Abstract
Autoimmune regulator (Aire) is one of the most well-characterized molecules in autoimmunity, but its function outside the immune system is largely unknown. The recent discovery of Aire expression in stem cells and early embryonic cells and its function in the self-renewal of embryonic stem (ES) cells highlight the importance of Aire in these cells. In this study, we present evidence that Aire promotes the expression of the pluripotent factor Lin28 and the self-renewal of ES cells. We presented the first evidence that the let-7 microRNA family contributed to the self-renewal promoting effect of Aire on ES cells. Moreover, we showed that Aire and Lin28 are co-expressed in the genital ridge, oocytes, and cleavage-stage embryos, and the expression level of Lin28 is correlated with the expression level of Aire. Although it is widely considered to be a promiscuous gene expression activator, these results indicated that Aire promotes the self-renewal of ES cells through a specific pathway (i.e., the activation of Lin28 and the inhibition of the let-7 microRNA family). The correlation between Aire and Lin28 expression in germ cells and early embryos indicated an in vivo function for Aire in toti- and pluripotent stem cells. This study presents the first molecular pathway that incorporates Aire into the pluripotency network. Moreover, it presents the first evidence that microRNAs contribute to the regulatory function of Aire and highlights a novel function of Aire in stem cell biology and reproduction. These functions reveal novel perspectives for studying the molecular mechanisms behind the establishment and sustenance of pluripotent identity.
Introduction
Self-renewal, defined as the sustenance of pluripotent differentiation potential while proliferating, is one of the most important properties of embryonic stem (ES) cells and the basis to acquire pluripotent stem cells for regenerative medicine [1]. The molecule mechanisms that promote the self-renewal of pluripotent stem cells have been subjected to intense study. A core of transcriptional circuits consisting of Oct4, Nanog, and Sox2 has been established to play central roles in the self-renewal process [2]. Recent data have extended this network to include much more transcriptional regulators [3]. Recent protein interaction studies have identified numerous interacting proteins with the core pluripotency factors [4]. A complex interacting network consisting of proteins from different functional categories such as transcriptional factors, chromatin structure modifiers, epigenetic modifiers, and RNA processors have been revealed as controlling the self-renewal of pluripotent stem cells [5]. In addition to the recent discovery of micro-RNAs as another important entity which regulates the self-renewal of pluripotent stem cells [6], the molecule network that sustains the self-renewal of pluripotent stem cells is complex and consists of molecules that regulate cell behavior at different levels. The integration of novel players into this network would greatly promote the understanding of how the pluripotency network sustains the self-renewal.
Autoimmune regulator (Aire) is considered a core regulator in immune tolerance, because it is an omnipotent gene that could activate thousands of genes with different tissue specificities in medullary thymic epithelium cells (mTECs) [7,8]. Mutation of the Aire gene compromises immune tolerance and causes an autoimmune syndrome called autoimmune polyendocrinopathy-candidiasis-ectodermal-dystrophy (APECED) [9,10]. However, we have recently identified the expression of Aire in ES cells and showed that the expression of Aire decreased with the differentiation of ES cells [11]. Data published by other groups and our unpublished observations have shown that Aire is expressed in blastomeres (from the 1-cell stage to blastocyst), induced pluripotent stem (iPS) cells, the embryonic genital ridge, and germ cells of both sexes [12]. Except for its restricted expression in mTECs and stromal cells of lymph nodes [13], Aire presented an expression profile that is very similar to the core pluripotency regulators such as Oct4 and Nanog. We have also shown that Aire knockdown attenuated the self-renewal of mouse ES (mES) cells and the expression of Oct4 and Nanog [11]. These data insinuated that the Aire gene is specific to pluripotent stem cells and actively participates in the pluripotency regulation network. Open chromatin state and global gene expression are considered specific characteristics for pluripotency, and general gene expression modulators, such as Wdr5 and TAF3, promote the self-renewal of ES cells [14–21]. Thus, it is worth investigating how an omnipotent expression activator such as Aire is integrated into the pluripotency regulation network and how it contributes to the self-renewal of ES cells.
Although the physiological role of Aire in autoimmunity is clear, the molecular mechanism of how Aire affects cellular behavior is not clear. Several models, including direct transcription regulation [22], chromatin modification [23], and activating genes by regulating developmental cell fate [24], have been proposed. Although there is supporting evidence for each model, a consensus has not been established. Moreover, Aire target genes are cell-type specific, with differing target profiles in mTECs, monocytes, and spermatogonias [7,25,26]. Therefore, pluripotent stem cell-specific target molecules and mechanisms could exist for Aire to regulate the gene expression and self-renewal of ES cells. Unraveling these targets and mechanisms would not only reveal new aspects of the pluripotency maintenance mechanism but also illuminate the deep implications of a general mechanism of Aire-dependent gene regulation.
Lin28 is a RNA binding protein that was originally characterized in a mutant screen of Caenorhabditis elegans as a development-regulating gene [27,28]. It has been proposed that Lin28 is an important mediator of the proliferation and self-renewal of ES cells [29,30]. More importantly, Lin28 is 1 of the 4 reprogramming factors initially used to produce human-induced pluripotent stem cells [31]. Unlike other core pluripotent regulators such as Oct4 and Nanog, which perform their functions by regulating transcription in the cell nucleus [2], Lin28 acts at the posttranscriptional level through 2 major mechanisms. First, Lin28 inhibits the maturation of the let-7 microRNA family by binding and modifying its precursors [32–34]. Let-7 microRNAs target and inhibit the expression of pluripotency and proliferation promoters such as HMGA2 and oppose the effects of a family of ESC cell-cycle-regulating miRNAs that maintain self-renewal [35,36]. Since these effects promoted the differentiation of ES cells, the inhibition of let-7 by Lin28 promotes the self-renewal of ES cells. Second, Lin28 promotes the self-renewal of ES cells by directly interacting with the mRNAs of pluripotency and cell-cycle regulators, including Oct4 and CDK4, to promote their ribosome recruitment and translation [30,37,38]. Although Lin28 is an important regulator of pluripotency and one of the few clearly characterized nodes that link the pluripotency network to microRNAs, the upstream regulators of Lin28 in pluripotent stem cells have not been determined. Delineating the regulators of Lin28 in pluripotent stem cells would significantly improve the understanding of the pluripotency sustenance network. Since Lin28 has been implicated as an active player in the reprogramming process that drives tumorgenesis [31,39,40], the characterization of regulators of Lin28 may also shed insight into the transforming mechanisms.
In this study, we report that Aire promotes the expression of Lin28 in ES cells. We demonstrate that Aire inhibits the expression of the let-7 microRNA family by activating Lin28, which sustains the undifferentiated state and promotes the proliferation of mES cells. Moreover, we show that Aire and Lin28 are co-expressed in germ cells and preimplantational mouse embryos, and the expression of Lin28 in genital ridges and oocytes correlates with the expression of Aire. To our knowledge, this is the first report of a molecular pathway that links Aire to pluripotency and the first link between Aire and the microRNA world. These results establish Aire as a novel player in the pluripotency network, present a new regulatory mechanism for the core pluripotency and reprogramming factor Lin28, and indicate microRNAs as novel targets of Aire.
Materials and Methods
Ethics statement
All animal work was conducted according to relevant national and international guidelines.
Cell lines and cell culture
Mouse embryonic fibroblast (MEF) feeder cells were isolated from 13.5 dpc ICR mice embryos. MEFs were routinely cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen) at 37°C in 5% CO2. MEFs were inactivated by Mitomycin C and plated at a density of 4×104 cells/cm2 for ES culture.
