Abstract
The NADPH oxidases (Nox) are a family of transmembrane oxidoreductases that produce superoxide and other reactive oxygen species (ROS). Nox5 was the last of the conventional Nox isoforms to be identified and is a calcium-dependent enzyme that does not depend on accessory subunits for activation. Recently, Nox5 was shown to be expressed in human blood vessels and therefore the goal of current study was to determine whether nitric oxide (NO) can modulate Nox5 activity. Endogenously produced NO potently inhibited basal and stimulated Nox5 activity and inhibition was reversible with chronic, but not acute exposure to L-NAME. Nox5 activity was reduced by NO donors, iNOS, eNOS and in endothelial cells and LPS-stimulated smooth muscle cells in a manner dependent on NO concentration. ROS production was diminished by NO in an isolated enzyme activity assay replete with surplus calcium and NADPH. There was no evidence for NO-dependent changes in tyrosine nitration, glutathiolation or phosphorylation of Nox5. In contrast, there was evidence for the increased nitrosylation of Nox5 as determined by the biotin-switch assay and mass spectrometry. Four S-nitrosylation sites were identified and of these, mutation of C694 dramatically lowered Nox5 activity, NO-sensitivity and biotin-labeling. Furthermore, co-expression of the denitrosylation enzymes thioredoxin (Trx1) and GSNO reductase (GSNOR) prevented NO-dependent inhibition of Nox5. The potency of NO against other Nox enzymes was Nox1≥Nox3>Nox5>Nox2 whereas Nox4 was refractory. Collectively, these results suggest that endogenously produced NO can directly S-nitrosylate and inhibit the activity of Nox5.
Keywords: Nitric oxide, NADPH oxidase, S-nitrosylation, Reactive Oxygen Species, Nox5
INTRODUCTION
Nitric Oxide (NO) is a pervasive molecule that regulates many aspects of cardiac and vascular physiology. It is produced primarily by the nitric oxide synthases (NOS) which include nNOS (NOS I), iNOS (NOS II) and eNOS (NOS III) and it regulates blood vessel homeostasis by modulating contractility, inflammation, atherosclerosis, remodeling and platelet aggregation (Nathan and Xie 1994; Alderton, Cooper et al. 2001). Reduced production or bioavailability of NO is an early marker of cardiovascular disease and correlates with the increased production of reactive oxygen species (ROS) (Cai and Harrison 2000). The mechanisms underlying the increased production of ROS are poorly understood and are thought to involve not only changes in gene expression but also post-translational modifications related to the loss of NO.
The binding of NO to soluble guanylate cyclase (sGC) is the best characterized endpoint of NO signaling (Murad 1986; Friebe and Koesling 2003). The activation of sGC by low concentrations of NO leads to an elevation of cGMP and increased protein kinase G (PKG) activity. Activated PKG phosphorylates a number of intracellular targets and mediates many of the biological activities of NO (Hofmann, Ammendola et al. 2000). However, it is becoming increasingly evident that the sGC-PKG signaling axis cannot account for all of the actions of NO (Lukowski, Weinmeister et al. 2008). The ability of NO metabolites to covalently modify sulfhydryl groups on proteins has revealed an alternative NO signaling pathway. Reversible S-nitrosylation of cysteine residues on substrate proteins has more recently been shown to be a prominent mode of NO-dependent signal transduction that modulates protein function in a manner analogous to phosphorylation (Stamler, Lamas et al. 2001). Indeed, like phosphorylation, a motif for S-nitrosylation has been postulated that consists of a cysteine residue located between an acidic and a basic amino acid combined within a hydrophobic environment (Hess, Matsumoto et al. 2001; Hess, Matsumoto et al. 2005; Foster, Forrester et al. 2009). S-nitrosylation is also reversible and two major enzymes have been shown to mediate denitrosylation, the S-nitrosoglutathione reductase (GSNOR), and thioredoxin 1 (Trx1) (Mitchell and Marletta 2005). S-nitrosylation has been demonstrated to regulate the activity of a wide variety of proteins and influence calcium and potassium channel conductivity, protein secretion, enzyme catalysis, cofactor binding and protein-protein interactions. (Xu, Eu et al. 1998; Matsushita, Morrell et al. 2003; Hess, Matsumoto et al. 2005; Morrell, Matsushita et al. 2005; Zimmet and Hare 2006; Qian, Zhang et al. 2010)
In vascular cells, significant sources of ROS are the NADPH oxidases (Nox) that produce superoxide and other reactive oxygen species (Lambeth 2007). Blood vessels have been shown to express Nox1, 2, 4 and more recently Nox5 (Lassegue, Sorescu et al. 2001; Guzik, Chen et al. 2008). The activity of Nox1-4 is broadly regulated by interactions with other subunits. In contrast, Nox5 diverges from this theme and possesses all of the enzymatic machinery required to produce ROS within a single gene. The N-terminus of Nox5 contains 4 calcium-binding EF hand motifs which enable calcium-dependent changes in activity. In addition, Nox5 is regulated by post-translational phosphorylation in response to protein kinase C activation(Fulton 2009). Nox5 was recently identified in both vascular cells (Guzik, Chen et al. 2008; Jay, Papaharalambus et al. 2008; Montezano, Burger et al. 2010) and human blood vessels and its expression is increased in advanced lesions (Guzik, Chen et al. 2008). Aside from phosphorylation, very little is known about the post-translational regulation of Nox5 and it is not yet known whether NO can modify Nox5 activity.
The interaction between superoxide and NO is very rapid and limited only by the diffusion of both molecules (Beckman and Koppenol 1996). The product of this interaction, peroxynitrite (ONOO−) is a potent oxidizing factor and is responsible for the nitration of susceptible tyrosine residues on proteins within close proximity. The consumption of NO by superoxide negates the normal physiological signaling of NO and promotes vascular dysfunction (Cai and Harrison 2000). Thus a balance between NO and superoxide is important for maintaining vascular homeostasis. Disruption of this balance, most commonly in the form of decreased levels of bioavailable NO, strongly correlates with both increased production of ROS and blood vessel dysfunction (Cai and Harrison 2000; Ali, Ketsawatsomkron et al. 2009).
Previous studies have shown that NO can inhibit the activity of Nox2 via S-nitrosylation of its subunits (Selemidis, Dusting et al. 2007) and this provides an additional mechanism for maintaining the balance between NO and superoxide in the vasculature. However, important questions remain unanswered. It is not yet known if NO can modulate the activity of the other Nox enzymes expressed in blood vessels such as Nox5 or whether NO can inhibit Nox enzyme activity by directly S-nitrosylating the catalytic Nox enzyme. Therefore, in the current study we pursued the novel question of whether NO can influence Nox5 activity. We also investigated the rank order potency of NO against other Nox enzymes including Nox1, Nox3 and Nox4, enzymes that have not previously been shown to be regulated by NO. As Nox5 is a self-contained Nox enzyme, we also sought to determine if NO can influence Nox activity by direct S-nitrosylation as opposed to the modification of other regulatory cofactors.
MATERIALS AND METHODS
Cell culture and transfection
COS-7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 100units/ml penicillin, 100mg/ml streptomycin, and 10% fetal calf serum. For transfection, COS-7 cells were seeded and transfected the next day using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Human aortic endothelial cells (HAECs) and human aortic smooth muscle cells (HASMC) were purchased from Cascade Biologics and grown in endothelial cell basal medium-2 (EBM-2, Clonetics) or smooth muscle cell basal medium (SmBM, Clonetics). HEK 293 cell line stably expressing HA-Nox5 was generated by Flp-In™ System (Invitrogen) and grown in complete DMEM medium.
Adenoviral generation and transduction
Adenoviruses encoding the control viruses RFP or HA-Nox5 were generated as described previously (Zhang, Malik et al. 2008). COS-7 cells were seeded and transduced the next day at a multiplicity of infection (MOI) of 30.
NO release
Medium (100μl) containing nitrite and nitrate (primarily NO2−) was precipitated with ethanol to remove proteins and refluxed in sodium iodide/glacial acetic acid to convert NO2− to NO for measurement of the basal NO. NO was measured via NO-specific chemiluminescence after reaction with ozone (Sievers NO analyzer; GE Analytical Instruments, Boulder, CO) as demonstrated previously (Jagnandan, Sessa et al. 2005). Net NO2− from cells transfected with eNOS or iNOS was calculated after subtracting NO2− levels from cells lacking NOS activity (RFP transfected group).
Biotin switch assay
S-nitrosylated Nox5 was detected by using the biotin-switch assay (Jaffrey and Snyder 2001; Forrester, Foster et al. 2009) with some modifications. In brief, 48h after transfection, cells were washed 2 times with cold PBS, and lysates prepared by incubation with HEN buffer containing 250 mM HEPES, 1 mM EDTA, 0.1 mM neocuproine [pH 7. 7]), SDS (2.5% final concentration) and methyl methanethiosulfonate (Sigma-Aldrich) at 50°C for 20 min. Proteins were precipitated with acetone, washed three times with 70% acetone, and mixed with 0.2 mM biotin-HPDP (Pierce) with or without 50mM ascorbate at ambient temperature for 1h. Biotinylated proteins were purified by using streptavidin-agarose beads (Sigma-Aldrich), separated by SDS-PAGE, and immunoblotted with anti-HA antibody. Alternatively biotinylated proteins were detected by non-reducing Western blot using anti-biotin antibody (Cell Signaling).
