Abstract
Background
Peripheral vascular disease and/or diabetic neuropathy represent one of the main etiologies for the development of lower leg and/or diabetic foot ulcerations, and especially after acute trauma or chronic mechanical stress. The reconstruction of such wounds is challenging due to the paucity of soft tissue resources in this region. Various procedures including orthobiologics, skin grafting (SG) with or without negative pressure wound therapy and local random flaps have been used with varying degrees of success to cover diabetic lower leg or foot ulcerations. Other methods include: local or regional muscle and fasciocutaneous flaps, free muscle and fasciocutaneous, or perforator flaps, which also have varying degrees of success.
Patients and methods
This article reviews 25 propeller perforator flaps (PPF) which were performed in 24 diabetic patients with acute and chronic wounds involving the foot and/or lower leg. These patients were admitted beween 2008 and 2011. Fifteen PPF were based on perforators from the peroneal artery, nine from the posterior tibial artery, and one from the anterior tibial artery.
Results
A primary healing rate (96%) was obtained in 18 (72%) cases. Revisional surgery and SG for skin necrosis was performed in six (24%) cases with one complete loss of the flap (4%) which led to a lower extremity amputation.
Conclusions
The purpose of this article is to review the use of PPF as an effective method for soft tissue coverage of the diabetic lower extremity and/or foot. In well-controlled diabetic patients that present with at least one permeable artery in the affected lower leg, the use of PPF may provide an alternative option for soft tissue reconstruction of acute and chronic diabetic wounds.
Keywords: diabetes mellitus, ulcers, lower leg, foot, propeller perforator flaps
The lifetime risk of developing an ulcer among patients with diabetes mellitus is about 12–25% (1, 2). The lower leg and foot ulcers are an important risk factor for amputation (3) with some authors reporting that 23–25% from the diabetic population undergo immediate amputation (4, 5) or even more than 55% in other statistics (6). Moreover, after major amputation in diabetics, the 5 year mortality rate can be up to 78% (7), and the risk of a contralateral limb amputation can be up to 50% (8). Despite the paucity of some authors in complex diabetic limb salvage techniques (9, 10), it has been proven that diabetic ulcers can be successfully treated through various reconstructive methods such as skin grafting (SG) with or without negative pressure wound therapy (NPWT), dermal substitutes (11–14) or by utilizing a large variety of pedicled and free flaps (12, 15–25).
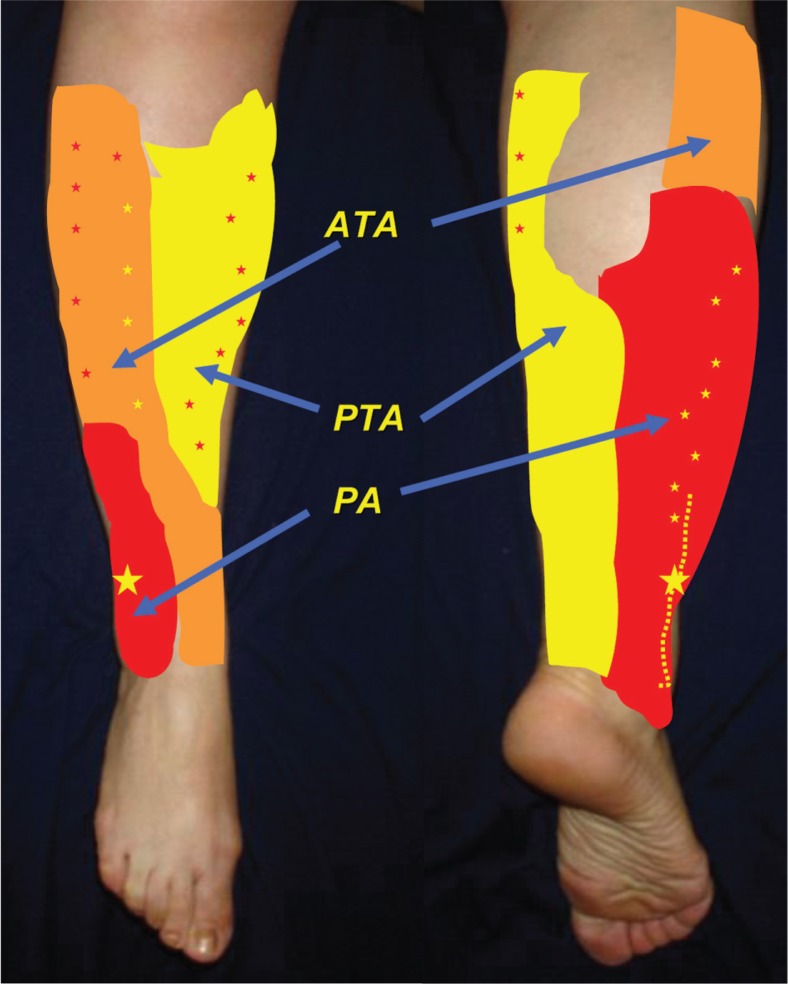
It is well-known that the blood supply of the distal lower leg and foot is ensured by the contribution of the peroneal artery (PA), anterior tibial artery (ATA), and posterior tibial artery (PTA) through their musculocutaneous (MC) and septocutaneous (SC) perforators (25–27) (Fig. 1). This vascular supply occupies three vascular territories organized as a series of longitudinal rows within the intermuscular septa of the lower leg (25–27). The most distally located perforators and especially those emerging from the PA approximately 5 cm above the lateral malleolus (26, 28) and from the PTA approximately 5 cm above the medial malleolus (28) represent a very good vascular source for perforator flaps enabling coverage of the foot and distal lower leg. The aim of this study was to retrospectively review the use of propeller perforator flaps (PPF) for soft tissue coverage of the diabetic lower extremity and foot in an overall period of 4 years.
