Abstract
Immune cell infiltration varies widely between different glioblastomas (GBMs). The underlying mechanism, however, remains unknown. Here we show that TGF-beta regulates proliferation, migration, and tumorigenicity of mesenchymal GBM cancer stem cells (CSCs) in vivo and in vitro. In contrast, proneural GBM CSCs resisted TGF-beta due to TGFR2 deficiency. In vivo, a substantially increased infiltration of immune cells was observed in mesenchymal GBMs, while immune infiltrates were rare in proneural GBMs. On a functional level, proneural CSC lines caused a significantly stronger TGF-beta-dependent suppression of NKG2D expression on CD8+ T and NK cells in vitro providing a mechanistic explanation for the reduced immune infiltration of proneural GBMs. Thus, the molecular subtype of CSCs TGF-beta-dependently contributes to the degree of immune infiltration.
Introduction
Glioblastoma (GBM) comprises distinct molecular subtypes [1,2]. While additional subtypes likely exist, only proneural and mesenchymal GBMs have been consistently identified in transcriptional [3], genetic [4], and proteomic studies [5]. We recently showed that CD133+ and CD133− cancer stem cells (CSCs) contribute to the heterogeneity of GBMs [6–8]. A 24-gene signature reliably differentiated between both CSC subtypes. In vivo, almost all proneural GBMs were identified by signature genes for CD133+ CSCs (proneural-like CSCs), while mesenchymal GBMs corresponded to CD133− CSCs (mesenchymal-like CSCs) [8].
The translational relevance of the molecular subtypes remains vague. A study investigating the response to immunotherapy with adjuvated autologous tumor lysate-pulsed dendritic cells found that patients with mesenchymal GBMs had more infiltrating immune cells and showed longer survival than patients with proneural GBMs. In addition, only mesenchymal GBMs responded to immunotherapy with increased immune infiltration. This finding, however, still needs to be confirmed by a larger number of samples [9]. Thus, the lack of infiltrating immune cells and productive immune responses in patients with proneural GBMs might be due to increased immune-paralyzing effects of the respective tumors [10]. To understand the different degrees of immune paralysis in both subtypes, we used the cell culture system of proneural-like and mesenchymal-like CSC lines and identified the functional role of TGF-beta signaling as a crucial difference.
Materials and Methods
Culture of primary GBM cells
Generation and culture of the CSC lines used has been described previously [7, 6]. Metabolic activity of CSC lines was determined using AlamarBlue (Biosource) according to the manufacturer's instructions. For clonogenicity assays, cells were seeded in 48-well plates and treated as indicated. Medium supplemented with TGF-beta was replaced twice a week.
In vivo tumor model
Cells were intracranially injected into T-lymphocyte-deficient NMRI(nu/nu)-mice as described previously [7]. Procedures were conducted in accordance with German laws governing animal care. Animals were sacrificed 10 weeks after tumor inoculation and 10-μm sections of snap-frozen tumor samples were analyzed. For the CSC line R28, the tumor size was determined by bioluminescence 3 weeks after implantation as described previously [11].
Quantitative real-time PCR
RNA was isolated and cDNA was synthesized using standard protocols. Real-time PCR was performed using the following primer pairs: TGFR2: forward 5′-AGCAACTGCAGCATCACCTC-3′; reverse 5′-GCACTTTGGAGAAGCAGCATC-3′; 18s-RNA: forward 5′-CGGCTACCACATCCAAGGAA-3′; reverse 5′-GCTGGAATTACCGCGGCT-3′. TGF-beta 1: forward 5′- GGCGATACCTCAGCAACCGG-3′; reverse 5′-TCGGCGGCCGGTAGTGAACC-3′; TGF-beta 2: forward 5′-CACCATAAAGACAGGAACCTG-3′; reverse 5′-GGAGGTGCCATCAATACCTGC-3′.
