Abstract
Interleukin-1β (IL-1β) has been shown to play an essential role in mediating intestinal inflammation of Crohn's disease and other inflammatory conditions of the gut. Previous studies from our laboratory have shown that IL-1β causes an increase in intestinal tight-junction permeability in Caco-2 monolayers in vitro. However, the IL-1β effect on the intestinal epithelial barrier in vivo remains unclear. Aims: the major aims of this study were to examine the effect of IL-1β on mouse intestinal epithelial barrier in vivo and to delineate the mechanisms involved using an in vivo model system consisting of a recycling perfusion of mouse small intestine. Intraperitonial injection of IL-1β at varying doses (0–10 μg) caused a concentration-dependent increase in mouse intestinal permeability to the paracellular marker dextran (10 KD), and the maximal increase in dextran flux occurred at IL-1β dose of 5 μg. IL-1β treatment caused an increase in myosin light-chain kinase (MLCK) mRNA and protein expression in the small intestinal tissue starting at 24 h, which continued up to 72 h. Additionally, IL-1β did not cause an increase in intestinal permeability in MLCK-deficient mice (C57BL/6 MLCK−/−). MLCK inhibitor ML-7 (2 mg/kg body weight) also inhibited the IL-1β-induced increase in small intestinal permeability. The IL-1β-induced increase in mouse intestinal permeability was associated with an increase in NF-κB activation. The intestinal tissue-specific silencing of NF-κB p65 inhibited the IL-1β-induced increase in intestinal permeability and increase in MLCK expression. These data show for the first time that IL-1β causes an increase in mouse intestinal permeability in vivo. These data suggested that the mechanism of IL-1β-induced increase in mouse intestinal permeability in vivo involved NF-κB p65-induced activation of the mouse enterocyte MLCK gene.
Introduction
Interleukin-1β (IL-1β) is a prototypical multifunctional cytokine playing a crucial role in the inflammatory process of the gut (O'Neill and Dinarello 2000; Ma and Anderson 2006), including in inflammatory bowel disease (IBD), ischemic–reperfusion injury, various types of infectious enteritis, celiac disease, and nonsteroidal anti-inflammatory drug (NSAID)-associated enteropathy (Dinarello 1996; O'Neill and Dinarello 2000; Dunne and O'Neill 2003; Ma and Anderson 2006). IL-1β is involved in both the initiation and amplification of the inflammatory response leading to intestinal injury. IL-1β has been shown to play an important role in the pathogenesis of intestinal inflammation in IBD and in animal models of intestinal inflammation. IBD patients have elevated levels of IL-1β in their intestinal tissue (Reinecker and others 1991), and a correlation between increasing levels of IL-1β and the level of intestinal inflammation has been demonstrated (Reinecker and others 1991; Heresbach and others 1997). An imbalance between IL-1β and its antagonist IL-1ra exists in the intestinal mucosa of IBD patients, suggesting that a lack of anti-inflammatory forms of IL-1 to counteract the elevated levels of IL-1β may be an important pathogenic defect (Hyams and others 1995; Cominelli and Pizarro 1996). Consistent with this possibility, administration of rIL-1ra prevented the intestinal inflammation in a rabbit model of colitis (Ferretti and others 1994). Recent studies have also demonstrated an existence of IL-1β gene polymorphisms in IBD patients that determines the course and the severity of intestinal inflammation in these patients (Nemetz and others 1999).
Defective intestinal epithelial tight-junction (TJ) barrier has been implicated to be an important pathogenic factor in a number of inflammatory conditions of the gut and systemic inflammatory conditions, including Crohn's disease (CD), necrotizing enterocolitis, NSAID-associated enteritis, ulcerative colitis, heat stroke, alcoholic hepatitis, and various infectious diarrheal syndromes (Hollander and others 1986; Hollander 1988; Ma and Anderson 2006). It has been postulated that the defective intestinal TJ barrier allows paracellular permeation of noxious luminal antigens that induce inflammatory response (Hollander and others 1986; Ma and Anderson 2006). In support of a central role in intestinal inflammation, previous studies in animal models of intestinal inflammation have shown that pharmacologic- or probiotic-induced enhancement of the intestinal epithelial TJ barrier prevents the development of intestinal inflammation in various murine models of IBD, including in dextran sodium sulfate-induced colitis, IL-10-deficient mice, and in T-cell-transfer mice (Sydora and others 2005; Poritz and others 2007; Su and others 2009; Chichlowski and others 2010; Zakostelska and others 2011). Additionally, clinical studies have also shown that therapeutic retightening of the intestinal TJ barrier in patients with active CD correlated with an improvement of the disease and long-term clinical remission, whereas a persistent increase in the intestinal permeability was associated with an early recurrence of the disease (Wyatt and others 1993; Miehsler and others 2001).
Previous studies from our laboratory have shown that IL-1β causes an increase in intestinal epithelial TJ permeability in filter-grown Caco-2 monolayers (Al-Sadi and Ma 2007; Al-Sadi and others 2008), suggesting the possibility that the elevated levels of IL-1β in IBD patients may contribute to the observed increase in intestinal permeability (Hollander and others 1986; Hollander 1988; Miehsler and others 2001; Vavricka and Rogler 2009). However, the effects of IL-1β on intestinal permeability in vivo in animal model systems remain unknown, and the clinical relevance of the IL-1β effect remains unclear. Thus, the major aim of this study was to examine the effect of IL-1β on intestinal permeability in vivo in an animal model system. Specifically, the IL-1β effect on intestinal permeability was determined by recycling perfusion of an isolated segment of mouse small intestine in vivo. Our data indicated that IL-1β at a physiologically relevant concentration causes an increase in mouse intestinal permeability. Additionally, our data suggested that the myosin light-chain kinase (MLCK) gene is an important target in IL-1β modulation of intestinal permeability in vivo.
Materials and Methods
Animals
The Laboratory Animal Care and Use Committee at the University of New Mexico approved all experimental protocols. Male C57BL/6 mice (9–10 weeks), IL-1R−/− and MLCK−/− mice, were purchased from Jackson Laboratories (Bar Harbor). The mice were kept 2 per cage in a temperature-controlled room at 25°C with a 12:12-h light–dark cycle (lights on at 07:00). Diet and drinking water were provided ad libitum.
Chemicals
IL-1β was purchased from Peprotech. Dextran 10 KD was purchased from Invitrogen. Anti-MLCK and IκB-α antibodies were obtained from Sigma-Aldrich. Anti-NF-κB p65 and anti-β-actin antibodies were obtained from Zymed/Invitrogen. HRP-conjugated secondary antibodies for Western blot analysis were purchased from Abcam. Cy-3 antibody for immunostaining was purchased from Jackson ImmunoResearch Laboratories. SiRNA NF-κB p65 and transfection reagents were from Dharmacon. All other chemicals were of reagent grade and were purchased from Sigma-Aldrich, VWR, or Fisher Scientific.
Ex vivo intestinal tissue epithelial resistance measurement in Ussing chambers
The small intestinal tissue epithelial resistance was measured using an Ussing chamber 24 h after i.p. injection of IL-1β. After a midline incision of the abdomen, the small intestine was dissected out, and a 1–1.5-cm sample was vertically mounted in Ussing chambers that provided an exposed area of 0.126 cm2, as described previously with modifications. The tissues were bathed with the Krebs-Ringer bicarbonate solution (128 mM NaCl, 5.1 mM KCl, 1.4 mM CaCl2, 1.3 mM MgCl2, 21 mM NaHCO3, 1.3 mM KH2PO4, 10 mM NaH2PO4, pH 7.4). Solutions were gassed with 95% O2/5% CO2. After a 15-min equilibration period, the transepithelial electrical voltage and current were measured at 5-min intervals until 30 min using an EVC 4000 Precision V/I clamp device (World Precision Instruments). Intestinal tissue resistance was calculated using Ohm's law (R=V/I). The experiment was repeated a minimum of 3 times using different tissue sample each time.