Fig. 1.
This figure demonstrates the vascular territories of the main arteries in the lower leg. The stars represent the main distribution of perforators in each territory. ATA – anterior tibial artery; PTA – posterior tibial artery; PA – peroneal artery.
Patients and methods
A retrospective review between 2008 and 2011 for diabetic patients who underwent PPF reconstruction for soft tissue coverage of the lower leg and foot was performed in our institution (Table 1). There were a total of 24 patients (25 ulcerations) with 12 in the foot and 13 in the lower leg.
Table 1.
Epidemiology, surgical treatment and outcomes
| No. | Etiology/wound site | Sex | Age | PPF | Size (cm) | Complication/treatment | Healing time, days | Limb salvage |
|---|---|---|---|---|---|---|---|---|
| 1 | PAOD/plantar aspect distal foot | M | 57 | PA/SC | 20×5 | − | 45 | + |
| 2 | PAOD/Achilles region | M | 60 | PA/SC | 8×3 | − | 31 | + |
| 3 | PAOD/plantar foot | F | 45 | PA/SC | 18×7 | Partial SN/SG | 63 | + |
| 4 | PAOD. Posttr. non-healing wound/dorsal ankle* | M | 56 | PA/SC | 28×10 (prox); 28×3 (distal) | − | 49 | + |
| 5 | Venous insufficiency/distal lower leg | F | 68 | ATA/SC | 12×4 | − | 28 | + |
| 6 | PAOD. Posttr. non-healing wound/calcaneum | M | 65 | PA/SC | 20×8 | − | 33 | + |
| 7 | PAOD. Venous insufficiency/distal lower leg* | M | 57 | PTA/MC | 15×7 | − | 35 | + |
| 8 | PAOD/Achilles region | M | 62 | PA/SC | 17×4 | Partial SN/SG | 91 | + |
| 9 | PAOD/calcaneum* | M | 58 | PTA/SC | 18×5 | Partial SN/SG | 78 | + |
| 10 | Frostbite/bilateral TMA stumps | M | 66 | PTA/SC; PA/SC | 28×10; 26×7 | − | 25; 37 | + |
| 11 | PAOD. Posttr. non-healing wound/distal lower leg | M | 74 | PA/MC | 17×7 | Partial SN/SG | 67 | + |
| 12 | PAOD/calcaneum | M | 70 | PTA/MC | 22×5 | − | 40 | + |
| 13 | PAOD. Posttr. non-healing wound/lateral malleolus | M | 72 | PTA/MC | 15×7 | Complete necrosis | 63 | Amputation |
| 14 | Venous insufficiency/distal lower leg | M | 66 | PA/MC | 12×6 | − | 27 | + |
| 15 | PAOD. Posttr. non-healing wound/distal lower leg* | M | 50 | PA/MC | 15/4 | − | 35 | + |
| 16 | Venous insufficiency/distal lower leg | M | 81 | PTA/SC | 10×5 | − | 47 | + |
| 17 | PAOD/plantar foot* | M | 39 | PA/SC | 31×12 (prox) 31×3 (distal) | − | 36 | + |
| 18 | PAOD. Non-healing wound after amputation for gangrene/TMA stump | M | 61 | PTA/SC | 21×9 | − | 21 | + |
| 19 | PAOD. Venous insufficiency. Posttr. non-healing wound/distal lower leg | M | 47 | PA/SC | 17×12 | Partial SN/SG | 82 | + |
| 20 | PAOD/Achilles region | F | 65 | PTA/SC | 17×6 | − | 23 | + |
| 21 | PAOD. Posttr. non-healing wound/lateral malleolus* | F | 52 | PA/SC | 13×5 | − | 39 | + |
| 22 | Venous insufficiency/distal lower leg | M | 65 | PTA/SC | 8×5 | − | 43 | + |
| 23 | PAOD/calcaneum* | M | 65 | PA/SC | 20×5 | Partial SN/SG | 88 | + |
| 24 | PAOD. Non-healing wound/lateral foot | F | 58 | PA/SC | 14×5 | − | 29 | + |
Patients with previous revascularization; PA – peroneal artery; ATA – anterior tibial artery; PTA – posterior tibial artery; SC – septocutaneous perforator; MC – musculocutaneous perforator; SN – superficial necrosis; SG – skin grafting; PAOD – peripheral arterial obstructive disease; M – male; F – female; Posttr. – Post-traumatic; TMA – transmetatarsal amputation; Prox – proximal.