Immunohistochemistry/immunocytochemistry/western blot
The tumor samples were graded by a senior neuropathologist (Joachim Weis) according to WHO classification criteria. The use of the samples had been approved by the local ethics committee. Four-micrometer sections of paraffin-embedded GBM samples were stained using the following antibodies: TGF-beta 1 (Acris:DM1047; 1:100), TGF-beta 2 (Acris:AP15815PU; 1:25), Olig 2 (Sigma-Aldrich; 1:1,250), CD8 (C8/144B, Dako; 1:100), CD68 (KP1, Dako; 1:1,000), DLL3 (St. Crzu; 1:200), NeuN (Millipore; 1:1,000), YKL-40 (Teco Medical group 1:500), CD44 (R&D; 1:200), and VEGF (St. Cruz 1:200). Antibodies used for immunocytochemistry are given in Ref. [7]. All GBM samples were scored blinded by either Markus Riemenschneider or Christoph P. Beier. The index to assign GBM as mesenchymal or proneural tumor was calculated as follows: For each marker (proneural: Olig2, DLL3, NeuN; mesenchymal: YKL-40, CD44, VEGF), 5 positions per slide were scored from 0 to 4 quantifying the number of tumor cells expressing the respective antigen (0: no expression; 1: <10% expression; 2: 10%–49% expression, 3: 50%–90%, 4: >90%). The sum of the means of the mesenchymal markers was then subtracted from the sum of the means of the proneural markers. Tumors with an index above 0 were assigned as proneural; if the index was below 0, the tumor was considered as mesenchymal. For scoring TGF-beta 1/2 and CD68, an identical semiquantitative score was employed. A different score was used for CD8+ cells: 0: no CD8+ cells, 1: 1–10 cells per visual field, 2: 20–29 cells per visual field, 3: 30–50 cells per visual field, 4: more than 50 CD8+ cells per visual field. For pSmad2 western blot, cell lysates were analyzed using pSmad2 (1:1,000; Calbiochem) and beta-actin (1:5,000; Abcam) antibodies.
Immunological assays and flow cytometry
Cells were stained using CD133/2-PE (clone293C3, Miltenyi Biotech; control: mIgG2b-PE; Invitrogen). To assess NKG2D expression, freshly isolated peripheral blood mononuclear cells were cocultured at a 10:1 ratio with CSCs or supernatant in a CSC medium [12]. About 48 h later, NKG2D levels were analyzed (BAT221; anti-NKG2D-PE; Miltenyi Biotech); control: IgG-PE isotype (BioLegend)). CD8+ T cells and NK cells were identified by costaining with anti-CD3-FITC, anti-CD8-PE, and anti-CD56-APC (Immunotools). SD-208 was obtained from Tocris.
Statistics and bioinformatics
Acquisition of microarray data is described in Ref. [7]. Analysis was performed using R and Bioconductor. Expression values were corrected and normalized using the robust multiarray average function (Affymetrix package). Differentially expressed genes were computed using linear models (Bioconductor package limma). We selected 100 genes (TGF target genes) showing the highest log FC (absolute logFC>1.47) and clustered gene samples [13] using complete linkage. Euclidean distances were computed between all samples only based on the TGF-beta target genes. To evaluate the stability of the clustering, consensus clustering was performed. A similar approach was used for the 24-gene signature [8]. The expression indices were calculated per sample by fitting a standard additive model with an independent gene, and sample effects using Tukey's median polish procedure. Parts of the analysis were performed using BRB ArrayTools developed by Dr. Richard Simon and BRB-ArrayTools Development Team.
