Abstract
Glioblastoma (glioblastoma multiforme; GBM; WHO Grade IV) accounts for the majority of primary malignant brain tumors in adults. Amplification and mutation of the epidermal growth factor receptor (EGFR) gene represent signature genetic abnormalities encountered in GBM. A range of potential therapies that target EGFR or its mutant constitutively active form, ΔEGFR, including tyrosine kinase inhibitors (TKIs), monoclonal antibodies, vaccines, and RNA-based agents, are currently in development or in clinical trials for the treatment of GBM. Data from experimental studies evaluating these therapies have been very promising; however, their efficacy in the clinic has so far been limited by both upfront and acquired drug resistance. This review discusses the current status of anti-EGFR agents and the recurrent problem of resistance to these agents that strongly indicates that a multiple target approach will provide a more favorable future for these types of targeted therapies in GBM.
Keywords: Epidermal growth factor receptor, EGFR-targeted therapy, Glioblastoma, therapeutic resistance
INTRODUCTION
Malignant gliomas, the most common primary intracranial brain tumors in adults, are among the deadliest of human cancers because they are highly invasive and neurologically destructive [1]. The median survival of patients with the most aggressive of these, WHO grade IV glioblastoma (GBM), is 12–15 months with a 5-year survival rate that remains at less than 5%, despite the use of intensive treatment modalities (i.e. surgical resection, radiotherapy, and chemotherapy) [1]. Thus, the development of novel efficacious therapies is greatly warranted to substantially improve the poor prognosis of patients afflicted with GBM.
One avenue towards achieving this has been a concerted effort at providing a global description of the genetic abnormalities that are present in GBM tumors [2, 3]. The most common genetic aberration associated with malignant glioma is amplification of the epidermal growth factor receptor (EGFR, also referred to as ERBB1 or HER1), with a frequency of about 50% [1]. EGFR is a member of the HER superfamily of receptor tyrosine kinases together with ERBB2, ERBB3, and ERBB4 [4]. The structure of each of the members comprises: a ligand-binding ectodomain with 2 cysteine-rich regions; a single transmembrane region; and, a cytoplasmic tyrosine kinase (TK) domain [5]. Binding of a cognate ligand to the ligand-binding site of HER receptors induces receptor homo- or heterodimerization, resulting in a conformational change that activates the intracellular TK domain. This results in autophosphorylation of the cytoplasmic tail of the receptor, induction of downstream signaling (through the phosphatidylinositol 3-kinase (PI3K)/Akt and the ras-raf-mitogen-activated protein kinase (MAPK) pathways, among others) [4], and transcription of genes controlling pleiotropic cellular responses [4]. The most common ligands for HER receptors are members of the EGF family of growth factors (i.e., heparin binding EGF-like growth factor, amphiregulin, epiregulin, betacellulin) and transforming growth factor α [6]. Interestingly, there is no known ligand for ERBB2, which is believed to undergo ligand-independent activation [4]. HER receptors are localized at the surfaces of many types of epithelial, mesenchymal, and neuronal cells such that signal transduction from these receptors into the intracellular compartment actuates many cellular processes including cell differentiation, metabolism, proliferation, and survival [7].
EGFR was the first receptor discovered to possess tyrosine kinase activity and to be sequenced [7]. In 1984, it was revealed that the sequence of EGFR was closely related to that of a known oncoprotein, the erbB tyrosine kinase, previously discovered to be associated with the onset of erythroleukemia [8]. Since then, EGFR has been shown to be frequently overexpressed or hyperactivated in a number of epithelial tumors [5]. The downstream signaling effects of these aberrations lead to impaired apoptosis, and/or enhanced proliferation, angiogenesis, necrosis, and treatment refractoriness, suggesting a causative relationship between receptor dysregulation and the pathobiology of many cancers. Several possible mechanisms are attributed to receptor dysregulation. These include gene amplification and intrinsic alterations of the receptor structure as a result of mutation. Indeed, many EGFR mutants have been described, and the most common mutant form associated with GBM is ΔEGFR (also named EGFRvIII, or de2-7EGFR) [9]. Other mutant forms, such as EGFRvII and EGFRvV are also found in GBM, but are infrequent and their clinical relevance is undefined [10]. Mutant ΔEGFR, arises through an in-frame deletion of 801 bp from the extracellular domain of EGFR and possesses ligand-independent constitutive (but low) tyrosine kinase activity [11]. Additionally, low level autophosphorylation of ΔEGFR results in defective receptor internalization due to reduced interaction with Casitas B-lineage (Cbl) proteins, resulting in increased stability of the receptor at the cell surface and amplified mitogenic effects [12, 13].