A handheld Doppler ultrasound and/or color Doppler examination was performed in all diabetic patients while an arteriography was performed in 15 patients. All patients had an acceptable vascular supply at the time of admission, even if in 13 patients only one artery was patent (PA in 11 cases and PTA in two cases). In seven patients, a previous revascularization was performed.
After hospital admission, sterile soft tissue cultures were obtained, broad-spectrum antibiotics were administered, and the ulcerations were locally treated for 3–7 days until the disappearance of inflammatory signs. In all cases, the surgical debridement and soft tissue defect coverage were performed at the initial operation.
The arterial choice based on which the PPF was performed was made according to the pre-operative Doppler and/or arteriographic examinations with the final decision made during operation and according to the intra-operative findings.
The results of the study were analyzed according to the type and dimensions of the flaps, post-operative complications, number of operations, wound healing rates, time to heal, number of limb salvage, and patient survival rates. The patient follow-up was between 6 and 51 months, with an average of 33.6 months.
Results
There were 24 diabetic patients with ulcerations in 25 limbs (Table 1). The male-to-female ratio was 19:5 with the mean age of 69.1 years old (range, 39–81 years). The ulcer location was found in the distal lower leg in 13 cases and in the foot in 12 cases, from which six were in the weight bearing area of the foot (Case Nos. 3, 6, 9, 12, 17, and 23). All of the patients except three (Case Nos. 3, 7, and 10) had associated risk factors of atherosclerosis with ischemic cardiomyopathy, hypertension, and peripheral arterial disease. In seven patients, a previous lower extremity revascularization was performed.
The 25 PPF performed consisted of 15 PA-PPF, nine PTA-PPF, and one ATA-PPF. The flaps were based on SC perforators in 19 cases, and on MC perforators in six cases. The dimensions of the flaps were averaged from 8×3 to 31×12 cm. The flap donor sites were directly closed in five cases while in the remaining cases the donor sites were closed in combination of suturing and SG.
In 18 cases the flaps healed without complications, with an overall primary healing success rate of 72%. Comparing this rate for each type of flap, we obtained a primary healing in one case from one ATA-PPF, in 10 cases from 15 PA-PPF, and in seven cases from nine PTA-PPF. There were six complications (24%) (five PA-PPF and one PTA-PPF) consisting in partial superficial necrosis (SN) of no more than 50% of their entire flap surface, which were revised by surgical debridement and SG. One full necrosis of the flap (PTA-PPF) led to a lower extremity amputation (4%). The overall success rate including the revisional surgeries was at 96% with a mean time to heal of 46.2 days (range, 21–91 days). The average follow-up was 33.6 months (6–51 months) with post-operative follow-ups of all patients being able to ambulate without any assistant devices and one patient using a prosthesis.
Discussion
It has been shown that the attempt for diabetic limb salvage should be encouraged since this is the single way to decrease the amputation rate and to increase the long-term survival in the diabetic population (23, 24). Once the necessity for reconstructive surgery in salvaging the diabetic foot has been better understood, a growing number of surgical options were introduced. The SG of such ulcerations is not generally a viable option since the grafts may not incorporate completely on the majority of diabetic wounds. In addition there is a high risk of ulcer recurrence after SG. Adjunctive therapeutic techniques such as negative pressure wound dressings (29), skin substitutes (11, 13, 30, 31), growth factors (32), hyperbaric oxygen (33–35), and extracorporeal shockwave therapy (35–37) have all been utilized to increase the chance of skin graft incorporation. However, the risk of ulcer recurrence still remains high. Yeh et al. (14) reported on SG as a salvage procedure for reconstruction of the diabetic foot with a wound healing rate of 74% at 1 month, 86.3% at 2 months, but with non-healing in five cases at 3 months, requiring stump revision in three cases.
Due to the limited amount of soft tissue resources for flap reconstruction in the lower leg and foot, it is generally considered that local or regional flaps could provide a viable option. However, there are several drawbacks including: the involvement of the neighboring tissue (12), the frequency of donor site SG including portions of the original defect (38, 39), and the high complication rate of some neurocutaneous flaps (40).
The use of local muscle flaps could be a good alternative for covering defects of small dimensions in the midfoot, hindfoot, or ankle (23), but larger defects require a free microsurgical flap. Both cutaneous and muscle free flaps can be used successfully in the diabetic foot. Oishi et al. (41) considered it more advantageous to use cutaneous free flaps due to the lower incidence of recipient site problems while Ducic and Attinger (23) considered muscle free flaps for better long-term durability and less functional complications. This surgical approach with muscle coverage is ideal for the plantar foot defects (23, 42) even if some surgeons have indicated that fasciocutaneous flaps can also be successfully used (20, 41, 43).