Measurement of Superoxide and Hydrogen Peroxide
Transfected cells were replated into white tissue culture-treated 96-well plates (Thermo Fisher Scientific) at a density of 5 × 104 cells/well. The cells were incubated at 37°C in phenol-free Dulbecco’s modified Eagle’s medium (Sigma) containing L-012 (400 ×M; Wako) for approximately 10 min prior to the addition of agonists (Daiber, August et al. 2004), and luminescence was quantified over time using a POLARstar luminometer (BMG Labtech). The relative light units(RLU) quantified from the luminescence of L-012 are reflective of changes in the superoxide produced by Nox enzymes (Jagnandan, Church et al. 2007). Increasing concentrations of peroxynitrite (EMD) or SIN-1 (EMD) was added to phenol free DMEM and L-012 chemiluminescence recorded in the presence and absence of SOD (100units/ml). Peroxynitrite was also added to HEK cells stably expressing Nox5 in a dose-dependent manner for 20minutes and L-012 chemiluminescence measured as described. In additional experiments lucigenin (5μM) was employed to corroborate measurements of superoxide obtained with L-012. Superoxide levels were also measured via the cytochrome C assay. Transfected cells were detached and incubated with acetylated cytochrome c (1mg/ml, Sigma) with or without PEG-SOD (100U/ml, Sigma) in Kreb’s buffer at 37°C for 30mins. Cytochrome c reduction was calculated using absorbance at 550 nm corrected for background readings at 540 and 560 nm. Superoxide levels were calculated from the difference between absorbance with or without PEG-SOD as previously described (Kelm, Dahmann et al. 1997; Landmesser, Dikalov et al. 2003; Fink, Laude et al. 2004; Dikalov, Griendling et al. 2007). Hydrogen peroxide was measured using the Amplex Red assay with excitation of 530 to 560 nm and emission detection at 590 nm. Cells were incubated at 37°C with 50 ×M Amplex Red, 0.125 U/ml horseradish peroxidase in phenol-free Dulbecco’s modified Eagle’s medium (Sigma) for 10 min. Relative fluorescent units were calculated after subtraction of control groups (lacZ).
Measurement of Peroxynitrite (ONOO−)
The formation of ONOO− was determined by the ONOO−-dependent oxidation of dihydrorhodamine (DHR) 123 to rhodamine 123 (Kooy, Royall et al. 1994; Song, Wu et al. 2007). Transfected cells were replaced into black tissue culture-treated 96-well plates (Thermo Fisher Scientific) at a density of 5 × 104 cells/well. Medium 200 (phenol red-free) was added containing 5μmol/L DHR 123. After 60-min incubation at 37°C, the fluorescence of rhodamine 123 was measured by excitation 485nm, emission 545 nm using a POLARstar reader. Results are expressed as % DHR oxidation compared to control transfected cells. Nitrotyrosine levels were measured as an index of ONOO− formation by immunoblotting. For analysis of total protein nitrotyrosine levels, cells were harvested 48h after transfection. Samples were immobilized onto nitrocellulose membranes using a Slot Blot apparatus (BioRad). Blocked (5% non-fat milk) membranes were exposed to antibodies raised against nitrotyrosine (Calbiochem) with comparison to a positive control generated from ONOO−-modified BSA (Calbiochem).
Intracellular Ca2+ Measurements
Intracellular calcium was measured by Fluo-4 NW (Invitrogen) as described previously (Church and Fulton 2006), and fluorescence was measured with a PolarSTAR Galaxy plate reader with excitation at 494 nm and emission at 516 nm. Calcium levels were calculated after the addition of ionomycin (1×M) and subtraction of basal fluorescence.
Nox5 Activity Assays
COS-7 cells expressing Nox5 were lysed in a MOPS (30 mM, pH 7.2)-based buffer containing KCl (100 mM), Triton X-100 (0.3%), and protease inhibitors (Sigma). Adherent cells were rocked gently, and the lysis buffer was aspirated and then washed with phosphate-buffered saline (4°C). Remaining fractions were resuspended in the MOPS buffer with 0.3 mM EGTA to remove residual calcium, sonicated at low power and centrifuged at 14,000 rpm (4°C). The supernatant was then aspirated, and the pellet was resuspended in MOPS buffer with mild sonication. The cell-free extract was aliquoted into buffers containing L-012 (400 ×M), 1 mM MgCl2, 100 ×M FAD (Sigma), and 1 mM CaCl2. After a brief period of equilibration with or without agonists, reduced NADPH (Sigma) was injected to a final concentration of 200 ×M, and the production of reactive oxygen species was monitored over time as described previously (Jagnandan, Church et al. 2007).
Western Blot Analysis
Cells were washed twice with phosphate-buffered saline, lysed on ice in 50 mM Tris-HCl, pH 6.8, 2% SDS, 30% glycerol, 6% β-mercaptoethanol, and 0.02% bromphenol blue. Lysates were clarified at 13,000 rpm for 10 min at 4°C and size-fractionated by 10% SDS-polyacrylamide gel electrophoresis and immunoblotted with corresponding antibodies.
MS Analysis
To identify sites of S-nitrosylation, the biotin switch assay was performed as described above on 2.5mg of cell lysate from Nox5-HA-expressing HEK cells treated 250μM CysNO for 30 min. Total Nox5 was immunoprecipitated with anti-HA-agarose (santa crutz), washed, resin-bound protein was trypsinized overnight using 5μg of sequencing-grade trypsin (Promega) at 37 °C. Trypsin was inactivated by addition of 1 mM AEBSF, and biotinylated peptides of Nox5 were precipitated with streptavidin-agarose (Sigma), washed with 4x with 50mM ammonium bicarbonate. Biotinylated peptides were eluted with 10 mM DTT @ 37 °C for 30 min and eluents were alkylated with 20 mM iodoacetamide for 30 min at room temp in the dark. Samples were evaporated to dryness and resuspended in 12 μl of water containing 2% acetonitrile and 1% trifluoroacetic acid. Peptide digests were analyzed using a nanoAcquity UPLC system coupled to a Synapt G2 HDMS mass spectrometer (Waters). 5 ×l of reconstituted peptide was trapped on a 20 ×m × 180 mm Symmetry C18 column (Waters) at 10 ×l/min for 5 minutes in water containing 0.1% FA, and further separated on a 75 ×m × 250 mm column with 1.7 ×m C18 BEH particles (Waters) using a gradient of 5 to 40% ACN/0.1% FA over 60 min at a flow rate of 0.3 ×l/min and a column temp of 45 °C. Samples were analyzed sensitivity mode by data-dependent acquisition using a 0.6 s precursor scan and allowing for MS/MS scans of the top three ions per precursor scan. Raw data was processed using Mascot Distiller and submitted for Mascot search (Matrix Science) against the Swissprot human database containing a reverse decoy database. Mascot search parameters were 10 ppm precursor and 0.04 Da product ion tolerance, with fixed carbidomethyl (C) and variable oxidized (M) and deamidation (NQ). Data was visualized using Scaffold 3 (Proteome Software).
Statistical analysis
Data are expressed as means ± S.E.M. Comparisons were made using two-tailed Student’s t test or analysis of variance (ANOVA) with a post-hoc test where appropriate. Differences were considered as significant at p < 0.05.