EGFR gene amplification and mutations are also found in breast, lung, and prostate cancers [14]. In spite of this, therapies that have been effective for solid tumors originating from these tissues have shown limited efficacy against GBM. EGFR-specific inhibitors have been approved for use in patients with non-small cell lung carcinoma (NSCLC), and are currently in clinical trials for GBM [15, 16, 17]. However, the clinical experience has been that many GBM patients do not respond to these therapies and those that do eventually show progression [13]. Thus, while knowledge of the inherent genetic alterations is pertinent in determining rational therapeutic targets, the biology of glioma has so far rendered it inadequate for predicting a durable drug response in GBM patients. In this review, we will focus on the current status of EGFR-targeted therapies as potential treatments for glioblastoma. We will then discuss the adeptness of GBMs at escaping the need for receptor function when challenged with receptor-targeted therapeutics, and how this complexity should be considered when exploring strategies to overcome the problem of therapeutic resistance.
GLIOBLASTOMA
Epidemiology
Each year approximately 6 to 12 out of 100,000 individuals are diagnosed with primary malignant brain tumors in the United States [18, 19]. These tumors represent ~ 2% of all cancers diagnosed each year and are the cause of 2% of all deaths from cancer in the U.S., with a death rate of ~13,000 Americans per year [18]. The World Health Organization (WHO) classification of Tumours of the Central Nervous System distinguishes gliomas based primarily on their histological appearances, where the grade indicates the level of malignancy [20, 21]. As already mentioned, the most malignant form is Grade IV glioblastoma, and is characterized by small areas of necrotic tissue surrounded by anaplastic cells [21, 22]. Tumors of this grade also display a number of hyperplastic blood vessels that facilitate rapid proliferation [21, 22]. Specifically, glioblastomas accounts for 60–70% of malignant gliomas diagnosed in American adults between the ages 46–74, and is more frequently diagnosed in men than in women [18]. Two major subclasses (primary and secondary) of GBM have been established based on the clinical properties and the chromosomal and genetic aberrations that are unique to each [1, 5]. Primary GBM appears to arise de novo from normal glial cells, or their precursors, and commonly occurs in patients above the age of 45 years [19]. In contrast, secondary GBM arises from the progressive transformation of lower grade tumors, are much less frequently encountered and are primarily seen in younger patients [1]. Given that the histopathology between primary and secondary GBM are identical at the grade IV stage, an exact estimation of frequency between the two is difficult to make. Nevertheless, primary GBM is believed to account for 95% of all GBMs, while only ~5% are believed to occur secondarily [23].
Genetic Alterations
The frequency of EGFR gene amplification and overexpression is more prevalent in the more common primary GBM compared to the secondary form [24]. In contrast, the frequency of inactivated p53 between the two mostly occurs in the less common secondary GBMs [24]. Thus, it appears that dysregulation of a variety of genetic pathways characterizes malignant gliomagenesis, with one subclass governed by overexpression or amplification of an oncogene and the other by inactivation of a tumor suppressor gene in the presence of a metabolic defect caused by mutations of the IDH1 and IDH2 genes [25]. Some of the other common genetic abnormalities encountered in GBM include deletion of p16Ink4a, phosphatase and tensin homolog (PTEN) mutations, loss of heterozygosity (LOH) at 10q, and amplification of the platelet-derived growth factor (PDGF) and receptors (PDGFRα/β), further highlighting the complexities of this disease that make it a challenge to provide effective therapies [1, 22].