In recent years, the free perforator flaps started to be extensively used in diabetic foot ulcers (20, 44–46). Despite the high success rate of free microsurgical flaps (even in diabetic patients; (20, 44–48)), there are several drawbacks in using them including: the necessity of a well-controlled diabetic patient, longer anesthesia and operative time, distant donor site, necessity of good recipient vessels, and microsurgical skills. Moreover, when a free flap has failed, further limb salvage reconstructive techniques may be limited to the diabetic patient.
Recent advances in the vascular anatomy of the lower leg (25–28, 49–56) have stimulated the interest in performing pedicled perforator flaps in the lower extremity.
Schaverien and Saint-Cyr (27) found in their study that, from distal to proximal, the location of perforators able to support a viable flap were within the 4–9 cm area emerging from the ATA and PTA with one to two perforators from the PA; in the 13–18 cm region, perforators were emerging from the PA and PTA; and in the 21–26 cm region, perforators were emerging from the ATA and PTA (Fig. 1). In diabetic patients with vascular compromise there is still usually a patent major vascular axis, most commonly being the PA (57) with viable perforators to support a perforator flap.
In our case series, the distal perforators (Fig. 2) as described by Schaverien and Saint-Cyr (27) emerging from the distal 4 to 9 cm region were mainly utilized. In this region, two very constant and reliable perforators, one at 5 cm proximal to the medial malleolus emerging from the PTA (28) and the other 5 cm proximal to the lateral malleolus emerging from the PA were founded in our patient population (25, 26, 28).
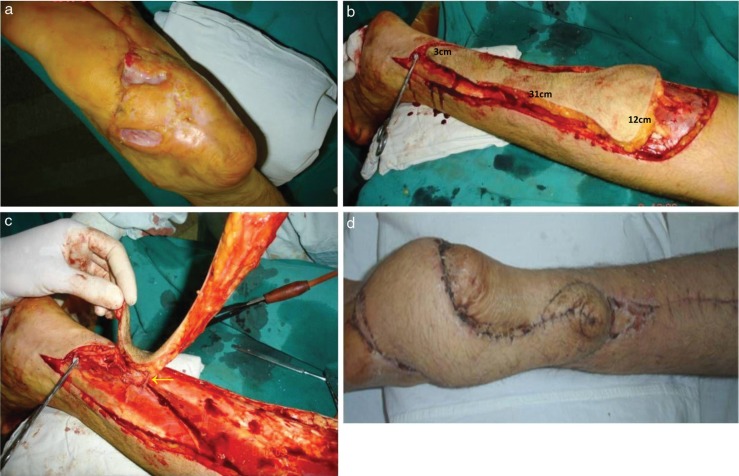
Fig. 2.
Pre-operative clinical view (a) of a non-healing plantar foot ulcerations for the patient in Case No. 17. Intra-operative view showing the harvesting of the perforator flap based on a distal perforator from the peroneal artery (PA) with dimensions of 31 cm in length and 12 cm of width in the proximal aspect and 3 cm of width in the distal aspect (b). Intra-operative view of the harvested flap with the arrow indicating the perforator emerging from the PA about 9 cm above the lateral malleolus (c). Clinical picture at 1-month post-operative follow-up (d).
In some cases, when the condition of the local tissues surrounding the defect were poor or when a perforator in the distal 4–9 cm region was not found, the flaps were based upon perforators emerging from the medial third of the tibia (located in the 13–18 cm region) (25). The vascularization of the lower limb was acceptable for all patients upon hospital admission, even if in 13 cases the vascular supply was provided by only one major arterial trunk (PA in 11 cases and PTA in two cases).
Considering the major complications for these types of flaps with post-operative venous congestion that can lead in some circumstances to partial or even complete flap necrosis, a design flap improvement was executed. This surgical artifice, called the perforator plus flap, was the preservation of a cutaneous bridge at the base of the flap, connecting the flap with the donor area (42, 58, 59). This surgical procedure was successfully utilized but unlike the original technique, the cutaneous bridge in our series was very narrow (25) (Fig. 3).