The mES D3 cell line was purchased from ATCC and cultured on mitomycin-inactivated MEFs in DMEM supplemented with 15% fetal bovine serum (Invitrogen) and 1000 ng/mL LIF (Millipore) at 37°C in 5% CO2. The pluripotency of the mES cells was routinely analyzed with an ALP staining Kit (Sigma-Aldrich) for SSEA-1 staining and teratoma formation. In addition, the karyotype was routinely checked.
Embryonic bodies were prepared by suspension culture in DMEM supplemented with 10% fetal bovine serum (Invitrogen) at 37°C in 5% CO2 and recovered after 6 days' differentiation for colony formation assay and real-time polymerase chain reaction (PCR) assay.
Animals and housing
The heterozygous Aire-knockout mice strain (C57BL6/J background; Jackson 004743) was purchased from Jackson Laboratories and bred in house [7]. Homozygous Aire-knockout mice were generated by breeding heterozygous pairs.
Antibodies
The following antibodies were used in this study: Rat anti-mouse Aire monoclonal antibody (eBioscience), rabbit anti-Lin28A monoclonal antibody (Cell Signaling), rabbit anti-mouse Aire polyclonal antibody (Huabio), rabbit anti-GAPDH polyclonal antibody (Huabio), rabbit anti-Oct4 polyclonal antibody (Huabio), Alexa 488-conjugated goat anti-rat IgG (Invitrogen), and Alexa 555-conjugated goat anti-rabbit IgG (Invitrogen).
Plasmid construction and lentivirus production
The full-length coding regions of mouse Aire and Lin28 genes were isolated from mES cDNAs by PCR amplification and cloned into a pCS-UBC lentivirus vector plasmid, which was constructed in house, derived from the pCS plasmid (Addgene plasmid 12158) by enzymatic digestion [41]. Cloned gene expression was under the control of the human ubiquitin promoter (UBC). ShRNA coding DNA sequences were obtained from the RNAi Consortium (www.broadinstitute.org/rnai/trc) and synthesized by Sangon (Shanghai) (Table 1). A pLKO TRC-cloning vector (plasmid 10878) was obtained from Addgene [42].
Table 1.
shRNA Sequences Used in this Study
| Name | Sequence |
|---|---|
| scramble | CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG |
| shAire-1 | CCGGCCAGTGGCAATTTGAAGAACACTCGAGTGTTCTTCAAATTGCCACTGGTTTTTG |
| shAire-2 | CCGGCCGAGACACCACCCTTCTCTTCTCGAGAAGAGAAGGGTGGTGTCTCGGTTTTTG |
| shLin28-1 | CCGGCCCAGTAAGAATGCAACTTAACTCGAGTTAAGTTGCATTCTTACTGGGTTTTTG |
| shLin28-2 | CCGGCACCTTTAAGAAGTCTGCCAACTCGAGTTGGCAGACTTCTTAAAGGTGTTTTTG |
shRNA sequences
For lentivirus packaging, plasmids were co-transfected with PsPAX (Addgene plasmid 12260), pMD2. G (Addgene plasmid 12259) packaging plasmids into HEK293T cells. Lentivirus was collected in an mES culture medium and used to infect mES cells after the supplementation of 4 μg/mL Polybrene (Sigma).
Genital ridge isolation
Aire+/+ C57BL6/J female mice were superovulated by consecutive injections, which were spaced 44–48 h apart, containing 5 IU PMSG and 5 IU hCG and crossed with Aire+/+ C57BL6/J males. Virginal plugs were checked to confirm successful mating. Genital ridges were isolated from 12.5 dpc embryos under a dissecting microscope, and the genders of genital ridges were identified with morphology criteria.
Oocyte and early embryo isolation and immunofluorescent staining
For oocyte isolation, Aire+/+ and Aire−/− female C57BL6/J mice were stimulated by an intraperitoneal injection of 7.5 IU of PMSG. After 46–48 h, germinal vesicle (GV) oocytes were isolated by puncturing follicles in M2 medium (Sigma). For preimplantational embryo isolation, Aire+/+ C57BL6/J female mice were superovulated by consecutive injections of 5 IU PMSG and 5 IU hCG (44–48 h between injections) and crossed with Aire+/+ C57BL6/J males. Twenty-four hours and 48 h after an injection of 5 IU hCG, successfully pregnant females were used for collecting zygotes (day 0.5) and 2-cell embryos (day 1.5), respectively.
GV oocytes, zygotes, and 2-cell embryos were fixed and permeabilized with Fix/Perm buffer from the Foxp3 staining buffer set (eBioscience) for 1 h at 37°C. The oocytes and embryos were blocked for 45 min at 37°C in a blocking buffer (Perm buffer; eBioscience) supplemented with 5% normal goat serum. After blocking, the oocytes and embryos were consecutively stained with the rat anti-mouse Aire monoclonal antibody (eBioscience), Alexa 488-conjugated goat anti-rat IgG (Invitrogen), rabbit anti-Lin28A monoclonal antibody (Cell Signaling), and Alexa 555-conjugated goat anti-rabbit IgG (Invitrogen). The oocytes and embryos were then mounted in VECTASHIELD HardSet Mounting Medium with 4′,6-diamidino-2-phenylindole. The samples were examined under an LSM 710 Confocal Microscope (Zeiss).
Colony formation assay
For the colony formation assay of ES cells, mES cells were seeded on 24-well cell culture plates on feeder cells at a density of 600 cells per well and grown for 4 days. Alkaline phosphatase (ALP) staining was performed with an ALP assay kit (Sigma). Macroscopic pictures were taken with a digital camera (Panasonic DMC-ZS1). The number of ALP-positive clones was counted under a phase-contrast microscope. The results are displayed as statistical averages of 3 independent experiments.
For colony formation assay of embryonic body (EB) cells differentiated for 6 days, embryonic bodies were collected and dissociated to single cells by trypsinization. The cells were then seeded on 24-well cell culture plates on feeder cells at a density of 3000 cells per well and grown for 8 days. ALP staining was performed with an ALP assay kit (Sigma). The number of ALP-positive clones was counted under a phase-contrast microscope. The results are displayed as statistical averages of 3 independent experiments.
Real-time reverse transcriptase-PCR
Total RNA was isolated with Trizol reagent, and cDNAs were synthesized with the ReverTra Ace® qPCR RT Kit (Toyobo). Tests were performed with SYBR Green reagents purchased from Toyobo and primers acquired from the Primer Bank (http://pga.mgh.harvard.edu/primerbank/), which are listed in Table 2.
Table 2.