Results
Differential responsiveness of proneural-like and mesenchymal-like GBM CSC lines
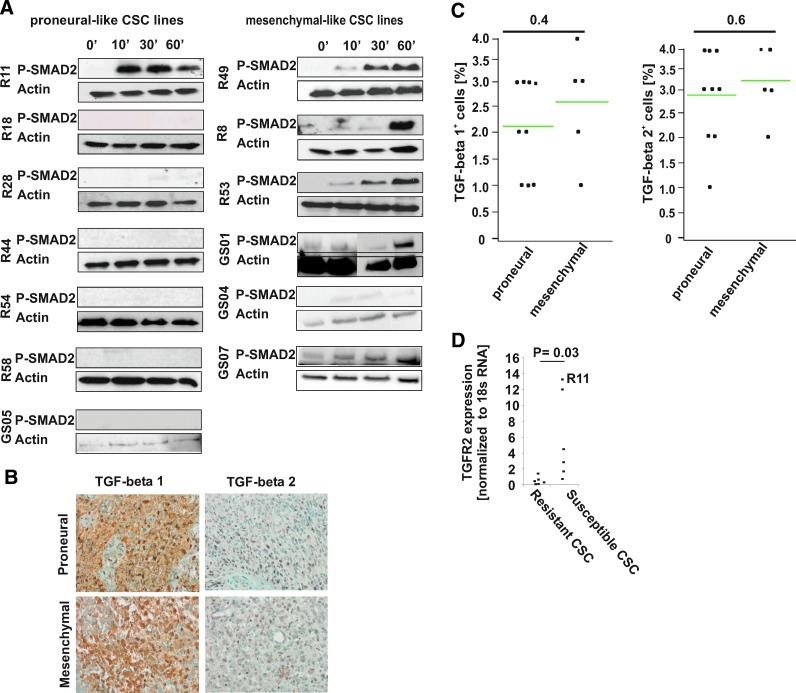
To characterize the role of TGF-beta signaling in both types of GBM CSC lines, we used the cell culture model of CSC lines that closely resembles the initial tumor and recapitulate the intertumoral heterogeneity in vitro [14,15]. TGF-beta-associated transcripts were differentially regulated between CD133+ (proneural-like, type I) CSC lines and CD133− (mesenchymal-like, type II) CSC lines [8]. To further understand these differences, we used 6 mesenchymal-like and 7 proneural-like CSC lines [7,8] that were assigned based on a recently published 24-gene signature (Supplementary Fig. S1C; Supplementary Data are available online at www.liebertpub.com/scd). As transcripts for TGF-beta-associated genes showed significant differences between proneural-like and mesenchymal-like CSC lines [8], we screened for TGF-beta-induced SMAD2-phosphorylation. This revealed a time-dependent SMAD2-phosphorylation in all mesenchymal-like CSC lines, but in only 1 proneural-like CSC line (Fig. 1A). Notably, despite the lack of autocrine SMAD2-phosphorylation, we observed TGF-beta 1 and 2 expression in vivo (Fig. 1B, C) as well as TGF-beta mRNA and unprocessed TGF-beta in all CSC lines in vitro (Supplementary Fig. S2A and data not shown). While there were no differences in vivo, we found a trend toward an increased TGF-beta 1/2 and SMAD7 expression in mesenchymal-like CSC lines (Supplementary Fig. S2A). The TGF-beta resistance of proneural-like CSC lines may be explained by a significantly decreased expression of TGFR2 in resistant CSC lines (Fig. 1D), while other key proteins of the TGF-beta signaling pathway (SMAD2, SMAD4, and p21) did not reveal consistent differential regulations in the CSC lines investigated (data not shown).
FIG. 1.