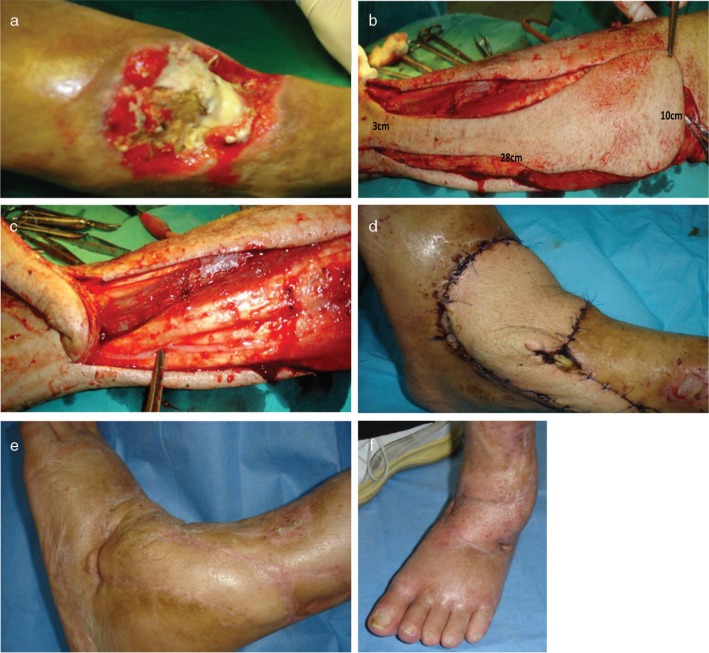
Fig. 3.
Pre-operative clinical view (a) of a non-healing dorsal ankle wound for the patient in Case No. 4. Intra-operative view showing the harvesting of the perforator plus flap based on a distal perforator from the peroneal artery with dimensions of 28 cm in length and 10 cm of width in the proximal aspect and 3 cm of width in the distal aspect (b). Intra-operative view of the harvested flap in continuity at its base with the donor site and forceps indicating the sural nerve (c). Post-operative clinical views at 2 weeks (d) and 1 year follow-up (e, f).
In the 24% complication rate reported in our diabetic patient population, the superficial partial skin necrosis (Fig. 4) was due to venous congestion which was revised by surgical debridement and SG that was performed within 7 days after the initial operation. In addition, one PTA-PPF had completely failed in a 72-year old male with a lateral malleolus defect that eventually led to an amputation. No mortality rates were recorded in the 4-year follow-up.
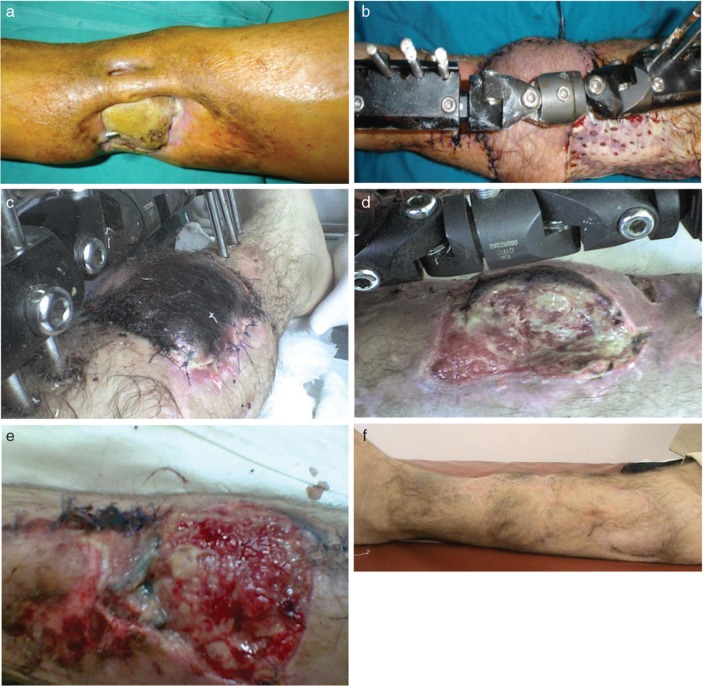
Fig. 4.
Pre-operative clinical view (a) of a non-healing distal lower extremity wound with tibia necrosis for the patient in Case No. 19. Intra-operative view showing the harvested peroneal based perforator flap with dimensions of 17 cm in length and 12 cm of width (b). Post-operative view showing the superficial necrosis of the distal third of the flap after venous congestion (c). The flap was revised and surgically debrided (d) with adequate granulation tissue 7 days after the revisional surgery (e) when skin grafting was performed. Post-operative clinical outcome at 6 months follow-up (f).
In conclusion, based on our results (primary healing in 72% of cases with an overall 96% healing rate), careful consideration on utilizing the PPF in reconstruction of the diabetic lower extremity should be based on the patient's overall medical status, wound size, status and location, arterial blood supply, and other surgical treatment options.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or from elsewhere to conduct this study.