Primers for Protein Coding Genes Used in this Study
| Name | Sense sequence | Anti-sense sequence |
|---|---|---|
| Aire FL | GGGGTACCATGGCAGGTGGGGATGGAATG | GCTCTAGATCAGGAAGAGAAGGGTGGTGTC |
| Lin28 FL | GCGAATTCATGGGCTCGGTGTCCAACCAG | GGATCGATTCAATTCTGGGCTTCTGGGAGCA |
| Aire qPCR | AGGTCAGCTTCAGAGAAAACCA | TCATTCCCAGCACTCAGTAGA |
| Oct4 qPCR | CAGCCAGACCACCATCTGTC | GTCTCCGATTTGCATATCTCCTG |
| Nanog qPCR | TTGCTTACAAGGGTCTGCTACT | ACTGGTAGAAGAATCAGGGCT |
| Lin28 qPCR | GGCATCTGTAAGTGGTTCAACG | CCCTCCTTGAGGCTTCGGA |
| Gata6 qPCR | CATCACCATCACCCGACCTAC | GGCCCTGTAAGCTGTGGAG |
| Brachyuary qPCR | GGATTCACATCGTGAGAGTTGG | GTCACAGCTATGAACTGGGTC |
| Gata4 qPCR | CAAACCTCAATGTGTCTCTTTGC | AGAGTAAAGCCTATCTCGCTGT |
| Sox7 qPCR | TGGACACGTATCCCTACGGG | TCCTGACATGAGGACGAGAAG |
| Nestin qPCR | CCCCTTGCCTAATACCCTTGA | GCCTCAGACATAGGTGGGATG |
| Fgf5 qPCR | GAAGCGTCTCACTCCCGAAG | GAAGAAAACGTCGCGCTACT |
Small RNAs were isolated with the mirVana™ miRNA Isolation Kit (Ambion), according to the manufacturer's instructions. The small RNAs were tailed with Escherichia coli polyA polymerase (Ambion) and reverse transcribed using the ReverTra Ace® qPCR RT Kit (Toyobo) with a tagged polyA primer (5′-ATTCTAGAGGCCGAGGCGGCCGACA TGT(24)VN-3′). MicroRNA qPCR was performed using SYBR Green reagents (Toyobo) with specific forward primers acquired from the Invitrogen database (Table 3) and a general reverse primer (5′-ATTCTAGAGGCCGAGGCGGC-3′).
Table 3.
Primers for microRNAs
| Name | Sense sequence | Anti-sense sequence |
|---|---|---|
| mmu-let-7a | GCGGTGAGGTAGTAGGTTGTATAGTT | ATTCTAGAGGCCGAGGCGGC |
| mmu-let-7b | CCTGAGGTAGTAGGTTGTGTGGTT | |
| mmu-let-7c | CGCTGAGGTAGTAGGTTGTATGGTT | |
| mmu-let-7d | GCAGAGGTAGTAGGTTGCATAGTT | |
| mmu-let-7e | GGCTGAGGTAGGAGGTTGTATAGTT | |
| mmu-let-7f | GCCCCTGAGGTAGTAGATTGTATAGTT | |
| mmu-let-7g | GGCCTGAGGTAGTAGTTTGTACAGTT | |
| mmu-let-7i | GCTGAGGTAGTAGTTTGTGCTGTT | |
| mmu-sno-U6 | TGCTCGCTTCGGCAGCACATATAC |
All real-time PCR analyses were performed on a RealPlex PCR apparatus (Eppendorf).
Western blotting
Western blotting was performed as previously described [43]. ES cells were harvested in lysis buffer consisting of 50 mM Tris-HCl pH 7.4, 1% NP-40, and 1% sodium dodecyl sulfate (SDS). Equal amounts of the protein lysate were separated by electrophoresis on a 12.5% Laemmli SDS-polyacrylamide gel and then transferred onto polyvinylidene fluoride membranes. The membranes were developed with an ECL kit purchased (GE Healthcare Life Sciences, Piscataway, NJ) after incubation with primary and secondary antibodies.
Immunofluorescence
Immunofluorescence was performed as previously described [43]. The subjects were observed, and images were acquired under an LSM 710 Confocal Microscope (Zeiss).
Quantifications and statistics
The fluorescence strength of the immunofluorescent stainings was measured by Magnification software (Orbicule). Intergroup differences were assessed by student's t-test using the StatView 5.0 program against control groups (SAS Institute Inc.); *P<0.05, **P<0.01. The column charts were drawn by Origin 8.0 software (OriginLab).
Results
Aire promoted the self-renewal of mES cells
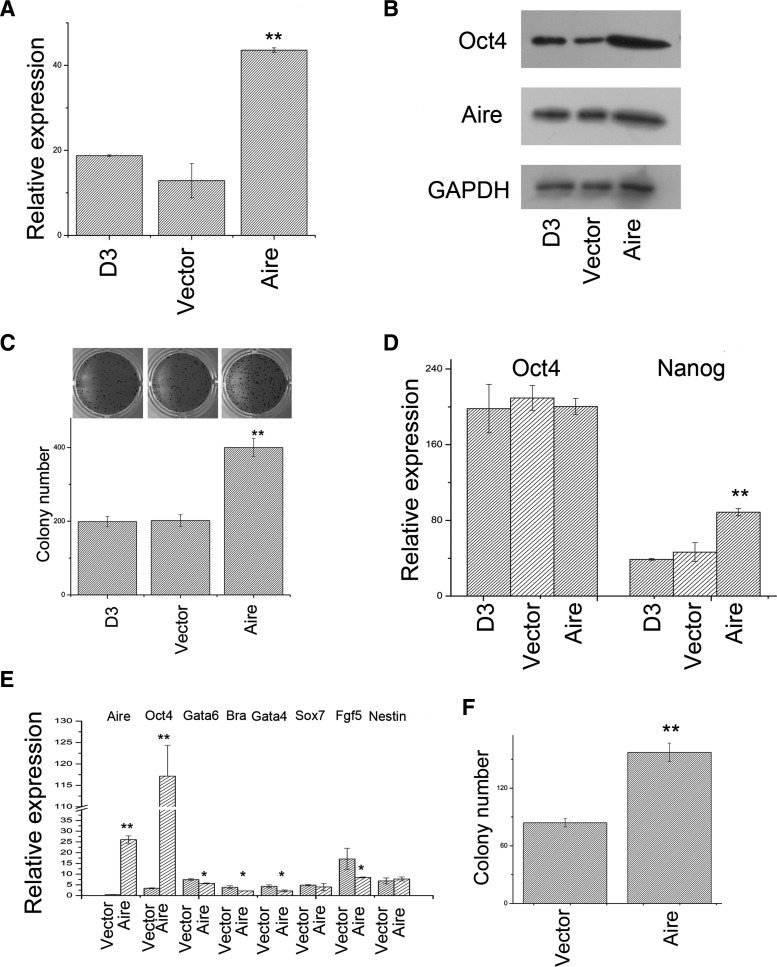
We explored the function of Aire in ES cells by gene overexpression. As shown in Fig. 1A, the infection of mES cells with lentiviral vector encoding the mouse Aire gene significantly enhanced the expression of Aire mRNA. Western blotting demonstrated that the Aire protein level significantly increased on gene overexpression in mES cells (Fig. 1B). ES cell self-renewal was examined with different assays. First, we examined the ability of mES cells to form ALP-positive colonies after Aire overexpression. As shown in Fig. 1C, Aire overexpression significantly enhanced the ability of mES cells to form ALP-positive colonies. Next, we examined the expression of 2 core pluripotent genes, Oct4 and Nanog, in the context of Aire overexpression. Real-time reverse transcriptase PCR (RT-PCR) analysis revealed that Aire overexpression significantly enhanced the mRNA expression of the pluripotent marker Nanog, while the Oct4 expression was not significantly altered (Fig. 1D). We further examined the Oct4 protein levels in mES cells on Aire overexpression with western blotting. As shown in Fig. 1B, the level of Oct4 protein in the mES cells significantly increased after Aire overexpression. Therefore, Aire overexpression enhanced the colony genicity of mES cells and promoted the transcriptional expression of some pluripotent genes (e.g., Nanog), while posttranscriptionally regulated other pluripotent genes (e.g., Oct4). We also examined the differentiation capacity of mES cells on Aire overexpression by examining the expression of several germ layer marker genes in embryonic bodies. Embryonic bodies were derived from vector-infected and Aire lentivirus-infected cells. As shown in Fig. 1E and consistent with our previous report, Aire expression was eliminated 6 days after differentiation [11], while in Aire-overexpressing cells, the expression level of Aire 6 days after differentiation is similar to that of undifferentiated cells. On Aire overexpression, the day 6 EBs possessed significantly lower expression of the trophoblast marker Gata6 [44], mesoderm marker Brachyuary [45], and endoderm marker Gata4 [46], while the expression level of ectoderm marker Fgf5 differed with marginal significance [47] (P=0.041). The expression levels of ectoderm marker Nestin and primitive endoderm marker Sox7 were not affected [48,49]. Since there is still a certain level of expression of these germ layer markers in EBs derived from Aire-overexpressing ES cells, these cells still possessed the ability to differentiate into the 3 germ layers and extraembryonic cells. We checked whether the pluripotent gene Oct4 was properly shut down during EB differentiation after Aire overexpression. As shown in Fig. 1E, the expression of Oct4 gene was significantly decreased after differentiation in vector-infected cells, but it is sustained at a high level in Aire-overexpressing cells. To further confirm that Aire promoted the self-renewal of ES cells in differentiation promoting conditions, we examined the ability of the cells from day 6 EBs to form ALP-positive colonies. As shown in Fig. 1F, the overexpression of Aire significantly enhanced the colony formation ability of d6 EB cells. These results indicated that Aire promoted the self-renewal proliferation of mES cells in conditions that encouraged self-renewal. Moreover, Aire quantitatively inhibited differentiation and promoted self-renewal in conditions that encouraged differentiation.
