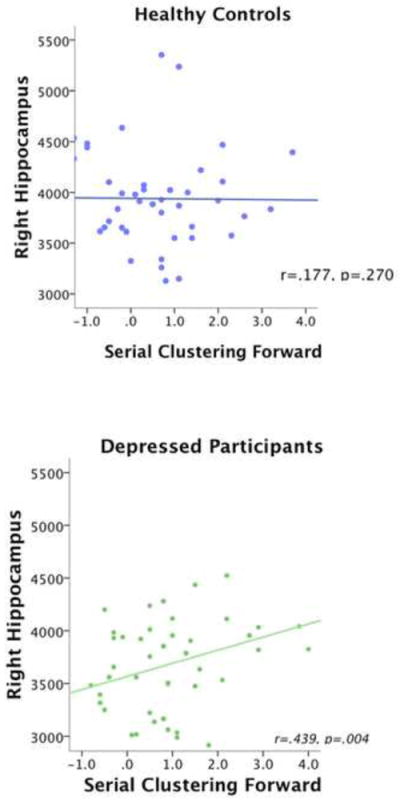
Scatter Plots Showing Correlations between Right Hippocampus and Serial Clustering Forward. Scatter plots are shown separately for healthy participants (A) and patients with depression (B),
Figure 2.
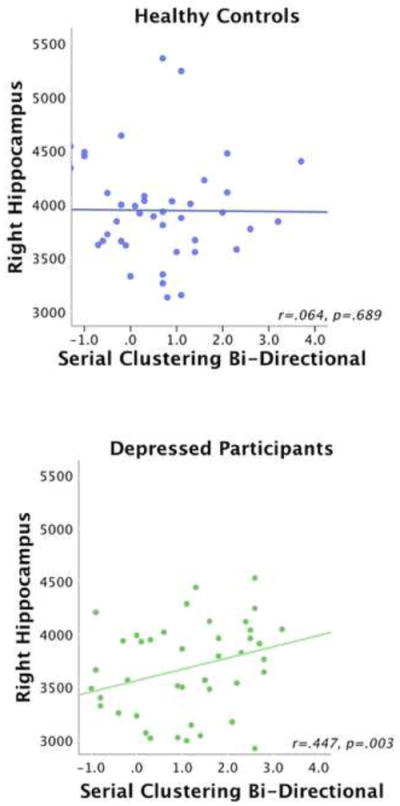
Scatter Plots of Correlations between Right Hippocampus and Serial Clustering Bidirectional, Scatter plots are shown separately for healthy participants (A) and patients with dpression (B).
No significant difference was found between groups in the correlation coefficients using the Fisher’s r-to-z transformation.
4. Discussion
The results of this study demonstrate that depressed participants and healthy controls differed on memory strategy employed while performing a verbal working memory task. Furthermore, recall based on serial clustering correlated with right hippocampus volume in the depressed group. Individuals who group items into the category to which they belong generally remember more words than individuals who do not (Buytenhuijs, Berger et al. 1994; Deckersbach, Savage et al. 2004) and thus semantic clustering is associated with better recall performance. In contrast, serial clustering is generally an ineffective learning strategy associated with poorer performance (Buytenhuijs, Berger et al. 1994; Delis 2000; Deckersbach, Savage et al. 2004). Indeed, our depressed subjects performed more poorly than controls on total free recall. Moreover, although the results did not meet the threshold for statistical significance, the participants with major depression tended to perform more poorly than the healthy controls on short delay cued recall but not on short delay free recall, consistent with an inferior recall strategy. As participants are not informed of the categorical organization of the word lists, applying a semantic clustering approach is a self-generated strategy, while employing serial recall by definition is an externally cued resource (Delis 2000; Berger, Cools et al. 2004). Internal or self-generated cues have stronger influence on memory than external cues (Berger, Cools et al. 2004). The inability to use semantic clustering may indicate deficits in developing internal cues. Therefore, this finding may indicate that depressed participants fail to use semantic clustering because they fail to generate the internal cue of category. Relative to the control group, the MDD group showed smaller hippocampal volumes, poorer total free recall, and more common use of serial, as opposed to semantic clustering. Nevertheless, the hippocampal volume was positively correlated with the degree to which serial clustering was used. Taken together these findings suggest that a complex relationship exists between these associated abnormalities in brain structure and cognitive function. For example, one potential interpretation of the positive correlation between hippocampal volume and serial clustering may be that MDD subjects with the most intact hippocampal structure are able to compensate for an underlying impairment in the use of semantic clustering by making greater use of serial clustering (albeit an inferior technique) to perform the task. Conversely, subjects who have the smallest hippocampal volumes may be less able to use this compensatory, serial clustering strategy, and thus show the poorest free recall performance because they are impaired at both semantic and serial clustering strategies
Our finding of a right lateralized difference in hippocampal volume contrasts with previous findings of bilateral hippocampal reductions, with the reduction on the left being the most pronounced (Sheline, Sanghavi et al. 1999; Sapolsky 2000; Videbech and Ravnkilde 2004; Kaymak, Demir et al. 2010). This is not, however, the only study to find significantly smaller hippocampal volumes only in the right side (Neumeister, et al., 2005; Amico, Meisenzahl et al. 2011). It should be noted that although the difference was not significant, mean left hippocampus volume was nominally lower in depressed subjects as compared to controls.
