Abstract
Although a variety of diagnostic imaging modalities are available for the evaluation of diabetes-related foot complications, the distinction between neuroarthropathy and osteomyelitis is still challenging. The early and accurate diagnosis of diabetic foot complications can help reduce the incidence of infection-related morbidities, the need for and duration of hospitalization, and the incidence of major limb amputation. Conventional radiography, computed tomography, nuclear medicine scintigraphy, magnetic resonance imaging, ultrasonography, and positron emission tomography are the main procedures currently in use for the evaluation of diabetes-related foot complications. However, each of these modalities does not provide enough information alone and a multimodal approach should be used for an accurate diagnosis. The present study is a review of the current concepts in imaging of diabetes-related foot complications and an analysis of the advantages and disadvantages of each method.
Keywords: diabetic foot, medical imaging, radiology, computed tomography, magnetic resonance imaging, nuclear imaging
Diabetes mellitus (DM) is a multi-systemic disease that is associated with significant complications affecting multiple organs. The worldwide prevalence of DM is currently estimated at 5.1% and is predicted to reach 7.7% by 2030 (1). DM is mainly a neurovascular disease that particularly affects the musculoskeletal system, especially the foot (2). Vasculopathy, neuropathy, and infection are the three major pathological processes that lead to the development of diabetes-related foot complications (3). An altered immune response (i.e. defective phagocytosis and microbicidal activity of granulocytes) is an equally responsible factor in the development of infectious foot complications (4).
The differentiation of soft tissue infection from an accompanying osteomyelitis and the early detection of abscess formation are the main goals of diagnostic imaging procedures (5). The prompt and efficient medical treatment of the diabetic foot is essential to avoid amputation (2). The most commonly used techniques for the multimodal imaging of the diabetic foot are plain radiography (PR), ultrasonography (US), magnetic resonance imaging (MRI), and nuclear medicine scintigraphy (NMS). Computed tomography (CT) is not sensitive enough but is widely used in combination with positron emission tomography (PET) (5–7).
Overview of imaging modalities
PR is one of the most common choices for radiological imaging owing to its lower cost and wide availability. PR provides information on arthropathic changes, osteomyelitis, and the presence of soft tissue gas and foreign bodies. If soft tissue gas accumulation is absent or minimal within the infected tissue, soft tissue changes such as cellulitis, fasciitis, sinus tracts, and abscess formation are difficult to detect (6–8). For the diagnosis of osteomyelitis, PR has relatively lower sensitivity (40–60%) and specificity rates (60–90%) than MRI and NMS studies. Therefore, PR is mainly used for the evaluation of major structural changes (6, 9, 10). When soft tissue gas is present, the proximal joint next to the infected area must be included in the PR to assess the extent of the infection (11).
US is another widely available and noninvasive imaging modality, although its role in the evaluation of diabetes-related foot complications is limited (12). It is especially useful for the detection of infectious/inflammatory soft tissue changes and localization of foreign bodies, diagnosis of tenosynovitis and joint effusion, and differentiation of infected/reactive collection. This modality is also important in providing guidance for the aspiration of abscesses, cystic lesions, and sterile collections (5, 7).
Although CT plays a limited role in the imaging of diabetes-related foot complications, it has certain advantages over PR, such as the generation of images with high tissue contrast. Furthermore, it is more sensitive and specific for the identification of cortical erosions, small sequestra, soft tissue gas, calcifications, and foreign bodies compared with PR and MRI (13). The three-dimensional nature of CT makes it a useful tool for the analysis of compartmental anatomy. The main disadvantages of CT are insufficient demarcation of healthy and infected tissues due to severe beam hardening artifacts caused by small and closely located foot bones, and the presence of ionizing radiation (5, 7).
MRI is currently considered as the best modality for the evaluation of soft tissue and bone marrow changes associated with the diabetic foot (12, 14, 15). Bone marrow edema as the early finding of neuroarthropathy and osteomyelitis can be detected using MRI with high sensitivity and specificity (90–100 and 40–100%, respectively) (2, 17, 16). High soft tissue contrast and multiplanar imaging capabilities are the major advantages of MRI for the detection and delineation of the extension of the infection. MRI also enables the differentiation of osteomyelitis from neuroarthropathy and reactive bone marrow edema and that of sterile joint effusion from septic arthritis, which require entirely different treatment regimens (16).
In NMS of the diabetic foot, the most commonly used methods are three-phase bone scintigraphy using 99m-technetium (99mTc) phosphonates, inflammation scintigraphy, and bone marrow scintigraphy (17). 99mTc-methylene diphosphonate bone scintigraphy was shown to have high sensitivity (94%), specificity (95%), and accuracy in the absence of an underlying abnormality such as neuroarthropathy, trauma, surgery, or tumor (18). In such cases, the specificity is markedly reduced to 33%, whereas the sensitivity remains high at 95% (19). The low specificity values of diphosphonate scans limit the utilization of the three-phase bone scan to the detection of early neuroarthropathic changes and the evaluation of soft tissue swelling in patients with no apparent skin ulcers. The four-phase bone scan, in which an additional 24-h static image is acquired, does not significantly improve the sensitivity of the scan for the detection of osteomyelitis (20).
Inflammation scintigraphy can be performed using radiolabeled granulocytes, radiolabeled polyclonal immunoglobulin, antigranulocyte antibodies, or gallium-67. The best results are obtained with radiolabeled antibodies, which have shown a sensitivity ranging from 72 to 100% and a specificity between 72 and 98% (21). 99mTc hexamethylpropyleneamineoxime leukocyte scintigraphy is highly specific (96%) for the detection of diabetic foot osteomyelitis (22). Special equipment and trained staff requirements are the major disadvantages of this time-consuming technique. Scintigraphy with 99mTc-labelled antigranulocyte monoclonal antibody fragment is an efficient technique for the detection of soft tissue and bone infections (9, 23, 24).
PET/CT with 18F-fluoro-2-deoxy-d-glucose an indicator of increased intracellular glucose metabolism that accumulates at the sites of infection, is associated with high sensitivity (80–95%) and specificity rates (90–100%) in the diagnosis of diabetes-related osteomyelitis and neuroarthropathy (10, 25). A precise diagnosis of osteomyelitis versus soft tissue infection with better anatomic localization is possible with PET/CT (26).
Although the presence of an underlying neuroarthropathy makes the diagnosis of osteomyelitis difficult, high accuracy and specificity rates have been achieved using PET/CT for the differentiation of osteomyelitis from neuroarthropathy (10, 27). Furthermore, PET/CT has been found to be superior to leukocyte-labeled and antigranulocyte monoclonal antibody fragment techniques in the diagnosis of chronic osteomyelitis (27). In patients with medical implants, which can cause distortion in MRI, PET/CT is a good alternative. This technique might also be preferred in the postoperative assessment of these patients (28).
Future directions for imaging modalities
High field MRI
Increased temporal and spatial resolution, decreased acquisition time and motion-related artifacts are the advantages of high filed MRI. Ultra-high field 7-Tesla systems may offer multinuclear applications with low gamma sodium and phosphorus nuclei. In the early stages of the disease, phosphorus imaging might show altered metabolic status of muscle and bone tissue in the patients with diabetes-related osteomyelitis (29).
Perfusion imaging
Assessment of tissue viability in the patients upon whom had been performed soft tissue flaps, and evaluation of perfusion features of tissue presenting ischemic changes are available via perfusion studies. CT perfusion imaging has some disadvantages such as contrast media and induced ionizing radiation. MR perfusion imaging with or without contrast agent seems to be the most appropriate technique. With MR perfusion imaging, the extent of ischemic changes may reliably be evaluated before surgery. Dynamic contrast enhanced perfusion and arterial spin labeling perfusion techniques will be able to find wide acceptance in the musculoskeletal imaging (9).