Dysregulation of EGFR has also been shown to enhance tumor growth, migration, angiogenesis, and metastatic spread [26]. Additionally, EGFR overexpression is a poor prognostic factor and correlates with decreased overall survival in GBM patients [27]. Most glial tumors that overexpress EGFR concomitantly overexpress the constitutively active mutant variant, ΔEGFR, with a frequency of ~40–50% [11]. Interestingly, ΔEGFR is tumor-specific, as it has not been found in normal tissues [14]. ΔEGFR overexpression correlates with induced tumor formation, increased proliferation, and inhibition of apoptosis [11, 28]. Similar to the wild-type (wt) receptor, the presence of ΔEGFR confers a less favorable prognosis for GBM patients [22]. Data from in vitro studies suggest that the cellular responses manifested due to the increased presence of wtEGFR and ΔEGFR result primarily from downstream activation of the MAPK/ERK and PI3K-Akt pathways, and in some cases, the co-expression of matrix metalloproteinases (MMPs) [29, 30]. Furthermore, it has been demonstrated in vivo that treatment with the ΔEGFR-specific monoclonal antibody (mAb 806) significantly decreases tumor growth and increases apoptosis [31]. This antibody functions relatively specifically to ablate ΔEGFR-mediated signaling, therefore, it seems that some glioma cells may require the intracellular signaling imparted by ΔEGFR for survival and should be susceptible to inhibitors of this process. Both wtEGFR and ΔEGFR are bonafide oncogenes that are prevalent in GBM and this nominates them as attractive targets for therapeutic strategies [9, 11]. Indeed, several EGFR-targeted therapies have been developed and are currently in various stages of clinical trials for the treatment of malignant glioma [9, 17].
CURRENT TREATMENT OPTIONS FOR GLIOBLASTOMA
Standard of Care
Standard treatment for almost all GBM cases begins with surgical resection with the goal of gross total tumor removal to alleviate GBM symptoms and to facilitate treatment of any residual tumor [32]. Despite recent technological developments in surgical techniques, the vast majority of patients are not cured by surgical resection. Tumors often infiltrate the normal brain parenchyma, and this invasive nature necessitates the use of adjuvant radiotherapy that is either combined with or is subsequently followed by chemotherapy [1]. Radiotherapy involves the administration of usually 50 to 60 Gy of irradiation to the whole brain following surgery [33]. For several decades, nitrosoureas, particularly carmustine (BCNU) and lomustine (CCNU), were the most common chemotherapeutics used alone or in combination with radiotherapy [32, 34]. Pivotal clinical trials conducted in the 1970s and 1980s evaluating the benefits of adjuvant therapy demonstrated that patients with malignant glioma treated after surgery with radiotherapy, or radiotherapy in combination with chemotherapy, displayed an increase in overall survival [35, 36]. Additionally, in 1999, an alkylating agent, temozolomide (TMZ), became commercially available as a treatment for glioblastoma, and has become a part of the standard treatment regime for most cases [37] largely because of a large randomized clinical trial, where it was shown that the median survival in newly diagnosed GBM patients treated with radiation plus TMZ was 14.6 months as opposed to 12.1 months for the radiation only group [38].
Despite these optimizations to the adjuvant therapy regimens, the overall survival rate for GBM patients has remained relatively the same as it was ten years ago. Tumors inevitably recur, and once they do, life expectancy drastically diminishes and only limited therapeutic options are available. Major advances in molecular and cell biology have afforded the development of novel second and third line therapies for cancer patients in the realm of targeted therapy. So far, targeted therapy has been used effectively against breast tumors driven by growth factor receptor dysregulation, and in the case of GBM, therapies directed against a number of growth factors and receptors (i.e. VEGF, EGFR, PDGFR) are in various stages of clinical development [39, 40, 41, 42, 43]. The most common and most clinically advanced of these therapies target EGFR and the ligand-independent mutant ΔEGFR. Table 1 lists the current EGFR/ΔEGFR targeted agents for the treatment of glioma, which include monoclonal antibodies, tumor-antigen specific vaccines, small molecule inhibitors, and RNA-based therapies [5, 9].
Table 1.
EGFR-Targeted Therapies in Clinical Trials for the Treatment of Malignant Glioma
| Therapy | Substance | Brand Name | Target(s) | Company/Institution | Phase in Clinical Trials |
|---|---|---|---|---|---|
| Monoclonal Antibodies | Cetuximab | Erbitux | EGFR | Merck KGaA | Phase I/ II (as GERT) |
| Panitumumab | Vectibix | EGFR | Amgen | Phase II (w/ Irinotecan) | |
| Nimotuzumab | Theraloc | EGFR | YM BioSciences Inc. | Phase III (w/ TMZ + radiotherapy) | |
| 125I-MAb 425 | EGFR | Fox Chase Cancer Center | Phase II | ||
| mAb 806 | ABT-806 | ΔEGFR (weakly EGFR) | Abbott | Phase I | |
| Vaccines | PEP-3-KLH | CDX-110 | ΔEGFR | Celldex Therapeutics | Phase II (w/ TMZ) |
| Tyrosine Kinase Inhibitors | Geftinib | Iressa | EGFR | AstraZeneca Pharmaceuticals | Phase II |
| Erlotinib | Tarceva | EGFR/ AEGFR | Genentech Inc. | Phase II | |
| Lapatinib | Tykerb | EGFR/HER2 | GlaxoSmithKline | Phase I/II |
EGFR-Targeted Therapy
Monoclonal Antibodies
Cancer immunotherapy seeks to manipulate a person's own immune system to recognize and specifically destroy tumor cells, using target-specific antigenic proteins and peptides [44]. Although, tumor immunotherapy has shown some success in the treatment of renal cell carcinoma, melanoma, and hematologic cancers, the application of this approach to glioma presents more of a challenge [44]. Limitations include possible hindrance in the drug's ability to cross the blood-brain barrier (BBB) and induction of potential autoimmunity, which could lead to severe and undesired effects, such as central nervous system toxicity [44, 45].