Responsiveness to TGF-beta in proneural- and mesenchymal-like cancer stem cell (CSC) lines. (A) SMAD2 phosphorylation after incubation with TGF-beta 1 and 2 (10 ng/mL) for 0, 10, 30, and 60 min was analyzed by immunoblotting. Beta-actin was included as loading control. (B, C) TGF-beta 1 and TGF-beta 2 expression in glioblastoma (GBM) (n=19, same tumors as in Fig. 5). The percentage of TGF-beta expressing cells was scored as described in the Materials and Methods section. There was no significant difference in the number of TGF-beta expressing cells between proneural and mesenchymal GBMs (one-sided t-test). (D) Relative TGF-betaR2 mRNA expression normalized to 18S RNA is given in CSC lines with and without SMAD2 phosphorylation (*P=0.03; one-sided t-test). Color images available online at www.liebertpub.com/scd
TGF-beta-induced effects on mesenchymal- and proneural-like CSC lines
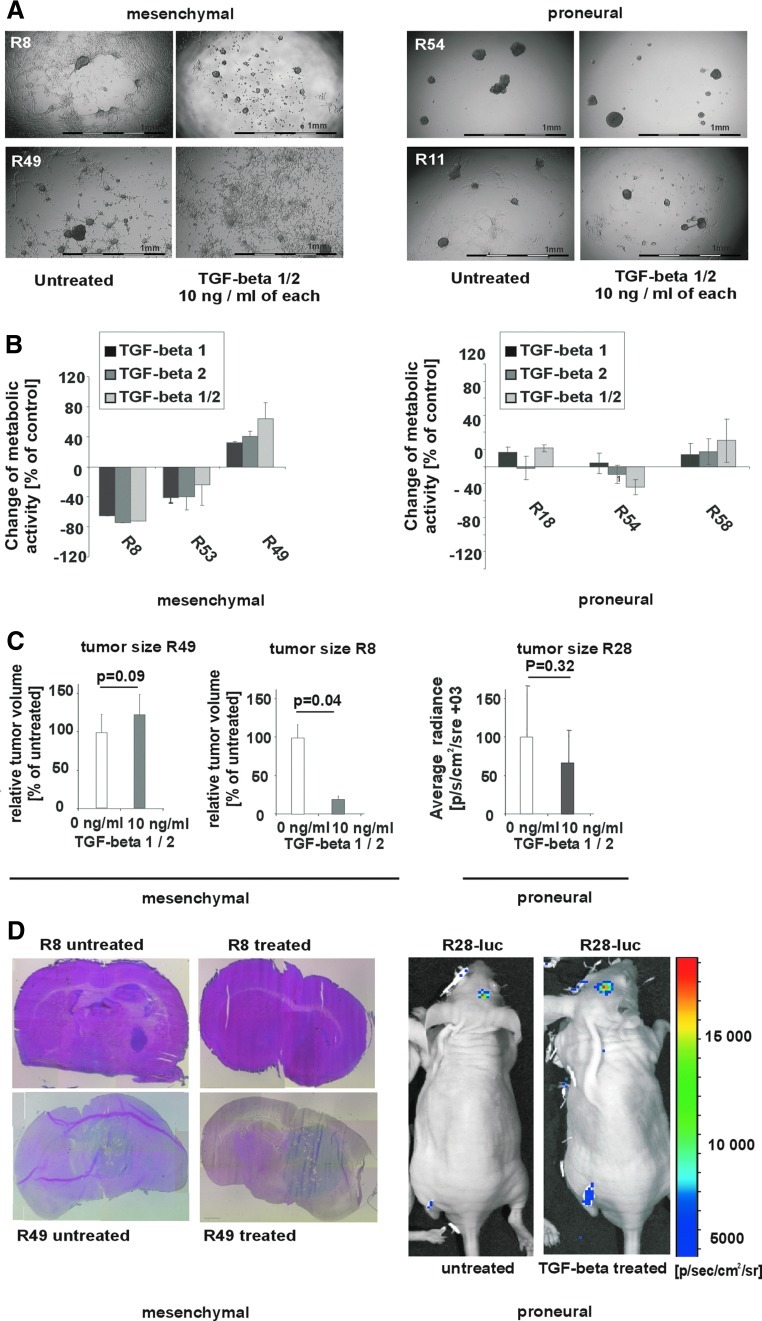
With regard to biological effects, TGF-beta 1, 2, or the combination of both behaved similarly in all experiments (not shown). TGF-beta incubation did affect neither migration, proliferation, or tumorigenicity, nor stem cell properties of those proneural-like CSC lines, which lacked SMAD2-phosphorylation and TGFR2 expression (Fig. 2A–D and Supplementary Fig. S3A–C). Also, the proneural-like CSC line R11, which expresses TGFR2 and showed SMAD2-phosphorylation, was found to resist TGF-beta-induced effects. In this cell line, however, TGF-beta might still modulate the self-renewal of GBM via induction of LIF, as recently described by Penuelas et al. [16]. Nevertheless, as the growth medium for GBM CSCs is routinely supplemented with LIF, LIF-dependent growth-promoting effects triggered by TGF-beta have been obscured for R11 (Supplementary Fig. S2B). In the mesenchymal-like CSC lines, in contrast, TGF-beta either suppressed or promoted migration, proliferation, and tumorigenicity (Fig. 2A–D). Moreover, as opposed to tumorigenicity, other specific stem cell properties like clonogenicity, proportion of CD133+ cells, or the differentiation profile remained unaltered after in vitro treatment with TGF-beta (Supplementary Fig. S3A–C). An analysis of factors like DAB2 that may decide whether TGF-beta acts as tumor suppressor or promoter [17] would, however, require a much larger number of cell lines and therefore exceeds the scope of the present study.
FIG. 2.