PET/MRI
With high resolution and capability of functional assessment such as spectroscopy, MRI is a good choice for combination with PET. The main technical limitation is impaired scintillation flash transfer in the high magnetic field. MRI compatible PET scanners with appropriate technology would seem to solve this problem in the future (21).
Medical imaging and diabetes-related foot complications
Soft tissue complications
Skin callus
Skin callus formation in atypical locations in the foot is a common problem in diabetic patients. In the forefoot, callus formation occurs beneath the first and fifth metatarsal heads and at the tip of the great toe. In the midfoot, callus formation occurs beneath the cuboid bone in patients with neuropathic disease and rocker-bottom deformity (Fig. 1), and in the hindfoot, most calluses develop at the heel (30). On MRI, a skin callus appears as a focal infiltration or mass within subcutaneous fat, with low signal intensity on T1-weighted images and low to intermediate signal intensity on T2-weighted images. On MR images obtained after intravenous gadolinium injection, the callus might resemble a soft tissue infection. Careful determination of the location of the lesion and the presence of associated soft tissue changes may help distinguish callus formation from infection. The formation of adventitial bursa may accompany callus development (9).
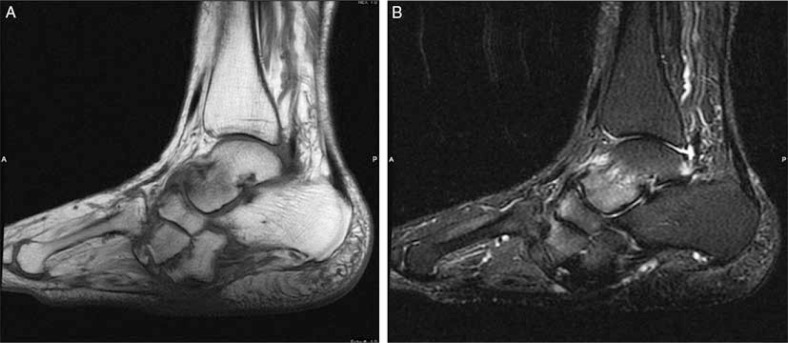
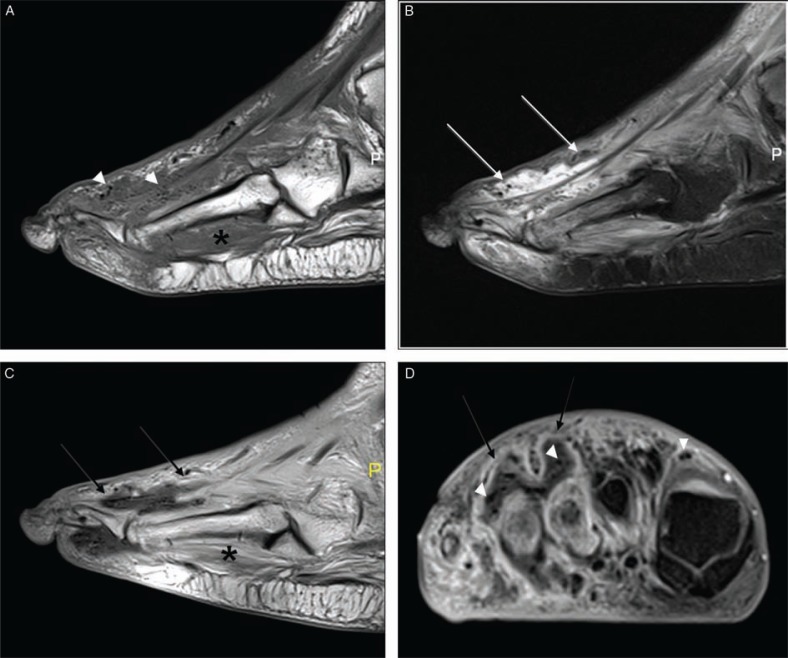
Fig. 1.
Skin callus and talar plantar flexion deformity in a diabetic patient with neuroarthropathy. Sagittal T1 (A), and T2-weighted with fat-suppressed (B) images revealed focal hypointense area in subcutaneous fat in the midfoot in both sequences (arrows) with no accompanying soft tissue changes consistent with callus. Subchondral marrow edema at intertarsal joints is a result of neuroarthropathy.
Skin ulcers
Skin ulcers are generally found in similar locations as calluses because ulcers are a result of callus breakdown. In the neuropathic foot, midfoot and dorsal toe ulcers commonly occur without preceding callus formation. Forefoot and midfoot ulcers are typically superficial and occur in ambulatory patients. In non-ambulatory patients, ulcers are mostly broad and deep and occur at the calcaneus and lateral malleolus because of chronic pressure on the externally rotated foot (30). Ulcers typically appear as focal skin interruptions with elevated margins and associated soft tissue defects (Fig. 2). Unlike calluses, ulcers are detected as hyperintense areas on T2-weighted images, with intense peripheral enhancement on T1-weighted images, a finding indicative of granulation tissue at the base of the ulcer (9).
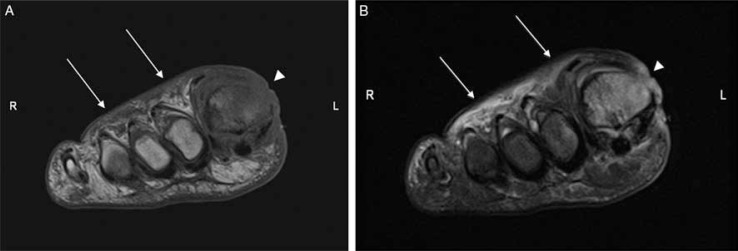
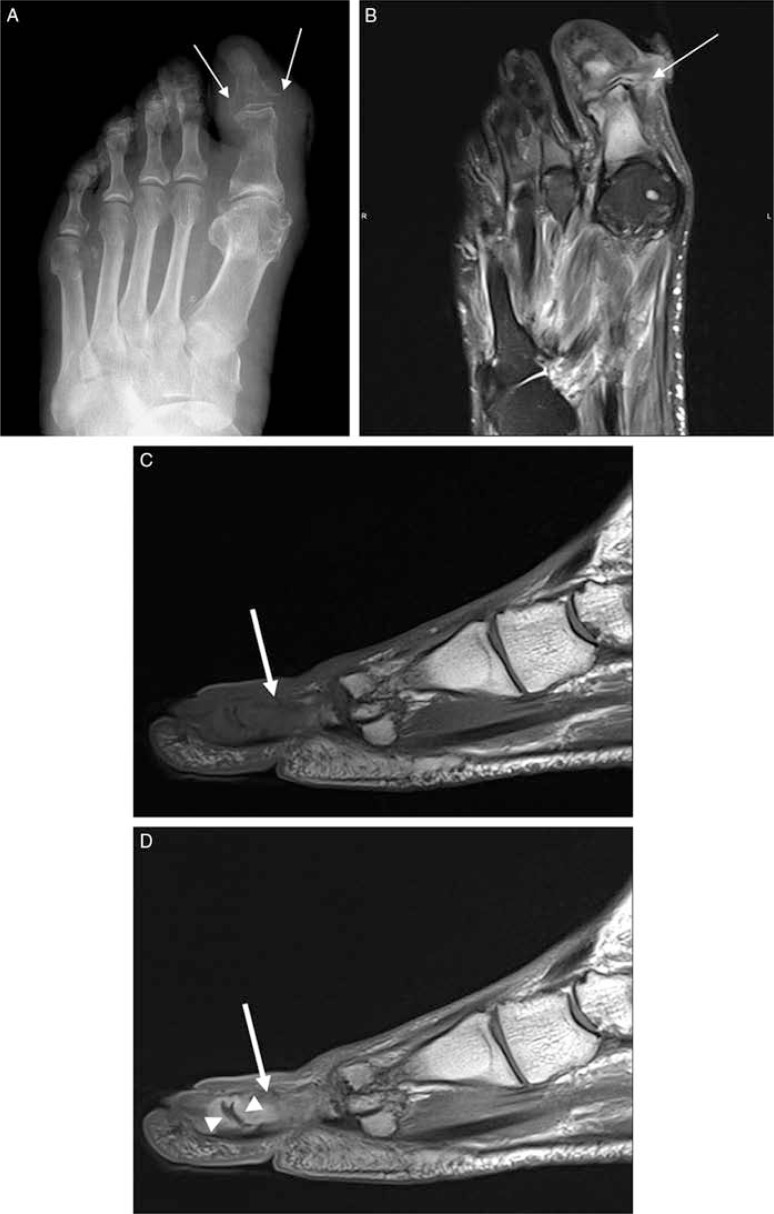
Fig. 2.
