Abstract
Diabetic foot infections are a high risk for lower extremity amputation in patients with dense peripheral neuropathy and/or peripheral vascular disease. When they present with concomitant osteomyelitis, it poses a great challenge to the surgical and medical teams with continuing debates regarding the treatment strategy. A cohort prospective study conducted between October 2005 and October 2010 included 330 diabetic patients with osteomyelitis mainly involving the forefoot (study group) and 1,808 patients without foot osteomyelitis (control group). Diagnosis of osteomyelitis was based on probing to bone test with bone cultures for microbiological studies and/or repeated plain radiographic findings. Surgical treatment included debridement, sequestrectomy, resections of metatarsal and digital bones, or toe amputation. Antibiotics were started as empirical and modified according to the final culture and sensitivities for all patients. Patients were followed for at least 1 year after wound healing. The mean age of the study group was 56.7 years (SD = 11.4) compared to the control group of 56.3 years (SD = 12.1), while the male to female ratio was 3:1. At initial presentation, 82.1% (n=271) of the study group had an ulcer penetrating the bone or joint level. The most common pathogens were Staphylococcus aureus (33.3%), Pseudomonas aeruginosa (32.2%), and Escherichia coli (22.2%) with an almost similar pattern in the control group. In the study group, wound healing occurred in less than 6 months in 73% of patients compared to 89.9% in the control group. In the study group, 52 patients (15.8%) had a major lower extremity amputation versus 61 in the control group (3.4%) (P=0.001). During the postoperative follow-up visits, 12.1% of patients in each group developed wound recurrence. In conclusion, combined surgical and medical treatment for diabetic foot osteomyelitis can achieve acceptable limb salvage rate and also reduce the duration of time to healing along with the duration of antibiotic treatment and wound recurrence rate.
Keywords: diabetic foot, osteomyelitis, ulcer, amputation, neuropathy
Diabetic foot infections and osteomyelitis are common pathological entities with serious complications that can result in a lower extremity amputation. There is uncertainty about optimal treatment and probably substantial variation in many clinical practices.
Osteomyelitis in the diabetic foot has been well described as a complication of a preexisting infected foot wound (1) and commonly found in diabetic patients with dense peripheral neuropathy. Staphylococcus aureus is the most common pathogen in diabetic foot osteomyelitis, followed by other aerobic Gram-positive cocci. Aerobic Gram-negative bacilli and anaerobes are occasionally isolated, often in mixed diabetic foot infections (2). The direct probing to bone test is fairly reliable in diagnosis of diabetic foot osteomyelitis, and in advanced cases, medical imaging studies may not be necessary in diagnosis.
Selection of the appropriate antimicrobial therapy is best directed by biopsy, cultures, and sensitivities of the infected bone obtained during surgery. Antibiotic therapy should usually be given parenterally, at least initially, and continued for at least 6 weeks. Surgical debridement or resection of the infected bone, when feasible, improves the outcome. With appropriate antibiotic therapy, most cases of diabetic foot osteomyelitis can be successfully managed (2).
Patients and methods
A cohort prospective study was conducted between October 2005 and October 2010 in Jabir AbuEliz Diabetic Center (JADC), Khartoum, Sudan. It included 330 diabetic patients with foot osteomyelitis (study group) and 1,808 diabetic patients with a foot ulcer but without foot osteomyelitis (control group). All patients had an informed consent, and the study was approved by the ethical committee in JADC.