Consistent with previous CVLT literature we found that depressed participants had a lower total free recall score, and relied more on serial clustering and less on semantic clustering, (Otto, Bruder et al. 1994; Hinkelmann, Moritz et al. 2009; Thomas, Gallagher et al. 2009). However, contrary to much of the literature, we did not find a difference between the two groups on delayed free recall. Previous literature shows that depressed participants increase their recall across trials just as healthy controls do, yet do so at a slower rate (Hinkelmann, Moritz et al. 2009). This similar pattern of learning suggests that it is encoding into memory rather than retrieval from memory that impairs depressed participants on the CVLT, particularly when coupled with impairments on the recognition task. Our observation that the depressed participants performed more poorly than healthy controls across the five learning trials but performed similarly on short and long delay free recall and long delay cued recall furthers the controversial claim that depressed participants have difficulties in verbal memory that stem from encoding rather than retrieval (Squire, 1987; Delis, Kramer, Kaplan, & Ober, 2000; Murray, Whitehouse, & Alloy, 1999). Though most research examining CVLT performance in depressed participants indicates that they performed more poorly than healthy controls across the five learning trials, the studies tend to assert that deficits stem from retrieval rather than encoding. In general this assertion is based on the sample of participants having impaired recall, but intact recognition. In addition, Murray and colleagues found that both dysphoric and non depressed groups recalled additional words under a forced recall task, which they assert indicates that these words were encoded and not retrieved (Murray, Whitehouse, & Alloy, 1999). However even Murray and associates indicate that while the later remembered words are available to the dysphoric group, they are less accessible to conscious recollection, which may be due to less elaborative processes being used at encoding. In fact, the extant literature generally suggests that depressed participants have intact recognition on the CVLT (Otto, Bruder et al. 1994; Delis 2000). Consistent with this conclusion, recognition differences between groups in the present study did not remain significant after controlling for multiple comparisons; however, the possibility of a type II error must be considered. It seems reasonable to infer from our data that deficits in verbal memory shown within this sample stem primarily from encoding rather than retrieval.
The depressed sample also exhibited a significantly smaller mean right thalamus volume compared to the control sample, although the correlation between verbal memory task performance and thalamic volume was not significant. The finding of smaller right thalamus volume is consistent with previous neuroimaging (Kim, Hamilton, & Gotlib, 2008) and postmortem (Bielau et al., 2005) literature that has reported reduced bilateral thalamus volumes in depression. The emphasis on a limbic-cortical-striatal-pallidal-thalamic circuit in mood regulation (Drevets, et al., 1992; Bielau et al., 2005; Kim, Hamilton, & Gotlib, 2008; Ystad, Eichele, Lundervold, & Lundervold, 2010) and recent research into structural abnormalities in this circuit (Kim, Hamilton, & Gotlib, 2008) implicate the thalamus in depression. In addition, a link between thalamic function and memory performance stems from lesion studies, which show that patients with thalamic lesions tend to have memory difficulties, which in most cases involve retrieval and encoding deficits, although the pattern of deficits can be variable (Van der Werf et al., 2000). Therefore, it is reasonable to expect a link between thalamic volume, CVLT performance, and depression. It is possible that the sample size was insufficient to display correlations between thalamic volume and CVLT performance. Although the other subcortical regions examined failed to show significant differences in this study, this is not altogether surprising given the relatively small sample size and the paucity of reports of abnormalities in the literature.