FIG. 1.
Aire promoted the self-renewal of embryonic stem (ES) cells: (A) Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of Aire expression level in normal mouse ES (mES) cells (D3), vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). The expression level is shown as relative to β-actin expression (n=3, *P<0.05, **P<0.01). (B) Western blotting analysis of GAPDH, Aire, and Oct4 protein expression in normal mES cells (D3), vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). (C) Colony genicity of normal mES cells (D3), vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). The upper panel shows macroscopic pictures of one representative well of each group. The lower panel shows statistics (n=3, *P<0.05, **P<0.01). (D) Real-time RT-PCR analysis of Oct4 and Nanog expression levels in normal mES cells (D3), vector-transfected mES cells (Vector), and Aire gene-transfected mES cells (Aire). The target gene expression level is shown as relative to β-actin expression (n=3, *P<0.05, **P<0.01). (E) Real-time RT-PCR analysis of the expression levels of Aire, Oct4, and germ layer marker genes in day 6 embryonic bodies (EBs) derived from vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). The expression levels are shown as relative to β-actin expression (n=3, *P<0.05, **P<0.01). (F) Colony genicity of day 6 EB cells derived from vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire) (n=3, *P<0.05, **P<0.01).
Aire promoted the expression of Lin28 in mES cells
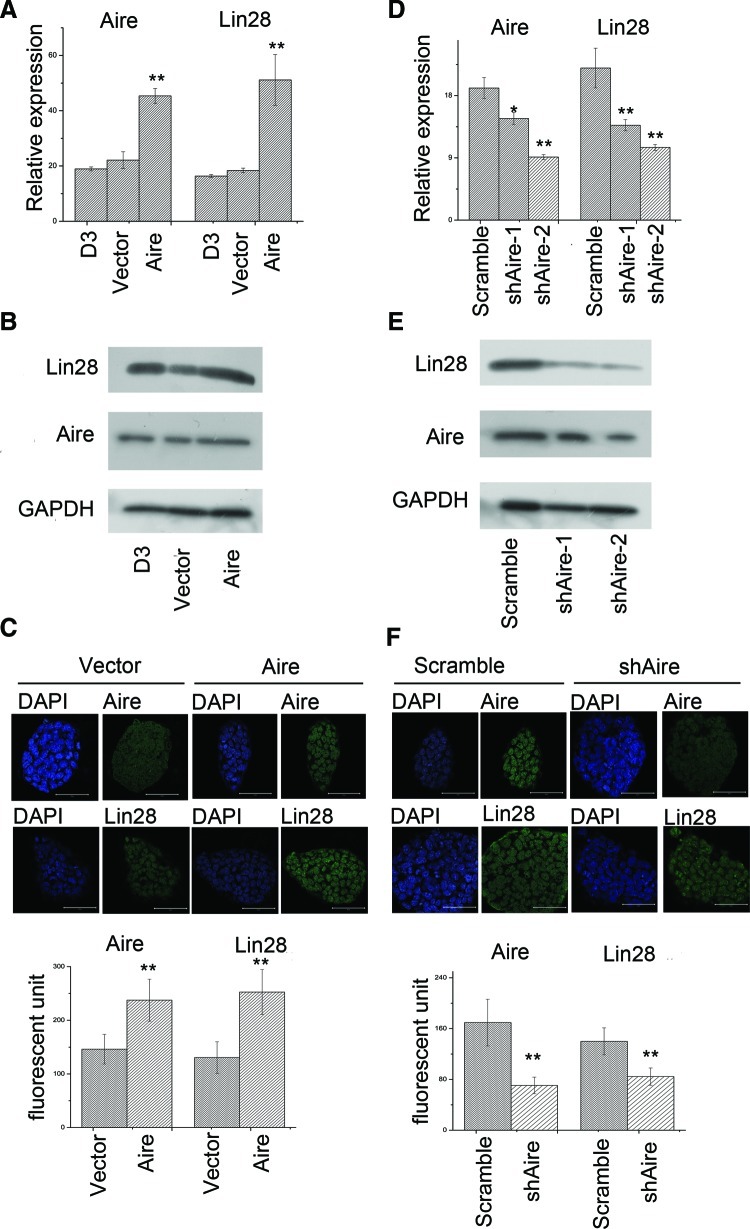
While dissecting the molecular mechanism by which Aire promoted the self-renewal of ES cells, we considered that Aire-induced posttranscriptional expression of Oct4 was a clue and searched for molecules that posttranscriptionally regulate the expression of Oct4. Since Lin28 has been proposed to promote the translation of Oct4 by triggering its ribosome recruitment [37], we examined the expression of Lin28 after Aire overexpression. As shown in Fig. 2A, the expression of Lin28 was significantly enhanced after Aire overexpression. Western blotting analysis revealed that Lin28 protein expression in the mES cell population increased significantly after Aire overexpression (Fig. 2B). Moreover, immunofluorescent analysis revealed that Aire overexpression significantly elevated the expression of Lin28 in situ. Since we have already demonstrated that Aire knockdown attenuated the self-renewal ability of ES cells [11], we sought to determine whether it also attenuated the expression of Lin28. As shown in Fig. 2D, the expression of Lin28 mRNA was significantly attenuated in ES cells by Aire knockdown. The expression of Lin28 protein was also attenuated by Aire knockdown, as shown in Fig. 2E. Immunofluorescence analysis determined that the Lin28 expression was significantly decreased on Aire knockdown. These results indicated that Aire promotes the expression of Lin28, and since Lin28 is vital for the self-renewal and reprogramming process [29–31], we speculated that the activation of Lin28 expression contributed significantly to the self-renewal promoting effect of Aire.
FIG. 2.