Forefoot ulcers associated with first metatarsal osteomyelitis in a diabetic patient (A, B). Short-axis T1 (A), and T2-weighted fat-suppressed (B) images showed two ulcers: one in the dorsum of the foot as evidenced by superficial skin discontinuity (arrows), and the other in the dorsal-medial portion of the first toe possibly resulted from callus breakdown (arrowheads). Abnormal bone marrow signal both in T1- and T2-weighted images is consistent with osteomyelitis.
Edema versus cellulitis
Diffuse, noninfectious soft tissue swelling is common in the foot and ankle in patients with diabetes. The role of imaging in the evaluation of soft tissue swelling is to determine whether neuropathic disease is present and assess the extent of soft tissue infection (30, 31). Focal or diffuse soft tissue swelling, which appears as increased opacity within the infected tissue, can be detected using PR. Gas accumulation within the infected tissue can be identified in PR and indicates the presence of severe infection with necrosis (Fig. 3). Unfortunately, in most patients, differentiation of diabetes-related soft tissue complications is not possible with PR alone (7).
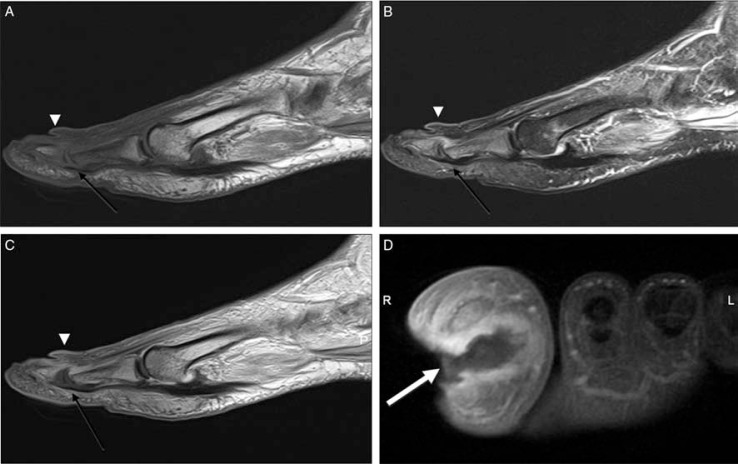
Fig. 3.
Cellulitis, fistula and associated osteomyelitis and septic arthritis of the first toe (A–D). Sagittal T1 (A), T2-weighted fat-suppressed (B), and post-contrast T1-weighted (C), and short-axis T1-weighted fat-suppressed (D) images demonstrate a high T2 signal and significant skin enhancement after administration of contrast demonstrating cellulitis (arrowheads). There is a deep ulcer in the medial portion of the first toe and fistula (white arrow) traversing the distal phalanx. Sagittal images also demonstrated the abnormal signal of the proximal and distal phalanges due to osteomyelitis and accompanying septic arthritis (black arrow).
In patients with cellulitis but without apparent skin ulcers, CT can detect skin edema and subcutaneous fat stranding. Small collections within the diffusely infected soft tissue planes might be detected. On CT images, homogeneous enhancement within the edematous soft tissue planes and/or superficial fascia after the administration of contrast material and fluid collection is more indicative of cellulitis than edema (7).
Skin thickening and edema within the subcutaneous soft tissues are characteristics of soft tissue edema and cellulitis. In both cases, there is an increased T2 signal intensity and reticulation of fat is more prominent on T1-weighted images (32). Contrast enhancement is a distinguishing feature of cellulitis and is not seen in diabetes-related edema or neuropathic disease (24). Nevertheless, a clinical evaluation is necessary to obtain an accurate diagnosis. The diagnosis of focal cellulitis in the presence of adjacent osteomyelitis, an abscess, or a sinus tract is easier (5–7, 9). In patients with cellulitis, a bone scan is particularly useful to exclude osteomyelitis. Increased bone marrow activity is generally observed in the first and second phases, whereas increased soft tissue activity without focal bone uptake is a crucial finding in the third phase and indicates cellulitis without osteomyelitis (7).
Abscess and sinus tract formation
The diagnosis of deep soft tissue abscesses is possible with CT and MRI. Abscesses often present as focal hypodense lesions with a surrounding enhancing rim on CT (7). On MRI, contrast enhancement along the wall of the fluid collection and a higher T2 signal intensity indicative of pus formation in comparison to the surrounding soft tissue edema are highly suggestive of an abscess. MRI also provides useful information about the localization and number of abscesses during the pre-surgical evaluation. The MRI features of a sinus tract resemble an abscess. Furthermore, a meandering sinus tract may appear round if viewed in cross-section and may be mistaken for an abscess. Therefore, sinus tracts should be evaluated in all imaging planes (9).
Necrotizing fasciitis, pyomyositis, and myonecrosis
The presence of air in soft tissues and fluid collections within the deep fascial planes indicate necrotizing fasciitis. This condition is characterized by thickening and enhancement of superficial and deep fascia, whereas subcutaneous fat tissues might be preserved (7). Because necrotizing fasciitis and pyomyositis are limb and life-threatening conditions in diabetic patients, their prompt diagnosis and immediate treatment is crucial.
In necrotizing fasciitis, liquefactive necrosis and edema cause fluid collection in the fascia, which is observed as an increase in peripheral band-like signal intensity on fat-suppressed T2-weighted images and post-contrast T1-weighted images of surrounding muscles and along the deep fascial planes. Intramuscular abscesses that present as focal lesions with an enhancing rim, a diffuse signal increase in the affected muscles on T2-weighted images, and an associated irregular enhancement of the deep fascia on postcontrast T1-weighted images are the major MRI features of pyomyositis (Fig. 4) (33).
Fig. 4.
Gas gangrene and pyomyositis in a patient with diabetes (A–C). Plain radiography (A), and sagittal T2-weighted fat-suppressed (B) demonstrate extensive soft tissue gas (arrowhead) in the dorsum of the foot. In T2-weighted image, diffuse signal increase in plantar muscles and fluid like focal areas (arrows) that demonstrated peripheral rim enhancement (arrows) in post-contrast T1-weighted images (C) consistent with pyomyositis.
Gas bubbles associated with soft tissue necrosis and certain bacteria appear as signal-void areas in all conventional sequences (5, 7, 33). The differentiation between soft tissue gas related to gangrene and that related to a skin ulcer, which may serve as a portal of entry of air to soft tissues, is critical. Gangrene is usually associated with non-enhancing devitalized tissue and is observed as a more extensive area of soft tissue gas than that found surrounding an ulcer (Fig. 5) (34). Clinically and radiologically, diabetes-related myopathy may not be distinguished from an infectious muscle abscess. Typical MRI findings in diabetic myonecrosis include marked edema and irregular enhancement surrounding necrotic muscle groups (Fig. 6) (35).