Intestinal permeability measurement
The intestinal permeability was measured by recycling small intestinal perfusion as previously described (Clayburgh and others 2005; Al-Sadi and others 2011; Ye and others 2011). After the experimental period, mice were anesthetized with isoflurane. After a midline incision of the abdomen, 5 cm of the intestine segment was isolated and cannulated at the proximal and distal ends with 0.76-mm-internal-diameter polyethylene tubing. The flushing solution (140 mM NaCl, 10 mM HEPES, pH 7.4) warmed to 37°C was first perfused through the intestine at 1 mL/min for 20 min followed by air flush to remove residual contents using an external pump (Bio-Rad Laboratories). This was followed by perfusion of 5 mL perfusate solution (85 mM NaCl, 10 mM HEPES, 20 mM sodium ferrocyanide, 5 mM KCl, 5 mM CaCl2, pH 7.4.) containing Texas Red-labeled dextran (10 KD) in a recirculating manner at 0.75 mL/min for 2 h. The abdominal cavity was covered with moistened gauze; the body temperature was measured via a rectal thermometer; and temperature was maintained at 37.5°C±0.5°C using a heating lamp. One-milliliter aliquots of the test solution were removed at the beginning and at the end of the perfusion. After perfusion, the animal was sacrificed, the perfused intestine segment excised, and the length measured. The excised intestinal loop was then snap-frozen in optimal cutting temperature or used for protein and RNA analysis. Ferrocyanide concentration in the perfusate was measured using a colorimetric assay. Texas Red-labeled dextran 10KD concentration was measured using an excitation wavelength of 595 nm and an emission wavelength of 615 nm in a microplate reader. Probe clearance was calculated as Cprobe=(CiVi–CfVf)/(CavgTL). In the equation, Ci represents the measured initial probe concentration; Cf represents the measured final probe concentration; Vi represents the measured initial perfusate volume; Vf was calculated as Vi([ferrocyanide]i/[ferrocyanide]f); Cavg was calculated as (Ci–Cf)/ln(Ci/Cf); T represents hours of perfusion, and L represents the length of the perfused intestine section in centimeters. Total of 128 wild-type mice (C57BL/6), 12 IL-1R knockout mice (C57BL/6 IL-1R−/−), and 12 MLCK knockout mice (C57BL/6 MLCK−/−) were used in the studies.
Assessment of protein expression by Western blot analysis
At the end of the experimental period, mouse intestinal tissues were rinsed twice with ice-cold phosphate-buffered saline (PBS), and sonicated and homogenized in a lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 500 μM NaF, 2 mM EDTA, 100 μM vanadate, 100 μM PMSF, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, 40 mM paranitrophenyl phosphate, 1 μg/mL aprotinin, and 1% Triton X-100). Total cell lysates were centrifuged to yield a clear lysate. Supernatant was collected, and protein was measured using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories). Laemmli gel-loading buffer was added to the lysate containing 10–20 μg of protein and boiled for 7 min, after which proteins were separated on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. Proteins from the gel were transferred to the membrane (Trans-Blot Transfer Medium, Nitrocellulose Membrane; Bio-Rad Laboratories) overnight. The membrane was incubated for 2 h in a blocking solution (5% dry milk in a TBS–Tween 20 buffer). The membrane was incubated with an appropriate primary antibody in the blocking solution. After being washed in the TBS–1% Tween buffer, the membrane was incubated in an appropriate secondary antibody and developed using the Santa Cruz Western Blotting Luminol Reagents (Santa Cruz Biotechnology) on the Kodak BioMax MS film (Fisher Scientific).
RNA isolation and reverse transcription
At the end of the experimental period, mouse intestinal tissues were washed twice with ice-cold PBS. Total RNA was isolated using a Qiagen RNeasy Kit (Qiagen), according to the manufacturer's protocol. The total RNA concentration was determined by absorbance at 260/280 nm using SpectraMax 190 (Molecular Devices). The reverse transcription (RT) was carried out using the GeneAmp Gold RNA PCR core kit (Applied Biosystems). Two micrograms of total RNA from each sample was reverse-transcribed into cDNA in a 40-μL reaction containing 1×RT-PCR buffer, 2.5 mM MgCl2, 250 μM of each dNTP, 20 U RNase inhibitor, 10 mM DTT, 1.25 μM random hexamer, and 30 U multiscribe RT. The RT reactions were performed in a thermocycler (MyCycler; Bio-Rad) at 25°C for 10 min, 42°C for 30 min, and 95°C for 5 min.
Quantification of gene expression using real-time polymerase chain reaction
The real-time polymerase chain reactions (PCRs) were carried out using the ABI prism 7900 sequence detection system and a TaqMan universal PCR master mix kit (Applied Biosystems), as previously described (Al-Sadi and Ma 2007; Al-Sadi and others 2009). Each real-time PCR contained 10 μL RT reaction mix, 25 μL 2×TaqMan universal PCR master mix, 0.2 μM probe, and 0.6 μM primers. Primer and probe design for the real-time PCR was made with Primer Express version 2 from Applied Biosystems [the primers used in this study are as follows: MLCK-specific primer pairs consisted of 5′-AGGAAGGCAGCATTGAGGTTT-3′ (forward), 5′-GCTTTCAGCAGGCAGAGGTAA-3′ (reverse); probe specific for MLCK consisted of FAM 5′-TGAAGATGCTGGCTCC-3′ TAMRA; the internal control GAPDH-specific primer pairs consisted of 5′ CCACCCATGGCAAATTCC-3' (forward), 5'-TGGGATTTCCATTGATGACCAG-3′ (reverse); probe specific for GAPDH consisted of JOE 5′-TGGCACCGTCAAGGCTGAGAACG-3′ TAMRA]. All runs were performed according to the default PCR protocol (50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min). For each sample, real-time PCRs were performed in triplicate, and the average threshold cycle (Ct) was calculated. A standard curve was generated to convert the Ct to copy numbers. Expression of MLCK mRNA was normalized with GAPDH mRNA expression. The average copy number of mRNA expression in control samples was set to 1.0.
Animal surgery and in vivo transfection of NF-κB p65 siRNA
The Laboratory Animal Care and Use Committee at the University of New Mexico approved all experimental protocols. Mice were fasted for 24 h before the surgery. Mice were anesthetized with isoflurane (4% for surgical induction; 1% for maintenance), using oxygen as carrier during surgical procedures. Surgical procedures were performed using a sterile technique. The abdomen was opened by a midline incision, and 6 cm of the intestine segment was isolated at the proximal and distal ends and tied with sutures. About 0.5 mL of siRNA transfection solution [containing the Accell medium; 2.5 nmol occludin siRNA, and 15 μL transfecting agent DharmaFect (Dharmacon)] was introduced into the isolated intestine segment (surface area 6 cm2) for a 1-h transfection period. Control animals underwent sham operation, where the siRNA transfection solution contained the Accell medium, 2.5 nmol nontarget siRNA, and 15 μL transfection reagent DharmaFect. The abdominal cavity was covered with moistened gauze. The body temperature was monitored continuously with a rectal probe and maintained at 37.5°C±0.5°C using a heating pad. After the 1-h transfection period, each end of the intestinal segment was untied and the intestine placed back in the abdominal cavity, and the abdomen was closed. Three days after transfection, functional studies of the intestinal epithelial barrier were performed. The surgery and the in vivo transfection procedures had no effect on the food intake and the body weight of the animals during the experimental period. The animal weight was averaged between 23 to 25 g during the experimental period. A total of 24 wild-type mice (C57BL/6) were used in these studies.