References
- 1.Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725–35. doi: 10.1016/S0140-6736(05)67699-4. [DOI] [PubMed] [Google Scholar]
- 2.Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ. North-West diabetes foot care study. Foot ulcer risk is lower in South-Asian and African-Caribbean compared with European diabetic patients in the UK: the north-west diabetes foot care study. Diabetes Care. 2005;28:1869–75. doi: 10.2337/diacare.28.8.1869. [DOI] [PubMed] [Google Scholar]
- 3.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen. 2005;13:230–6. doi: 10.1111/j.1067-1927.2005.130303.x. [DOI] [PubMed] [Google Scholar]
- 4.Wrobel JS, Mauyfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the medicare population. Diabetes Care. 2001;24:860–4. doi: 10.2337/diacare.24.5.860. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 6.Malmstedt J, Leander K, Wahlberg E, Karlström L, Alfredsson L, Swedenborg J. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population-based cohort study. Diabetes Care. 2008;31:887–92. doi: 10.2337/dc07-2424. [DOI] [PubMed] [Google Scholar]
- 7.Ikonen TS, Sund R, Venermo M, Winell K. Fewer major amputations among individuals with diabetes in Finland in 1997–2007: a population-based study. Diabetes Care. 2010;33:2598–603. doi: 10.2337/dc10-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldner MG. The fate of the second leg in the diabetic amputee. Diabetes. 1960;9:100–3. doi: 10.2337/diab.9.2.100. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BL, Glickman MH, Bandyk DF, Esses GE. Failure of foot salvage in patients with end-stage renal disease after surgical revascularization. J Vasc Surg. 1995;22:280–5. doi: 10.1016/s0741-5214(95)70142-7. [DOI] [PubMed] [Google Scholar]
- 10.Ger R, Schessel ES. Prevention of major amputations in nonischemic lower limb lesions. J Am Coll Surg. 2005;201:898–905. doi: 10.1016/j.jamcollsurg.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg. 2005;113:1339–46. doi: 10.1097/01.prs.0000112746.67050.68. [DOI] [PubMed] [Google Scholar]
- 12.Attinger CE, Ducic I, Hess CL, Basil A, Abbruzzesse M, Cooper P. Outcome of skin graft versus flap surgery in the salvage of the exposed Achilles tendon in diabetics versus nondiabetics. Plast Reconstr Surg. 2006;117:2460–7. doi: 10.1097/01.prs.0000219345.73727.f5. [DOI] [PubMed] [Google Scholar]
- 13.Iorio ML, Goldstein J, Adams M, Steinberg J, Attinger C. Functional limb salvage in the diabetic patient: the use of a collagen bilayer matrix and risk factors for amputation. Plast Reconstr Surg. 2011;127:260–7. doi: 10.1097/PRS.0b013e3181f95c4b. [DOI] [PubMed] [Google Scholar]
- 14.Yeh JT, Lin CH, Lin YT. Skin grafting as a salvage procedure in diabetic foot reconstruction to avoid major limb amputation. Chang Gung Med J. 2010;33:389–96. [PubMed] [Google Scholar]
- 15.Lai CS, Lin SD, Yang CC, Chou CK, Wu SF, Chang CH. Limb salvage of infected diabetic foot ulcers with microsurgical free-muscle transfer. Ann Plast Surg. 1991;26:212–20. doi: 10.1097/00000637-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Pallua N, Di Benedetto G, Berger A. Forefoot reconstruction by reversed island flaps in diabetic patients. Plast Reconstr Surg. 2000;106:823–7. doi: 10.1097/00006534-200009040-00011. [DOI] [PubMed] [Google Scholar]
- 17.Giraldo F, De Haro F, Ferrer A. Opposed transverse extended V-Y plantar flaps for reconstruction of neuropathic metatarsal head ulcers. Plast Reconstr Surg. 2001;108:1019–24. doi: 10.1097/00006534-200109150-00034. [DOI] [PubMed] [Google Scholar]
- 18.Attinger CE, Ducic I, Cooper P, Zelen CM. The role of intrinsic muscle flaps of the foot for bone coverage in foot and ankle defects in diabetic and nondiabetic patients. Plast Reconstr Surg. 2002;110:1047–54. doi: 10.1097/01.PRS.0000021448.57210.52. [DOI] [PubMed] [Google Scholar]
- 19.Chen SL, Chen TM, Chou TD, Chang SC, Wang HJ. Distally based sural fasciomusculocutaneous flap for chronic calcaneal osteomyelitis in diabetic patients. Ann Plast Surg. 2005;54:44–8. doi: 10.1097/01.sap.0000141377.00807.16. [DOI] [PubMed] [Google Scholar]
- 20.Hong JP. Reconstruction of the diabetic foot using the anterolateral thigh perforator flap. Plast Reconstr Surg. 2006;117:1599–608. doi: 10.1097/01.prs.0000207057.16292.8f. [DOI] [PubMed] [Google Scholar]
- 21.Vega SJ, Sbitany H, Bossert RP. Vastus lateralis free flap for soft tissue coverage of a lower extremity diabetic ulcer. J Reconstr Microsurg. 2007;23:51–3. doi: 10.1055/s-2006-958703. [DOI] [PubMed] [Google Scholar]