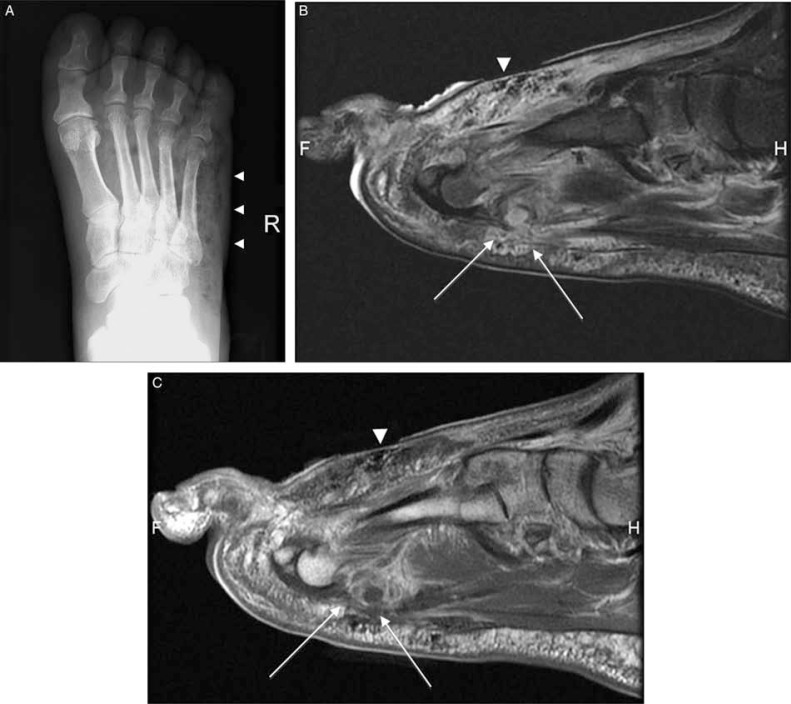
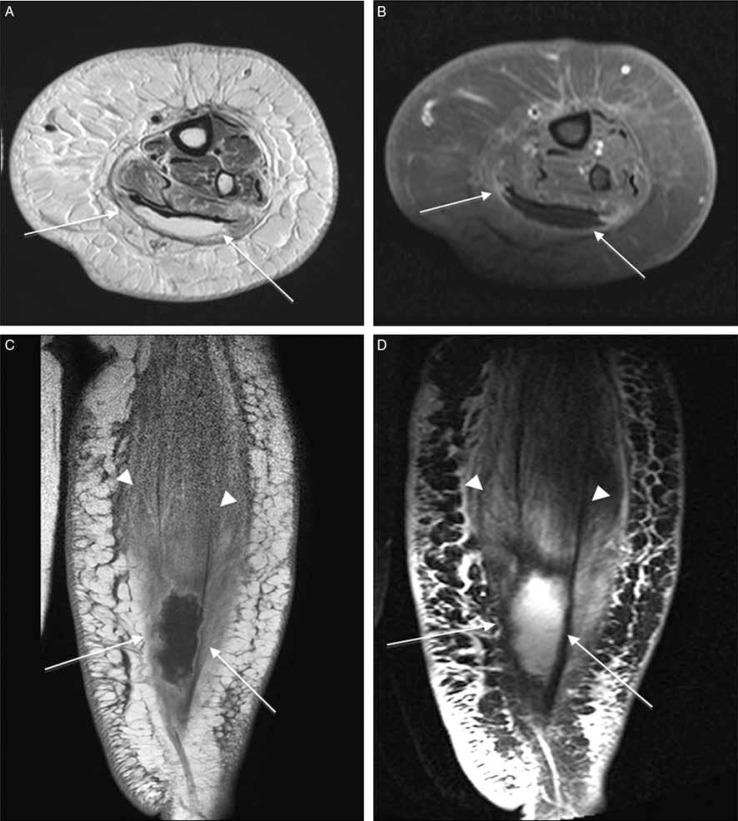
Fig. 5.
Gas gangrene in a patient with crepitation of the foot and signs of infection and osteomyelitis (A–D). Sagittal T1 (A), T2-weighted fat-suppressed (B), post-contrast, sagittal T1-weighted (C), and short-axis T1-weighted (D) MRI show widespread low-signal-intensity foci demonstrating blooming artifact (arrowheads) along the dorsum of the foot indicating soft-tissue gas. Furthermore, fluid collections (white arrows), that show peripheral enhancement (black arrows) and accompanying subcutaneous tissue, fascial and muscular enhancement (asterisk) are present.
Fig. 6.
Diabetic myonecrosis of the left posterior calf muscles in a poorly controlled diabetes patient in an extremely painful and tender left leg. Transverse fat-saturated T2–weighted (A), post-contrast, transverse fat-suppressed T1 (B), and coronal T1-weighted (C), and T2-weighted-fat suppressed (D) images shows a cystic non-enhancing area within gastrocnemius muscle (arrows) and swollen edematous posterior calf muscles (arrowheads) consistent with myonecrosis. Note the extensive cellulitis and superficial fascial inflammation but no signs of soft tissue ulcer or sinus tract.
Tenosynovitis
Septic tenosynovitis most commonly occurs in the peroneal and Achilles tendons, originating from lateral and calcaneal ulcers, respectively. In the forefoot, most infections involve the flexor tendons and are the result of plantar forefoot ulcerations (9). Tendon thickening, hyperintensity on T2-weighted images, and enhancement around the tendon sheath are nonspecific findings, except in the presence of peritendinous enhancement coursing through an area of cellulitis and adjacent to an infected ulcer (Fig. 7) (36, 37).
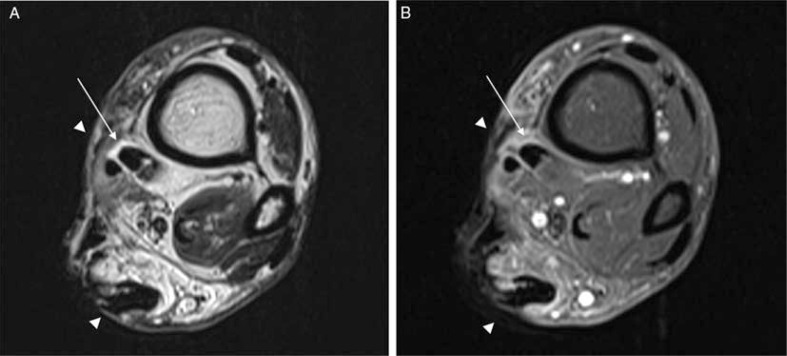
Fig. 7.
Tenosynovitis in a diabetic patient with deep, extensive soft tissue ulceration in the medial portion of the proximal ankle (A, B). Transverse T2-weighted (A) post-contrast T1-weighted fat-suppressed (B) image demonstrated tiny fluid collection around the posterior tibial tendon that demonstrates contrast enhancement (arrows). The tendon is closely related with to the deep ulcer in subcutaneous tissue (arrowheads).
US may be useful for differentiating between infected and uninfected fluid surrounding the tendon sheath, as the presence of abnormal internal echogenicity is characteristic of infected fluid collection, demonstrating a localized infection. US also provides a dynamic evaluation of tendon integrity (7).
Osseous complications
Neuroarthropathy
Peripheral neuropathy causes reduced perception of trauma, and peripheral vascular disease results in ischemia, causing poor healing, joint instability, deformity, and increased new bone formation. Cartilage damage is also a feature of this condition, and it induces progressive arthropathy with erosions and subchondral cysts. The midfoot or tarsometatarsal (Lisfranc) joints are typically affected in neuropathic osteoarthropathy, leading to a collapse of the longitudinal arch and increased load bearing on the cuboid, ultimately causing a rocker-bottom deformity. Neuropathic arthropathy can present in acute and chronic forms (38).