RESULTS
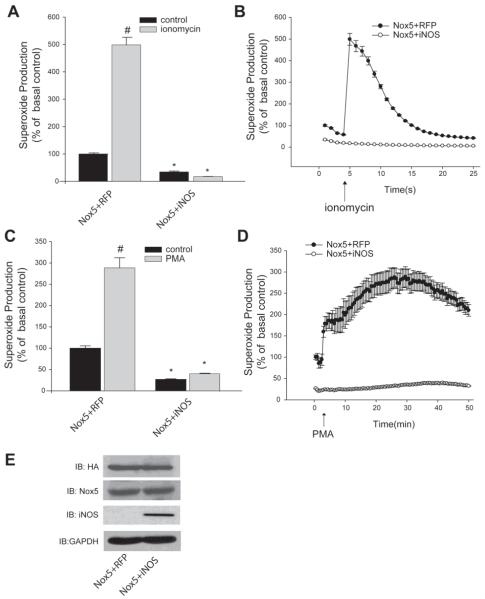
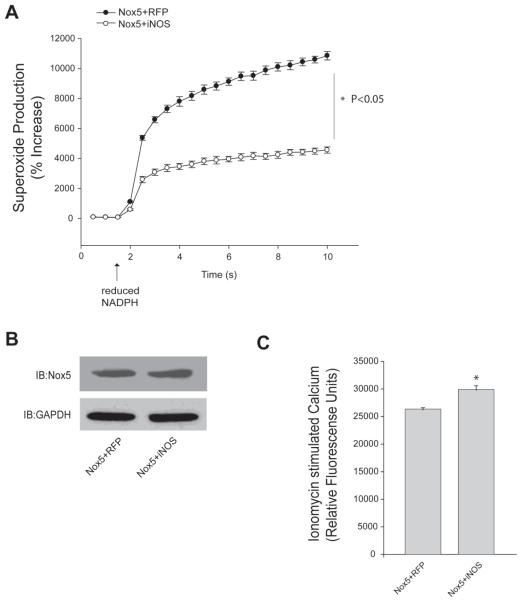
To investigate whether NO can influence Nox5 activity, we co-expressed iNOS with Nox5. We employed iNOS because it produces relatively large amounts of NO within the cell, yet much smaller and more biologically relevant amounts than the typical concentration of NO donor used. In COS-7 cells co-transfected with Nox5 and iNOS, there was substantially less superoxide produced in response to the calcium ionophore, ionomycin compared to that produced from cells expressing Nox5 and a control plasmid, RFP (Figure 1A, B). Maximal superoxide production is shown in panel A and the levels of superoxide over time are shown in B. In addition to ionomycin, PMA is known to stimulate Nox5 activity by increasing its phosphorylation through the activation of PKC pathways (Jagnandan, Church et al. 2007; Serrander, Jaquet et al. 2007), without modifying the level of intracellular calcium (Jagnandan, Church et al. 2007). There was dramatically less superoxide produced in cells expressing both Nox5 and iNOS in response to PMA compared to Nox5 co-transfected with a control plasmid (Figure 1C, D). In addition to stimulated production of superoxide, basal or unstimulated levels of superoxide were also lower in cells expressing both Nox5 and iNOS (Figure 1A-D). Importantly, the co-expression of iNOS did not modify the expression level of Nox5 as detected by either immunoblotting for the HA epitope tag (Figure 1E, upper panel) or using a polyclonal anti-Nox5 antibody (middle panel), iNOS was detected by an anti-iNOS antibody, relative to the level of GAPDH (lower panel). As there was no significant change in the expression of Nox5, these results suggest that the reduced activity of Nox5 is occurring due to a NO-dependent post-translational mechanism.
Figure 1. Endogenous NO suppresses basal and stimulated Nox5 activity.
COS-7 cells were co-transfected with Nox5 and either iNOS or RFP (control) and stimulated with (A-B) ionomycin (1μM) or (C-D) PMA (100nM). Production of superoxide was determined via chemiluminescence and expressed as % of control (means ± S.E., n =6 * p<0.05 versus RFP, # p<0.05 versus ionomycin or PMA). Maximal superoxide is shown in the bar graph (left) and superoxide recorded over time in the line graph (right). (E) Expression of Nox5 was detected by Western blot with anti-HA and total Nox5 polyclonal antibody, iNOS expression was detected by iNOS antibody GAPDH was used as loading control.
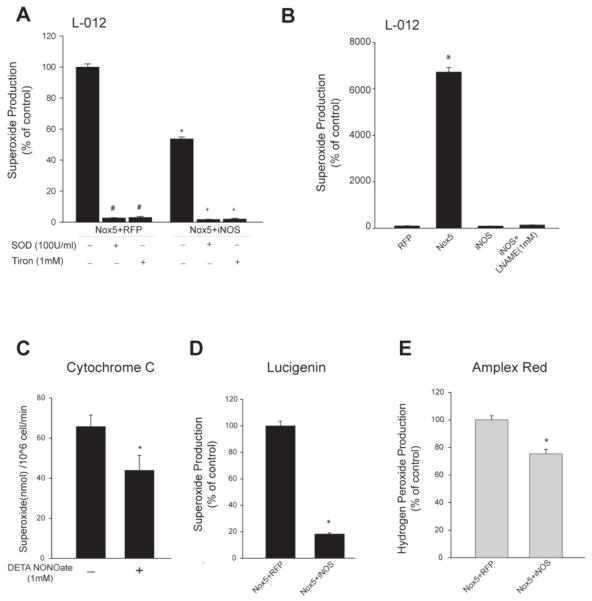
To address whether L-012 is specifically measuring superoxide, SOD (100U/ml) or Tiron (1mM) were added to cells co-expressing Nox5 and RFP or Nox5 and iNOS. The levels of superoxide detected with L-012 were abolished by the presence of the superoxide scavengers (Figure 2A). The complete loss of L-012 signal in the presence of SOD suggests that it does not detect hydrogen peroxide which is also a significant product of Nox5 (Serrander, Cartier et al. 2007). Indeed, using amplex red to selectively detect hydrogen peroxide, we observed a significant increase in hydrogen peroxide levels in the presence of SOD (Supplemental Figure1) that was not detected with L-012 (Figure 2A). The specificity of L-012 for superoxide (Daiber, August et al. 2004) is further supported by previous reports showing that Nox4, which emits only hydrogen peroxide, does not increase L-012 chemiluminescence (Serrander, Cartier et al. 2007; Chen, Pandey et al. 2011). To determine if L-012 can detect reactive nitrogen species (NO or ONOO−) produced from iNOS, L-012-chemiluminescence in cells expressing iNOS alone was examined in the presence and absence of the NOS inhibitor L-NAME(1mM). There is no change in L-012-mediated chemiluminescence in cells expressing iNOS versus a control plasmid (Figure 2B). Superoxide was also detected from Nox5 by measuring the reduction of cytochrome c to ferrocytochrome c (Fe2+) (Dikalov, Griendling et al. 2007; Chen, Pandey et al. 2011). Cytochrome c reduction was significantly decreased in cells exposed to NO (Figure 2C). Lucigenin was also employed to measure superoxide. Although results with lucigenin can be complicated by the potential for redox cycling, at low concentrations it is considered a reliable and sensitive method for the measurement of superoxide (Liochev and Fridovich 1997; Dikalov, Griendling et al. 2007). As detected by lucigenin (5μM), Nox5 activity was reduced by the co-expression of iNOS (Figure 2D). Nox5 also produces hydrogen peroxide (H2O2) and the measurement of H2O2 levels using the Amplex Red assay also revealed a significant decrease in H2O2 production in the presence of iNOS (Figure 2E). In media alone, the addition of authentic peroxynitrite or SIN-1 resulted in a dose-dependent increase in L-012 chemiluminescence at concentrations above 10 and 50 μM, respectively. Increases in L-012 chemiluminescence to SIN-1 were abolished by SOD, whereas increases to peroxynitrite were sensitive to SOD at concentrations below 2mM (Supplemental Figure 2A, B).
Figure 2. Nox5-derived superoxide and hydrogen peroxide are reduced by NO.
(A) 48hr after transfection in COS-7 cells, SOD(100U/ml) or Tiron(1mM) were added to the cells. Superoxide was measured by chemiluminescence and expressed as % of control. (B) Superoxide release in COS-7 cells expressing RFP, Nox5 or iNOS in the presence and absence of L-NAME (1mM) (C) COS-7 cells expressing Nox5 were treated with or without DETA NONOate (1mM), and superoxide was measured by cytochrome c reduction. Data is expressed as nmol/10^6 cells/min. (D) Superoxide was measured in COS-7 cells expressing Nox5 with or without iNOS using lucigenin-chemiluminescence. (E) H2O2 was measured by Amplex Red 48hrs after co-transfection. Data is expressed as % of control (means ± S.E., n =6 * p<0.05 versus Nox5+RFP).
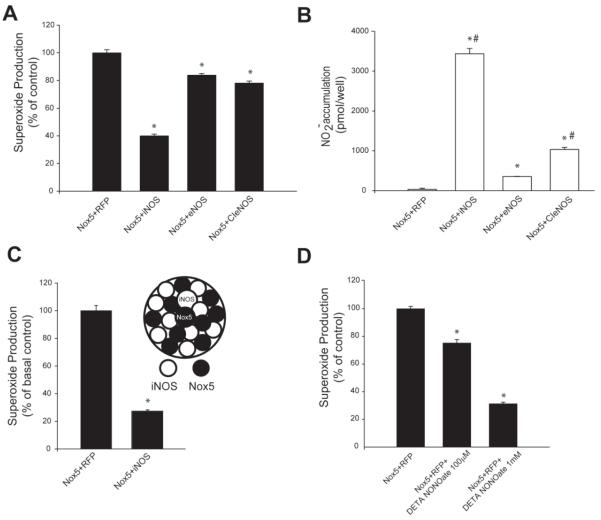
To determine whether the amount of NO produced is important for the inhibition of Nox5 activity, COS-7 cells were co-transfected with either iNOS, eNOS or a calcium insensitive form of eNOS (CI-eNOS) that produce different amounts of NO (Church and Fulton 2006). As shown in Figure 3A, Nox5 activity was significantly reduced by all three forms of nitric oxide synthase (NOS) with iNOS being the most potent followed by CI-eNOS and wt eNOS. The degree of Nox5 inhibition was proportional to the amount of NO produced by each NOS with greater NO production causing the most inhibition (Figure 3A, B). To exclude a non-specific interaction between a NOS enzyme and Nox5, we first expressed iNOS and Nox5 in separate cells and following transfection, cells expressing iNOS or Nox5 were detached, mixed together in equal proportions, replated and the relative superoxide production measured 24h later. As shown in Figure 3C, we found that despite being formed in separate cells, iNOS-derived NO was able to potently reduce Nox5 activity in adjacent cells and these results support the concept that NO itself is the crucial determinant of reduced Nox5 activity. Exogenous NO was also an effective inhibitor of Nox5 activity. COS-7 cells expressing Nox5 were exposed to the NO donor, DETA NONOate (100μM and 1mM) for 24h and superoxide production was measured. As shown in Figure 3D and consistent with previous results with co-transfected NOS, we found that exogenous NO can elicit dose-dependent inhibition of Nox5. We also assessed whether nitrite, a primary metabolite of NO in aqueous media (Lewis and Deen 1994) could influence Nox5 activity. As shown Supplemental Figure 5A, progressively higher concentrations of nitrite (1-1000μM) did not influence Nox5 activity.