In the early 1900s, Paul Erhlich was the first to propose the process of using monoclonal antibodies to target tumors, and later advances in antibody technology afforded the production of human monoclonal antibodies [45, 46]. One way to inhibit EGFR-mediated signaling is to disrupt receptor-activating ligand binding [45]. Blocking ligand binding to its cognate receptor could normalize growth rates, induce apoptosis, and increase tumor susceptibility to chemotherapeutic agents. Monoclonal antibodies, both unconjugated and conjugated, directed towards wtEGFR and ΔEGFR have been developed for therapeutic use in GBM. The most developed of the unconjugated antibodies is cetuximab (Erbitux®; Merck KGaA), which functions to prevent EGFR-mediated signal transduction by interfering with ligand binding and EGFR extracellular dimerization [45]. Additionally, cetuximab is also believed to trigger EGFR receptor internalization and destruction [47]. Pre-clinical data from in vitro studies suggest that treatment with cetuximab alone has a minimal impact on glioma tumorigenicity; however, it is synergistic with cytostatic drugs and radiotherapy [47, 48]. Mouse xenograft (intracranial and subcutaneous) studies have demonstrated that treatment with cetuximab decreases proliferation and increases cell death and overall survival [49]. Despite the fact that antibodies are large in molecular weight, these data suggest that cetuximab can traverse the blood-brain barrier. Currently, a phase I/II clinical trial is ongoing to study the efficacy of combining radiotherapy, TMZ and cetuximab, together known as GERT, to treat patients with primary GBM [50]. Other unconjugated monoclonal antibodies that are in initial stages of clinical development for GBM include panitumumab (Vectibix®; Amgen) and nimotuzumab (Theraloc®; YM BioSciences Inc.), which all function similarly to cetuximab [5, 45]. More recently, cetuximab has been shown to be effective against GBM tumors harboring a novel exon 27 deletion mutation in the carboxyl-terminus domain of EGFR, EGFR-CTD [51]. Particularly, cetuximab was effective in impairing tumorigenicity and prolonging the survival of xenograft mice with oncogenic EGFR-CTD deletion mutants [51]. Thus, while it appears that cetuximab alone has had limited clinical effectiveness among GBM patients, these data suggest that it may be a more promising therapeutic for patients harboring this specific EGFR mutation.
With respect to conjugated antibodies, the specific binding properties of antibodies are exploited to deliver the toxic effects of either conjugated toxins or radioisotopes. 125I-MAb 425 is one of the most advanced of the radioisotope-conjugated monoclonal anti-EGFR antibodies. Various phase II clinical trials have reported that 125I-MAb 425, either administered alone or concomitant with radiotherapy or temozolomide, significantly improves median survival in GBM patients [52, 53, 54]. The ligand-conjugate toxin composed of EGF and diphtheria toxin, DAB389EGF, represents another means to specifically transport toxins to EGFR, which involves the use of cognate ligands as vectors. Additionally, treatment with DAB389EGF was associated with significant tumor regression in subcutaneous glioblastoma xenografts [55]. Some monoclonal antibodies have been engineered to specifically target ΔEGFR. One such antibody is mAb806, which attenuates receptor autophosphorylation by binding to the short cysteine loop of the extracellular domain that is always exposed in ΔEGFR, and may also weakly target amplified wt EGFR, which transiently exposes this epitope during the switch from the inactive to the ligand-activated conformation [31, 56]. Pre-clinical data show that mAb806 strongly inhibits the growth of tumor xenografts that express ΔEGFR and more weakly those that express wt EGFR. In the clinic, it is well tolerated and displays excellent biodistribution and specificity for its target in GBM patients [57, 58]. These promising results have prompted a Phase I clinical trial with a humanized version of mAb806 (ABT-806; Abbott).