Comparison of TGF-beta effects in proneural-like and mesenchymal-like CSC lines. (A) The growth pattern and (B) proliferation of mesenchymal-like and proneural-like CSC lines after incubation with 10 ng/mL TGF-beta 1 and 2 or solvent control for 7 days is shown. The following CSC lines showed a similar response pattern: R8, R53, GS01, GS04, GS07 (growth inhibition); R49 (growth promotion); R11, R18, R28, R44, R54, R58, GS05 (no response). The change of proliferation and images of representative CSC lines were given. (B) To determine the growth rate, 5,000 cells per well were seeded in a 96-well plate. The relative change of the metabolic activity (as determined by AlamarBlue) after treatment with TGF-beta for 7 days as compared to untreated control is given (R8/R53: also reported in [8]). (C) CSC lines R49 (left panel), R8 (middle panel), and R28-luc (described in Ref. [11], right panel) were treated or not with TGF-beta for 7 days before 2×105 viable cells were inoculated into nude mice. After 10 weeks (R8, R49), animals were sacrificed, 10-μm tumor sections were stained with hematoxylin and eosin, and the maximum tumor area was determined using a caliper. Tumor volumes obtained with TGF-beta treated cells are given relative to tumor volumes of lesions grown from untreated GBM CSC. The number of tumor cells of R28-luc was determined by bioluminescence ([11], P values: two-sided t-test). (D) Representative pictures of the tumors are shown. Color images available online at www.liebertpub.com/scd
Mesenchymal, but not proneural GBMs express TGF-beta-induced transcripts
To identify TGF-beta responsive GBMs, we compared gene expression profiles of TGF-beta responsive CSC lines (R8/R53) with and without TGF-beta treatment and determined a set of 100 TGF-beta target genes (Supplementary Table S1). We then analyzed 80 previously published microarrays from well-characterized GBM samples [13]. Clustering based on the TGF-beta target genes unveiled 2 groups: a TGF-betaresponsive cluster characterized by expression patterns typical for activated TGF-beta and a TGF-betaunresponsive cluster, suggesting that autocrine TGF-beta signaling is only active in a subgroup (Fig. 3A). We then clustered the same set of 80 GBMs based on the 24-gene signature differentiating between proneural-like and mesenchymal-like CSCs in vivo and in vitro [8] and again obtained 2 stable clusters. To compare the expression of TGF-beta target genes and 24 signature genes, we calculated 2 expression indices. The TGF-beta target genes and the 24-gene signature index anticorrelate (r=−0.885) with most TGF-betaresponsive GBMs resembling mesenchymal-like CSC lines. Conversely, TGF-betaunresponsive GBMs mainly displayed a proneural-like CSC background (Fig. 3B).
FIG. 3.
Only GBMs resembling mesenchymal-like CSCs express TGF-beta-induced transcripts. (A) To identify TGF-beta-induced transcripts, 100 differentially regulated transcripts were determined by comparing microarrays (Affymetrix U133 plus 2.0) of TGF-beta treated and untreated R8/R53 cells (TGF-beta target genes). Clustering experiments were then performed on the data set comprising 80 primary GBMs, as published by Murat et al. [13]. To show that clusters were not driven by random expression fluctuations, we used consensus clustering ([8]). Dark color corresponds to pairs that were never clustered together, while bright color corresponds to pairs that were always clustered together. The infrequent intermediate counts are represented by a color gradient. (B) The same 80 GBMs as in (A) were sorted according to the mesenchymal versus proneural 24-gene signature index in increasing order (lower bar). The color of the upper bar gives the index of the TGF-beta target genes with dark colored/red indicating TGF-betaresponsive and bright colored/green indicating TGF-betaunresponsive GBMs. Color images available online at www.liebertpub.com/scd