- 22.Jiga LP, Barac S, Taranu G, Blidisel A, Dornean V, Nistor A, et al. The versatility of propeller flaps for lower limb reconstruction in patients with peripheral arterial obstructive disease: initial experience. Ann Plast Surg. 2010;64:193–7. doi: 10.1097/SAP.0b013e3181a72f8c. [DOI] [PubMed] [Google Scholar]
- 23.Ducic I, Attinger CE. Foot and ankle reconstruction: pedicled muscle flaps versus free flaps and the role of diabetes. Plast Reconstr Surg. 2011;128:173–80. doi: 10.1097/PRS.0b013e3182173d3a. [DOI] [PubMed] [Google Scholar]
- 24.Yan H, Yang M, Lineaweaver WC, Myers RS, Chen H, Zhang F. Flap reconstruction of distal lower extremity wounds in diabetic patients. Ann Plast Surg. 2011;67:426–8. doi: 10.1097/SAP.0b013e3181fab99e. [DOI] [PubMed] [Google Scholar]
- 25.Georgescu AV. Propeller perforator flaps in distal lower leg: evolution and clinical applications. Arch Plast Surg. 2012;39:94–105. doi: 10.5999/aps.2012.39.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geddes CR, Tang M, Yang D, Morris S. Anatomy of the integument of the lower extremity. In: Blondeel PN, Morris SF, Hallock GG, Neligan PC, editors. Perforator flaps: anatomy, technique & clinical applications. St. Louis, MO: Quality Medical Publishing, Inc; 2006. pp. 541–78. [Google Scholar]
- 27.Schaverien M, Saint-Cyr M. Perforators of the lower leg: analysis of perforator locations and clinical application for pedicled perforator flaps. Plast Reconstr Surg. 2008;122:161–70. doi: 10.1097/PRS.0b013e3181774386. [DOI] [PubMed] [Google Scholar]
- 28.Koshima I, Itoh S, Nanba Y, Tsutsui T, Takahashi Y. Medial and lateral malleolar perforator flaps for repair of defects around the ankle. Ann Plast Surg. 2003;51:579–83. doi: 10.1097/01.sap.0000095654.07024.65. [DOI] [PubMed] [Google Scholar]
- 29.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment. Clinical experience. Ann Plast Surg. 1997;38:563–77. [PubMed] [Google Scholar]
- 30.Gentzkow GD, Prendergast JJ, Iwaski S, Mengel M, Prendergast JJ, Ricotta JJ, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350–4. doi: 10.2337/diacare.19.4.350. [DOI] [PubMed] [Google Scholar]
- 31.Veves A, Falanga V, Armstrong DG. Graftskin, a human skin equivalent, is effective in the management of non-infected neuropathic diabetic foot ulcers. Diabetes Care. 2001;24:290–5. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 32.Steed DL. Clinical evaluation of recombinant human platelet derived groth factor for treatment of lower extremity diabetic ulcers. Diabetic ulcer study group. J Vasc Surg. 1995;21:71–8. doi: 10.1016/s0741-5214(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 33.Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, et al. The role of hyperbaric oxygen therapy in ischemic diabetic lower extremity ulcers: a double-blind randomized-controlled trial. Eur J Vasc Endovasc Surg. 2004;25:513–8. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- 34.Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Oriani G, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer a randomized study. Diabetes Care. 1996;19:1338–43. doi: 10.2337/diacare.19.12.1338. [DOI] [PubMed] [Google Scholar]
- 35.Wang CJ, Wu RW, Yang YJ. Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res Clin Pract. 2011;92:187–93. doi: 10.1016/j.diabres.2011.01019. [DOI] [PubMed] [Google Scholar]
- 36.Schaden W, Thiele R, Kolpl C, Pusch M, Nissan A, Attinger CE, et al. Shock wave therapy for acute and chronic soft tissue wounds: a feasible study. J Surg Res. 2007;143:1–12. doi: 10.1016/j.jss.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Wang CJ, Kuo YR, Wu RW, Liu RT, Hsu CS, Wang FS, et al. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J Surg Res. 2009;152:96–103. doi: 10.1016/j.jss.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Masquelet AC, Beveridge J, Romana C. The lateral supramalleolar flap. Plast Reconstr Surg. 1988;81:74–81. doi: 10.1097/00006534-198801000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Grabb WC, Argenta LC. The lateral calcaneal artery skin flap. Plast Reconstr Surg. 1981;68:723–30. doi: 10.1097/00006534-198111000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Baumeister SP, Spierer R, Erdmann D, Sweis R, Levin LS, Germann GK. A realistic complication analysis of 70 sural artery flaps in a multimorbid patient group. Plast Reconstr Surg. 2003;112:129–40. doi: 10.1097/01.PRS.0000066167.68966.66. [DOI] [PubMed] [Google Scholar]