In addition to the subcortical regions studied herein, previous literature indicates that some areas of the prefrontal cortex are involved in performing tasks of verbal memory. Numerous studies show that individuals with frontal lobe lesions are impaired on tasks of free recall, largely because they fail to use strategies and cues (Hirst & Volpe, 1988; Gershberg & Shimamura, 1995; Baldo, Delis, Kramer, & Shimamura, 2002). The functional neuroimaging literature has also shown that activation of the prefrontal cortex during encoding was indicative of semantic clustering during recall (Savage et al., 2001; Long, Oztekin, & Badre, 2010). Results of the current study suggest that depressed participants rely on serial clustering more than semantic clustering, compared to the controls and there was a positive correlation between the use of serial clustering and right hippocampal volume in the depressed group. Though the previous studies have focused on cortical regions such as the prefrontal cortex, together these findings suggest both hippocampus and prefrontal cortex involvement of the limbic-cortical-striatal-pallidal-thalamic circuitry in verbal memory.
This study has several limitations. First, the study has a relatively small sample size. In addition, the fact that because most of our depressed participants exhibited moderate depression, correlations between severity of depressive symptoms and structural or memory abnormalities could not be fully explored. In addition, our results may not generalize to patient samples that are more mild or severe with respect to illness severity. As evidence indicates that depressed mood itself can impact memory (O’Jile, Schrimsher et al. 2005), including a range of mild to severe depression would shed light on the impact of depression severity on verbal memory. An important consideration for examinations of verbal memory impairment in depressed individuals is whether the impairment is a part of a stable underlying neurobiological vulnerability to major depressive disorder, or whether it occurs only in the context of depressed mood. Some data indicate that memory impairment is primarily state dependent. For example, Douglas and Porter (2009) propose a relationship between improvements in depressive symptomatology and improvements of verbal memory in major depression. In addition, neurocognitive deficits have been found in those suffering from their first episodes of depression (Kaymak, Demir et al. 2010). In contrast, evidence showing that there are declarative memory impairments in those at risk (genetically) of developing major depressive disorder would argue for trait dysfunction (Mannie et. al., 2008). Moreover, verbal memory impairment persists after the alleviation of the symptoms of depression, (Douglas and Porter, 2009), and the more chronic the illness the more pronounced cognitive deficits tend to be (Fossati, et al., 2004). Taken together, these data suggest that there are both state and trait components to the verbal memory impairment in depression. Unfortunately, the cross sectional design of the current study could not disentangle these effects. In addition, the current study did not include an examination of amotivation, which is a core component of depression. Performance differences between healthy controls and depressed participants have been rendered non-significant when patients exhibiting low effort are removed from the sample (Rohling, Green, Allen, and Iverson, 2002; Benitez, Horner and Bachman, 2011). We cannot rule out that performance measures of the CVLT were influenced by amotivation in our sample.
Future studies should include more clinical assessments and information at the time of testing such as measures to determine state and trait levels of depression, for example the State-Trait Depression Scale, as well as full medication history, to increase the interpretability of our results. It would also be valuable to examine the effects of a range of clinical variables such as severity of illness on hippocampal volumes. Additionally, a measure of motivation/effort, such as the forced recognition trial of the CVLT or the Test of Memory Malingering, should be included so that participants who are not giving optimal effort on the cognitive task can be removed from the analysis.
This is the first study that directly compares the impairments in learning and memory with structural abnormalities found in major depression in an unmedicated sample. Studies such as the current one not only elucidate brain structures involved in the pathophysiology of depression, but also provide insight into how these structural differences manifest cognitively. Examinations such as these can lead to a clearer understanding of the pathophysiology of depression. In addition, the finding that depressed participants rely on an inferior memory strategy and that recall based on this strategy is correlated with right hippocampal volume may impact efforts to address memory deficits faced by depressed participants.
Highlights.
-
>
Depressed participants have smaller bilateral thalamus and right hippocampus.