On PR, the flattening of the metatarsal head is often the first sign of diabetes-related neuroarthropathy. Involvement of the interphalangeal joints and ankle is less common (5). Early changes such as bone resorption and tiny fragmentation are considered as atrophic. Delayed hypertrophic changes are compatible with new bone formation and joint deformities (Fig. 8) (39). The earliest finding of neuroarthropathy in PR is focal demineralization. In the absence of soft tissue involvement, subchondral or periarticular changes in the midfoot with polyarticular distribution strongly indicate diabetes-related neuroarthropathy (16). However, subtle changes associated with neuroarthropathy such as occult fractures and bone marrow edema is not detected by PR.
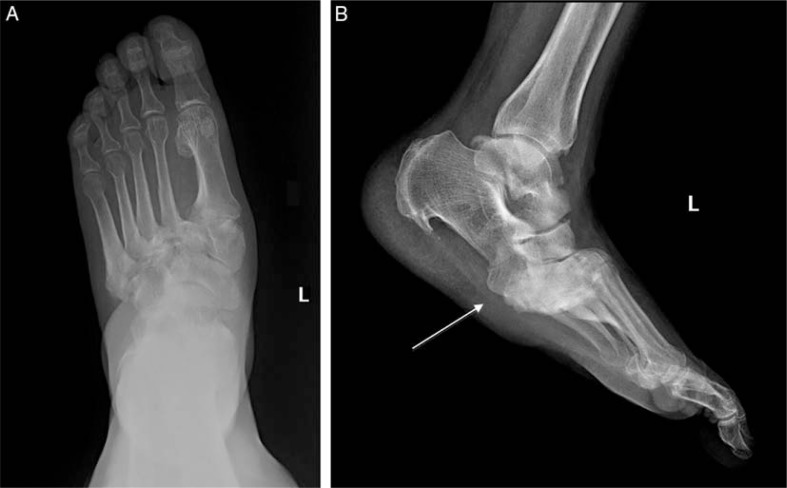
Fig. 8.
Neuropathic arthropathy (A, B). Anteroposterior (A), and lateral non-weight bearing (B) views show osseous and articular disorganization, midfoot collapse, and talar plantarflexion (arrow) with development of a rocker-bottom deformity.
On CT, the early changes of neuroarthropathy such as bone marrow edema and microfractures cannot be distinguished, as they are subtle. However, in the chronic stages, bone fragments and accompanying soft tissue swelling can easily be observed (Fig. 9). In the early stages of diabetic neuroarthropathy, when there are no radiographic findings, neuroarthropathy can mimic osteomyelitis on bone scans, clinically, and on MRI images (9).
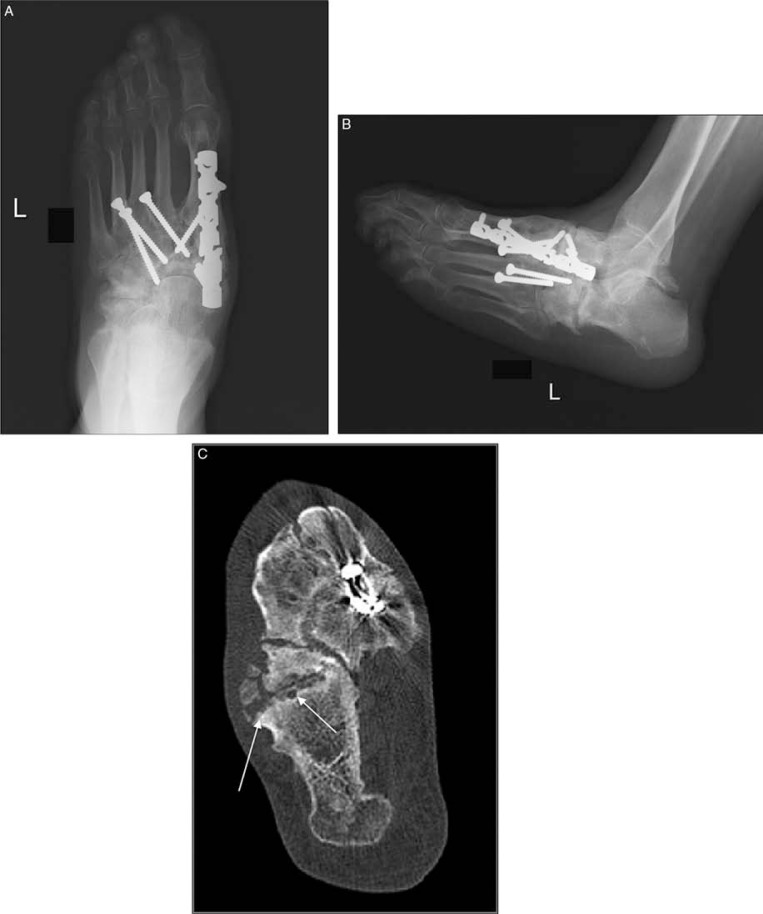
Fig. 9.
Midfoot reconstruction in a patient with unstable neuropathic osteoarthropathy (A–C). Anteroposterior (A), and lateral (B) plain radiographs demonstrated neuropathic changes in the midfoot region and complex realignment and fusion (A). Joint destruction, osseous fragmentation and subchondral cyst formation (arrows) better delineated compared to plain radiographs in transverse CT image (C).
The symptoms associated with the early stages of neuroarthropathy, including soft tissue edema, fluid collections, effusions, and bone marrow abnormalities, can be detected by MRI (40, 41). Periarticular soft tissue and bone marrow enhancement are observed by contrast-enhanced MRI (42). Bone resorption is a common finding in the subacute stages of neuroarthropathy. In the chronic stage, there is no substantial soft tissue edema or osseous resorption, yet the foot is deformed (43, 44). Deformity, osseous fragmentation, and joint effusion may be observed in MRI, with little marrow edema (Fig. 10).
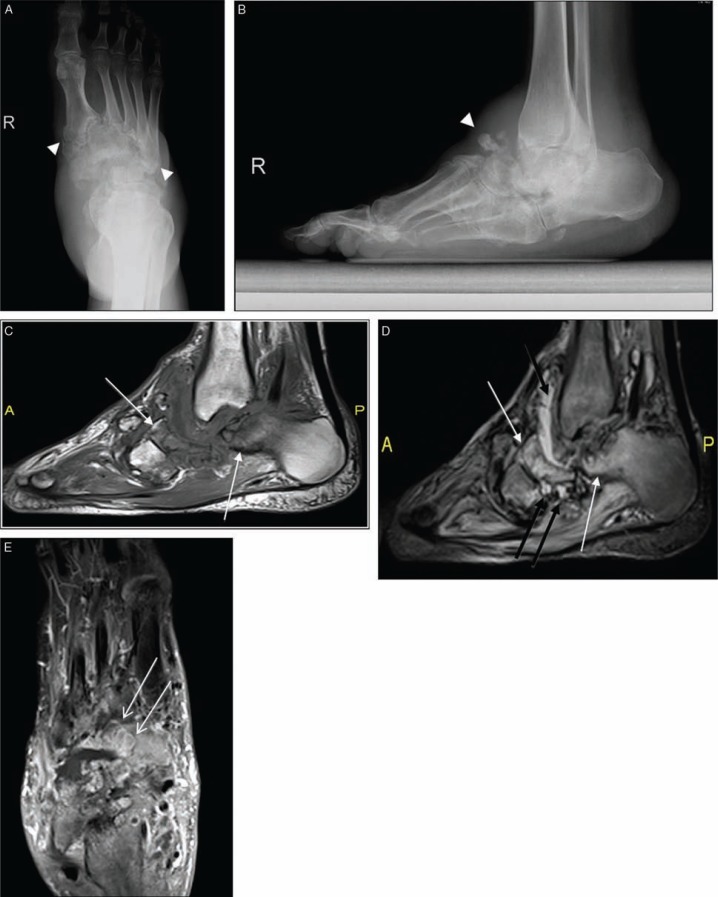
Fig. 10.