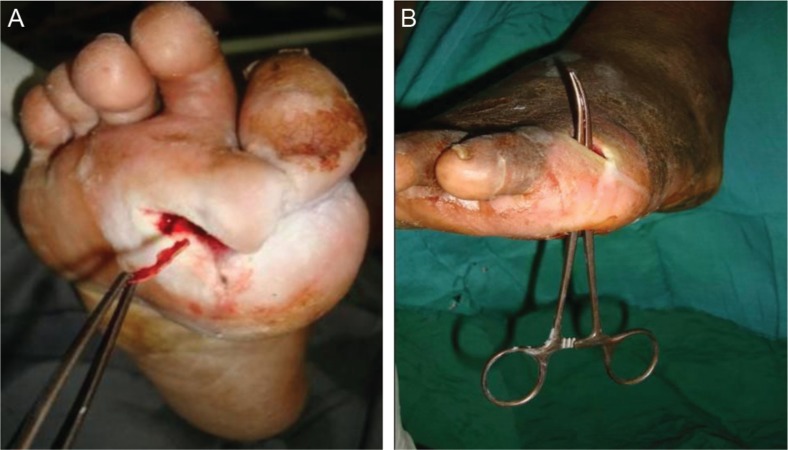
Clinical assessment of all patients who presented to our outpatient clinic with diabetic foot ulcers included the probe-to-bone (PTB) test. The PTB test was performed using a sterile probe to palpate the suspicious osteomyelitic bone at the base of the wound. The diagnosis of osteomyelitis was based on the PTB (Fig. 1) along with a surgical bone specimen taken for microbiological studies. Repeated plain radiographic views were used to diagnose osteomyelitis and demonstrate bone destruction in addition to the follow-up for resolution of infection. Magnetic resonance imaging (MRI) and bone scintigraphy were only used selectively to differentiate between osteomyelitis and non-infective bone destruction in diabetic neuropathic patients.
Fig. 1.
Clinical pictures (A, B) demonstrating the probe-to-bone test in our study.
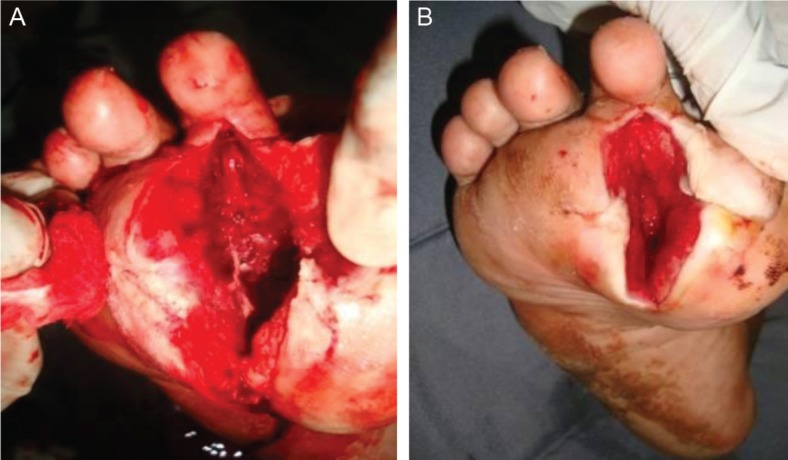
Surgical treatment included debridement, sequestrectomy, metatarsal and/or phalangeal resection, or toe amputation (Fig. 2). Surgical debridement was performed in patients who had no critical limb ischemia, and minor amputations were performed for those with localized gangrene. In all patients, major limb ischemia was diagnosed both clinically and with vascular non-invasive studies of ankle brachial index < 0.9. Wound healing was allowed by secondary intention or in cases where extensive tissue and skin loss or the wound edges were too far apart for closure, acceleration of wound healing was achieved by minor plastic reconstructive procedures such as secondary sutures, skin grafts, and flaps (Figs. 3 and 4). Postoperative wound off-loading was initiated with fiberglass casting, initial bed rest, accommodative dressings, external fixation, assistant devices, therapeutic shoes, or total contact casting.
Fig. 2.
Surgical resection and debridement of infected metatarsal and phalangeal bones (A, B).