Vaccines
Antitumor vaccines have also been developed with the promise of precisely eradicating tumor cells while limiting toxicity. To date, vaccines in clinical development for the treatment of glioma consist of different combinations of dendritic cells (DCs), peptides, adjuvants, and even autologous tumors [44]. The most promising of these vaccines are dendritic cell and peptide-based; other combinations have resulted in either no significant improvement over standard therapy or the induction of several adverse events [44]. The ΔEGFR-specific vaccines are directed against a novel glycine epitope at the fusion junction that arises as a consequence of the in frame deletion of exons 2–7 from the extracellular domain of wtEGFR [59]. The proposed mechanism of action for achieving tumor regression is believed to initiate with capture of the peptide by the antigen-presenting complex (APC). The peptide is then relocated into the most proximal lymph nodes, where the antigenic peptide is presented to circulating cytotoxic T-lymphocytes (CTLs), which are then activated upon recognition, and finally infiltrate the tumor to eliminate the respective cancer cells [60, 61]. Tumorigenicity studies in rodents show that intracerebral treatment with a ΔEGFR synthetic peptide conjugated to keyhole limpet hemocyanin (PEP-3-KLH/CDX-110®, Celldex Therapeutics) reduces tumor size and increases overall survival [62]. Although major clinical responses were rarely obtained from previous peptide-based vaccine studies, the PEP-3-KLH vaccine in combination with TMZ has been shown to improve both progression free survival (14.2 months vs 7.3 months) and median survival (26 months vs. 15. 2 months) in GBM patients [5, 60]. Additionally, a vaccine comprised of DCs pulsed with the PEP-3-KLH peptide has been tested in the clinic and was shown to increase overall median survival time (22.8 months vs. 15.2 months) without severe adverse events [63]. From these results, it has been proposed that vaccination with PEP-3-KLH is safe and capable of inducing ΔEGFR-specific immune responses in patients with newly diagnosed primary GBM.
A vaccine that recognizes wtEGFR has also been designed, in which an EGFR binding peptide is conjugated with a lytic-type peptide containing cationic-rich amino acids that kills the cancer cell by disintegration of the cell membrane [64]. The goal of this vaccine is to serve as an adjuvant for either treatment with monoclonal antibodies or tyrosine kinase inhibitors to eradicate the cancer cells that are intractable to signaling inhibition, which renders them resistant to these therapies. Recent in vitro studies show that the EGFR-lytic peptide destroys cancer cells as quickly as ten minutes after exposure and exerts strong cytotoxic activity on TKI-resistant glioma cells. Although, this therapy has not been tested specifically in a glioblastoma xenograft model, it was shown to be effective in suppressing the growth of both breast and pancreatic xenografts [64]. Futhermore, more recent evidence has demonstrated that a single replacement of histidine to arginine in the lytic peptide sequence enhances its anticancer activity [65]. Taken together, these data suggest a potential value for this therapy against GBMs that should be further explored.
In spite of these advancements, a number of obstacles involving the efficacy of epitope-specific vaccines remain, including the notion that targeting of a heterogeneously expressed tumor antigen may potentially select for the survival and proliferation of antigen-negative cells. In fact, in a more recent phase II clinical trial to assess the immunogenicity of the PEP-3-KLH peptide vaccine, tumor recurrence following a significant period of progression free survival was observed [66]. Indeed, 82% of the relapse tumors were completely ΔEGFR negative, demonstrating that this vaccine can effectively eliminate ΔEGFR-expressing cells; however, it currently lacks the efficacy that is needed to eradicate this disease [66].
Small Molecule Inhibitors
Small molecule tyrosine kinase inhibitors (TKIs) are the most clinically advanced of the EGFR-targeted therapies, and both reversible and irreversible inhibitors are in clinical trials. Some of the reversible inhibitors include erlotinib (Tarceva®/OSI774; Genentech/Roche/OSI), gefitinib (Iressa®/ZD1839; AstraZeneca), lapatanib (Tykerb®; GlaxoSmithKline) and PKI166 (Novartis), and the irreversible inhibitors include canertinib (CI1033; Pfizer/Warner-Lambert) and pelitinib (EKB-569; Wyest-Ayerst) [42]. Mechanistically, these TKIs compete with ATP for binding to the tyrosine kinase domain of EGFR [42]. The irreversible and reversible nature of these inhibitors lead to the ablation of both phosphorylation of the receptor and downstream signaling [5]. Though a number of these inhibitors have been developed, gefitinib and erlotinib represent the best explored TKI inhibitors in the clinic for the treatment of GBM [5].