Figure 3. Inhibition of Nox5 is proportional to the amount of diffusible NO.
(A) COS-7 cells were co-transfected with Nox5 and either iNOS, eNOS or a calcium-insensitive eNOS (CI-eNOS) and superoxide was measured by L-012 chemiluminescence. (B) NO release was measured by chemiluminescent detection of NO2−. (C) Superoxide release from cells expressing either iNOS or Nox5. COS-7 cells were transfected separately with iNOS and Nox5, detached, cells mixed together and superoxide production measured 24h later. (D) COS-7 cells expressing Nox5 were exposed to the NO donor, DETA NONOate (100μM and 1mM) and 24h later superoxide measured. Superoxide release is expressed as % of control (means ± S.E., n =6 * p<0.05 versus Nox5+RFP). NO release is presented as pmol/well (means ± S.E., n =6 * p<0.05 versus Nox5+RFP, # p<0.05 versus Nox5+eNOS)
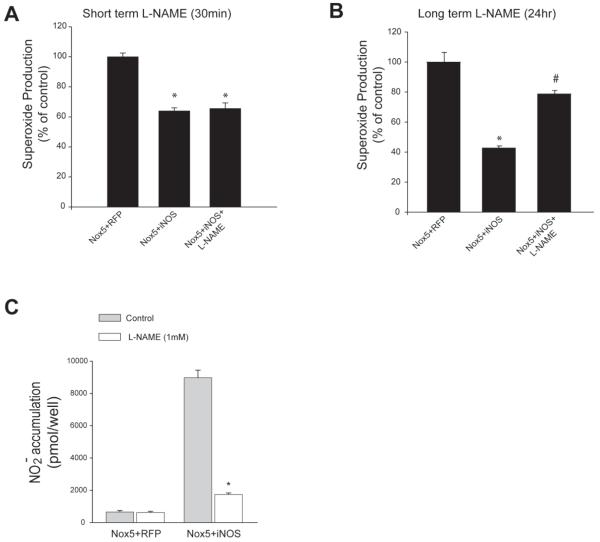
Our next goal was to determine whether the decreased levels of superoxide measured from cells expressing Nox5 and iNOS was due to an actual reduction in superoxide production or simply a reduction in the ability to accurately measure superoxide levels i.e. a competition between the binding of superoxide:NO and superoxide:L-012. COS-7 cells were co-transfected with Nox5 and either iNOS or RFP (control) and 0.5 hours prior to measuring superoxide, iNOS activity was abrogated with the NOS-inhibitor, L-NAME. As shown in Figure 4A, the acute (0.5h) inhibition of iNOS with L-NAME was not able to reverse the reduced activity of Nox5. To assess whether more chronic inhibition of iNOS is able to reverse or negate inhibition of Nox5 we co-transfected Nox5 with iNOS and added L-NAME to select wells 24h prior to measurement of superoxide. As shown in Figure 4B, longer term inhibition of iNOS was able to reverse the inhibition of Nox5 activity. At the concentration employed, L-NAME efficiently inhibited NO production from iNOS (Figure 4C). To exclude a non-specific effect of L-NAME in the absence of iNOS, cells expressing Nox5 were treated with or without L-NAME and superoxide levels measured. L-NAME did not have a significant effect on superoxide levels (Supplemental Figure 5B).
Figure 4. Inhibition of Nox5 can be reversed by persistent, but not acute inhibition of NO production.
(A) COS-7 cell were co-transfected with Nox5 and either iNOS or RFP (control). 48h after transfection, cells were treated with L-NAME (1mM) for 1h and superoxide was measured (B) L-NAME (100μM) was added for 24h following transfection. Data is expressed as % of control (means ± S.E., n =6 * p<0.05 versus Nox5+RFP, # p<0.05 versus Nox5+iNOS). (C) After 48hr transfection, cells were treated with L-NAME(1mM), NO release was measured by chemiluminescence (means ± S.E., n =6 * p<0.05 versus no L-NAME).
To determine whether iNOS-derived NO can directly modify the catalytic activity of Nox5 and whether Nox5 subcellular location is important for the inhibitiory actions of NO, we employed a partially purified enzyme activity assay. The addition of reduced NAPDH initiated robust superoxide production from the extracts of cells containing Nox5 (Figure 5A). In cells co-expressing iNOS and Nox5 there was significantly less superoxide produced despite surplus levels of calcium, FAD and NADPH. Similar to our observation in cells, the reduction in enzymatic activity occurred without a change in the relative expression of Nox5 (Figure 5B). As Nox5 activity has an absolute requirement for calcium (Jagnandan, Church et al. 2007), we also measured the level of intracellular calcium in transfected COS-7 cells. As shown in Figure 5C, there was no reduction in intracellular calcium in the presence of iNOS and these results further suggest a direct effect of NO on Nox5 activity rather than other pathways.
Figure 5. NO reduces Nox5 enzyme activity in a cell-free activity assay but does not reduce intracellular calcium levels.
(A) COS-7 were co-transfected with Nox5 and either iNOS or RFP (control). Cells were lysed and cell-free activity of Nox5 was measured in the presence of CaCl2 (1mM), NADPH (200μM) and FAD (100μM). Superoxide levels were determined via L-012 chemiluminescence and expressed as % increase above unstimulated levels (means ± S.E., n =6 * p<0.05 versus RFP). (B) Expression of Nox5 was determined by Western blot with anti-HA and GAPDH was used as loading control. (C) Intracellular Ca2+ was measured after addition of ionomycin (1μM) in transfected COS-7 cells using the Fluo-4. Results are presented as mean ± S.E. (n = 8 * p<0.05 versus RFP).
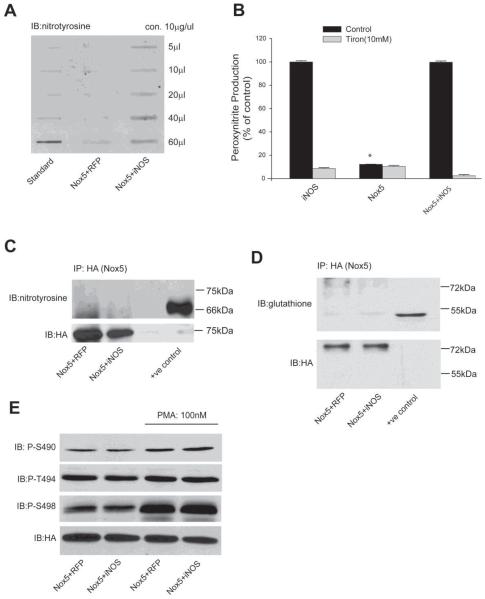
As the reduced activity of Nox5 is most likely due to post-translational modifications, we next investigated whether NO alters the tyrosine nitration, phosphorylation or glutathiolation of Nox5. COS-7 cells were co-transfected with Nox5 and either iNOS or RFP and 48h post-transfection the amount of tyrosine nitration was determined in total cell lysates using an anti-nitrotyrosine antibody. As shown in Figure 6A, cells expressing both Nox5 and iNOS had higher levels of tyrosine nitration. Indeed, more direct measurements of ONOO− using DHR revealed that cells expressing iNOS produced significantly higher levels of ONOO− compared to cells transfected with Nox5 and a control plasmid. Interestingly, there was no significant increase in ONOO− levels in cells expressing both iNOS and Nox5 (Figure 6B), and changes in the relative expression of iNOS and Nox5 revealed levels of ONOO− that were proportional to iNOS expression (Supplemental Figure 4). To determine whether DHR fluorescence was reflective of ONOO− concentrations, cells were incubated with Tiron (Figure 6B) or uric acid (Supplemental Figure 6). DHR fluorescence was reduced to background levels in the presence of both antioxidants. To assess whether ONOO− can directly influence Nox5 activity, cells expressing Nox5 were exposed to increasing doses of ONOO−. As shown in Supplemental Figure 3, the addition of ONOO− did not decrease Nox5 activity and at higher concentrations an increase in L-012 chemiluminescence was observed.
Figure 6. Endogenously produced NO does not alter the nitration, phosphorylation or glutathiolation of Nox5.