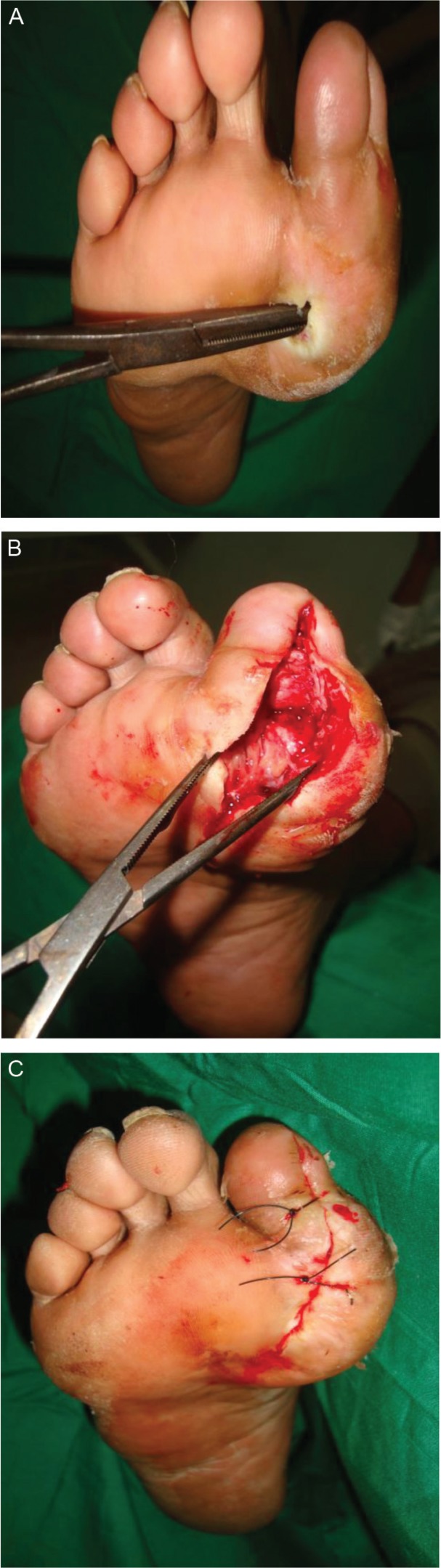
Fig. 3.
Intraoperative clinical pictures demonstrating the surgical probing to bone (A), partial bone resection (B), and wound closure (C).
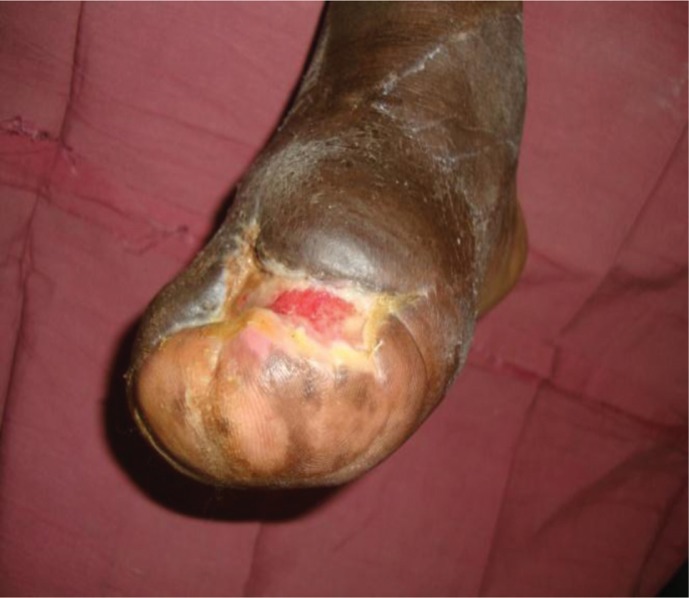
Fig. 4.
Surgical example of wound healing with local flap closure.
The minimal duration of antibiotic treatment was 6 weeks, and it varied according to the clinical and radiological evidence of infection resolution. The commonly used antibiotics were ceftriaxone, ceftazidime, gentamicin, amikacin, amoxicillin/clavulanic acid, and ciprofloxacin. An intravenous dose was administered initially for 3–7 days followed by oral intake. After wound healing, patients were followed for at least 1 year, and limb salvage was considered when no major lower extremity amputation was required.
The data were analyzed using the SPSS program version 12. Statistical tests using student's t-test for numerical values and Chi square (χ 2) tests were utilized. A P value was considered significant when it was <0.05.