In pre-clinical in vitro studies, erlotinib was shown to inhibit anchorage-independent growth of glioblastoma cell lines [67]. More importantly, this inhibition was shown to correlate with suppressed induction of EGFR mRNA [67]. Additionally, long-term exposure to erlotinib was found to down-regulate the expression of both ΔEGFR and molecular effectors of tumor invasion in transformed glioblastoma cell lines [30]. The efficacy of erlotinib, however, is more characterized in other cancer cell types, where it has been shown to inhibit cell-cycle progression by inducing G1/S phase arrest [68, 69]. Moreover, erlotinib is able to induce apoptosis in colon and pancreatic cancer cell lines by stimulating DNA fragmentation and decreasing the expression of the anti-apoptotic Bcl-2 family members, respectively [68, 69]. Data from in vivo studies demonstrate that erlotinib also displays anti-angiogenic activity by suppressing vessel formation in pancreatic tumor xenografts [69]. Erlotinib was first clinically tested for the treatment of advanced and metastatic NSCLC, and to date was shown to significantly improve median survival in these patients by 42.5% in a phase III randomized trial [5]. In contrast, in a recent phase II clinical trial for GBM therapy, erlotinib was well tolerated, but only demonstrated a modest effect over placebo [15]. These results underscore the notion that differences in tissue-specific biology and/or signaling networks coupled to EGFR greatly influence TKI efficacy.
Interestingly, the antitumor activity of gefitinib is independent of the expression level of EGFR, but is heavily determined by its ability to inhibit anti-apoptotic signals [70]. Similar to erlotinib, gefitinib was shown to be effective at inhibiting the in vitro growth of a variety of human cancer cell lines by similar mechanisms [71]. Treatment with gefitinib was shown to inhibit cell survival and proliferation and by inducing G0/G1 arrest in adenocarcinoma and pancreatic cancer cells, respectively [69]. Additionally, gefitinib is able to hinder the in vivo growth of human breast and ovarian tumor xenografts, but despite its success in other cancer cell types, gefitinib has been less effective against glioblastoma [71]. Gefitinib and erlotinib appear to work best against tumors expressing EGFR with mutations in exons 19 and 21 of the TK domain, but to date, such EGFR mutants have not been found in GBM [5]. Interestingly, in the same study testing the efficacy of cetuximab against GBM tumors harboring novel EGFR mutations, erlotinib was also shown to be very effective against these EGFR CTD mutant GBM tumors [51]. Unfortunately, the clinical efficacy of gefitinib reflects the pre-clinical studies, in which in one phase II clinical trial, gefitinib was well tolerated and displayed anti-tumor activity, but the median overall survival time in GBM patients was only 38.4 weeks from treatment initiation [16]. A more recent phase II trial revealed that gefitinib reaches high concentrations in tumor tissue and efficiently dephosphorylates its target; however, regulatory circuits that promote sustained downstream signal transduction independent of EGFR phosphorylation appear to be more dominant [72]. Thus, it appears that gefitinib alone may prove to have limited therapeutic value in treating GBM patients.
The reality is that these EGFR-specific tyrosine kinase inhibitors have been relatively ineffective against gliomas, with response rates only reaching as high as 25% in the case of erlotinib [9]. Though both TKIs are well tolerated and display some antitumor activity in GBM patients, the recurrent problem of resistance to receptor inhibition has limited their efficacy [73, 74]. The presence of ΔEGFR with intact PTEN has been suggested as a molecular determinant of glioma sensitivity to TKIs [70], but many tumors that harbor this signature show only a modest response to TKIs or are resistant [70]. Furthermore, this signature is not predictive of TKI sensitivity in some serially passaged GBM xenografts [75]. These findings suggest that additional molecular determinants are relevant, and discovering them will be critically important to the rational design of more effective GBM therapies. Additionally, though the molecular weights of small molecule inhibitors are within the size limit for molecules that are allowed to cross the BBB, recent studies have shown that plasma concentrations of gefitinib and erlotinib following therapy were only 6–11% of the starting dose [5]. Thus, insufficient delivery to the target may be another cause of the disappointing clinical responses to these TKIs.