Chronic neuropathic osteoarthropathy (A–E). Anteroposterior (A), and lateral (B) plain radiographs of the foot and ankle illustrate fragmentation and subluxation (arrowheads) at the midfoot with dorsal soft tissue swelling. There is an extensive edematous bone marrow changes (black arrows) in the midfoot in T1 (C), and T2-weighted fat-suppressed images (D). Multiple fluid collections (black arrows) mostly in the midfoot articulations were also demonstrated. Diffuse bone marrow enhancement and associated periarticular subchondral cysts (white arrows) in post-contrast long axis T1 weighted fat-suppressed (E) images are all suggestive for neuroarthropathy only. Note there is no associated ulcer, sinus tract or abscess formation. Clinical evaluation revealed no signs of infection.
In the diagnosis of acute neuroarthropathy, radionuclide studies are generally used as the complementary methods to MRI. The routine triple-phase bone scan is used as an initial step owing to its low specificity. Leukocyte-labeled scintigraphic studies enable the exclusion of osteomyelitis in the presence of a positive bone scan. However, the leukocyte scan may be positive in the early stages of diabetic neuroarthropathy owing to joint effusion induced by periarticular microfractures. A decrease in leukocyte accumulation within the involved area in the follow-up scan indicates acute neuropathic changes (7).
PET/CT can provide accurate data about neuropathic changes, and in routine clinical practice, it is mainly used to enhance the diagnostic value of PR and MRI. Prominent bone remodeling in the postsurgical stages decreases the specificity of the triple-phase bone scan; therefore, PET/CT or leukocyte-labeled scintigraphy studies are more appropriate (10, 21).
Osteomyelitis
Osteomyelitis is observed in 20–65% of patients with diabetes-related foot complications (45, 46). Although bone biopsy is still considered the criterion standard (40, 47), the multimodality diagnosis of osteomyelitis includes PR, nuclear scintigraphy, CT and MRI. The earliest evidence of osteomyelitis on PR includes focal areas of demineralization of the affected bone, obscuration of the fat planes, and soft tissue swelling. The classical triad of osteomyelitis consists of periosteal reaction, osteolysis, and bone destruction. Despite the high sensitivity and specificity rates (60 and 80%, respectively) of PR in the detection of acute osteomyelitis, the classic findings may not be present in the early stages of the disease (up to 10–20 days) (9). Replacement of intramedullary fat, periosteal reaction, and cortical resorption-destruction can be identified using CT (9). CT is also superior to PR and MRI in the detection of subtle cortical erosions, foreign bodies, gas accumulation, small sequestra, and vascular/dystrophic calcifications (5, 7).
Leukocyte-labeled radionuclide imaging and its combination with other imaging techniques provide accurate information for the diagnosis and differentiation of osteomyelitis from acute neuroarthropathy (7, 9, 10, 21). After an abnormal bone scan finding, leukocyte scans with either 111I or 99mTc may be accurate for the diagnosis of osteomyelitis and arthritis. One to 3 days after the onset of symptoms of acute osteomyelitis, a triple-phase bone scan showed increased activity in the first two phases (angiographic and blood pool phases) and focal increased uptake in the late phase (2–3 h; Fig. 11). Although the addition of a fourth phase to the bone scan provides a high target-background ratio and reduces noise, its use is controversial. On the other hand, the combination with a leukocyte scan may result in higher sensitivity and specificity rates in comparison with the four-phased bone scan (48). When cost-effectiveness is considered, the triple-phase bone scan combined with a labeled leukocyte scan seems to be reasonable (7). In the diagnosis of osteomyelitis, the highest sensitivity and specificity rates have been reported for inflammatory scintigraphy with radiolabeled antibodies (72–100 and 72–98%, respectively). Bone marrow scintigraphy can be performed to show bone marrow infection in the presence of severe arthropathic changes (17).
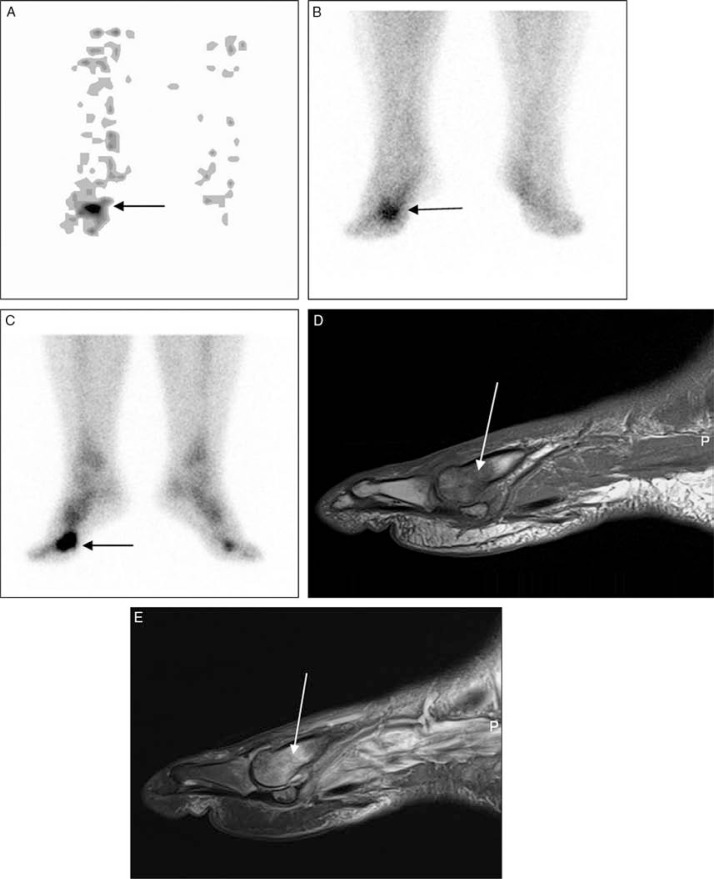
Fig. 11.
Positive three-phase hydroxymethanediphosphonate (99mTc-HDP) bone scan in a male with a clinical suspicion of osteomyelitis. First phase (angiographic) (A) bone scan demonstrating asymmetric blood flow with markedly increased tracer delivery to the region of the right toe (black arrow). Second phase (blood pool) (B), and third phase (delayed) (C) bone scan images illustrating marked increase in tracer activity (black arrows) in the same region consistent with osteomyelitis. Bone marrow changes in sagittal T1-weighted (D) and T2-weighted fat-suppressed (E) images also indicate presence of osteomyelitis in the first metatarsal head (white arrows).
Several studies, including one conducted by the American College of Radiologists Study Group, underlined the usefulness and value of MRI in the evaluation of diabetes-related foot complications and its accuracy for detecting osteomyelitis even in the presence of neuropathic arthropathy (14). It has been recommended that MRI should be the first modality of choice in diabetic patients with soft tissue swelling without an apparent skin ulcer, followed by leukocyte-labeled scintigraphy (12). The MRI-based diagnosis of osteomyelitis hinges on the identification of abnormal bone marrow signals, typified by hypointensity on T1-weighted images and hyperintensity on fluid-sensitive images. Although T2-weighted images are considered the most sensitive, it is the abnormal T1-weighted signal that is more indicative of osteomyelitis because the loss of the fatty marrow signal represents true infiltration of the bone by the infectious process (9, 30). A careful evaluation of the signal intensity of any bone adjacent to detectable ulcers and sinus tracts is a precise way of assessing the presence of osteomyelitis (31).