Results
Three hundred and thirty diabetic patients with foot osteomyelitis mainly in the forefoot (study group) and 1,808 diabetic patients without foot osteomyelitis (control group) were studied. Table 1 shows that both groups were matched for age only with more male predominance in the study group. Among the study group, 60.1% had diabetes for more than 10 years. Toes were involved in 1,192 patients from both groups (51%), metatarsal heads in 37.5%, and the heel in only 3%.
Table 1.
Comparison between diabetic foot ulcerations with osteomyelitis (study group) and without osteomyelitis (control group)
| Study group | Control group | Tests of significance and risk ratio | |
|---|---|---|---|
| Mean age (years)+standard deviation | 56.7+11 | 56.3+12.1 | |
| Male:female ratio | 3:1 | 1:1.3 | |
| Type of diabetes | Yates χ 2=5.13; P=0.0235 | ||
| Type 2 | N=267 (80.7%) | N=1350 (74.7%) | |
| Type 1 | N=64 (19.3%) | N=458(25.3%) | |
| Duration of wound healing | Yates χ 2=20.02; P=0.0002 | ||
| < 3 months | 42.4% | 72.7% | |
| 3–6 months | 30.6% | 17.2% | |
| 6 months to 1 year | 18.8% | 7.9% | |
| > 1 year | 8.2% | 2.2% | |
| Outcome | Yates χ 2=85.41; P<0.0001 | ||
| Limb salvage | N=270 (81.8%) | N=1735 (96%) | |
| Major amputation | N=52 (15.8%) | N=61 (3.4%) | |
| Deaths | N=8 (2.4%) | N=12 (0.7%) | |
| Wound recurrence | N=40 (12.1%) | N=218 (12.1%) | P=0.97; odds ratio = 1.005; CI = 0.732–1.378 |
At initial presentation, 82.1% (n=271) of the osteomyelitis patients in the study group had an ulcer penetrating the bone or joint level (Wagner Grade 3 ulcer). The most common causative organisms for the study group were Staphylococcus aureus (33.3%), Pseudomonas aeruginosa (32.2%), and Escherichia coli (22.2%). In the remaining 59 patients from the study group (17.9%), the diagnosis of osteomyelitis was based on clinical finding of increased size of the toe (sausage toe) with repeated findings on plain radiographic views. Bone scintigraphy and MRI were used only in 53 patients from both study and control groups. In the control group, 87.2% of the ulcers initially presented with a Grade 2 or less Wagner ulcer, and the most common bacterial isolates were similar to that of the study group.
Out of the patients with osteomyelitis, 61.2% had peripheral neuropathy compared to 50% in the control group (P=0.05; χ 2=3.84). Peripheral vascular disease was reported in 32.7% of the study group and in 28.8% of the control group (P=0.007). Diabetic neuropathic osteoarthropathy was reported in 11 cases (3.3%) of the study group and in 48 patients in the control group (2.7%). Management entailed partial sequestrectomy for necrotic bone combined with long-term antibiotics for 6–8 weeks based on the results of bacteriological cultures and sensitivities. The degree of bone resection varied from minor excision of necrotic bone up to complete removal of the digital bones. In the study group, toes were affected in 237 patients (71.6%) versus 955 in the control group (53%). Toe amputation was performed in 116 patients in the study group (35%) versus 355 in the control group (19%) (P=0.001). The hallux was the most common digit to be amputated (n=35), which represented 10.6% of the study group.
Wound healing in the study group occurred within less than 6 months in 73% of patients compared to 89.9% in the control group (P=0.0002) (Table 1).
In the study group, limb salvage was achieved in 81.8% compared to 96% in the control group (P=0.0001). In the study group, 52 patients (15.8%) had a major lower extremity amputation caused by deep infection and extensive tissue necrosis in 40 patients, and peripheral vascular disease in the remaining 12 patients. In addition, there was no significant difference in the incidence of wound recurrence between the two groups during 1 year of follow-up (Table 1).
Discussion
Diabetic foot infections account for a substantial global burden of morbidity, psychosocial disruption, and economic cost. Recommendations for best practice are continuously evolving in parallel with improvements in medical imaging modalities, development and clinical use of new antimicrobial agents, and data surrounding novel wound-healing adjunctive strategies (3).