Laser-capture microdissection of intestinal epithelial cells
Frozen mouse tissue sections were fixed with 75% ethanol for 30 s, hematoxylin and eosin stained for 20 s, and dehydrated with 75% ethanol, 30 s; 95% ethanol, 30 s; 100% ethanol, 30 s; and xylene, 5 min. After dehydration, sections were air-dried for 5 min. The Arcturus PixCell II system (Molecular Devices) was used for microdissection and laser capture. The intestinal epithelial cells from the mucosal surface were captured using a 7.5-μM-diameter laser beam typically at 80- to 100-mV power with pulse duration of 0.5 to 1.0 ms. On average, about 500 shots were taken per cap, and approximately 1000 enterocytes were obtained per cap. Microdissection caps were inserted into 0.5-mL microcentrifuge tubes containing 350 μL of lysis buffer, and total RNA was isolated.
Immunostaining of NF-κB p65
Cytoplasmic-to-nuclear translocation of NF-κB p65 in mouse intestinal tissue was assessed by immunofluorescent antibody labeling. At the end of the experimental period, mouse tissue sections were fixed with 75% ethanol for 30 s and dehydrated with 75% ethanol, 30 s; 95% ethanol, 30 s; 100% ethanol, 30 s; and xylene, 5 min. After dehydration, sections were air-dried for 5 min. The sections were washed twice in cold PBS and were fixed with 2% paraformaldehyde for 20 min. After being permeabilized with 0.1% Triton X-100 in PBS at room temperature for 20 min, tissues were then incubated in a blocking solution composed of bovine serum albumin and normal donkey serum in PBS for 1 h. Tissues were then labeled with primary antibodies in the blocking solution overnight at 4°C. After being washed with PBS, the tissues were incubated in fluorescein isothiocyanate and Cy-3-conjugated secondary antibodies for 1 h at room temperature. ProLong Gold antifade reagent (Invitrogen) was used to mount the tissues. Immunolocalization of NF-κB p65 was visualized using a fluorescence microscope (Meta LSM 510, University of New Mexico Imaging center) equipped with a Hamamatsu digital camera (Hamamatsu Photonics). Images were processed with Zen software (Zeiss).
Statistical analysis
Results are expressed as means±SE. Statistical significance of differences between mean values was assessed with Student's t-tests for unpaired data and analysis of variance whenever required (Graph Pad Prism 4.00 for Windows; GraphPad Software). All reported significance levels represent 2-tailed P values. A P value of<0.05 was used to indicate statistical significance. Each experiment in mouse intestine in vivo was performed at least 4 times to ensure reproducibility.
Results
Effect of IL-1β on mouse intestinal permeability in vivo
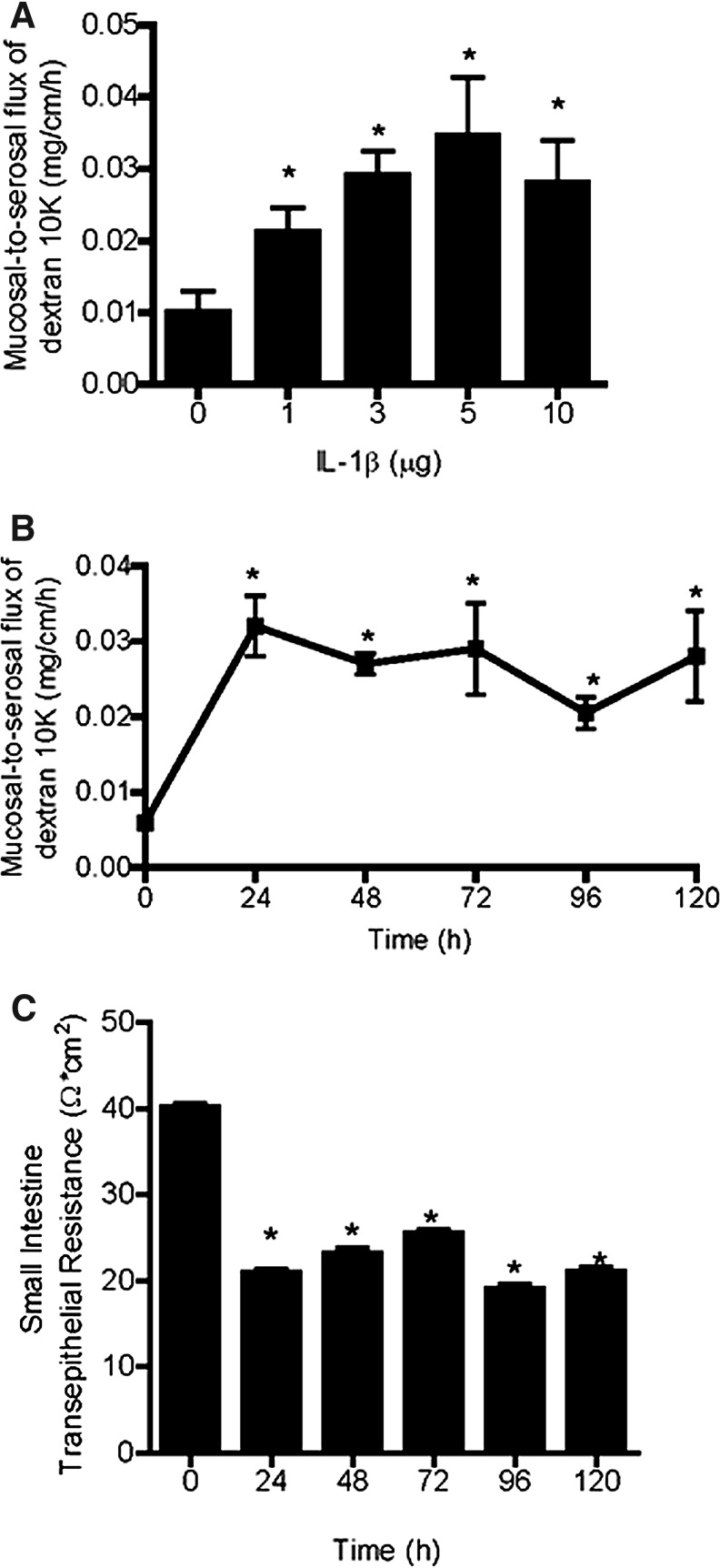
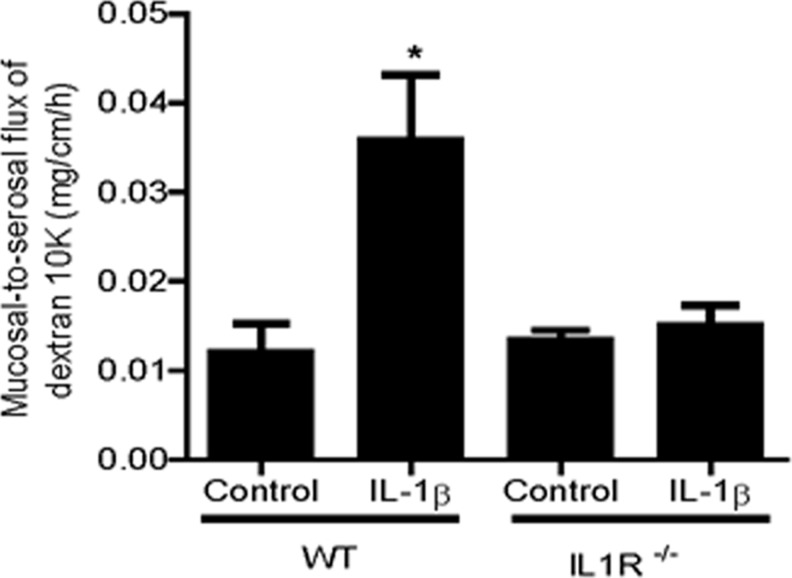
The IL-1β effect on mouse intestinal permeability was determined by recycling perfusion of an isolated segment of the small intestine in vivo, as previously described. The intestinal permeability was determined by measuring the flux rate of Texas Red-labeled dextran (m.w.=10,000), a commonly used paracellular marker. Mice (C57BL/6) were injected with IL-1β intraperitoneally at doses (1–10 μg) used in previous animal studies (Vindenes and others 1998; Yoshida and others 2010). Intraperitonial (i.p.) injection of IL-1β (0–10 μg) caused a concentration-dependent increase in mouse intestinal permeability to the paracellular marker dextran (10 KD) (Fig. 1A). The maximal increase in dextran flux was reached at an IL-1β concentration of 5 μg, and increasing the concentration up to a 10-μg dose did not produce a further increase in intestinal permeability. The time course of IL-1β (5 μg) effect on mouse intestinal permeability is shown in Fig. 1B. IL-1β caused a significant increase in intestinal permeability by 24 h, and the increase in intestinal permeability persisted up to day 5 of the experimental period. In separate