(A) COS-7 cells were co-transfected with Nox5 and either iNOS or RFP, and 48h post-transfection, total protein nitration was measured via slot blot and anti-nitrotyrosine immunoblotting. (B) Peroxynitrite (ONOO−) was measured by DHR 123 fluorescence in the presence or absence of the antioxidant Tiron (10mM). Results are presented as % of control (means ± S.E., n =6 * p<0.05 versus iNOS). (C-D) Nox5 was immunoprecipitated from COS-7 cells co-transfected with iNOS or RFP and immunoblotted for nitrotyrosine (C) or glutathione (D). Nitrated BSA (bovine serum albumin) was used as a positive control for nitrotyrosine and oxidized actin from HeLa cell extracts for glutathiolated proteins (Virogen). (E) Nox5 was co-transfected with iNOS or RFP and 48h after transfection, cells were treated with vehicle or PMA (100nM) for 30mins, and the phosphorylation of Nox5 on S490, T494 and S498 was detected by phosphorylation state specific antibodies. Total Nox5 expression was detected using anti-HA antibody. Results are representative of at least 3 separate experiments.
To assess whether Nox5 is tyrosine nitrated, we immunoprecipitated Nox5 from cells co-expressing Nox5 and either control protein (RFP) or iNOS and immunoblotted for nitrotyrosine. As shown in Figure 6C, we did not observe tyrosine nitration of Nox5 despite robust detection of a commercially purchased positive control (nitrated BSA, right lane) and total Nox5 (lower panel). We performed similar experiments to assess whether Nox5 was glutathiolated and found no evidence for this modification despite detection of a positive control (Figure 6D). As phosphorylation is an important post-translational modification that has been established to potently regulate Nox5 activity and calcium sensitivity (Jagnandan, Church et al. 2007), we also probed for changes in the phosphorylation of serine 490, threonine 494 and serine 498. As shown in Figure 6E, the co-expression of iNOS did not modify the baseline or PMA-stimulated phosphorylation of Nox5.
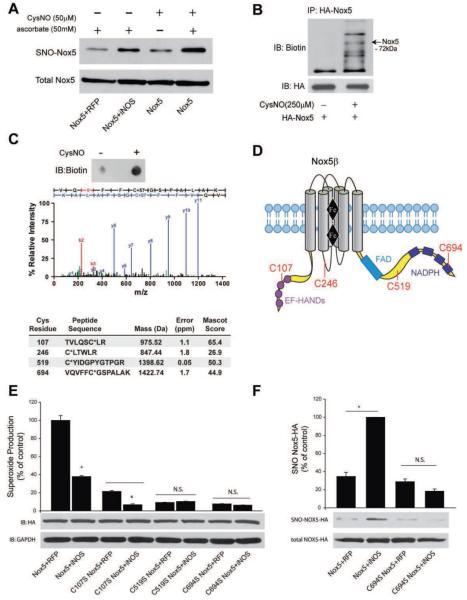
To investigate the possibility of Nox5 S-nitrosylation, the biotin switch assay was performed on Nox5-transfected COS-7 cells which co-expressed iNOS or were treated with CysNO. In cells co-expressing Nox5 and iNOS, there was evidence for significantly more S-nitrosylation of Nox5 versus control transfected cells (Figure 7A, upper panel versus total Nox5 in the lower panel). The cell-permeable transnitrosylating agent CysNO also increased Nox5 S-nitrosylation versus control, and assay specificity was confirmed by ascorbate-dependence (Figure 7A).
Figure 7. iNOS increases the S-nitrosylation of Nox5.
COS-7 cells were transfected with Nox5 and either control (RFP) or iNOS. 48h post-transfection, cells were either not treated or treated with CysNO and the S-nitrosylation of Nox5 determined via the biotin switch assay in the presence and absence of asorbate. Biotinylated proteins were concentrated by streptavidin-agarose and immunoblotted for HA (SNO-Nox5, upper panel) versus immunoblotting for HA in total cell lysates (total Nox5, lower panel). (B) COS-7 cells expressing Nox5 were exposed to CysNO, lysed, the biotin switch assay performed and Nox5 immunoprecipitated via the HA-tag. Immune complexes were immunoblotted with anti-biotin antibody (upper panel) and then reprobed for total Nox5 (HA, lower panel) under non-reducing conditions. (C) MS/MS spectrum of Cys694-carbidomethylated tryptic peptide isolated from HA-Nox5 expressing COS-7 after CysNO treatment (see Methods). The b- and y-series ions are in red and blue, respectively.) Summary of Cys-containing Nox5 peptides identified from CysNO-treated COS-7 following biotin switch assay and LC-MS/MS analysis, including mass and error of parent ion, and Mascot ion score. Asterix indicates the site of carbidomethylation. (D) Geographical illustration of the sites of Nox5 S-nitrosylation. (E) Relative production of superoxide from Nox5 WT, C107S, C519S and C694S in the presence and absence of iNOS. Results are presented as % of control (means ± S.E., n =6 * p<0.05 versus RFP). (F) The S-nitrosylation of Nox5 WT and C694S was detected via the biotin switch assay. Biotinylated proteins were concentrated by streptavidin-agarose and immunoblotted for HA (SNO-Nox5, upper panel) versus HA in total cell lysates (total Nox5, lower panel). The relative densitometry of SNO:total HA is shown as a % of the maximum (means ± S.E., n =3 * p<0.05).
Nox5β contains 15 cysteine residues, 9 of which are predicted to be localized within the intracellular regions of Nox5. To determine the sites of S-nitrosylation, Nox5-expressing COS-7 cells were treated with vehicle or CysNO and lysates subjected to the biotin switch assay followed by immunoprecipitation of Nox5. Non-reducing Western blot analysis of a fraction of the precipitated immune complexes confirmed an increase in biotin incorporation into Nox5 following CysNO treatment (Figure 7B). The identities of the other proteins in the anti-biotin Western blot are not known, but their presence is likely due to a combination of factors including other proteins co-precipitating, the non-reducing conditions necessary to observe incorporated biotin and the specificity of the anti-biotin antibody. The remaining Nox5-bound immune complexes were digested on-resin with trypsin, and the preservation of biotin in the released peptide fragments was confirmed by dot blot analysis (Figure 7C, top panel). The free biotinylated peptides were further precipitated with streptavidin-agarose and eluted by reduction of the Cys-S-S-biotin disulfide. After alkylation with iodoacetamide, peptides were sequenced by 1D-LC-MS/MS. Five Nox5 peptides were identified from CysNO-treated cells, including four Cys-containing peptides, demonstrating a high degree of assay specificity (Figure 7C, lower panel). Of these cysteine residues, 3 (C107, C519, C694) are located within the functional cytoplasmic regions of Nox5 (Figure 7D). Additional spectra for the other peptides are shown in Supplemental Figure 7.
To identify the functional significance of identified cysteine residues, we first looked for conservation in the Nox5 sequences from other species and found that C107, 519 and C694 are highly conserved. Many studies have shown that the cytoplasmic C-terminus of the Nox enzymes is important for electron flow and can influence the extent of ROS production (Nisimoto, Jackson et al. 2010; von Lohneysen, Noack et al. 2010; Takac, Schroder et al. 2011). We have also shown that phosphorylation of Nox5 within this region is important for overall enzyme activity and in particular, calcium-sensitivity (Jagnandan, Church et al. 2007). Thus our a priori bias was towards sites that may have more functional relevance versus C246 which exists on a transmembrane loop and may potentially be of less significance. Mutation of each of these Cys residues resulted in a reduction of Nox5 activity (Figure 7E). The C107S mutant exhibited a substantial reduction in Nox5 activity. Despite the reduced activity, ROS production remained sensitive to inhibition by iNOS, suggesting that additional sites of S-nitrosylation may also be of importance in the NO-dependent regulation of Nox5 activity. In contrast, mutation of C519 or C694 dramatically reduced Nox5 activity, and iNOS had no additional effect on activity of these mutants (Figure 7E). C694 is predicted to reside within the NADPH-binding domain of Nox5, and the S-nitrosylation of Nox5 in the presence of iNOS was abolished by mutation of this site (Figure 7F). Thus, while CysNO may S-nitrosylate at least four cysteine residues on Nox5, iNOS likely inhibits Nox5 via S-nitrosylation of the catalytically essential C694.