In developing countries, patients usually present late for treatment with devastating complications of the advanced diabetes mellitus disease. In those cases of diabetic foot osteomyelitis with an ulcer presentation that can be probed to the bone level, imaging studies may rarely be used. The PTB test with bone specimen collection taken for microbiological culture, sensitivities, and/or biopsy is very reliable, and the diagnosis of osteomyelitis can confidently be established particularly if the underlying bone is necrotic and friable in consistency (1, 4–6). PTB testing should be included in the initial assessment of all diabetic patients with infected pedal ulcers (7). In our study, a common presentation we encountered was a sizable sausage-shaped toe, the appearance of which should alert the physician to the possibility of underlying osteomyelitis (8).
In few cases, there is a diagnostic difficulty to differentiate between osteomyelitis and neuropathic osteoarthropathy especially when there is no wound and PTB testing cannot be performed. In these cases, repeated plain radiographs, MRI, and/or bone scintigraphy may be needed. In many previous studies, MRI is thought to be the most accurate imaging tool to diagnose osteomyelitis (9–14).
It has been shown that surgical resection of the infected and necrotic bone favors a good outcome in chronic osteomyelitis (15–18) and that careful assessment of the patient's lower extremity vascular and neurological status is necessary before any surgical intervention (19, 20). Surgical treatment in our study group included sequestrectomy and debridement of necrotic and friable bone to healthy bone margins. In some cases, more extensive surgical debridement in the form of partial resection of the metatarsal bone or even phalangeal bones was performed. Surgical excision of the infected bone provided reliable specimens for microbiological and histopathological analysis. Minor amputations were performed if there was an extensive localized soft tissue necrosis provided that there was no critical ischemia.
Published data have reported that diabetic foot osteomyelitis, in the absence of extensive necrosis or gangrene, usually responds to antimicrobial therapy without the need for an ablative surgical procedure (21). Venkatesan et al. have also reviewed the management of osteomyelitis in a multidisciplinary diabetic foot clinic and reported that the success of conservative therapy with prolonged course of oral antibiotics challenges conventional advice that excision of infected bone is essential in the management of osteomyelitis in diabetic patients (22). In our study, we have found that surgical sequestrectomy combined with prolonged oral antibiotic therapy gave the best results for the patients with diabetic foot osteomyelitis. This combined method may reduce the changes in biomechanical alterations of the diabetic foot and minimize the duration of antibiotic therapy. However, more research including studies of adjunctive therapies in cases of bone infection in the feet of diabetic patients is required (23).
The choice of antibiotic therapy is best guided by a bone biopsy or debridement culture results (19). Parenteral therapy is needed for severe infections, but oral therapy is adequate for most mild or moderate diabetic foot infections (24). In our study group, bone specimens for culture and sensitivities to identify the causative pathogens were obtained after the surgical wound debridement in order to avoid contamination results. Staphylococcus aureus was the most common pathogen to be isolated from our specimens, which was in agreement with most international reports (1, 15, 22, 25), but a higher than usual incidence of Pseudomonas aeruginosa osteomyelitis was noticed. Patients received antibiotics according to the results of culture and sensitivity for at least 6 weeks. Initially, broad-spectrum intravenous antibiotics were used in the presence of a severe diabetic foot infection until the final microbiological results were received. In moderate or mild diabetic foot infections, oral antibiotics were used. The duration of antibiotics depended on the clinical, microbiological, and radiological evidence of infection control. When infection was adequately eradicated, the antibiotics treatment was discontinued even in the presence of a wound. Non-infected wounds can heal if appropriate wound care with regular wound dressings are performed, and antibiotic therapy is not usually indicated in clinically non-infected wounds (17, 26).
The duration of antibiotic therapy may be shortened considerably after surgical intervention (27). Split thickness skin grafting has also been shown to be an effective method of managing diabetic foot ulcers as compared to conservative wound dressings by reducing healing times and length of hospital stay with minimal donor-site morbidity (28).