Aire promoted the expression of Lin28 in ES cells. (A) Real-time RT-PCR analysis of the Aire and Lin28 expression in normal mES cells (D3), vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). The expression level was shown as relative to β-actin expression (n=3, *P<0.05, **P<0.01). (B) Western blotting analysis of the expression of GAPDH, Aire, and Lin28 protein in normal mES cells (D3), vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). (C) Immunofluorescence analysis of the expression of Aire and Lin28 in vector-infected mES cells (Vector), and Aire encoding lentivirus-infected mES cells (Aire). The upper panel displays figures of representative colonies (bar=50 μm). The lower panel shows statistical analysis according to the same random fluorescent unit (n=30, *P<0.05, **P<0.01). (D) Real-time RT-PCR analysis of Aire and Lin28 expression in scrambled shRNA-infected mES cells (scramble) and mES cells infected with lentivirus encoding 2 Aire shRNAs (shAire-1 and shAire-2). The expression level was shown as relative to β-actin expression (n=3, *P<0.05, **P<0.01). (E) Western blotting analysis of GAPDH, Aire, and Lin28 protein expression in scrambled shRNA-infected mES cells (scramble) and mES cells infected with lentivirus encoding 2 Aire shRNAs (shAire-1 and shAire-2). (F) Immunofluorescence analysis of Aire and Lin28 expression in scrambled shRNA-infected mES cells (scramble) and mES cells infected with lentivirus encoding shAire-2 shRNA (shAire). The upper panel shows figures of representative colonies (bar=50 μm). The lower panel shows the statistical analysis according to the same random fluorescent unit (n=30, *P<0.05, **P<0.01). Color images available online at www.liebertpub.com/scd
Aire promoted the self-renewal of mES cells by activating Lin28
With the aim of validating that Lin28 activation contributed to the effect of Aire on mES cell self-renewal, we performed genetic rescue experiments. First, we knocked down Lin28 expression in Aire-overexpressing mES cells. As shown in Fig. 3A and B, Lin28 mRNA and protein levels were recovered by 2 shRNAs targeting Lin28 to the level of vector-transfected mES cells. A colony genicity assay was performed to examine self-renewal ability. As shown in Fig. 3C, the colony genicity of the Aire-overexpressing cells was significantly attenuated after Lin28 knockdown. A further confirmation was made by overexpressing Lin28 in Aire-knockdown mES cells. As shown in Fig. 3D and E, after infection with lentiviral vectors encoding mouse Lin28, the Lin28 expression in Aire-knockdown mES cells was similar to or a little higher than in the scrambled shRNA transgenic mES cells. The colony genicity was assayed to examine self-renewal ability. As shown in Fig. 3F, while the colony genicity of Aire knockdown mES cells was significantly lower than in scrambled shRNA transgenic cells, Lin28 overexpression in these mES cells rescued colony genicity from Aire knockdown. These results indicated that the activation of Lin28 expression significantly contributed to the self-renewal promoting effect of Aire in mES cells.
FIG. 3.
Aire promoted the self-renewal of ES cells through Lin28: (A). Real-time RT-PCR analysis of the expression level of Lin28 in vector-infected mES cells (Vector), Aire encoding lentivirus-infected mES cells (Aire), mES cells co-infected with Aire-encoding lentivirus and scrambled shRNA-encoding lentivirus (Aire+scr), and mES cells co-infected with Aire encoding lentivirus and 2 Lin28 shRNAs encoding lentivirus (Aire+shL1 and Aire+shL2) cells. The expression levels are displayed as relative to β-actin expression (n=3, *P<0.05, **P<0.01). (B) Western blotting analysis of GAPDH and Lin28 protein expression in vector-infected mES cells (Vector), Aire encoding lentivirus-infected mES cells(Aire), mES cells co-infected with Aire encoding lentivirus and scrambled shRNA encoding lentivirus (Aire+scr), or mES cells co-infected with Aire encoding lentivirus and one of 2 Lin28 shRNA encoding lentiviruses mES cells (Aire+shL1 and Aire+shL2). (C) Colony genicity of vector-infected mES cells (Vector), Aire encoding lentivirus-infected mES cells (Aire), mES cells co-infected with Aire encoding lentivirus and scrambled shRNA encoding lentivirus (Aire+scr), mES cells co-infected with Aire encoding lentivirus, and 2 Lin28 targeting shRNA encoding lentivirus (Aire+shL1 and Aire+shL2) cells (n=3, *P<0.05, **P<0.01) (D) Real-time RT-PCR analysis of the expression level of Lin28 in scrambled shRNA encoding lentivirus-infected ES cells (scramble), shAire-2 encoding lentivirus-infected ES cells(shAire), ES cells co-infected with 2 lines of shAire-2 encoding lentivirus, and Lin28 encoding lentivirus co-infected(shAire+L1 and shAire+L2). The expression level is shown relative to β-actin expression (n=3, *P<0.05, **P<0.01). (E) Western blotting analysis of the GAPDH and Lin28 protein expression in scrambled shRNA encoding lentivirus-infected ES cells (scramble), shAire-2 encoding lentivirus-infected ES cells (shAire), and ES cells co-infected with 2 lines of shAire-2 encoding lentivirus along with Lin28 encoding lentivirus (shAire+L1 and shAire+L2). (F) Colony genicity of scrambled shRNA encoding lentivirus-infected ES cells (scramble), shAire-2 encoding lentivirus-infected ES cells (shAire), and 2 lines of shAire-2 encoding lentivirus and Lin28 encoding lentivirus co-infected (shAire+L1 and shAire+L2) ES cells (n=3, *P<0.05, **P<0.01).
Aire regulated let-7 micro RNAs
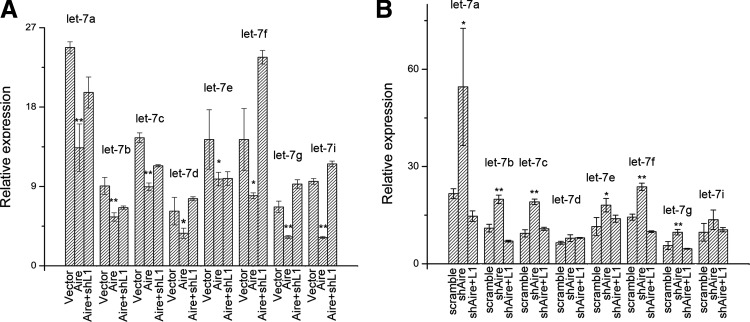
Previous reports demonstrated that Lin28 inhibits the maturation of the differentiation-promoting let-7 microRNA family and promotes ES cell self-renewal [29,32]. The inhibition of the let-7 microRNA family by Lin28 has also been considered vital for its function as a reprogramming factor and oncogene [31,39,40]. Since we have shown that Aire promoted Lin28 expression in mES cells, we investigated whether Aire regulated the levels of mature let-7 microRNAs. First, we examined the expression of let-7 microRNAs in mES cells after Aire overexpression. As displayed in Fig. 4A, the expression of all 8 let-7 family microRNAs was significantly lower in Aire-overexpressing mES cells compared with vector-infected cells. Subsequently, we examined the level of let-7 microRNAs in mES cells after Aire knockdown. As shown in Fig. 4B, the Aire knockdown in mES cells resulted in a significant increase of the let-7 microRNA family except for the let-7d and let-7i, which also showed an increasing trend. To further confirm that Aire regulated the let-7 microRNA family indirectly through Lin28, genetic rescue experiments were performed. As shown in Fig. 4A, when Lin28 was knocked down in Aire overexpressing mES cells, the level of most let-7 microRNAs increased and approached the level in vector-infected cells. As depicted in Fig. 4B, when Lin28 was overexpressed in Aire knockdown mES cells, the level of most let-7 microRNAs decreased and approached the level in scramble shRNA-infected cells. These results indicated that Aire inhibited the maturation of the let-7 microRNA family through Lin28, and the inhibition of the let-7 microRNA family contributed to the self-renewal promoting effect of Aire.
FIG. 4.