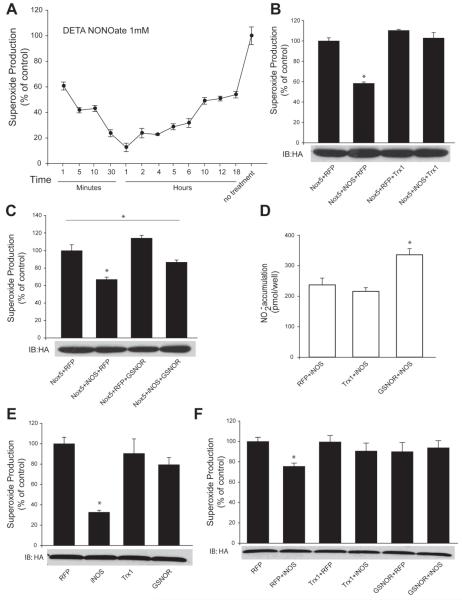
As S-nitrosylation is a reversible modification, the next experiments investigated the timing of the reversibility of NO-dependent inhibition of Nox5. In cells expressing Nox5, the NO donor DETA NONOate was applied and Nox5 activity was measured over time as shown in Figure 8A. One minute after exposure to the NO donor, the activity of Nox5 was reduced by approximately 40%. Maximal inhibition was seen at 1h (85%) and the activity of Nox5 then proceeded to recover over a period of several hours. Several enzymes have been shown to accelerate the de-nitrosylation of S-nitrosothiols, in particular thioredoxin (Trx1) and GSNO reductase (GSNOR). In cells expressing both Nox5 and iNOS, the addition of Trx1 negated the ability of iNOS to reduce Nox5 activity (Figure 8B). The addition of GSNOR blunted, but did not prevent, the iNOS mediated reduction in Nox5 activity in COS-7 cells (Figure 8C). We also measured nitric oxide production in these cells to ensure that Trx1 and GSNOR do not modify iNOS function and NO release. As shown in Figure 8D, Trx1 did not modify iNOS activity. Surprisingly however, GSNOR significantly increased iNOS-derived NO. We also investigated whether Trx1 and GSNOR can influence Nox5 activity in the absence of NO. As shown in Figure 8E, in cells co-expressing Nox5 and Trx1 or Nox5 and GSNOR there was no significant difference in superoxide production versus cells expressing Nox5 and a control plasmid (RFP). Lastly in a stable cell line the reduced activity of Nox5 in the presence of iNOS was absent in cells co-expressing either Trx1 or GSNOR (Figure 8F).
Figure 8. NO-inhibition of Nox5 is reversible and is prevented by the denitrosylases Trx1 and GSNOR.
(A) COS-7 cells expressing Nox5 were exposed to 1mM DETA NONOate and superoxide production was monitored over time. (B-C) COS-7 cells were co-transfected with Nox5, RFP or iNOS with or without the denitrosylases, thioredoxin1 (Trx1) or GSNO reductase (GSNOR). Superoxide production is expressed as % of control (means ± S.E., n =6 * p<0.05). (D) Measurement of NO release in cells expressing iNOS with or without Trx1 and GSNOR. (E) Superoxide release from HEK cells stably expressing Nox5 with or without either iNOS, Trx1 or GSNOR. (F) HEK cells stably expressing Nox5 were co-transfected with iNOS and either control (RFP), Trx1 or GSNOR. Data are presented as means ± SE (n = 4-6 * p<0.05).
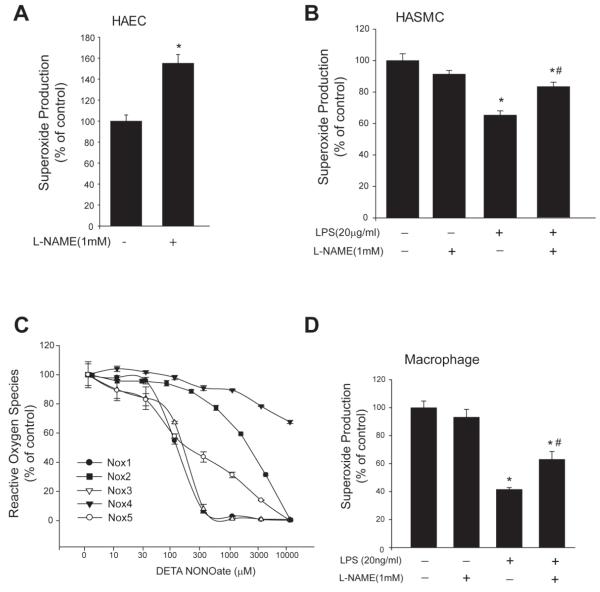
To determine the physiological significance of the S-nitrosylation of Nox5, we next investigated whether endogenous NO, produced in human aortic endothelial cells (HAEC, eNOS) or human aortic smooth muscle cells (HASMC) following LPS stimulation, can influence Nox5 activity. HAECs were transduced with Nox5 adenovirus and cells were treated with L-NAME for 24h and superoxide was measured. In Nox5-transduced HASMCs, LPS (20μg/ml) was used to induce iNOS expression and cells were treated with or without L-NAME for 24h. As shown in Figure 9A, inhibition of endogenous NO in HAEC with L-NAME significantly potentiates superoxide production from Nox5. Similarly in HAVSMC, Nox5 activity is reduced by LPS treatment and increased by the NO synthase inhibitor, L-NAME (Figure 9B). To assess whether NO can inhibit other isoforms of Nox that are expressed in vascular tissue, we expressed Nox1, Nox3, Nox4 in COS7 cells and stimulated Nox2 activity with PMA in HL60 cells, and assessed the relative degree of inhibition of ROS production in response to increasing doses of a NO donor. NO significantly reduced the activity of Nox 1-5 isoforms in a dose-dependent manner (Figure 9C). Nox1 was the most sensitive Nox isoform to NO, closely followed by Nox3 and Nox5 was intermediate followed by Nox2. Interestingly, the activity of Nox4 was the most resistant to the inhibitory actions of NO exhibiting only 35% inhibition at the highest NO concentration versus 100% inhibition for the other isoforms. In isolated mouse peritoneal macrophages which primarily express Nox2, the addition of LPS reduced superoxide production and this was reversed with L-NAME (Figure 9D).
Figure 9. Endogenous NO from HAEC, HASMC or murine macrophages inhibits Nox5 or Nox2-dependent superoxide production and relative susceptibility of Nox isoforms to NO.
(A) HAECs were transduced with Nox5 adenovirus (MOI of 5) and 24h later treated with or without L-NAME (1mM) for an additional 24h and superoxide was measured. (B) HASMCs were transduced with Nox5 adenovirus (MOI of 5) for 24h and LPS (20μg/ml) was used to induce iNOS production. Cells were treated with or without L-NAME (1mM) for an additional 24h and superoxide was measured by chemiluminescence. (C) COS-7 cells were transfected with different Nox isoforms; Nox4 and Nox5 were transfected alone and Nox1 and Nox3 were co-transfected with NoxO1 and NoxA1. Nox2 activity was stimulated with PMA(100nM) in HL60 cells. Cells were exposed to the NO donor, DETA NONOate (1mM) for 24h and superoxide production (HL60, COS-7 cells transfected with Nox1, 3 and 5) and hydrogen peroxide (Nox4) was measured over time and expressed as % of control (means ± S.E., n =6). (D) Peritoneal macrophages were exposed to LPS(20μg/ml) with or without L-NAME(1mM) for 24h. Superoxide was measured using L-012 mediated chemiluminescence. Data is expressed as % of control (means ± S.E., n =6 * p<0.05 versus control, # p<0.05 versus LPS alone).
DISCUSSION
In the current study, we show that both exogenous and endogenously produced NO can reduce the activity of Nox5 in a dose-dependent manner. Furthermore, blockade of endogenously produced NO in human endothelial and smooth muscle cells increases Nox5-dependent ROS production. The inhibitory effects of NO are seen under basal conditions and following stimulation with both calcium-dependent and independent agonists. Reduced activity is also observed in an isolated enzyme activity assay and is independent of changes in Nox5 expression, intracellular calcium levels or insufficient concentrations of NADPH or FAD. The relative phosphorylation of Nox5 on Ser490, 498 and Thr494 was unchanged by exposure to NO and tyrosine nitration and glutathiolation of Nox5 were not detected. In contrast, we found that Nox5 was robustly S-nitrosylated. Consistent with this, NO-dependent inhibition was reversible and was prevented by co-expression of the denitrosylating enzymes, GSNOR and Trx1. Indeed, MS analysis revealed that Nox5 is S-nitrosylated on at least 4 sites, C107, C246, C519 and C694. Of these sites, mutation of the highly conserved C694, dramatically reduced Nox5 activity and NO sensitivity and also reduced the S-nitrosylation of Nox5. In addition to Nox5, we found that different Nox isoforms exhibited a range of sensitivities to NO, with Nox1 and Nox3 being the most sensitive followed by Nox5, Nox2 and that Nox4 was largely resistant to NO-dependent inhibition. We conclude that NO potently suppresses the activity of Nox5 via a reversible increase in S-nitrosylation.
The ability of NO to suppress Nox5 activity is consistent with previous reports showing that the activity of Nox2 is suppressed by NO (Clancy, Leszczynska-Piziak et al. 1992; Fujii, Ichimori et al. 1997; Lee, Miura et al. 2000; Selemidis, Dusting et al. 2007). Indeed, we confirmed these findings with data that the activity of Nox2 (in HL60 and macrophages) was indeed regulated by NO. However, the prior studies have identified the regulatory subunits of Nox2 as the target of S-nitrosylation and not Nox2 itself (Fujii, Ichimori et al. 1997; Selemidis, Dusting et al. 2007). Nox5 differs from the other Nox isoforms, including Nox2, in that all of the elements required to produce superoxide are contained within the single enzymatic unit (Fulton 2009). Accordingly, the ability of NO to reduce Nox5 activity without a change in protein expression is indicative of either changes in the availability of substrates or post-translational modifications. In an isolated enzyme activity assay with surplus calcium, NADPH and FAD, the activity of Nox5 continued to be reduced by NO and this data suggests that diminished access to co-factors or substrates or Nox5 subcellular location cannot account for the loss of activity. By exclusion, we reasoned that there must be changes in the post-translational regulation of Nox5. We found no change in phosphorylation or detectable tyrosine nitration or glutathiolation of Nox5. This data is consistent with previous studies who have found no role for ONOO− in mediating NO-dependent inhibition of Nox2 (Fujii, Ichimori et al. 1997; Selemidis, Dusting et al. 2007).