After surgical debridement, off-loading played an important role for our postoperative care. Relieving pressure on the ulcerated area is absolutely crucial in the treatment of the diabetic foot to prevent further trauma, reduce any shearing forces to the area, and facilitate wound healing. Diabetic limb salvage was obtained in a considerable number of patients with osteomyelitis with a combined surgical and antibiotic treatment. However, we reported a significantly higher incidence of major amputation in the study group. This may be considered due to the more extensive tissue necrosis, bone and joint destruction, and loss of normal foot structure that presented in the study group with diabetic foot osteomyelitis. The hallux was also found to be the most common site affected in the study group (29). In addition, both groups had almost similar wound recurrence rates during the postoperative period.
Conclusion
In developing countries, due to the delayed presentation of a diabetic foot complication, initial medical imaging studies may rarely be used. Combined surgical and medical treatment can achieve acceptable diabetic limb salvage rates and also reduce the duration of time to healing, duration of antibiotic treatment, and wound recurrence.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or from elsewhere to conduct this study.
References
- 1.Hartemann-Heurtier A, Senneville E. Diabetic foot osteomyelitis. Diabetes Metab. 2008;34:87–95. doi: 10.1016/j.diabet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis. 1997;25:1318–26. doi: 10.1086/516148. [DOI] [PubMed] [Google Scholar]
- 3.Matthews PC, Berendt AR, Lipsky BA. Clinical management of diabetic foot infection: diagnostics, therapeutics and the future. Expert Rev Anti Infect Ther. 2007;5:117–27. doi: 10.1586/14787210.5.1.117. [DOI] [PubMed] [Google Scholar]
- 4.Aragón-Sánchez J, Lipsky BA, Lázaro-Martínez JL. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med. 2011;28:191–4. doi: 10.1111/j.1464-5491.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 5.Morales Lozano R, González Fernández ML, Martinez Hernández D, Beneit Montesinos JV, Guisado Jiménez S, Gonzalez Jurado MA. Validating the probe-to-bone test and other tests for diagnosing chronic osteomyelitis in the diabetic foot. Diabetes Care. 2010;33:2140–5. doi: 10.2337/dc09-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270–4. doi: 10.2337/dc06-1572. [DOI] [PubMed] [Google Scholar]
- 7.Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273:721–3. [PubMed] [Google Scholar]
- 8.Rajbhandari SM, Sutton M, Davies C, Tesfaye S, Ward JD. ‘Sausage toe’: a reliable sign of underlying osteomyelitis. Diabet Med. 2000;17:74–7. doi: 10.1046/j.1464-5491.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 9.Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519–27. doi: 10.1086/590011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine SE, Neagle CE, Esterhai JL, Wright DG, Dalinka MK. Magnetic resonance imaging for the diagnosis of osteomyelitis in the diabetic patient with a foot ulcer. Foot Ankle Int. 1994;15:151–6. doi: 10.1177/107110079401500311. [DOI] [PubMed] [Google Scholar]
- 11.Rozzanigo U, Tagliani A, Vittorini E, Pacchioni R, Brivio LR, Caudana R. Role of magnetic resonance imaging in the evaluation of diabetic foot with suspected osteomyelitis. Radiol Med. 2009;114:121–32. doi: 10.1007/s11547-008-0337-7. [DOI] [PubMed] [Google Scholar]
- 12.Croll SD, Nicholas GG, Osborne MA, Wasser TE, Jones S. Role of magnetic resonance imaging in the diagnosis of osteomyelitis in diabetic foot infections. J Vasc Surg. 1996;24:266–70. doi: 10.1016/s0741-5214(96)70102-7. [DOI] [PubMed] [Google Scholar]