experiments, transepithelial resistance (TER) of mouse small intestinal tissue was measured ex vivo by mounting intestinal tissue (1-cm diameter) in an Ussing chamber. IL-1β (5 μg) caused a time-dependent drop in intestinal tissue TER (Fig. 1C). In subsequent studies, the effect of IL-1β on mouse intestinal permeability was examined in IL-1 receptor type I-deficient mice (C57BL/6 IL-1R−/−). Intraperitonial injection of IL-1β (5 μg) did not cause an increase in mouse intestinal permeability in IL-1R−/− mice (Fig. 2), suggesting that the IL-1β effect on mouse intestinal permeability required IL-1β binding to its membrane receptor IL-1R.
FIG. 1.
Effect of i.p. interleukin (IL)-1β on mouse intestinal permeability. (A) IL-1β caused a concentration-dependent (0–10 mg/kg body weight) increase in mouse intestinal mucosal-to-serosal flux of dextran 10KD. (B) IL-1β caused a time-course (0–120 h) increase in mouse intestinal mucosal-to-serosal flux of dextran 10K. (C) IL-1β caused a time-dependent (0–120 h) drop in mouse intestinal transepithelial resistance (TER) as measured in Ussing chambers. *P<0.001 versus control.
FIG. 2.
Effect of IL-1β (5 μg/kg body weight) on mouse intestinal mucosal-to-serosal flux of dextran 10KD in IL-1R−/− mice. IL-1β (5 μg/kg body weight) did not cause an increase in mouse intestinal mucosal-to-serosal flux of dextran 10KD in IL-1R−/− mice. *P<0.001 versus WT control.
Role of MLCK on IL-1β-induced increase in intestinal permeability
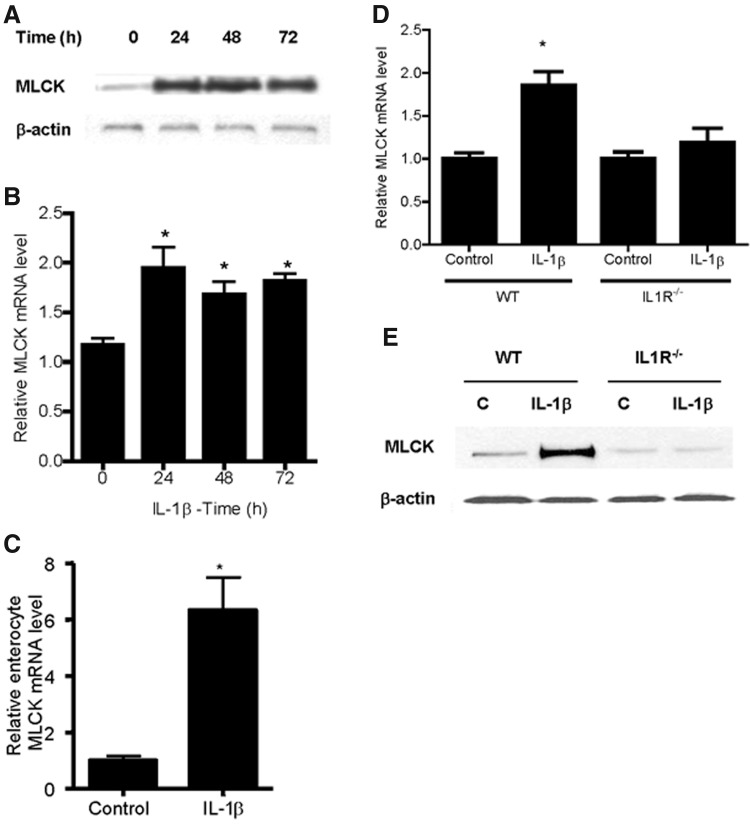
Previous studies have shown that MLCK plays an important role in the regulation of intestinal TJ barrier function (Schwarz and others 2007; Su and others 2009; Weber and others 2010). In the following studies, the in vivo effect of IL-1β on the MLCK gene and protein expression was examined. Intraperitoneal IL-1β injection resulted in a time-dependent upregulation in MLCK protein expression in the mouse small intestinal tissue, which persisted up to a 72-h experimental period (Fig. 3A). IL-1β also caused an increase in intestinal tissue MLCK mRNA as assessed by real-time PCR (Fig. 3B). Since intestinal tissue consists of many different cell types and the increase in the intestinal tissue MLCK level could have resulted from the nonenterocyte cell population, we examined the IL-1β effect on the enterocyte MLCK mRNA level using laser-capture microdissection (LCM) to capture a pure population of enterocytes from the intestinal mucosal surface. The in vivo IL-1β treatment resulted in a 6-fold increase in the MLCK mRNA level in the LCM-isolated enterocytes (Fig. 3C). In subsequent experiments, the IL-1β effect on MLCK expression in C57BL/6 IL-1R−/− mice was also examined. IL-1β (5 μg) did not cause an increase in MLCK mRNA or protein expression in IL-1R−/− mice (Fig. 3D, E), suggesting that the IL-1β effect on intestinal tissue MLCK expression was mediated by an IL-1β receptor signal transduction pathway.
FIG. 3.
Effect of IL-1β on myosin light-chain kinase (MLCK) expression in vivo. (A) IL-1β (5 μg/kg body weight) caused a time-dependent increase in mouse intestinal tissue MLCK protein expression as assessed by Western blot analysis. (B) IL-1β caused an increase in mouse intestinal tissue MLCK mRNA transcript as assessed by real-time polymerase chain reaction (PCR); IL-1β 24-h treatment. *P<0.001 versus control. (C) IL-1β caused an increase in the enterocyte MLCK mRNA transcript after isolating a pure population of mouse enterocytes by laser-capture microdissection (LCM); IL-1β 24-h treatment. *P<0.001 versus control. Effect of IL-1β on mouse intestinal MLCK expression in IL-1R−/− mice. (D) IL-1β did not cause an increase in mouse intestinal MLCK mRNA expression in IL1R−/− mice as assessed by real-time PCR. *P<0.001 versus WT control. (E) IL-1β did not cause an increase in mouse intestinal MLCK protein expression in IL1R−/− mice as assessed by Western blot analysis.