In cells expressing iNOS there was significant production of ONOO− which was detected by both DHR fluorescence and indirectly by increased tyrosine nitration of total cellular proteins. An unexpected observation was that the co-expression of Nox5 with iNOS did not lead to the detection of additional ONOO−. This was surprising given that the products of both enzymes react avidly when in close proximity (Thomas, Liu et al. 2001). One possible explanation could be a physical separation or compartmentalization of Nox5 within membranes of the ER or plasma membrane that excludes an interaction between superoxide and nitric oxide. However, this is unlikely as we have compelling evidence that NO can directly access and modify Nox5 via S-nitrosylation which suggests that there is sufficient NO or S-nitrosothiols in the vicinity. In addition, NO produced in separate cells is capable of diffusing to and penetrating adjacent cells to inhibit Nox5. Alternatively, it is possible that the stoichiometry of NO and superoxide is not sufficiently favorable to elicit a significant change in the amount of detectable ONOO− produced endogenously from iNOS (Jourd’heuil, Jourd’heuil et al. 2001). To address this relationship, we also performed a dose-response with lower amounts of transfected iNOS together with fixed amounts of Nox5 and found that the production of ONOO− was in direct proportion to the level of iNOS expression and unaffected by the presence of Nox5. While the interaction between NO and superoxide is well characterized in in vitro biochemical assays (Espey, Thomas et al. 2002; Zielonka, Sikora et al. 2010), the interaction of NO with Nox-derived superoxide is, by contrast, poorly understood. Genetic deletion of Nox2 has been shown to reduce overall tyrosine nitration (Kawano, Kunz et al. 2007) and increased expression of Nox1 increases tyrosine nitration (Chiera, Meccia et al. 2008) which suggests that Nox-derived superoxide can be important in the formation of ONOO−. However, the majority of experiments in support of a role of Nox enzymes in ONOO− formation have been performed in vivo and thus could be influenced by other variables. In addition to iNOS, Nox5 did not modify the level of ONOO− produced from eNOS (data not shown) and previous studies have shown that Nox5-derived superoxide does reacts with eNOS-derived NO and prevents both cGMP production and relaxation (Zhang, Malik et al. 2008). A further interpretation of our data is that the probe employed to detect ONOO− levels is not sensitive to ONOO− formed by the interaction of Nox-derived superoxide and NO. The functional significance of ONOO− on Nox5 activity was also investigated and ONOO−, even at high concentrations, did not significantly reduce the activity of Nox5. This data suggests that even though iNOS and eNOS produce increased levels of peroxynitrite, the most likely mediator of reduced Nox activity is NO.
In contrast to the other posttranslational modifications studied, both endogenous and exogenous NO induced a robust S-nitrosylation of Nox5. S-nitrosylation has been compared to phosphorylation in part because it is a reversible post-translational modification that alters protein function (Hess, Matsumoto et al. 2005). We observed a maximal effect of NO on Nox5 activity within 1h which was steadily reversed over a period of hours. This relatively slow recovery is consistent with the time course of GSNO decay in endothelial cells (Zeng, Spencer et al. 2001). Indeed, the rate of denitrosylation of S-nitrosylated proteins is variable and some substrates display significant stability in the presence of reducing agents which may suggest alternative mechanisms of NO removal (Paige, Xu et al. 2008). S-nitrosoglutathione reductase (GSNOR) or alcohol dehydrogenase 5 (ADH5) was one of the first enzymes shown to facilitate the removal of NO from S-nitrosylated glutathione and the loss of GSNOR leads to the accumulation of S-nitrosylated proteins (Jensen, Belka et al. 1998; Liu, Hausladen et al. 2001). In addition, thioredoxin 1 (Trx1) has been shown to facilitate the denitrosylation of both stable S-nitrosylated proteins (Kahlos, Zhang et al. 2003; Mitchell and Marletta 2005) as well as a variety of other substrates (Benhar, Thompson et al. 2010). We found that co-expression of the denitrosylases, GSNOR and Trx1 blunted the ability of iNOS to suppress Nox5 activity. Importantly, when expressed alone, GSNOR and Trx1 did not reduce the activity of iNOS indicating that the improved performance of Nox5 in the presence of GSNOR and Trx1 was not related to a non-specific reduction in the amount of NO produced. Similarly, GSNOR and Trx1 did not modify Nox5 activity in the absence of iNOS. Collectively these results suggest that GSNOR and Trx1 promote the denitrosylation of Nox5. An interesting and unexpected finding was that GSNOR significantly potentiated the activity of iNOS. To our knowledge, this has not been previously reported and the loss of GSNOR in mice does not appear to alter overall NO biosynthesis (Que, Liu et al. 2005). It is not yet known if the effects of GSNOR on iNOS are due to increased catalytic activity, stability or increased expression or whether this effect is specific for iNOS versus other NOS enzymes. Certainly the increased production of NO could explain the reduced ability of GSNOR to restore Nox5 activity relative to Trx1, which did not modify iNOS activity. We also observed that supplemental nitrite did not modify Nox5 activity which suggests that it must need to be converted to NO first before it can influence Nox function or perhaps that higher concentrations are necessary.
The mechanism by which NO and S-nitrosylation inhibits Nox5 activity is not yet known. The weight of evidence suggests that it is not an indirect effect on other variables that can impact Nox function and instead is likely to be due to the direct S-nitrosylation of the enzyme. Indeed, MS analysis of S-nitrosylated Nox5β revealed 4 target cysteine residues out of a total of 15, C107, C246, C519 and C694. All of these cysteine residues are well conserved in the reported sequences of Nox5 from different Nox isoforms (Nox1-4). However, there is no conservation of C107, C246 and C519 in other Nox species. In contrast, C694 is highly conserved (Kawahara, Quinn et al. 2007) and is equivalent to C531 in Nox1, C537 in Nox2, C535 in Nox3 and C547 in Nox4. C694 is predicted to lie within the NADPH binding region of Nox5 and other Nox isoforms (Fulton 2009) and possibly could interfere with NADPH binding. Mutation of C694 to serine, reduced the S-nitrosylation of Nox5 and severely diminished Nox5-dependent ROS production, suggesting that C694 is indeed critical for Nox enzymatic activity. Mutation of C107 reduced overall ROS production but did not confer protection from NO-mediated decreases in Nox5 activity. Many studies have shown that the cytoplasmic C-terminus of the Nox enzymes is important for electron flow and can influence the extent of ROS production (Nisimoto, Jackson et al. 2010; von Lohneysen, Noack et al. 2010; Takac, Schroder et al. 2011). We have also shown that phosphorylation of Nox5 within this region is important for overall enzyme activity and in particular, calcium-sensitivity (Jagnandan, Church et al. 2007). Similar to C694, loss of C519 severely reduced baseline Nox5 activity and further decreases in activity in the presence of NO were not observed. This site is predicted to lie between the C-terminal NADPH binding regions and the FAD binding site and thus could possibly interfere with electron traffic to the heme residues. Overall, the most likely scenario is that the S-nitrosylation of Nox5 at multiple cysteine residues confers different mechanisms of inhibition.
In addition to Nox5, NO suppressed the activity of Nox 1-4 in a dose-dependent manner. Nox1 and Nox3 were the most sensitive to NO and Nox4 the most refractory. These results support the concept that NO is a general inhibitor of Nox enzymes. The mechanisms underlying the relative resistance of Nox4 to NO are not known. The sites of S-nitrosylation on the other Nox isoforms are not known and are beyond the scope of the present study. It is possible that S-nitrosylation of C694, which is highly conserved in the other Nox isoforms and lies within the NADPH binding domain, is a general mechanism for decreased Nox activity. The additional S-nitrosylation of other sites and also the possibility of S-nitrosylation of accessory subunits could contribute to the difference in relative NO-sensitivity of the Nox isoforms that we observed. However, this is unlikely as Nox2 in HL60 cells was less sensitive to NO. In addition, Nox4 does not require accessory subunits beyond p22phox and emits only detectable hydrogen peroxide (Martyn, Frederick et al. 2006; Serrander, Cartier et al. 2007; Helmcke, Heumuller et al. 2009) versus a combination of both superoxide and hydrogen peroxide which are produced by the other enzymes (Chen, Pandey et al. 2011). It is not yet known if NO differentially modifies the ability of the other Nox enzymes (Nox1, 3 and 5) to produce superoxide versus hydrogen peroxide.