TGF-beta-dependent immunosuppression by proneural-like CSCs
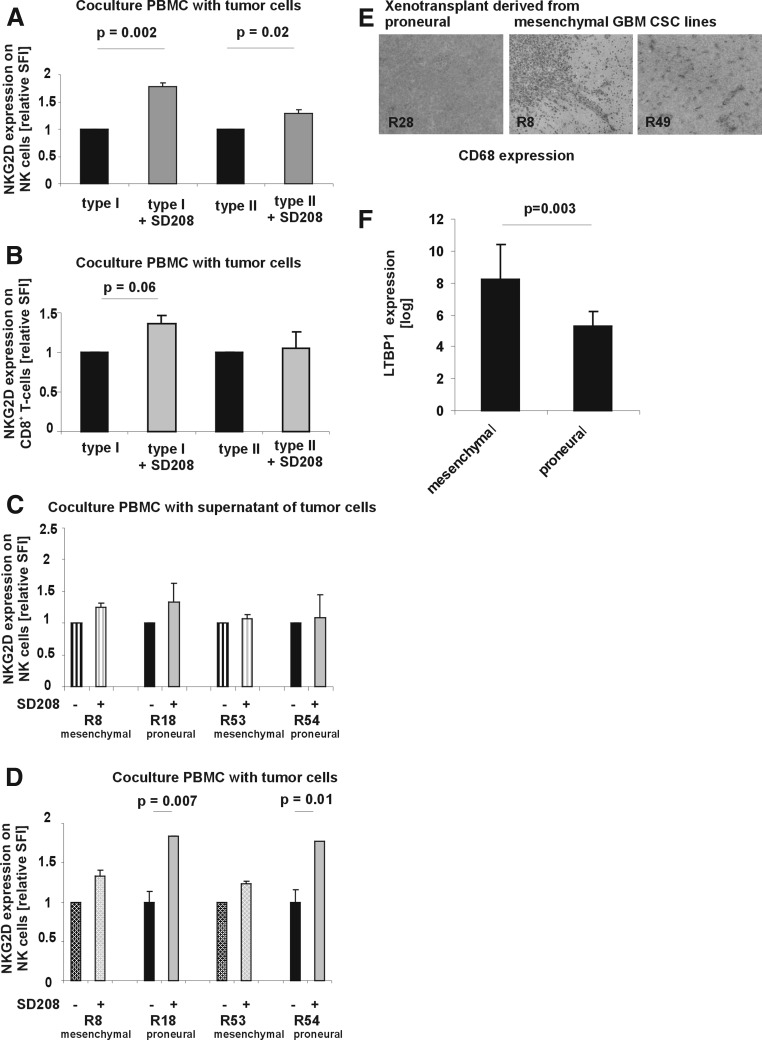
The combination of TGF-beta resistance and TGF-beta expression in proneural-like CSCs suggests an immunosuppressive function of TGF-beta in this subtype. As the respective GBM patients are deceased, we could not compare cytotoxic and proliferative T-cell assays in a syngeneic context. Instead, we investigated the TGF-beta-induced downregulation of NKG2D receptor expression on CD8+ T-cells and NK-cells in vitro [18]. The TGFR1 kinase inhibitor SD-208 specifically blocks TGF-beta signaling and thereby unmasks TGF-beta-dependent effects [19]. In the absence of immune cells, CSC lines did not secrete active TGF-beta, because coculture of PBMC with supernatants of CSC lines remained without effect (Fig. 4C). Cocultures of PBMC with CSC lines revealed an increased TGF-beta-dependent downregulation of NKG2D when cocultured with proneural-like CSC lines (Fig. 4A, B, D). Thus, TGF-beta-resistant proneural-like CSC lines exert enhanced, TGF-beta-dependent immunosuppression in 2 highly sensitive functional tests in vitro. We then investigated xenotransplants (Fig. 2D) from mesenchymal-like and proneural-like CSC lines for infiltration with CD68+ microglia. Tumors formed by both mesenchymal-like CSC lines, but not the tumors formed by the proneural-like CSC line were infiltrated by microglia further supporting the concept of differential immunosuppressive properties of proneural- and mesenchymal-like CSCs (Fig. 4E). One possible explanation for the different bioactivity of TGF-beta between the 2 subtypes could be the differential expression of Latent TGF-binding protein 1 (LTBP1), which retains TGF-beta in the extracellular matrix in an inactivate state (Fig. 4F).
FIG. 4.
Proneural-like CSC lines downregulate NKG2D receptor expression on CD56+ NK and CD8+ T cells in a TGF-beta-dependent manner. 1.5×106 PBMC were cocultured with 1.5×105 proneural-like (R18, R54) or mesenchymal-like CSC lines (R8, R53) (A, B, D) or supernatant of the GBM CSC lines (C) for 48 h in 500 μl of the CSC medium in the absence or presence of SD-208 (1 μM) as indicated (two-sided t-test). NKG2D receptor expression on NK cells (A, C, D) and CD8 T cells (B) was determined by flow cytometry (two-sided t-test). Expression levels are indicated as specific fluorescence intensities (SFI) obtained by dividing the fluorescence intensity detected with the specific antibody by the fluorescence signal measured with the isotype control antibody. (E) Xenograft tumors from R8, R49, and R28 CSC lines were stained for infiltrating CD68+ microglia cells. Representative pictures are shown. (F) The expression of latent TGF-beta binding protein 1 (which sequesters latent TGF-beta in the extracellular matrix of GBM cells) is given. Shown are average values from 6 mesenchymal and 7 proneural GBM CSC lines (P value, two-sided Student's-t-test).