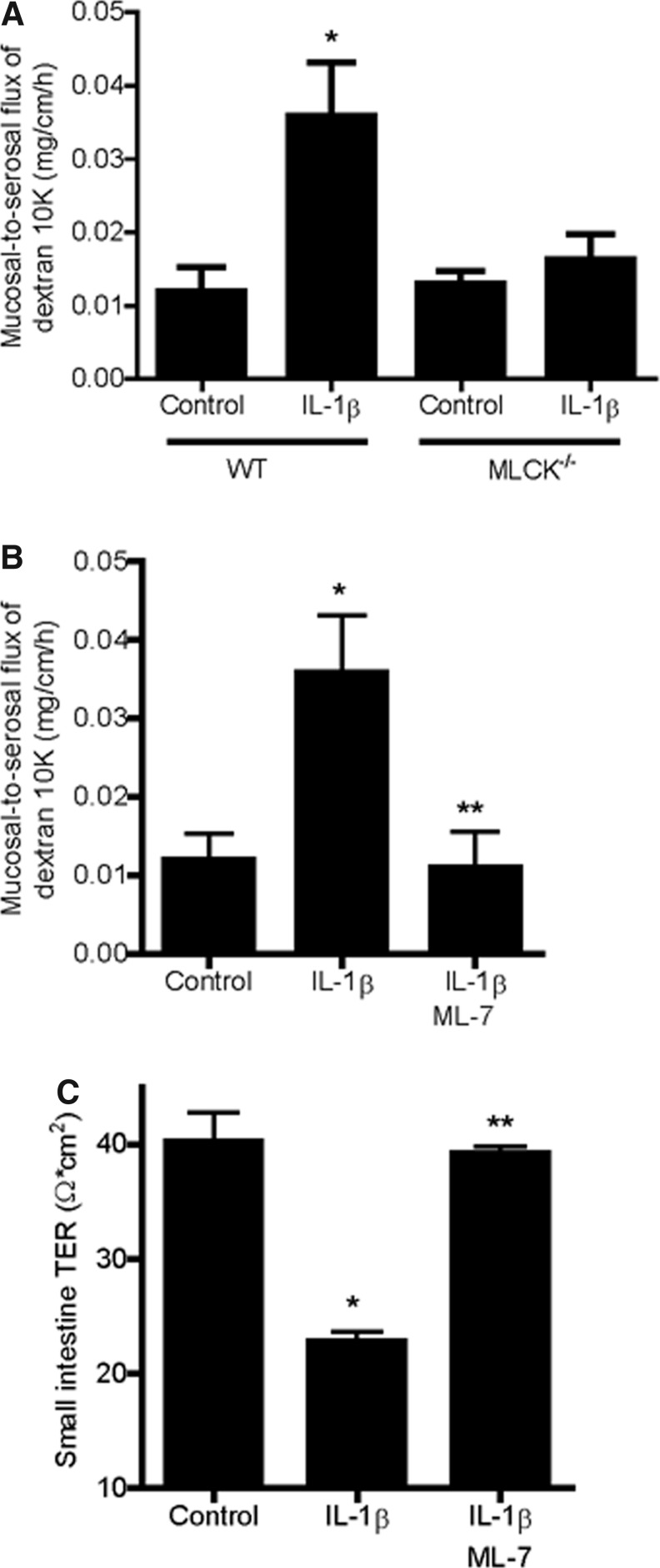
Next, the possible causal relationship between MLCK expression and increase in mouse intestinal permeability was examined. To determine the requirement of MLCK, the IL-1β effect on intestinal permeability was examined in MLCK-deficient mice (C57BL/6 MLCK−/−). IL-1β administration did not cause an increase in intestinal permeability in MLCK−/− mice (Fig. 4A). To further confirm the regulatory role of MLCK in IL-1β- induced increase in intestinal permeability in vivo, the effect of the MLCK inhibitor ML-7 was also examined. The ML-7 pretreatment (2 mg/kg i.p.) prevented the IL-1β-induced increase in intestinal permeability (Fig. 4B). Additionally, ML-7 also prevented the IL-1β-induced drop in small intestinal tissue TER (Fig. 4C). Together, these results suggested that the IL-1β-induced increase in mouse intestinal permeability in vivo was mediated by an increase in MLCK expression and activity.
FIG. 4.
Effect of MLCK inhibition on IL-1β-induced increase in mouse intestinal tight-junction (TJ) permeability in vivo. (A) IL-1β (5 μg/kg body weight) did not cause an increase in the mouse intestinal mucosal-to-serosal flux of dextran 10KD in MLCK−/− mice. *P<0.001 versus WT control. (B) Inhibition of MLCK with ML-7 (2 mg/kg) prevented the IL-1β-induced increase in the mouse mucosal-to-serosal flux of dextran 10KD. *P<0.001 versus control; **P<0.001 versus IL-1β treatment. (C) ML-7 (2 mg/kg) prevented the IL-1β-induced drop in mouse intestinal TER measured in Ussing chambers. *P<0.001 versus control; **P<0.001 versus IL-1β treatment.