Nitric oxide is a ubiquitous signaling molecule that influences organ function by a number of different post-translational modifications. It is well established that NO-dependent signaling is compromised in cardiovascular diseases states and occurs alongside progressive vascular dysfunction (Cai and Harrison 2000; Forstermann and Munzel 2006). A number of mechanisms have been proposed to mediate these changes including deficiencies of arginine, BH4, hsp90 binding and increased Nox enzyme expression (Heinzel, John et al. 1992; Xia, Dawson et al. 1996; Shinozaki, Kashiwagi et al. 1999; Griendling, Sorescu et al. 2000; Stepp, Ou et al. 2002; Berkowitz, White et al. 2003). The results of the current study suggest an additional mechanism that could further exacerbate the reduced bioavailability of NO that is observed in cardiovascular disease states. Thus, physiological production of NO would constrain or restrict ROS production via the S-nitrosylation of NADPH oxidases. Loss of biologically active NO, such as occurs in disease, would remove this restraint and enable higher levels of ROS production from more active Nox enzymes. This concept is supported by evidence in this study that human vascular cells exposed to L-NAME, which by inhibiting endogenous NO production, potentiates superoxide production. In addition, both endogenously produced and exogenously administered NO have been shown to robustly protect from ischemia reperfusion (Bolli 2001) and this is largely due to suppression of abundant ROS production (McCord 1985). The physiological production of NO is also considered to be anti-inflammatory and has been shown to reduce production of ROS from inflammatory cells (Rodenas, Mitjavila et al. 1998).
CONCLUSIONS
Thus the ability of NO to suppress Nox2 activity is clearly only part of the story. Nox5, Nox1 and Nox3 must be added to the list of NO-sensitive ROS generating oxidoreductases. Nox5 is directly modified by reversible S-nitrosylation and future studies are needed to identify whether the other Nox enzymes are similarly S-nitrosylated and to identify the mechanism by which Nox4 resists NO inhibition. Collectively, this mechanism may contribute to the redox balance observed in blood vessels and may account, at least in part, for the elevated ROS production observed under conditions of diminished NO.
Supplementary Material
Highlights.
Nitric oxide (NO) at biologically relevant concentrations inhibits both the basal and stimulated activity of NADPH oxidases.
Reduction of Nox activity was also observed in an isolated enzyme activity assay and was independent of changes in Nox5 expression, intracellular calcium levels or insufficient concentrations of NADPH or FAD.
The relative phosphorylation of Nox5 on Ser490, 498 and Thr494 was unchanged by exposure to NO and tyrosine nitration and glutathiolation of Nox5 were not detected.
Biotin-switch and MS analysis revealed that Nox5 was S-nitrosylated on 4 cysteine residues. Mutation of the highly conserved C694 reduced Nox5 activity, NO sensitivity and S-nitrosylation.
The NADPH oxidase isoforms exhibited a varied sensitivity to NO inhibition with a rank order potency of Nox1>Nox3>Nox5>Nox2>>Nox4
Acknowledgments
This work was supported by NIH grants RO1 HL085827 (DJRF, RCV), R01HL092446 (DF, DWS) and P01 HL101902-01A1 (DF, SMB) and an established investigator award from the AHA (DF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alderton WK, Cooper CE, et al. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MI, Ketsawatsomkron P, et al. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res. 2009;105(10):1013–1022. doi: 10.1161/CIRCRESAHA.109.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Benhar M, Thompson JW, et al. Identification of S-nitrosylated targets of thioredoxin using a quantitative proteomic approach. Biochemistry. 2010;49(32):6963–6969. doi: 10.1021/bi100619k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz DE, White R, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108(16):2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33(11):1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Chen F, Pandey D, et al. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid Redox Signal. 2011;14(11):2107–2119. doi: 10.1089/ars.2010.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiera F, Meccia E, et al. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med. 2008;44(3):332–342. doi: 10.1016/j.freeradbiomed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281(3):1477–1488. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Leszczynska-Piziak J, et al. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90(3):1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, August M, et al. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36(1):101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Dikalov S, Griendling KK, et al. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49(4):717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG, Thomas DD, et al. Focusing of nitric oxide mediated nitrosation and oxidative nitrosylation as a consequence of reaction with superoxide. Proc Natl Acad Sci U S A. 2002;99(17):11127–11132. doi: 10.1073/pnas.152157599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink B, Laude K, et al. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287(4):C895–902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Forrester MT, Foster MW, et al. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic Biol Med. 2009;46(2):119–126. doi: 10.1016/j.freeradbiomed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Foster MW, Forrester MT, et al. A protein microarray-based analysis of S-nitrosylation. Proc Natl Acad Sci U S Arx. 2009;106(45):18948–18953. doi: 10.1073/pnas.0900729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93(2):96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Fujii H, Ichimori K, et al. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997;272(52):32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal. 2009;11(10):2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, et al. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Chen W, et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52(22):1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel B, John M, et al. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281(Pt 3):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmcke I, Heumuller S, et al. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11(6):1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, et al. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, et al. S-nitrosylation: spectrum and specificity. Nat Cell Biol. 2001;3(2):E46–49. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- Hofmann F, et al. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113(Pt 10):1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001(86):pl1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- Jagnandan D, Church JE, et al. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282(9):6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- Jagnandan D, Sessa WC, et al. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol. 2005;289(4):C1024–1033. doi: 10.1152/ajpcell.00162.2005. [DOI] [PubMed] [Google Scholar]
- Jay DB, Papaharalambus CA, et al. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45(3):329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DE, Belka GK, et al. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J. 1998;331(Pt 2):659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourd’heuil D, Jourd’heuil FL, et al. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem. 2001;276(31):28799–28805. doi: 10.1074/jbc.M102341200. [DOI] [PubMed] [Google Scholar]
- Kahlos K, Zhang J, et al. Thioredoxin restores nitric oxide-induced inhibition of protein kinase C activity in lung endothelial cells. Mol Cell Biochem. 2003;254(1-2):47–54. doi: 10.1023/a:1027380828645. [DOI] [PubMed] [Google Scholar]
- Kawahara T, Quinn MT, et al. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Kunz A, et al. iNOS-derived NO and nox2-derived superoxide confer tolerance to excitotoxic brain injury through peroxynitrite. J Cereb Blood Flow Metab. 2007;27(8):1453–1462. doi: 10.1038/sj.jcbfm.9600449. [DOI] [PubMed] [Google Scholar]
- Kelm M, Dahmann R, et al. The nitric oxide/superoxide assay. Insights into the biological chemistry of the NO/O-2. interaction. J Biol Chem. 1997;272(15):9922–9932. doi: 10.1074/jbc.272.15.9922. [DOI] [PubMed] [Google Scholar]
- Kooy NW, Royall JA, et al. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994;16(2):149–156. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43(3):332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, Sorescu D, et al. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88(9):888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Lee C, Miura K, et al. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275(50):38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Deen WM. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem Res Toxicol. 1994;7(4):568–574. doi: 10.1021/tx00040a013. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. Lucigenin luminescence as a measure of intracellular superoxide dismutase activity in Escherichia coli. Proc Natl Acad Sci U S A. 1997;94(7):2891–2896. doi: 10.1073/pnas.94.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hausladen A, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410(6827):490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- Lukowski R, Weinmeister P, et al. Role of smooth muscle cGMP/cGKI signaling in murine vascular restenosis. Arterioscler Thromb Vasc Biol. 2008;28(7):1244–1250. doi: 10.1161/ATVBAHA.108.166405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, et al. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18(1):69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115(2):139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1(3):154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- Montezano AC, Burger D, et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106(8):1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell CN, Matsushita K, et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci U S A. 2005;102(10):3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y, Jackson HM, et al. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49(11):2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Xu G, et al. Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol. 2008;15(12):1307–1316. doi: 10.1016/j.chembiol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Zhang Q, et al. Role of local production of endothelium-derived nitric oxide on cGMP signaling and S-nitrosylation. Am J Physiol Heart Circ Physiol. 2010;298(1):H112–118. doi: 10.1152/ajpheart.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que LG, Liu L, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas J, Mitjavila MT, et al. Nitric oxide inhibits superoxide production by inflammatory polymorphonuclear leukocytes. Am J Physiol. 1998;274(3 Pt 1):C827–830. doi: 10.1152/ajpcell.1998.274.3.C827. [DOI] [PubMed] [Google Scholar]
- Selemidis S, Dusting GJ, et al. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007;75(2):349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Serrander L, Cartier L, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406(1):105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrander L, Jaquet V, et al. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89(9):1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Kashiwagi A, et al. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes. 1999;48(12):2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- Song P, Wu Y, et al. Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation. 2007;116(14):1585–1595. doi: 10.1161/CIRCULATIONAHA.107.716498. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Lamas S, et al. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Ou J, et al. Native LDL and minimally oxidized LDL differentially regulate superoxide anion in vascular endothelium in situ. Am J Physiol Heart Circ Physiol. 2002;283(2):H750–759. doi: 10.1152/ajpheart.00029.2002. [DOI] [PubMed] [Google Scholar]
- Takac I, Schroder K, et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286(15):13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Liu X, et al. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98(1):355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lohneysen K, Noack D, et al. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol. 2010;30(4):961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Dawson VL, et al. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93(13):6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Eu JP, et al. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279(5348):234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Zeng H, Spencer NY, et al. Metabolism of S-nitrosoglutathione by endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281(1):H432–439. doi: 10.1152/ajpheart.2001.281.1.H432. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Malik P, et al. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28(9):1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka J, Sikora A, et al. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285(19):14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114(14):1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.