Aire regulated the level of let-7 group microRNA in ES cells. (A) Real-time RT-PCR analysis of let-7 family microRNA expression in vector-infected mES cells (Vector), Aire encoding lentivirus-infected mES cells (Aire), and mES cells co-infected with Aire encoding lentivirus and a Lin28 targeting shRNA encoding lentivirus (Aire+shL1). Target gene expression level is shown relative to sno-U6 small RNA (n=3, *P<0.05, **P<0.01) (B) Real-time RT-PCR analysis of let-7 family microRNA expression in scrambled shRNA encoding lentivirus-infected ES cells (scramble), shAire-2 encoding lentivirus-infected ES cells (shAire), and mES cells co-infected with shAire-2 encoding lentivirus and a Lin28 encoding lentivirus (shAire+L1). Target gene expression level is shown relative to sno-U6 small RNA (n=3, *P<0.05, **P<0.01).
In vivo relationships between Aire and Lin28
The embryonic genital ridge is one of the most important sources of pluripotent embryonic germ cells [50,51]. Since Aire is expressed in germ cells and Lin28 possesses the ability to sustain a pluripotent state [26,29], we examined whether Aire regulates Lin28 in germ cells. Aire and Lin28 expression was examined with real-time RT-PCR in genital ridges isolated from 12.5 dpc embryos of both sexes. As shown in Fig. 5A, Aire and Lin28 were expressed in the genital ridges of both sexes at a much higher level than in ES cells. Moreover, Aire expression in the female genital ridges was significantly higher than in the male ones. Concomitantly, Lin28 expression levels in female genital ridges were significantly higher than in the male ones. This positive correlation between Aire and Lin28 in genital ridges indicated a positive effect of Aire on Lin28 in embryonic germ cells. Subsequently, we compared the Aire and Lin28 expression by immunofluorescent staining in oocytes in wild-type (Wt) and Aire-knockout (Mut) mice. As shown in Fig. 5B, Aire was present in oocytes from wild-type mice but was absent in oocytes from Aire-knockout mice. This finding implied that Aire was maternally stored in the oocytes of normal mice, while maternal proteins in Aire-knockout mice were depleted. A direct observation revealed a weaker Lin28 staining in oocytes from Aire-knockout mice relative to wild-type mice, and statistical analysis revealed a significantly lower fluorescent strength of Lin28 staining in Aire-knockout oocytes than in wild-type mice. However, Lin28 was still present in Aire-knockout oocytes, which suggests that Aire was not exclusively required for the expression of Lin28 but quantitatively regulated the expression of Lin28 in oocytes. Since Aire and Lin28 were stored in oocytes as maternal proteins, we examined the presence of Aire and Lin28 in totipotent mouse blastomeres before the major maternal embryo transition. As shown in Fig. 5C, Aire and Lin28 were observed in the cytoplasm of fertilized mouse eggs and 2-cell embryos. Interestingly, Aire and Lin28 demonstrated foci-shaped colocalization in the early embryos, which suggested functional interactions. These results indicated that Aire and Lin28 are co-expressed in germ cells, totipotent cells, and pluripotent stem cells. Moreover, Aire quantitatively regulated the expression of Lin28.
FIG. 5.
Aire and Lin28 in germ cells and early embryos. (A) Real-time RT-PCR analysis of Aire and Lin28 expression in the 12.5 dpc male genital ridge (M) and the 12.5 dpc female genital ridge (F). Target gene expression level is shown relative to β-actin expression (n=3, *P<0.05, **P<0.01). (B) Immunofluorescence analysis of Aire and Lin28 expression in germinal vesicle (GV) stage oocytes from wild-type (Wt) and Aire-knockout (Mut) mice. The left panel shows staining of a representative oocyte. The right panel shows statistical analysis according to the same random fluorescent unit (n=9, *P<0.05, **P<0.01). (C) Immunofluorescence analysis of Aire and Lin28 expression in 1-cell-stage and 2-cell-stage mouse embryos. Embryos from both stages expressed Aire and Lin28. Color images available online at www.liebertpub.com/scd
Discussion
A novel player in the pluripotency network
The molecular network that defines and sustains pluripotency has been intensely studied. There had been a widely accepted network centered on a self-regulated circuit, comprising Oct4, Nanog, and Sox2, which served as a major sustaining force of the pluripotent state of stem cells [2]. However, recent studies have revealed many novel sides of the pluripotency network. Molecular entities such as histone modifiers [20,52], chromatin structure remodelers [53,54], general transcription factors [21], and microRNAs [6] have been shown to critically regulate pluripotency maintenance and reprogramming. These results indicated that the pluripotency network is a multifaceted network that comprises regulators functioning at various levels of regulation. The discovery of novel players in this network would greatly improve the understanding of pluripotency. Since open chromatin structure and promiscuous transcription have been proposed to be a basic property of the pluripotent state [14], it is expected that the regulator of these properties should function in the pluripotency network. In this study, we have demonstrated that the promiscuous transcription regulator Aire is a novel player in the pluripotency network. Along with our previous observation that Aire promoted the expression of tissue-specific genes in ES cells [11], a link between the pluripotency networks, open chromatin, and promiscuous transcription is now established. We also highlight the functional significance of the first phase of the biphasic Aire expression pattern in development: preimplantational totipotent cells and pluripotent stem cells [12]. Moreover, we have unexpectedly observed that despite being a promiscuous transcription regulator, Aire is integrated into the pluripotency network though a specific transmitter, Lin28. This finding suggests that promoting promiscuous transcription and pluripotency maintenance are 2 parallel functions of the Aire gene. Our data indicated that the promiscuous transcription regulator Aire specifically promoted the expression of Lin28 at the transcriptional level. Although many different mechanisms including direct transcription initiation activation through CBP interaction [22], transcription elongation sustenance through p-TEFb interaction [55], and epigenetic activation have been proposed to be responsible for the transcription activating effect of Aire [23], but most of them seemed not to be specific to ES cells. It is possible that this specific effect is related to the specific transcriptional network of ES cells, but more confirmation is needed. The parallel function of Aire in ES cells for promiscuous transcription promotion and pluripotency sustenance also begs several questions. Why has evolution integrated these 2 seemingly independent functions into a single gene? How are these 2 functions differentially conducted and regulated? Do these 2 functions still co-exist in adult cells, similar to mTECs, and, consequently, support the developmental model of Aire function in the thymus? Since Lin28 is a major mediator of reprogramming of differentiated cells into induced pluripotent stem cells [31], the results suggested the potential of Aire as a new component of the cocktail used by regenerative medicine researchers to reprogram adult cells. Since Aire is in the center of a multi-molecular network consisting of chromatin remodelers, transcription regulators, and RNA processors [56], another important contribution of this work to the understanding of pluripotency network is that the integration of Aire into the pluripotency network would significantly expand the network and emphasize more new players.