Immune infiltration of GBM correlates to the molecular subtype
To further substantiate this hypothesis of an increased immunosuppression by proneural CSCs, we stained a series of GBMs against markers for cytotoxic T-lymphocytes (CD8+) and CD68+ microglia (Fig. 5A). The degree of immune infiltration varied. To differentiate between proneural and mesenchymal GBMs, we used a set of marker genes (proneural: Olig2, DLL3, NeuN; mesenchymal: YKL-40, CD44, VEGF, Supplementary Fig. S1A). We calculated an index based on the average expression of the marker genes (Supplementary Fig. S1B), indicating the assignment to proneural and mesenchymal GBMs. The assignment to the mesenchymal phenotype significantly correlated with the amount of CD8+ (CD8: r=0.68) and CD68+ cells (r=0.78, Fig. 5B–D), suggesting that proneural and mesenchymal GBMs differ with respect to infiltration by immune cells.
FIG. 5.
Proneural and mesenchymal GBMs show different levels of infiltration by immune cells. (A) Human GBMs were stained for infiltrating CD8+ and CD68+ cells. Counting of the stained cells revealed substantial differences. (B) Expression of markers for proneural GBMs (Olig2, DLL3, NeuN), mesenchymal GBMs (YKL-40, CD44, VEGF), and infiltrating immune cells (CD8 and CD68) were quantified in 19 human GBMs. We then calculated a proneural/mesenchymal index (see Material and Methods section and Supplementary Fig. S1). The representative stainings for Olig 2 expression, infiltrating CD8, and CD68 cells are displayed. (C) The variable immune infiltration score observed in the stained GBM was correlated with the proneural/mesenchymal index. (D) The number of infiltrating CD8+ and CD68+ immune cells per high-power field was plotted for mesenchymal (n=10) and proneural (n=9) GBM. Statistical differences were assessed by the Student's two-sided t-test. Color images available online at www.liebertpub.com/scd
Discussion
The relationship of GBM to proneural-like or mesenchymal-like CSCs indicates a profound biological difference in the function of TGF-beta in these tumors. While tumors expressing transcripts of proneural-like CSCs show TGF-beta expression in vitro and in vivo, they are not susceptible toward pro- or antiproliferative effects of TGF-beta. As TGF-beta is the most potent immunosuppressive cytokine known, it may suppress the function and invasion of immune cells in proneural GBMs [9,20]. We could substantiate this hypothesis using functional assays showing a significantly stronger TGF-beta-dependent suppression of NKG2D receptor expression on NK- and T-cells by proneural-like CSC lines as compared to mesenchymal controls (Fig. 4A–D). In line with these results, proneural GBMs suppressed invasion of immune cells more efficiently as compared to mesenchymal GBMs (Fig. 5B–D). These findings bear substantial implication, because they suggest that therapies aiming at the attenuation of TGF-cells beta-mediated immunosuppression are likely to be essential for every immunotherapy applied to tumors maintained by proneural-like CSCs. Conversely, the strong immunosuppression by TGF-beta provides a possible explanation for the completely refractory phenotype of proneural GBMs in an otherwise highly promising vaccination study on GBM patients [9].
Conversely, the functions of TGF-beta in GBMs maintained by mesenchymal-like CSCs are manifold and TGF-beta may either inhibit or promote tumor growth [17]. While the suppression of invading immune cells may be reduced due to the increased expression of Latent TGF-beta binding protein 1, it may also function as tumor suppressor in at least a subgroup, which calls for an extremely cautious application of TGF-beta targeting therapies to mesenchymal GBMs.
In summary, the CSC subtype determines the degree of immune infiltration and local immune paralysis via a different degree of TGF-beta-mediated suppression of infiltrating immune cells. Thus, the identification of the CSC subtype in GBMs based on the 24-gene signature will help to guide both TGF-beta targeting and immune therapies by identifying GBMs with strong TGF-beta-dependent immune paralysis. The CSC-guided subclassification of GBMs therefore provides a new approach to guide both immune and TGF-beta targeting therapies.