Nuclear transcription factor NF-κB p65 regulates IL-1β-induced increase in MLKC gene expression and intestinal permeability
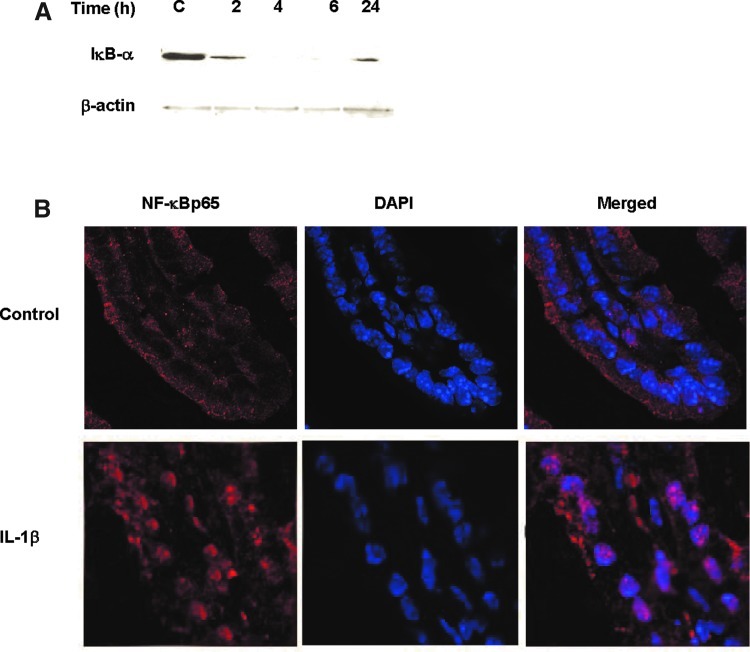
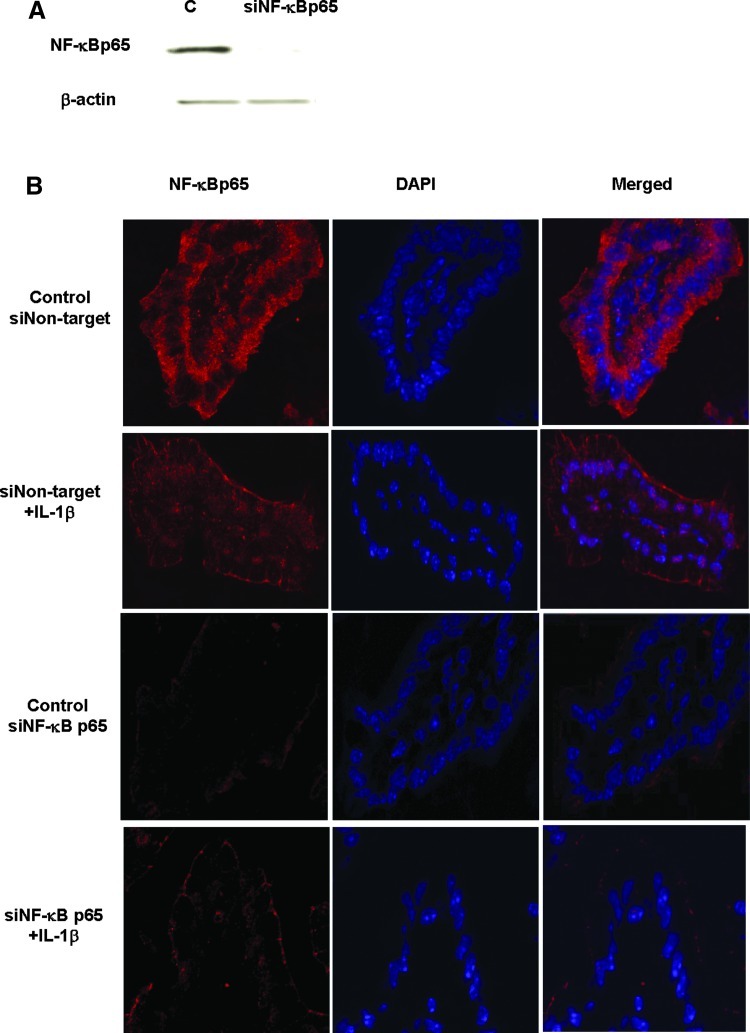
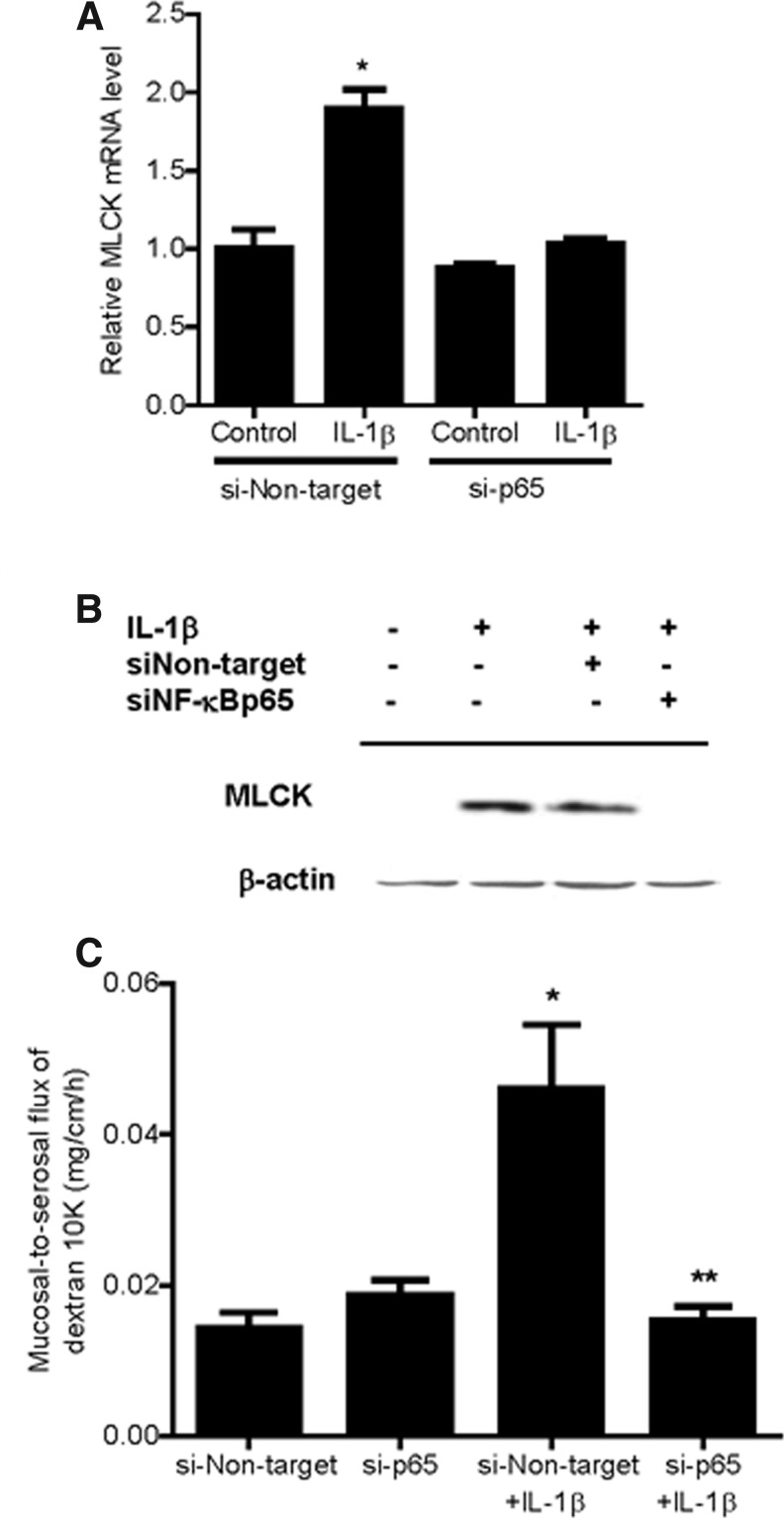
Previous studies suggested that the nuclear transcription factor NF-κB p65 plays a regulatory role in the MLCK gene activity in Caco-2 cells (Graham and others 2006; Al-Sadi and Ma 2007; Al-Sadi and others 2008;Ye and Ma 2008). In the following studies, we examined the possibility that NF-κB p65 mediates the IL-1β regulation of MLCK gene expression and intestinal permeability in vivo. Intraperitoneal IL-1β injection resulted in a time-dependent degradation of IκB-α in the intestinal tissue, and by 4 h of treatment, there was almost a complete disappearance of IκB-α in the intestinal tissue (Fig. 5A). The diminished IκB-α level persisted throughout the 24-h experimental period (Fig. 5A). The immunostaining studies of the intestinal tissue indicated that NF-κB p65 was located mostly in the enterocyte cytoplasm in the control mouse intestinal tissue (Fig. 5B). After IL-1β treatment, there was an intense staining of NF-κB p65 in the nucleus, consistent with activation and cytoplasmic-to-nuclear translocation of NF-κB p65 (Fig. 5B). To determine the possible regulatory role of NF-κB p65 in mediating the IL-1β-induced increase in MLCK gene expression and mouse intestinal permeability, the expression of mouse enterocyte NF-κB p65 was selectively silenced in vivo, using a recently introduced method of selective silencing of a target gene in the intestine in vivo (Ye and others 2011). In these studies, NF-κB p65 expression in mouse enterocytes was silenced by exposing the isolated segment (6 cm in linear length) of the intestinal mucosal surface with an in vivo transfection solution containing NF-κB p65 siRNA, as described in the Methods section. (Following siNF-κB p65 in vivo, the transfected intestinal segment was placed back into its original location in the abdomen and the mouse abdominal cavity closed with sutures.) Three days later, mouse was administered IL-1β intraperitoneally, and the IL-1β effect on enterocyte MLCK gene expression and intestinal permeability determined in the transfected intestinal segment. The NF-κB p65 siRNA transfection resulted in a near complete depletion of NF-κB p65 protein expression by day 3 after siRNA transfection, as determined by Western blot analysis and immunostaining (Fig. 6A, B). The siRNA-induced depletion of enterocyte p65 expression prevented the IL-1β-induced increase in enterocyte MLCK mRNA (as determined by LCM) and intestinal tissues MLCK expression (Western blot analysis) (Fig. 7A, B). The siRNA-induced knockdown of NF-κB p65 also inhibited the IL-1β increase in intestinal permeability (Fig. 7C). Together, these data suggested that the nuclear transcription factor NF-κB p65 plays a key regulatory role in MLCK gene expression and intestinal permeability in vivo.