-
>
Depressed participants perform worse than healthy controls on a verbal memory task.
-
>
Depressed participants use the inferior memory strategy serial clustering.
-
>
In depressed participants serial clustering is correlated with hippocampal volume.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health. Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government.
Footnotes
There are no relationships, financial or otherwise, that could be considered conflicts of interests affecting this manuscript. We would like to thank the participants for their patience and cooperation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Arlener D. Turner, Email: adanielleturner@gmail.com.
Wayne C. Drevets, Email: wdrevets@laureateinstitute.org.
Carlos Zarate, Jr, Email: zaratec@mail.nih.gov.
Allison Nugent, Email: nugenta@mail.nih.gov.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: APA; 2000. Text Revision. [Google Scholar]
- Amico F, Meisenzahl E, et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. Journal of Psychiatry and Neuroscience. 2011;36(1):15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual for Mental Disorders. Washington, DC: American Psychological Association; 2000. [Google Scholar]
- Ashtari M, Greenwald BS, et al. Hippocampal/amygdala volumes in geriatric depression. Psychological Medicine. 1999;29(3):629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test II: findings from patients with focal frontal lesions. Journal of the International Neuropsychological Society. 2002;8(4):539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Baumann B, Danos P, et al. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11(1):71–78. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- Berger HJ, Cools AR, et al. Striatal dopamine and learning strategy-an (123)I-FP-CIT SPECT study. Neuropsychologia. 2004;42(8):1071–1078. doi: 10.1016/j.neuropsychologia.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Bielau H, Trubner K, et al. Volume deficits of subcortical nuclei in mood disorders A postmortem study. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(6):401–412. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Changes in Brain Volume in Major Depression. Depression: Mind and Body. 2005;2(2):38–46. [Google Scholar]
- Buytenhuijs EL, Berger HJ, et al. Memory and learning strategies in patients with Parkinson’s disease. Neuropsychologia. 1994;32(3):335–342. doi: 10.1016/0028-3932(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Current Opinion in Psychiatry. 2006;19(1):25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, et al. Neurocognitive mechanisms in depression: implications for treatment. Annual Review of Neuroscience. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, et al. Episodic memory impairment in bipolar disorder and obsessive-compulsive disorder: the role of memory strategies. Bipolar Disord. 2004;6(3):233–244. doi: 10.1111/j.1399-5618.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual Review of Medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Declarative Memory: Insights from Cognitive Neurobiology. Annual Review of Psychology. 1997;48:547–572. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/D, Version 2.0) New York: Biometrics Research Department; 1995. [Google Scholar]
- Fossati P, Harvey P, Le Bastard G, Ergis A, Jouvent R, Allilaire J. Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. Journal of Psychicatric Research. 2004;38:137–145. doi: 10.1016/j.jpsychires.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP. The neuropsychology of human learning and memory. In: Martinez JL Jr, Kesner RP, editors. Neurobiology of Learning and Memory. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Geuze E, Vermetten E, et al. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Molecular Psychiatry. 2005;10(2):160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Gold PW, Drevets WC, et al. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biological Psychiatry. 2002;52(5):381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Current Opinion in Neurobiology. 2005;15(6):638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, et al. Declarative memory following anterior temporal lobectomy in humans. Behavioral Neuroscience. 1994;108(1):3–10. doi: 10.1037//0735-7044.108.1.3. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, et al. Cognitive impairment in major depression: association with salivary cortisol. Biological Psychiatry. 2009;66(9):879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Hirst W, Volpe BT. Memory strategies with brain damage. Brain and Cognition. 1988;8:379–408. doi: 10.1016/0278-2626(88)90060-7. [DOI] [PubMed] [Google Scholar]
- Husain MM, McDonald WM, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Research. 1991;40(2):95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- Kaymak SU, Demir B, et al. Hippocampus, glucocorticoids and neurocognitive functions in patients with first-episode major depressive disorders. European Archives of Psychiatry and Clinical Neuroscience. 2010;260(3):217–223. doi: 10.1007/s00406-009-0045-x. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Hamilton JP, et al. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Research. 2008;164(2):114–122. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizilbash AH, Vanderploeg RD, et al. The effects of depression and anxiety on memory performance. Archives of Clinical Neuropsychology. 2002;17(1):57–67. [PubMed] [Google Scholar]