EGFR RNA-based Therapies
Interference with genetic transcription or translation is another mechanism through which receptor inhibition may be achieved, and some methods that have been developed over the last decade include antisense RNA, RNA interference (RNAi), and ribozymes [76]. Antisense oligonucleotides, such as OGX-011, are already in advanced clinical development for the treatment of NSCLC and prostate cancer, and thus far, the clinical responses are very promising [77]. Antisense RNAs hybridize to the sense mRNA of the target, resulting in inhibition of translation and protein synthesis. In an orthotopic xenograft model of human glioblastoma, intratumoral injection of a plasmid or viral vector expressing ΔEGFR-targeted antisense RNA was shown to cause a significant decrease in tumor growth [78]. In RNA interference methods, the suppression of homologous genes by small interfering RNAs (siRNAs) leads to sequence-specific target mRNA degradation. EGFR-specific siRNAs are directed against the TK domain, and were shown to cause 90% knockdown of EGFR mRNA in U251 glioma cells [79]. Furthermore, siRNA mediated-knockdown of EGFR resulted in G2/M arrest and reduced proliferation [79]. These findings were confirmed in vivo in an intracranial xenograft model, where treatment with EGFR-specific siRNAs increased overall survival by almost 90% [79]. However, the safety of siRNAs as therapeutics has been the biggest concern. In other in vivo studies, a vast number of mice fatalities were observed due to oversaturation of RNAi pathways [76]. One strategy in place to overcome this obstacle is to use the lowest possible concentration of siRNAs that provides therapeutic efficacy by designing exogenous siRNAs with increasing length [76]. This would introduce them into the RNAi pathway upstream of RISC directly at the step of Dicer cleavage, resulting in enhanced RNAi activity at lower concentrations [76]. Another strategy in place is the use of cyclodextrin-modified dendritic polyamine complexes (DexAMs) as a vehicle for translocating siRNAs [80]. Recently, DexAMs were shown to deliver EGFRvIII siRNAs efficiently and selectively to glioblastoma cells with minimal toxicity [80]. Futhermore, codelivery of EGFRvIII-siRNA and erlotinib in GBM was found to significantly inhibit cell proliferation and induce apoptosis in glioblastoma cells [80]. Finally, a third method of interference that has been explored involves the use of anti-ΔEGFR hairpin ribozymes. Ribozymes catalytically cleave certain RNA substrates in a sequence-specific manner, in which cleavage is mediated by a catalytic core [5]. In pre-clinical in vitro studies, treatment with anti-ΔEGFR hairpin ribozymes was shown to reduce ΔEGFR mRNA by 90% and inhibit anchorage-independent growth of U87MGΔEGFR glioma cells [5]. These encouraging pre-clinical outcomes along with the success of this approach in other cancers suggest that RNA-based therapies should be further explored in clinical trials for the treatment of GBM.
MALIGNANT GLIOMA AND THERAPEUTIC RESISTANCE
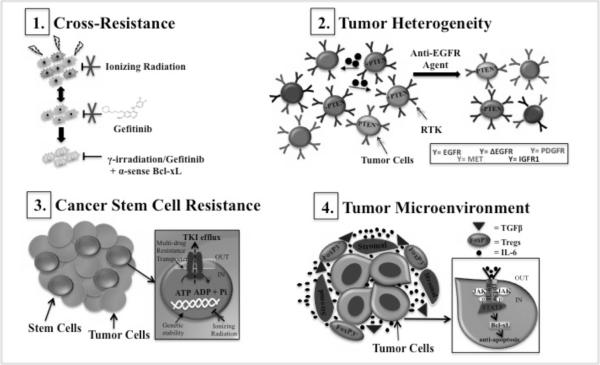
In spite of the advances in our current knowledge of glioma biology and genetics, this disease remains largely incurable. For the reasons discussed above, some EGFR-targeted agents hold great potential, however, successful treatment of malignant gliomas continues to be a major therapeutic challenge due to both inherent and acquired resistance [81, 82]. Mechanisms causing resistance to EGFR TKIs have been studied extensively in a number of solid tumors. Some of the documented mechanisms include the acquisition of secondary EGFR point mutations, co-activation and/or amplification of other RTKs, and up-regulation of drug efflux pumps [82]. A few of these mechanisms have been established for NSCLC and breast cancer; however, mechanisms of resistance that are unique to glioma are not clearly defined [82, 83]. Malignant gliomas are recalcitrant by nature and are able to escape the need for receptor function by activating alternative compensatory pathways when challenged with receptor-targeted therapeutics. Though, the specific compensatory mechanisms that are essential to this escaper phenotype have not been delineated, a few generalizations (see Figure 1.) can be drawn from established knowledge in the field of glioma biology and therapeutic resistance [70].