- 41.Oishi SN, Levin LS, Pederson W. Microsurgical management of extremity wounds in diabetics with peripheral vascular disease. Plast Reconstr Surg. 1993;92:485–92. doi: 10.1097/00006534-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 42.May JW, Jr, Halls MH, Simon SR. Free microvascular muscle flaps with skin graft reconstruction of extensive defects of the foot: a clinical and gait analysis study. Plast Reconstr Surg. 1985;75:627–41. doi: 10.1097/00006534-198505000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Ulusal BG, Lin YT, Ulusal AE, Lin CH, Yen JT. Reconstruction of foot defects with free lateral arm fasciocutaneous flaps: analysis of fifty patients. Microsurgery. 2005;25:581–8. doi: 10.1002/micr.20176. [DOI] [PubMed] [Google Scholar]
- 44.Duffy FJ, Jr, Brodsky JW, Royer CT. Preliminary experience with perforator flaps in reconstruction of soft-tissue defects of the foot and ankle. Foot Ankle Int. 2005;26:191–7. doi: 10.1177/107110070502600302. [DOI] [PubMed] [Google Scholar]
- 45.Yang WG, Chiang YC, Wei FC, Feng GM, Chen KT. Thin anterolateral thigh perforator flap using a modified perforator microdissection technique and its clinical application for foot resurfacing. Plast Reconstr Surg. 2006;117:1004–8. doi: 10.1097/01.prs.0000200615.77678.f1. [DOI] [PubMed] [Google Scholar]
- 46.Hong JP, Shin HW, Kim JJ, Wei FC, Chung YK. The use of anterolateral thigh perforator flap in chronic osteomyelitis of the lower extremity. Plast Reconstr Surg. 2005;115:142–7. [PubMed] [Google Scholar]
- 47.Verhelle NA, Lemaire V, Nelissen X, Vandamme H, Heymans O. Combined reconstruction of the diabetic foot including revascularization and free-tissue transfer. J Reconstr Microsurg. 2004;20:511–7. doi: 10.1055/s-2004-836121. [DOI] [PubMed] [Google Scholar]
- 48.Wettstein R, Schurch R, Banic A, Erni D, Harder Y. Review of 197 consecutive free flap reconstructions in the lower extremity. J Plast Reconstr Aesthet Surg. 2008;61:772–6. doi: 10.1016/j.bjps.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 49.Koshima I, Moriguchi T, Ohta S, Hamanaka T, Inoue T, Ikeda A. The vasculature and clinical application of the posterior tibial perforator-based flap. Plast Reconstr Surg. 1992;90:643–9. doi: 10.1097/00006534-199210000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Tang M, Mao Y, Almutairi K, Morris SF. Three-dimensional analysis of perforators of the posterior leg. Plast Reconstr Surg. 2009;123:1729–38. doi: 10.1097/PRS.0b013e3181a3f376. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Wang X, Wen S, Zhu H, Ning Z, Mi X, et al. Posterior tibial artery-based multilobar combined flap free transfer for repair of complex soft tissue defects. Microsurgery. 2008;28:643–9. doi: 10.1002/micr.20529. [DOI] [PubMed] [Google Scholar]
- 52.Quaba O, Quaba AA. Pedicled perforator flaps for the lower limb. Semin Plast Surg. 2006;20:103–11. [Google Scholar]
- 53.Wu WC, Chang YP, So YC, Yip SF, Lam YL. The anatomic basis and clinical applications of flaps based on the posterior tibial vessels. Br J Plast Surg. 1993;46:470–9. doi: 10.1016/0007-1226(93)90220-6. [DOI] [PubMed] [Google Scholar]
- 54.Carriquiry C, Costa MA, Vasconez LO. An anatomic study of the septocutaneous vessels of the leg. Plast Reconstr Surg. 1985;76:354–63. doi: 10.1097/00006534-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Heitmann C, Khan FN, Levin LS. Vasculature of the peroneal artery: an anatomic study focused on the perforator vessels. J Reconstr Microsurg. 2003;19:157–62. doi: 10.1055/s-2003-39828. [DOI] [PubMed] [Google Scholar]
- 56.Wei FC, Chen HC, Chuang CC, Noordhoff MS. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg. 1986;78:191–200. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Hansen T, Wikstrom J, Johansson LO, Lind L, Ahlström H. The prevalence and quantification of atherosclerosis in an elderly population assessed by whole-body magnetic resonance angiography. Arterioscler Thromb Vasc Biol. 2007;27:649–54. doi: 10.1161/01.ATV.0000255310.47940.3b. [DOI] [PubMed] [Google Scholar]
- 58.Mehrotra S. Perforator-plus flaps: a new concept in traditional flap design. Plast Reconstr Surg. 2007;119:590–8. doi: 10.1097/01.prs.0000239570.18647.83. [DOI] [PubMed] [Google Scholar]
- 59.Parrett BM, Winograd JM, Lin SJ, Borud LJ, Taghinia A, Lee BT. The posterior tibial artery perforator flap: an alternative to free flap closure in the comorbid patient. J Reconstr Microsurg. 2009;25:105–9. doi: 10.1055/s-0028-1090616. [DOI] [PubMed] [Google Scholar]