Differentiation of osteomyelitis from neuroarthropathy
The differentiation of acute neuroarthropathy from acute osteomyelitis is one of the most challenging issues in the evaluation of diabetes-related foot complications. Several MRI features have been described that are helpful in differentiating between these entities. Osteomyelitis develops almost exclusively by the contiguous spread of infection from skin ulcerations at predictable sites, whereas neuropathic arthropathy is primarily periarticular (30). The presence of a bone marrow abnormality in the periarticular region without an adjacent ulceration is indicative of neuroarthropathy. The location of bone marrow signal changes is the most useful distinguishing feature of osteomyelitis. Neuroarthropathy most commonly affects the tarsometatarsal and metatarsophalangeal joints, whereas osteomyelitis occurs distal to the tarsometatarsal joint, in the calcaneus and malleolus (9). The midfoot presents the greatest diagnostic difficulty, although secondary signs of infection such as direct spread from an ulcer over a rocker-bottom deformity and the presence of a sinus tract are indicative of osteomyelitis (9, 14, 49).
Septic arthritis
Midfoot septic arthritis is characteristic of patients with neuroarthropathy and is the most challenging issue to diagnose because its appearance can be nearly identical to that of midfoot neuroarthropathy (9). Periarticular demineralization and joint space enlargement may be observed in PR in the early stages of septic arthritis. MRI may show complex joint effusion and thick synovial enhancement in the affected joint. Perisynovial edema within adjacent soft tissue and subchondral reactive bone marrow edema are also common findings. It is important to distinguish reactive bone marrow changes that might be seen in septic arthritis from an associated osteomyelitis. Proximal extension of the edema beyond the subchondral bone and a diffuse, overt hypointense signal in the adjacent marrow on T1-weighted images are likely indicative of osteomyelitis (Fig. 12) (50).
Fig. 12.
Septic arthritis and associated osteomyelitis in a diabetic patient. Plain radiograph (A) demonstrates focal soft tissue swelling, demineralization (arrows) in periarticular region in distal interphalangial joint of the first toe. Long-axis T2-weighted fat-suppressed image (B) demonstrated an ulcer and sinus tract (thin white arrow) extending to the joint space. There is a synovial enhancement (arrowhead) and abnormal intramedullary signal (thick white arrow) that is extending from the joint surface in pre-(C) and post-contrast T1-weighted (D) images consistent with septic arthritis and accompanying osteomyelitis.
Postsurgical changes
A detailed history of previous trauma and treatment (surgery, interventional procedure, etc.) is essential for the diagnosis of any diabetic foot pathology. In such patients, PR might provide limited information, especially in the presence of orthopedic hardware. On the other hand, CT with its multiplanar capability provides high spatial resolution images for the assessment of non-union, fracture, osteolysis, and dystrophic soft tissue calcifications (7). PET/CT can provide an accurate diagnosis when used alone and is the method of choice for the evaluation of patients who have MR-incompatible surgical devices. Scintigraphy enhances the diagnostic value of PET/CT and MRI, especially in patients with a suspected infection. Postsurgical prominent bone remodeling decreases the specificity of the triple-phase bone scan; therefore, leukocyte-labeled scintigraphy studies should be performed in these cases (7, 21). The evaluation of myocutaneous flaps is another difficult issue for physicians and radiologists. Muscle perfusion scintigraphy with 99mTc-methoxyisobutrylisonitryl or thalium-201 may be performed in these cases, but these scintigraphy methods have not been met with wide acceptance (21).
Summary
The medical imaging of diabetes-related foot complications remains challenging. There are advantages and disadvantages to each currently available modality, suggesting that multimodal imaging is the strategy of choice for accurate diagnosis in diabetic patients. MRI has been shown to play an integral role in the evaluation of diabetes-related foot complications.
Conflict of interest and funding
The authors have received no funding or benefits from industry to conduct this literature review.
References
- 1.Lam D, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–6. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 2.Roug IK, Pierre-Jerome C. MRI spectrum of bone changes in the diabetic foot. Eur J Radiol. 2012;81:1625–9. doi: 10.1016/j.ejrad.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Gouveri E, Papanas N. Charcot osteoarthropathy in diabetes: a brief review with an emphasis on clinical practice. World J Diabetes. 2011;2:59–65. doi: 10.4239/wjd.v2.i5.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard JL, Lavigne JP, Sotto A. Diabetes and foot infection: more than double trouble. Diabetes Metab Res Rev. 2012;28:S46–53. doi: 10.1002/dmrr.2234. [DOI] [PubMed] [Google Scholar]
- 5.Loredo RA, Garcia G, Chhaya S. Medical imaging of the diabetic foot. Clin Podiatr Med Surg. 2007;24:397–424. doi: 10.1016/j.cpm.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Dinh T, Snyder G, Veves A. Current techniques to detect foot infection in the diabetic patient. Int J Low Extrem Wounds. 2010;9:24–30. doi: 10.1177/1534734610363004. [DOI] [PubMed] [Google Scholar]
- 7.Loredo R, Rahal A, Garcia G, Metter D. Imaging of the diabetic foot diagnostic dilemmas. Foot Ankle Spec. 2010;3:249–64. doi: 10.1177/1938640010383154. [DOI] [PubMed] [Google Scholar]
- 8.Hartemann-Heurtier A, Senneville E. Diabetic foot osteomyelitis. Diabetes Metab. 2008;34:87–95. doi: 10.1016/j.diabet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Donovan A, Schweitzer ME. Current concepts in imaging diabetic pedal osteomyelitis. Radiol Clin North Am. 2008;46:1105–24. doi: 10.1016/j.rcl.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Nawaz A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol. 2010;12:335–42. doi: 10.1007/s11307-009-0268-2. [DOI] [PubMed] [Google Scholar]
- 11.Zgonis T, Stapleton JJ, Girard-Powell VA, Hagino RT. Surgical management of diabetic foot infections and amputations. AORN J. 2008;87:935–46. doi: 10.1016/j.aorn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer ME, Daffner RH, Weissman BN, Bennett DL, Blebea JS, Jacobson JA, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881–6. doi: 10.1016/j.jacr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Murphy W, Totty W, Destouet J. Musculoskeletal system. In: Lee J, Sagel S, Stanley R, editors. Computed body tomography with MRI correlation. New York, NY: Raven Press; 1989. pp. 899–987. [Google Scholar]
- 14.Kapoor A, Page S, LaValley M, Gale D, Felson D. Magnetic resonance imaging tor diagnosing foot osteomyelitis. Arch Intern Med. 2007;167:125–32. doi: 10.1001/archinte.167.2.125. [DOI] [PubMed] [Google Scholar]
- 15.Rozzanigo U, Tagliani A, Vittorini E, Pacchioni R, Brivio LR, Caudana R. Role of magnetic resonance imaging in the evaluation of diabetic foot with suspected osteomyelitis. Radiol Med. 2009;114:121–32. doi: 10.1007/s11547-008-0337-7. [DOI] [PubMed] [Google Scholar]