FIG. 5.
Effect of IL-1β on NF-κB p65 activation on mouse intestinal tissue in vivo. (A) IL-1β caused a time-dependent increase in IκB-α degradation starting at 2 h and continuing up to 24 h, as assessed by Western blot analysis. (B) Effect of IL-1β on NF-κB p65 localization as assessed by immunostaining. IL-1β (4-h treatment) caused cytoplasmic-to-nuclear translocation of NF-κB p65 in mouse intestinal tissue.
FIG. 6.
Effect of NF-κB p65-induced knockdown by siRNA transfection in vivo on an IL-1β-induced increase in mouse intestinal TJ permeability. (A) NF-κB p65 siRNA transfection in vivo caused a near-complete depletion of NF-κB p65 protein expression as assessed by Western blot analysis. (B) NF-κB p65 siRNA transfection in vivo caused a near-complete depletion of NF-κB p65 protein expression as assessed by immunostaining.
FIG. 7.
Effect of NF-κB p65-induced knockdown by siRNA transfection in vivo on IL-1β-induced increase in mouse intestinal MLCK expression. (A) NF-κB p65 siRNA transfection in vivo prevented the IL-1β-induced increase in the mouse intestinal MLCK mRNA transcript as assessed by real-time PCR. *P<0.001 versus control. (B) NF-κB p65 siRNA transfection in vivo prevented the IL-1β-induced increase in mouse intestinal MLCK protein expression as assessed by Western blot analysis. (C) NF-κB p65 siRNA transfection in vivo prevented the IL-1β-induced increase in mouse intestinal mucosal-to-serosal flux of dextran 10KD. *P<0.001 versus siRNA nontarget; **P<0.001 versus siRNA NF-κB p65.
Discussion
The defective intestinal epithelial TJ barrier is an important pathogenic factor in IBD (Hollander and others 1986; Ma and Anderson 2006). In both clinical and animal studies, maintenance of intestinal epithelial barrier has been shown to be an important factor in attenuating intestinal inflammation (Fink 2003; Hogan and others 2006). In various animal models of IBD, including in IL-10-deficient mice and dextran sodium sulfate-induced colitis, pharmacologic or probiotic enhancement of the intestinal TJ barrier prevented the development of intestinal inflammation (Sydora and others 2005; Chichlowski and others 2010). Similarly, in patients with severe active CD, a normalization of intestinal barrier after medical therapy corresponded to an improvement of the active disease and long-term clinical remission (Wyatt and others 1993; Miehsler and others 2001). In contrast, a persistent elevation in intestinal permeability predicted a poor outcome (Hollander 1988; Wyatt and others 1993; Ma and Anderson 2006). IL-1β levels are markedly elevated in IBD and play an important role in promoting intestinal inflammation (Hollander 1988; Ma and Anderson 2006). Recent studies from our laboratory have shown that IL-1β causes an increase in Caco-2 TJ permeability (Al-Sadi and Ma 2007; Al-Sadi and others 2008), raising the possibility that the elevated levels of IL-1β in IBD may contribute to the observed intestinal barrier defect. However, the effects of IL-1β on intestinal barrier in animal model systems or humans have yet to be reported, and the in vivo relevance remains unclear. Herein, using a live mouse intestinal perfusion system, we show that IL-1β induces a selective increase in intestinal permeability without causing an acute intestinal mucosal damage, but by targeting MLCK gene expression.
MLCK has been demonstrated to play an important role in intestinal TJ barrier regulation in various murine models of intestinal inflammation (Schwarz and others 2007; Su and others 2009; Weber and others 2010). Previous studies have shown that immune- and stress-mediated increases in intestinal permeability and the subsequent development of intestinal inflammation were mediated in part by an increase in MLCK expression (Schwarz and others 2007; Weber and others 2010), and treatment with MLCK inhibitors prevented the increase in intestinal permeability and the development of intestinal inflammation (Su and others 2009; Weber and others 2010). Su et al. also reported that transgenic mice overexpressing constitutively active MLCK in intestinal epithelial cells have an increase in intestinal permeability (Su and others 2009), suggesting that the overexpression of MLCK was sufficient to cause an increase in intestinal permeability. Although these transgenic mice did not develop spontaneous intestinal inflammation, when challenged with CD4+CD45+Rbhi lymphocytes, they developed more rapid and severe colitis (Su and others 2009), suggesting that the MLCK-induced intestinal barrier defect was an important factor contributing to the development of more severe colitis. The pathophysiologic importance of MLCK expression in the intestinal barrier defect and intestinal inflammation in IBD was also suggested by a study showing marked elevation of MLCK expression in intestinal epithelial cells from IBD patients (Blair and others 2006). The increase in enterocyte MLCK expression correlated with an increase in MLCK enzymatic activity and the degree of MLCK expression correlated directly with the severity of intestinal inflammation, leading the investigators to conclude that “MLCK upregulation may contribute to barrier dysfunction and IBD pathogenesis” (Blair and others 2006). In the present study, we tested the hypothesis that MLCK plays a central role in an IL-1β-induced increase in intestinal permeability in vivo. Our data indicated that IL-1β causes an increase in MLCK gene and protein expression in mouse enterocytes, and that knockout of MLCK or inhibition of MLCK activity prevented the IL-1β-induced increase in mouse intestinal permeability. These results suggested that the IL-1β-induced increase in mouse intestinal permeability in vivo required an increase in enterocyte MLCK expression and protein activity. These results also suggested that the marked elevated levels of IL-1β seen in patients with active IBD are likely to contribute to the observed increase in MLCK expression in enterocytes and increase in intestinal permeability in these patients.
Additionally, the present study showed that an IL-1β-induced increase in mouse intestinal permeability in vivo was prevented in IL-1R-deficient mice, suggesting the requirement of IL-1β binding to its membrane receptor IL-1R to induce the increase in intestinal permeability. Moreover, the IL-1β effect on mouse intestinal permeability was shown to increase significantly at day 1, and the effect persisted up to day 5 with no additional increase in intestinal permeability, suggesting that the changes in intestinal permeability was directly related to the IL-1β effect through its binding to its receptor and subsequent activation of downstream signaling pathways.