- Kramer JH, Yaffe K, et al. Age and gender interactions on verbal memory performance. Journal of the International Neuropsychological Society. 2003;9(1):97–102. doi: 10.1017/s1355617703910113. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Archives of General Psychiatry. 1992;49(7):553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- Long NM, Oztekin L, Badre D. Swparable Prefrontal Cortex Contributions to Free REcall. The Journal of Neuroscience. 2010;30(33):10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie ZN, Barnes J, Bristow GC, Harmer CJ, Cowen PJ. Memory impairment in young women at increased risk of depression: influence of cortisol and 5-HTT genotype. Psychological Medicine. 2008;39(5):757–62. doi: 10.1017/S0033291708004248. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murray LA, Whitehouse WG, Alloy LB. Mood congruence and depressive deficits in memory: A forced-recall analysis. Memory. 1999;7:175–196. doi: 10.1080/741944068. [DOI] [PubMed] [Google Scholar]
- Nadel L, Ryan L, Hayes SM, Gilboa A, Moscovitch M. The role of the hipocampal complex in long-term episodic memory. International Congress Series. 2003;1250:215–234. [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biological Psychiatry. 2005;57(8):935–7. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA, Jr, Drevets WC. Automated Subcortical Segmentation using FIRST: Test-retest reliability, inter-scanner reliability, and Comparison to manual segmentation. Human Brain Mapping. doi: 10.1002/hbm.22068. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Jile JR, Schrimsher GW, et al. The relation of self-report of mood and anxiety to CVLT-C, CVLT, and CVLT-2 in a psychiatric sample. Archives of Clinical Neuropsychology. 2005;20(4):547–553. doi: 10.1016/j.acn.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Otto MW, Bruder GE, et al. Norms for depressed patients for the California verbal learning test: associations with depression severity and self-report of cognitive difficulties. Archives of Clinical Neuropsychology. 1994;9(1):81–88. [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith S, et al. Improved Surface Models for FIRST. Human Brain Mapping; Melbourne, Australia: 2008. [Google Scholar]
- Reppermund S, Ising M, et al. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychological Medicine. 2009;39(4):603–614. doi: 10.1017/S003329170800411X. [DOI] [PubMed] [Google Scholar]
- Ring HA, Serra-Mestres J. Neuropsychiatry of the basal ganglia. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72(1):12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneousand directed application of verbal learning strategies. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Alpert MS. Consious recollection and the human hippocampal formation: Evidence from Positron Emission Tomography. Psychology. 1996;48:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Grabulosa JM, Junque C, et al. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(1):129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48(8):791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, et al. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows. Chicago: SPSS, Inc; 2010. [Google Scholar]
- Squire LR. Memory and brain. New York: Oxford University Press; 1987. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, et al. Differential mood changes following basal ganglia vs thalamic lesions. Archives of Neurology. 1988;45(7):725–730. doi: 10.1001/archneur.1988.00520310031013. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Gallagher P, et al. A comparison of neurocognitive impairment in younger and older adults with major depression. Psychological Medicine. 2009;39(5):725–733. doi: 10.1017/S0033291708004042. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, et al. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38(5):613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Voerman NC, Petersson KM, Daudey L, Weber B, van Spaendonck KP, Kremer HPH, Fernandez G. Interaction between the Human Hippocampus and the Caudate Nucleus during Route Recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biological Psychiatry. 2004;56(2):101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Barnhofer T, Crane C, hermans D, Raes F, Watkins E, Dalgleish T. Autobiographical Memory Specificity and Emotional Disorder. Psychological Bulletin. 2007;33(1):122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad MA, Lundervold AJ, et al. Hippocampal volumes are important predictors for memory function in elderly women. BMC Med Imaging. 2009;9:17. doi: 10.1186/1471-2342-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Erickson K, Nugent A, Fromm SJ, Mallinger AG, Furey ML, Drevets WC. Functional Anatomy of Autobiographical Memory Recall Deficits in Depression. Psychological Medicine. 2011;29:1–13. doi: 10.1017/S0033291711001371. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, et al. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11(3):111–119. [PubMed] [Google Scholar]