- 16.Toledano TR, Fatone EA, Weis A, Cotten A, Beltran J. MRI evaluation of bone marrow changes in the diabetic foot: a practical approach. Semin Musculoskelet Radiol. 2011;15:257–68. doi: 10.1055/s-0031-1278425. [DOI] [PubMed] [Google Scholar]
- 17.Palestro C, Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med. 2009;39:53–65. doi: 10.1053/j.semnuclmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. Radiographics. 2003;23:341–58. doi: 10.1148/rg.232025103. [DOI] [PubMed] [Google Scholar]
- 19.Schauwecker D. The scintigraphic diagnosis of osteomyelitis. AJR Am J Roentgenol. 1992;158:9–18. doi: 10.2214/ajr.158.1.1727365. [DOI] [PubMed] [Google Scholar]
- 20.Alazraki N, Dries D, Datz F. Value of a 24-hour image (four-phase bone scan) in assessing osteomyelitis in patients with peripheral vascular disease. J Nucl Med. 1985;26:711–7. [PubMed] [Google Scholar]
- 21.Ranachowska C, Lass P, Korzon-Burakowska A, Dobosz M. Diagnostic imaging of the diabetic foot. Nucl Med Rev Cent East Eur. 2010;13:18–22. [PubMed] [Google Scholar]
- 22.Poirier JY, Garin E, Derrien C, Devillers A, Moisan A, Bourquet P, et al. Diagnosis of osteomyelitis in the diabetic foot with a 99mTc-HMPAO leucocyte scintigraphy combined with a 99mTc-MDP bone scintigraphy. Diabetes Metab. 2002;28:485–90. [PubMed] [Google Scholar]
- 23.Quigley AM, Gnanasegaran G, Buscombe JR, Hilson AJ. Technetium-99m-labelled sulesomab (LeukoScan) in the evaluation of soft tissue infections. Med Princ Pract. 2008;17:447–52. doi: 10.1159/000151565. [DOI] [PubMed] [Google Scholar]
- 24.Sousa R, Massada M, Pereira A, Fontes F, Amorim I, Oliveira A. Diagnostic accuracy of combined 99mTc-sulesomab and 99mTc-nanocolloid bone marrow imaging in detecting prosthetic joint infection. Nucl Med Commun. 2011;32:834–9. doi: 10.1097/MNM.0b013e3283496695. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Zhuang H, Alavi A. Imaging of lower extremity artery atherosclerosis in diabetic foot: FDG-PET imaging and histopathological correlates. Clin Nucl Med. 2007;32:567–8. doi: 10.1097/RLU.0b013e3180646ac0. [DOI] [PubMed] [Google Scholar]
- 26.Keidar Z, Militianu D, Melamed E, Bar-Shalom R, Israel O. The diabetic foot: initial experience with 18F-FDG PET/CT. J Nucl Med. 2005;46:444–9. [PubMed] [Google Scholar]
- 27.Kumar R, Basu S, Torigian D, Anand V, Zhuang H, Alavi A. Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev. 2008;21:209–24. doi: 10.1128/CMR.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopfner S, Krolak C, Kessler S, Tiling R, Brinkbäumer K, Hahn K, et al. Preoperative imaging of Charcot neuroarthropathy in diabetic patients: comparison of ring PET, hybrid PET, and magnetic resonance imaging. Foot Ankle Int. 2004;25:890–5. doi: 10.1177/107110070402501208. [DOI] [PubMed] [Google Scholar]
- 29.Regatte RR, Schweitzer ME. Ultra-high-field MRI of the musculoskeletal system at 7.0T. J Magn Reson Imaging. 2007;25:262–9. doi: 10.1002/jmri.20814. [DOI] [PubMed] [Google Scholar]
- 30.Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223:747–55. doi: 10.1148/radiol.2233011279. [DOI] [PubMed] [Google Scholar]
- 31.Schweitzer ME, Morrison WB. MR imaging of the diabetic foot. Radiol Clin North Am. 2004;42:61–71. doi: 10.1016/S0033-8389(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 32.Moore TE, Yuh WT, Kathol MH, el-Khoury GY, Corson JD. Abnormalities of the foot in patients with diabetes mellitus: findings on MR imaging. AJR Am J Roentgenol. 1991;157:813–16. doi: 10.2214/ajr.157.4.1892042. [DOI] [PubMed] [Google Scholar]
- 33.Seok JH, Jee WH, Chun KA, Kim JY, Jung CK, Kim YR, et al. Necrotizing fasciitis versus pyomyositis: discrimination with using MR imaging. Korean J Radiol. 2009;10:121–8. doi: 10.3348/kjr.2009.10.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panchbhavi V, Hecox S. All that is gas is not gas gangrene: mechanical spread of gas in the soft tissues. A case report. J Bone Joint Surg Am. 2006;88:1345–8. doi: 10.2106/JBJS.E.01172. [DOI] [PubMed] [Google Scholar]
- 35.Schulze M, Kötter I, Ernemann U, Fenchel M, Tzaribatchev N, Claussen CD, et al. MRI findings in inflammatory muscle disease and their noninflammatory mimics. AJR Am J Roentgenol. 2009;192:1708–16. doi: 10.2214/AJR.08.1764. [DOI] [PubMed] [Google Scholar]
- 36.Boutin R, Brossmann J, Sartoris D, Reilly D, Resnick D. Update on imaging of orthopedic infections. Orthop Clin North Am. 1998;29:41–66. doi: 10.1016/s0030-5898(05)70006-7. [DOI] [PubMed] [Google Scholar]
- 37.Ledermann HP, Morrison WB, Schweitzer ME, Raikin SM. Tendon involvement in pedal infection: MR analysis of frequency, distribution, and spread of infection. AJR Am J Roentgenol. 2002;179:939–47. doi: 10.2214/ajr.179.4.1790939. [DOI] [PubMed] [Google Scholar]
- 38.Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg. 1999;38:34–40. doi: 10.1016/s1067-2516(99)80086-6. [DOI] [PubMed] [Google Scholar]
- 39.Trepman E, Nihal A, Pinzur MS. Current topics review: Charcot neuroarthropathy of the foot and ankle. Foot Ankle Int. 2005;26:46–63. doi: 10.1177/107110070502600109. [DOI] [PubMed] [Google Scholar]
- 40.Marcus CD, Ladam-Marcus VJ, Leone J, Malgrange D, Bonnet-Gausserand FM, Menanteau BP. MR imaging of osteomyelitis and neuropathic osteoarthropathy in the feet of diabetics. Radiographics. 1996;16:1337–48. doi: 10.1148/radiographics.16.6.8946539. [DOI] [PubMed] [Google Scholar]
- 41.Morrison WB, Ledermann HP. Work-up of the diabetic foot. Radiol Clin North Am. 2002;40:1171–92. doi: 10.1016/s0033-8389(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 42.Yuh WT, Corson JD, Baraniewski HM, Rezai K, Shamma AR, Kathol MH, et al. Osteomyelitis of the foot in diabetic patients: evaluation with plain film, 99mTc-MDP bone scintigraphy, and MR imaging. AJR Am J Roentgenol. 1989;152:795–800. doi: 10.2214/ajr.152.4.795. [DOI] [PubMed] [Google Scholar]
- 43.Brower AC, Allman RM. Pathogenesis of the neurotrophic joint: neurotraumatic vs. neurovascular. Radiology. 1981;139:349–54. doi: 10.1148/radiology.139.2.7220879. [DOI] [PubMed] [Google Scholar]
- 44.Clouse ME, Gramm HF, Legg M, Flood T. Diabetic osteoarthropathy. Clinical and roentgenographic observations in 90 cases. Am J Roentgenol Radium Ther Nucl Med. 1974;121:22–34. doi: 10.2214/ajr.121.1.22. [DOI] [PubMed] [Google Scholar]
- 45.Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis. 1997;25:1318–26. doi: 10.1086/516148. [DOI] [PubMed] [Google Scholar]
- 46.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 47.Wu JS, Gorbachova T, Morrison WB, Haims AH. Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? AJR Am J Roentgenol. 2007;188:1529–34. doi: 10.2214/AJR.06.1286. [DOI] [PubMed] [Google Scholar]
- 48.Tronco G, Tomas M, Palestro C. Fourphase bone scintigraphy and osteomyelitis: a reevaluation. Clin Nucl Med. 1992;24:216. [Google Scholar]
- 49.Tan PL, Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol. 2007;80:939–48. doi: 10.1259/bjr/30036666. [DOI] [PubMed] [Google Scholar]
- 50.Graif M, Schweitzer ME, Deely D, Matteucci T. The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol. 1999;28:616–20. doi: 10.1007/s002560050562. [DOI] [PubMed] [Google Scholar]