- 13.Marcus CD, Ladam-Marcus VJ, Leone J, Malgrange D, Bonnet-Gausserand FM, Menanteau BP. MR imaging of osteomyelitis and neuropathic osteoarthropathy in the feet of diabetics. Radiographics. 1996;16:1337–48. doi: 10.1148/radiographics.16.6.8946539. [DOI] [PubMed] [Google Scholar]
- 14.Yuh WT, Corson JD, Baraniewski HM, Rezai K, Shamma AR, Kathol MH, et al. Osteomyelitis of the foot in diabetic patients: evaluation with plain film, 99mTc-MDP bone scintigraphy, and MR imaging. AJR Am J Roentgenol. 1989;152:795–800. doi: 10.2214/ajr.152.4.795. [DOI] [PubMed] [Google Scholar]
- 15.Rao N, Lipsky BA. Optimising antimicrobial therapy in diabetic foot infections. Drugs. 2007;67:195–214. doi: 10.2165/00003495-200767020-00003. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA, Peters EJ, Senneville E, Berendt AR, Embil JM, Lavery LA, et al. Expert opinion on the management of infections in the diabetic foot. Diabetes Metab Res Rev. 2012;28S:163–78. doi: 10.1002/dmrr.2248. [DOI] [PubMed] [Google Scholar]
- 17.Zgonis T, Roukis TS. A systematic approach to diabetic foot infections. Adv Ther. 2005;22:244–62. doi: 10.1007/BF02849934. [DOI] [PubMed] [Google Scholar]
- 18.Ertugrul BM, Oncul O, Tulek N, Willke A, Sacar S, Tunccan OG, et al. A prospective, multi-center study: factors related to the management of diabetic foot infections. Eur J Clin Microbiol Infect Dis. 2012;31:2345–52. doi: 10.1007/s10096-012-1574-1. [DOI] [PubMed] [Google Scholar]
- 19.Mader JT, Ortiz M, Calhoun JH. Update on the diagnosis and management of osteomyelitis. Clin Podiatr Med Surg. 1996;13:701–24. [PubMed] [Google Scholar]
- 20.Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999;177:282–6. doi: 10.1016/s0002-9610(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 21.Bamberger DM, Daus GP, Gerding DN. Osteomyelitis in the feet of diabetic patients. Long-term results, prognostic factors, and the role of antimicrobial and surgical therapy. Am J Med. 1987;83:653–60. doi: 10.1016/0002-9343(87)90894-1. [DOI] [PubMed] [Google Scholar]
- 22.Venkatesan P, Lawn S, Macfarlane RM, Fletcher EM, Finch RG, Jeffcoate WJ. Conservative management of osteomyelitis in the feet of diabetic patients. Diabet Med. 1997;14:487–90. doi: 10.1002/(SICI)1096-9136(199706)14:6<487::AID-DIA373>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Aragón-Sánchez J. Treatment of diabetic foot osteomyelitis: a surgical critique. Int J Low Extrem Wounds. 2010;9:37–2. doi: 10.1177/1534734610361949. [DOI] [PubMed] [Google Scholar]
- 24.Lipsky BA. Empirical therapy for diabetic foot infections: are there clinical clues to guide antibiotic selection? Clin Microbiol Infect. 2007;13:351–3. doi: 10.1111/j.1469-0691.2007.01697.x. [DOI] [PubMed] [Google Scholar]
- 25.Wheat J. Diagnostic strategies in osteomyelitis. Am J Med. 1985;78:218–24. doi: 10.1016/0002-9343(85)90388-2. [DOI] [PubMed] [Google Scholar]
- 26.Bader MS. Diabetic foot infection. Am Fam Physician. 2008;78:71–9. [PubMed] [Google Scholar]
- 27.Snyder RJ, Cohen MM, Sun C, Livingston J. Osteomyelitis in the diabetic patient: diagnosis and treatment. Part 2: medical, surgical, and alternative treatments. Ostomy Wound Manage. 2001;47:24–30. 32–41. [PubMed] [Google Scholar]
- 28.Mahmoud SM, Mohamed AA, Mahdi SE, Ahmed ME. Split-skin graft in the management of diabetic foot ulcers. J Wound Care. 2008;17:303–6. doi: 10.12968/jowc.2008.17.7.30522. [DOI] [PubMed] [Google Scholar]
- 29.ElMakki Ahmed M, Tamimi AO, Mahadi SI, Widatalla AH, Shawer MA. Hallux ulceration in diabetic patients. J Foot Ankle Surg. 2010;49:2–7. doi: 10.1053/j.jfas.2009.07.005. [DOI] [PubMed] [Google Scholar]