It is well established that the expression and activation of the nuclear transcription factor NF-κB is markedly increased in both intestinal epithelial cells and immune cells in IBD (Dijkstra and others 2002; Atreya and others 2008; Vavricka and Rogler 2009). NF-κB is a key proinflammatory mediator that targets a number of proinflammatory genes, leading to an increased production of proinflammatory cytokines, including tumor necrosis factor-α, IL-1β, IL-6, IL-12, and IL-23 (Atreya and others 2008). Immunostaining studies have shown a direct correlation between expression and activation of NF-κB p65 and degree of mucosal inflammation in IBD (Rogler and others 1998). Thus, an important proposed therapeutic strategy in IBD is to block NF-κB activation in the affected intestinal tissue (Atreya and others 2008; Neurath and others 1998). In addition to its direct immune-regulating effects, recent in vitro studies in Caco-2 cells suggested that NF-κB may also play a regulatory role in the TJ barrier function (Al-Sadi and others 2008; Ye and Ma 2008). The involvement of NF-κB in the intestinal barrier regulation in vivo in animal systems has not been previously reported. This is in part due to a lack of appropriate animal model systems and technical challenges in inducing enterocyte-specific inhibition or silencing of NF-κB. In the present study, we used a recently introduced novel in vivo intestinal transfection approach to selectively silence NF-κB p65 expression in mouse enterocytes (Ye and others 2011). Our results indicated that IL-1β causes a progressive degradation of IκB-α and corresponding activation of NF-κB p65 in mouse enterocytes. The selective siRNA-induced silencing of NF-κB p65 in mouse enterocytes in vivo inhibited the IL-1β-induced increase in MLCK gene and protein expression and the subsequent increase in mouse intestinal permeability. These data suggested that the IL-1β-induced increase in MLCK gene expression was regulated by NF-κB p65, and that the NF-κB p65-induced increase in MLCK expression was required for the increase in intestinal permeability. These findings suggest that in addition to its known effects on proinflammatory gene activation, NF-κB may also promote intestinal inflammation by regulating MLCK gene expression and intestinal permeability. As for the mechanism of MLCK regulation of intestinal TJ barrier function, previous studies have shown that an increase in the MLCK protein level is accompanied by an increase in MLCK activity, which in turn leads to an increase in MLCK-induced phosphorylation of myosin light chain (Turner and others 1997; Clayburgh and others 2005; Ma and Anderson 2006). The myosin light-chain phosphorylation then in a step-wise manner leads to the activation of myosin-Mg+2-ATPase, and ATP-dependent contraction of perijunctional actomyosin filaments, and mechanical tension-induced retraction of junctional membranes and proteins, and resultant opening of the TJ barrier and increase in TJ permeability (Turner and others 1997; Turner and others 1999).
In conclusion, our data show for the first time that IL-1β causes an increase in mouse intestinal permeability in vivo. The IL-1β effect on intestinal permeability was mediated by an activation and nuclear translocation of NF-κB in mouse enterocytes, leading to an increase in MLCK gene and protein expression and an MLCK-dependent increase in intestinal permeability. Our studies also demonstrate the feasibility of targeting enterocyte NF-κB p65 in vivo to prevent the cytokine-induced increase in intestinal permeability.
Acknowledgments
Images in this article were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/Facility.html
This research project was supported by a Veterans Affairs (VA) Merit Review grant from the VA Research Service and the National Institute of Diabetes and Digestive and Kidney Diseases Grant RO 1-DK-81429-03.
Author Disclosure Statement
No competing financial interests exist.
References
- Al-Sadi R. Boivin M. Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R. Ye D. Dokladny K. Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R. Khatib K. Guo S, et al. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi RM. Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya I. Atreya R. Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- Blair SA. Kane SV. Clayburgh DR. Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- Chichlowski M. Westwood GS. Abraham SN. Hale LP. Role of mast cells in inflammatory bowel disease and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS One. 2010;5:e12220. doi: 10.1371/journal.pone.0012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh DR. Barrett TA. Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F. Pizarro TT. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10(Suppl 2):49–53. doi: 10.1046/j.1365-2036.1996.22164020.x. discussion 54. [DOI] [PubMed] [Google Scholar]
- Dijkstra G. Moshage H. Jansen PL. Blockade of NF-kappaB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease? Scand J Gastroenterol Suppl. 2002;236:37–41. doi: 10.1080/003655202320621436. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dunne A. O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- Ferretti M. Casini-Raggi V. Pizarro TT, et al. Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest. 1994;94:449–453. doi: 10.1172/JCI117345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–151. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Graham WV. Wang F. Clayburgh DR, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- Heresbach D. Alizadeh M. Dabadie A, et al. Significance of interleukin-1beta and interleukin-1 receptor antagonist genetic polymorphism in inflammatory bowel diseases. Am J Gastroenterol. 1997;92:1164–1169. [PubMed] [Google Scholar]
- Hogan SP. Seidu L. Blanchard C, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. Vadheim CM. Brettholz E, et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Hollander D. Crohn's disease—a permeability disorder of the tight junction? Gut. 1988;29:1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams JS. Fitzgerald JE. Wyzga N, et al. Relationship of interleukin-1 receptor antagonist to mucosal inflammation in inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1995;21:419–425. doi: 10.1097/00005176-199511000-00008. [DOI] [PubMed] [Google Scholar]
- Ma TY. Anderson JM. Physiology of the gastrointestinal tract. 4th. Vol. 2. Burlington, MA: Elsevier Academic Press; 2006. Tight junctions and the intestinal barrier. [Google Scholar]
- Miehsler W. Puspok A. Oberhuber T. Vogelsang H. Impact of different therapeutic regimens on the outcome of patients with Crohn's disease of the upper gastrointestinal tract. Inflamm Bowel Dis. 2001;7:99–105. doi: 10.1097/00054725-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Nemetz A. Nosti-Escanilla MP. Molnar T, et al. IL1B gene polymorphisms influence the course and severity of inflammatory bowel disease. Immunogenetics. 1999;49:527–531. doi: 10.1007/s002510050530. [DOI] [PubMed] [Google Scholar]
- Neurath MF. Fuss I. Schurmann G, et al. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci. 1998;859:149–159. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–209. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- Poritz LS. Garver KI. Green C, et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- Reinecker HC. Steffen M. Doehn C, et al. Proinflammatory cytokines in intestinal mucosa. Immunol Res. 1991;10:247–248. doi: 10.1007/BF02919700. [DOI] [PubMed] [Google Scholar]
- Rogler G. Brand K. Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Schwarz BT. Wang F. Shen L, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L. Shen L. Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydora BC. Tavernini MM. Doyle JS. Fedorak RN. Association with selected bacteria does not cause enterocolitis in IL-10 gene-deficient mice despite a systemic immune response. Dig Dis Sci. 2005;50:905–913. doi: 10.1007/s10620-005-2663-0. [DOI] [PubMed] [Google Scholar]
- Turner JR. Angle JM. Black ED, et al. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol. 1999;277:C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- Turner JR. Rill BK. Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Vavricka SR. Rogler G. New insights into the pathogenesis of Crohn's disease: are they relevant for therapeutic options? Swiss Med Wkly. 2009;139:527–534. doi: 10.4414/smw.2009.12520. [DOI] [PubMed] [Google Scholar]
- Vindenes HA. Ulvestad E. Bjerknes R. Concentrations of cytokines in plasma of patients with large burns: their relation to time after injury, burn size, inflammatory variables, infection, and outcome. Eur J Surg. 1998;164:647–656. doi: 10.1080/110241598750005525. [DOI] [PubMed] [Google Scholar]
- Weber CR. Raleigh DR. Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. Vogelsang H. Hubl W, et al. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- Ye D. Guo S. Al-Sadi R. Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D. Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12:1331–1346. doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H. Russell J. Senchenkova EY, et al. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am J Pathol. 2010;177:2774–2781. doi: 10.2353/ajpath.2010.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakostelska Z. Kverka M. Klimesova K, et al. Lysate of probiotic lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One. 2011;6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]