Supplementary Material
Acknowledgments
This work was supported by a grant of the Bavarian Research Foundation to D.B., U.B., and P.K. and a career award to young female scientists of the RWTH Aachen, Medical School to D.B. Parts of the project were funded by BayGene and the NGFNplus Brain Tumor Network to C.P.B. and P.H. (Subproject 7A and 7B No. 01GS0887 and 01GS1105) and the in-house research support of the RWTH Aachen, Medical School (START-Program) to D.B.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Li A. Walling J. Ahn S. Kotliarov Y. Su Q. Quezado M. Oberholtzer JC. Park J. Zenklusen JC. Fine HA. Unsupervised analysis of transcriptomic profiles reveals six glioma subtypes. Cancer Res. 2009;69:2091–2099. doi: 10.1158/0008-5472.CAN-08-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huse JT. Phillips HS. Brennan CW. Molecular subclassification of diffuse gliomas: seeing order in the chaos. Glia. 2011;59:1190–1199. doi: 10.1002/glia.21165. [DOI] [PubMed] [Google Scholar]
- 3.Phillips HS. Kharbanda S. Chen R. Forrest WF. Soriano RH. Wu TD. Misra A. Nigro JM. Colman H, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Verhaak RG. Hoadley KA. Purdom E. Wang V. Qi Y. Wilkerson MD. Miller CR. Ding L. Golub T, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan C. Momota H. Hambardzumyan D. Ozawa T. Tandon A. Pedraza A. Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunther HS. Schmidt NO. Phillips HS. Kemming D. Kharbanda S. Soriano R. Modrusan Z. Meissner H. Westphal M. Lamszus K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27:2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 7.Beier D. Hau P. Proescholdt M. Lohmeier A. Wischhusen J. Oefner PJ. Aigner L. Brawanski A. Bogdahn U. Beier CP. CD133+ and CD133− glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 8.Lottaz C. Beier D. Meyer K. Kumar P. Hermann A. Schwarz J. Junker M. Oefner PJ. Bogdahn U, et al. Transcriptional profiles of CD133+ and CD133− glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res. 2010;70:2030–2040. doi: 10.1158/0008-5472.CAN-09-1707. [DOI] [PubMed] [Google Scholar]
- 9.Prins RM. Soto H. Konkankit V. Odesa SK. Eskin A. Yong WH. Nelson SF. Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weller M. Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev. 1995;21:128–151. doi: 10.1016/0165-0173(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 11.Schraivogel D. Weinmann L. Beier D. Tabatabai G. Eichner A. Zhu JY. Anton M. Sixt M. Weller M. Beier CP. Meister G. CAMTA1 is a novel tumour suppressor regulated by miR-9/9(*) in glioblastoma stem cells. EMBO J. 2011;30:4309–4322. doi: 10.1038/emboj.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisele G. Wischhusen J. Mittelbronn M. Meyermann R. Waldhauer I. Steinle A. Weller M. Friese MA. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 13.Murat A. Migliavacca E. Gorlia T. Lambiv WL. Shay T. Hamou MF. de Tribolet N. Regli L. Wick W, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 14.Lee J. Kotliarova S. Kotliarov Y. Li A. Su Q. Donin NM. Pastorino S. Purow BW. Christopher N, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Pollard SM. Yoshikawa K. Clarke ID. Danovi D. Stricker S. Russell R. Bayani J. Head R. Lee M, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Penuelas S. Anido J. Prieto-Sanchez RM. Folch G. Barba I. Cuartas I. Garcia-Dorado D. Poca MA. Sahuquillo J. Baselga J. Seoane J. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Hannigan A. Smith P. Kalna G. Lo Nigro C. Orange C. O'Brien DI. Shah R. Syed N. Spender LC, et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-beta from a tumor suppressor to a tumor promoter. J Clin Invest. 2010;120:2842–2857. doi: 10.1172/JCI36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friese MA. Wischhusen J. Wick W. Weiler M. Eisele G. Steinle A. Weller M. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 19.Uhl M. Aulwurm S. Wischhusen J. Weiler M. Ma JY. Almirez R. Mangadu R. Liu YW. Platten M, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzbaum JA. Huang K. Lawler S. Ding B. Yu J. Chiocca EA. Allergy and inflammatory transcriptome is predominantly negatively correlated with CD133 expression in glioblastoma. Neuro Oncol. 2010;12:320–327. doi: 10.1093/neuonc/nop035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.