Aire interacts with microRNAs
Protein coding genes and microRNAs are 2 major clades of functional molecules in biological systems. MicroRNAs regulate a wide range of biological processes, including but not excluded to cell proliferation [57], stem cell self-renewal [6], embryonic development [58], and immune response [59]. Specifically, it had been proposed that pluripotent stem cells possess characteristic microRNA expression profiles, and microRNAs (e.g., mir-294 and mir-295) promote the self-renewal of pluripotent stem cells and cell reprogramming [6, 60]. Although long held as a promiscuous activator of the expression of protein coding genes, there is no evidence of a functional relationship between Aire and microRNAs. In this work, we presented the first evidence that Aire could regulate the level of the let-7 microRNA family though the regulation of the microRNA binding protein Lin28. As we have shown, the regulation of let-7 miRNAs by Aire is functionally transmitted to the pluripotency maintenance effect on ES cells. These results added a new level to the complexity of the regulation mechanism of Aire on its targets and could help explain some observations in the field. For example, Gillard et al. have shown that the pluripotency marker genes Oct4 and Sox2 were expressed in normal mouse mTECs, while expression was abrogated in Aire-knockout mTECs [24]. We have also observed that Aire knockdown in mES cells resulted in attenuated expression of Oct4 and Nanog [11,24]. Since Oct4, Sox2, and Nanog were not among the canonical Aire-dependent tissue-specific genes, our discovery might indicate that the regulation of miRNAs, similar to the let-7 family, potentially contributes to these observations. Whether Aire also regulated miRNAs other than the let-7 family and in cell types other than ES cells remains to be determined. miRNAs have been linked to autoimmune diseases, and circulating miRNAs function in immunological processes [59,61]. Thus, establishing the relationship between Aire and miRNAs provides a new perspective for understanding the function and regulatory mechanisms of Aire. Further investigation would potentially reveal the major regulatory nature of Aire on immunotolerance and other processes.
Implications into the extra-thymus function of Aire
Except for the mTECs, germ cells and early blastomeres were the other major expression entities of Aire [12]. Although Aire had been linked to the early wave of apoptosis during male germ cells development [26], no other clear functional relationship had been established between Aire and these cell types. Interestingly, except for blastocyst inner cell mass, germ cells and early blastomeres are important cellular sources for pluripotent stem cells, including embryonic germ cells, testis-derived pluripotent stem cells, and blastomere-derived pluripotent stem cells [62–64]. Moreover, core pluripotent genes, such as Oct4 and Nanog, are expressed in germ cells and early blastomeres, and Oct4 protein have been shown to be maternally stored in oocytes, which played critical roles in preimplantational development [65,66]. It is possible that the Aire-Lin28 pathway contributed to the potential to become pluripotent stem cells of these cell types, based on the similar expression pattern between Aire and pluripotent factors, the promotional effect of Aire on ES cell self-renewal, and the consistent regulatory relationship between Aire and Lin28 in ES cells, germ cells, and blastomeres. It will be interesting to see whether Aire is expressed in new pluripotent stem cell types, such as gonad-derived pluripotent stem cells or very small embryonic like stem cells, and explore the potential usage of Aire as a marker that isolates new pluripotent cell types [63,64,67]. Interestingly, homozygous Aire-knockout mice suffered a high incidence of infertility [68]. Although autoimmune defects have been considered a major cause of this phenotype [7], other possibilities, including a germ cell developmental defect, defects in oocyte genomic reprogramming during fertilization, and defects in preimplantational development, have not been strictly excluded. Since Lin28 has been implicated in germ cell development [69], cellular reprogramming, and self-renewal of pluripotent stem cells, our discovery of the quantitative regulatory effect of Aire on Lin28 indicated that the effect of Aire on germ cell development and early development could contribute to the partial infertility phenotype in Aire-knockout mice. Extending this hypothesis, as a fraction of women suffering APECED suffer from infertility, the findings provide new insights into the development of diagnoses and therapeutics for this type of infertility.
Conclusion
In conclusion, our findings integrated Aire as a novel participant of the pluripotent network through Lin28 and presented the first functional interaction between Aire and microRNAs. These findings would shed deep implications on both the structure and function of the pluripotent networks and the molecule networks that Aire regulate gene expression and cellular behaviors.
Acknowledgment
The authors thank Dr. Inder Verma, Dr. David Root, and Dr. Didier Trono for their kind provision of plasmids. This work is supported by grants from the National Natural Science Foundation (31071201/C0704) and (30971625/C060604).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Shenghui H. Nakada D. Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Develop Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 2.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J. Chu J. Shen X. Wang J. Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg DLC. Snoek T. Mullin NP. Yates A. Bezstarosti K. Demmers J. Chambers I. Poot RA. An oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardo M. Lang B. Yu L. Prosser H. Bradley A. Babu MM. Choudhary J. An expanded oct4 Interaction network: implications for stem cell biology, development, and disease. Cell Stem Cell. 2010;6:382–395. doi: 10.1016/j.stem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houbaviy HB. Murray MF. Sharp PA. Embryonic stem cell-specific microRNAs. Develop Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MS. Venanzi ES. Klein L. Chen Z. Berzins SP. Turley SJ. von Boehmer H. Bronson R. Dierich A. Benoist C. Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 8.Mathis D. Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey C. Winqvist O. Puhakka L. Halonen M. Moro A. Kämpe O. Eskelin P. Pelto-Huikko M. Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 10.Aaltonen J. Bjorses P. Perheentupa J. Horelli-Kuitunen N. Palotie A. Peltonen L. Lee YS. Francis F. HenningSteffen , et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 11.Gu B. Zhang J. Chen Q. Tao B. Wang W. Zhou Y. Chen L. Liu Y. Zhang M. Aire regulates the expression of differentiation-associated genes and self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2010;394:418–423. doi: 10.1016/j.bbrc.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishikawa Y. Hirota F. Yano M. Kitajima H. Miyazaki J-i. Kawamoto H. Mouri Y. Matsumoto M. Biphasic aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med. 2010;207:963–971. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubert F-X. Kinkel SA. Webster KE. Cannon P. Crewther PE. Proeitto AI. Wu L. Heath WR. Scott HS. A specific anti-aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar-Maia A. Alajem A. Meshorer E. Ramalho-Santos M. Open chromatin in pluripotency and reprogramming. Nat Rev Mol Cell Biol. 2011;12:36–47. doi: 10.1038/nrm3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efroni S. Duttagupta R. Cheng J. Dehghani H. Hoeppner DJ. Dash C. Bazett-Jones DP. Le Grice S. McKay RDG, et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell. 2008;2:437–447. doi: 10.1016/j.stem.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meshorer E. Yellajoshula D. George E. Scambler PJ. Brown DT. Misteli T. Hyperdynamic Plasticity of chromatin proteins in pluripotent embryonic stem cells. Develop Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guenther MG. Levine SS. Boyer LA. Jaenisch R. Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu B. Zhang J. Wang W. Mo L. Zhou Y. Chen L. Liu Y. Zhang M. Global expression of cell surface proteins in embryonic stem cells. PLoS ONE. 2010;5:e15795. doi: 10.1371/journal.pone.0015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu B. Zhang J. Wu Y. Zhang X. Tan Z. Lin Y. Huang X. Chen L. Yao K. Zhang M. Proteomic analyses reveal common promiscuous patterns of cell surface proteins on human embryonic stem cells and sperms. PLoS ONE. 2011;6:e19386. doi: 10.1371/journal.pone.0019386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang Y-S. Tsai S-Y. Lee D-F. Monk J. Su J. Ratnakumar K. Ding J. Ge Y. Darr H, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z. Scannell DR. Eisen MB. Tjian R. Control of embryonic stem cell lineage commitment by core promoter factor, TAF3. Cell. 2011;146:720–731. doi: 10.1016/j.cell.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitkänen J. Rebane A. Rowell J. Murumägi A. Ströbel P. Möll K. Saare M. Heikkilä J. Doucas V. Marx A. Peterson P. Cooperative activation of transcription by autoimmune regulator AIRE and CBP. Biochem Biophys Res Commun. 2005;333:944–953. doi: 10.1016/j.bbrc.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 23.Org T. Rebane A. Kisand K. Laan M. Haljasorg U. Andreson R. Peterson P. AIRE activated tissue specific genes have histone modifications associated with inactive chromatin. Hum Mol Genet. 2009;18:4699–4710. doi: 10.1093/hmg/ddp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillard GO. Dooley J. Erickson M. Peltonen L. Farr AG. Aire-dependent alterations in medullary thymic epithelium indicate a role for aire in thymic epithelial differentiation. J Immunol. 2007;178:3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 25.Sillanpää N. Magureanu CG. Murumägi A. Reinikainen A. West A. Manninen A. Lahti M. Ranki A. Saksela K, et al. Autoimmune regulator induced changes in the gene expression profile of human monocyte-dendritic cell-lineage. Mol Immunol. 2004;41:1185–1198. doi: 10.1016/j.molimm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Schaller CE. Wang CL. Beck-Engeser G. Goss L. Scott HS. Anderson MS. Wabl M. Expression of aire and the early wave of apoptosis in spermatogenesis. J Immunol. 2008;180:1338–1343. doi: 10.4049/jimmunol.180.3.1338. [DOI] [PubMed] [Google Scholar]
- 27.Ambros V. Horvitz H. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 28.Moss EG. Lee RC. Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 29.Viswanathan SR. Daley GQ. Lin28: a microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Xu B. Zhang K. Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 32.Hagan JP. Piskounova E. Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol. 2009;16:1021–1025. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanathan SR. Daley GQ. Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo I. Joo C. Kim Y-K. Ha M. Yoon M-J. Cho J. Yeom K-H. Han J. Kim VN. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138:696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Nishino J. Kim I. Chada K. Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melton C. Judson RL. Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu C. Ma Y. Wang J. Peng S. Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38:1240–1248. doi: 10.1093/nar/gkp1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin J. Jing W. Lei X-X. Feng C. Peng S. Boris-Lawrie K. Huang Y. Evidence that Lin28 stimulates translation by recruiting RNA helicase A to polysomes. Nucleic Acids Res. 2011;39:3724–3734. doi: 10.1093/nar/gkq1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Büssing I. Slack FJ. Großhans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14:400–409. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Viswanathan SR. Powers JT. Einhorn W. Hoshida Y. Ng TL. Toffanin S. O'Sullivan M. Lu J. Phillips LA, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi H. Blömer U. Takahashi M. Gage FH. Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moffat J. Grueneberg DA. Yang X. Kim SY. Kloepfer AM. Hinkle G. Piqani B. Eisenhaure TM. Luo B, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y. Gu B. Wu R. Zhang J. Li Y. Zhang M. Development of a rabbit monoclonal antibody group against Smads and immunocytochemical study of human and mouse embryonic stem cells. Stem Cells and Development. 2007;26:387–391. doi: 10.1089/hyb.2007.0517. [DOI] [PubMed] [Google Scholar]
- 44.Hay DC. Sutherland L. Clark J. Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- 45.Kitaguchi T. Mizugishi K. Hatayama M. Aruga J. Mikoshiba K. Xenopus Brachyury regulates mesodermal expression of Zic3, a gene controlling left–right asymmetry. Develop Growth Diff. 2002;44:55–61. doi: 10.1046/j.1440-169x.2002.00624.x. [DOI] [PubMed] [Google Scholar]
- 46.Rojas A. Schachterle W. Xu S-M. Martín F. Black BL. Direct transcriptional regulation of Gata4 during early endoderm specification is controlled by FoxA2 binding to an intronic enhancer. Develop Biol. 2010;346:346–355. doi: 10.1016/j.ydbio.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelton TA. Sharma S. Schulz TC. Rathjen J. Rathjen PD. Transient pluripotent cell populations during primitive ectoderm formation: correlation of in vivo and in vitro pluripotent cell development. J Cell Sci. 2002;115:329–339. doi: 10.1242/jcs.115.2.329. [DOI] [PubMed] [Google Scholar]
- 48.Maye P. Becker S. Siemen H. Thorne J. Byrd N. Carpentino J. Grabel L. Hedgehog signaling is required for the differentiation of ES cells into neurectoderm. Develop Biol. 2004;265:276–290. doi: 10.1016/j.ydbio.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 49.Frankenberg S. Gerbe F. Bessonnard S. Belville C. Pouchin P. Bardot O. Chazaud C. Primitive endoderm differentiates via a three-step mechanism involving nanog and RTK signaling. Develop Cell. 2011;21:1005–1013. doi: 10.1016/j.devcel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Leitch HG. Blair K. Mansfield W. Ayetey H. Humphreys P. Nichols J. Surani MA. Smith A. Embryonic germ cells from mice and rats exhibit properties consistent with a generic pluripotent ground state. Development. 2010;137:2279–2287. doi: 10.1242/dev.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shamblott MJ. Axelman J. Wang S. Bugg EM. Littlefield JW. Donovan PJ. Blumenthal PD. Huggins GR. Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adamo A. Sese B. Boue S. Castano J. Paramonov I. Barrero MJ. Belmonte JCI. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 53.Yan Z. Wang Z. Sharova L. Sharov AA. Ling C. Piao Y. Aiba K. Matoba R. Wang W. Ko MSH. BAF250B-associated SWI/SNF chromatin-remodeling complex is required to maintain undifferentiated mouse embryonic stem cells. Stem Cells. 2008;26:1155–1165. doi: 10.1634/stemcells.2007-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaniel C. Ang Y-S. Ratnakumar K. Cormier C. James T. Bernstein E. Lemischka IR. Paddison PJ. Smarcc1/Baf155 Couples self-renewal gene repression with changes in chromatin structure in mouse embryonic stem cells. Stem Cells. 2009;27:2979–2991. doi: 10.1002/stem.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oven I. Brdickova N. Kohoutek J. Vaupotic T. Narat M. Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abramson J. Giraud M. Benoist C. Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y. Baskerville S. Shenoy A. Babiarz JE. Baehner L. Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plasterk RHA. Micro RNAs in animal development. Cell. 2006;124:877–881. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 59.Contreras J. Rao DS. MicroRNAs in inflammation and immune responses. Leukemia. 2011;26:404–413. doi: 10.1038/leu.2011.356. [DOI] [PubMed] [Google Scholar]
- 60.Judson RL. Babiarz JE. Venere M. Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotech. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegel S. Mackenzie J. Chaplin G. Jablonski N. Griffiths L. Circulating microRNAs involved in multiple sclerosis. Mol Biol Rep. 2012;39:6219–6125. doi: 10.1007/s11033-011-1441-7. [DOI] [PubMed] [Google Scholar]
- 62.Tesar PJ. Derivation of germ-line-competent embryonic stem cell lines from preblastocyst mouse embryos. Proc Natl Acad Sci U S A. 2005;102:8239–8244. doi: 10.1073/pnas.0503231102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conrad S. Renninger M. Hennenlotter J. Wiesner T. Just L. Bonin M. Aicher W. Bühring H-J. Mattheus U, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 64.Kanatsu-Shinohara M. Inoue K. Lee J. Yoshimoto M. Ogonuki N. Miki H. Baba S. Kato T. Kazuki Y, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Hayashi K. de Sousa Lopes SMC. Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- 66.Foygel K. Choi B. Jun S. Leong DE. Lee A. Wong CC. Zuo E. Eckart M. Reijo Pera RA. Wong WH. Yao MWM. A novel and critical role for oct4 as a regulator of the maternal-embryonic transition. PLoS ONE. 2008;3:e4109. doi: 10.1371/journal.pone.0004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ratajczak M. Zuba-Surma E. Machalinski B. Ratajczak J. Kucia M. Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev Rep. 2008;4:89–99. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- 68.Jiang W. Anderson MS. Bronson R. Mathis D. Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.West JA. Viswanathan SR. Yabuuchi A. Cunniff K. Takeuchi A. Park I-H. Sero JE. Zhu H. Perez-Atayde A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]