Figure 1.
Malignant glioma and therapeutic resistance. GBMs are adept at evading inhibition of EGFR receptor function through several possible mechanisms. (1) Brain tumor cells that are intractable to DNA damage-induced apoptosis may also tolerate apoptotic cues driven by TKI-mediated inhibition of EGFR. Combinatorial therapy using inhibitors of anti-apoptotic activity may overcome this cross-resistance. (2) Intratumoral diversity within GBM tumors may drive resistance to single based anti-EGFR agents due to: RTK co-activation, PTEN deletion/mutations, and tumor cell-tumor cell interactions via secreted molecules. PTEN* denotes mutation (3) Efflux of EGFR TKIs and increased genetic stability may lead to the maintenance of CSC populations and tumor relapse. (4) Enhanced immunosuppression mediated by circulating growth factors, cytokines and suppressor T cells can antagonize the systemic immune responses generated by anti-EGFR immunotherapies. Additionally, circulating IL-6 in the tumor microenvironment can facilitate resistance intracellularly via activation of the JAK/STAT3/Bcl-xL pathway.
Cross-Resistance
Malignant brain tumors are typified by resistance to apoptosis and diffuse invasion into the normal brain parenchyma [84]. During invasion, brain tumors cells often arrest in mitosis, rendering them refractory to radiotherapy and chemotherapy, whose antitumor activity triggers DNA damage-induced apoptosis [85]. Additionally, once tumor cells disseminate into the normal brain regions, they are protected by an intact BBB, which can also significantly limit the efficacy of therapeutic agents that cannot traverse this barrier [85]. Furthermore, despite being robustly angiogenic, the tortuous vasculature in malignant brain tumors critically limits drug penetration [85]. Hence, overcoming the obstacles that lie within the inherent biology of gliomagenesis will be necessary if these tumors are to become completely eradicated. A primary mechanism by which some EGFR TKIs (i.e., gefitinib) exert their effects is also through induction of apoptosis [70]. In essence, brain tumor cells that have developed a way to tolerate chemotherapy and radiotherapy may also be intractable to these TKIs or vice versa, as a result of cross-resistance [86]. In fact, oncogenic ΔEGFR has been demonstrated to confer resistance to chemotherapy by inducing the expression of the anti-apoptotic protein Bcl-xL [86]. Surprisingly, despite sustained repression of ΔEGFR expression, some GBM tumors eventually recur [87]. These ΔEGFR-independent tumors were significantly less apoptotic and had restored Bcl-xL protein expression [87]. This result and other findings that demonstrate that some tumors are also maintained in the absence of EGFR kinase activity suggest that treatment with EGFR-targeted therapies alone may not reach maximum therapeutic efficacy [87, 88]. The most clinically advanced approach to inhibit Bcl-xL anti-apoptotic activity involves the use of a bi-specific antisense Bcl-2/Bcl-xL oligonucleotide [89]. In pre-clinical studies, this therapy has already been shown to be effective in inducing apoptosis in glioblastoma cell lines that overexpress these two proteins [90]. Thus, concomitant treatment with Bcl-xL-specific antisense oligonucleotides may enhance the efficacy of both chemotherapeutic agents and EGFR-directed TKIs in the treatment of GBM [91].
Tumor Heterogeneity
GBM tumors are considerably heterogeneous, and this intratumoral diversity represents a second probable cause of anti-EGFR therapeutic resistance. Within these tumors lie mixed cytological subtypes, regional differences in gene expression, and variable representation of key genetic mutations and chromosomal alterations [84]. The maintenance of individual cell growth within GBM tumors could result from (1) mutation and amplification of multiple RTKs, or, (2) deletions or decreased function in tumor suppressor genes. In essence, some tumors may be inherently resistant to EGFR-targeted therapy simply because they are not dependent on its receptor function. In fact, in other solid tumors such as colorectal cancer, activation of ERBB2 signaling was shown to be a mechanism of resistance to cetuximab [92]. Indeed, other RTKs and GPI-linked receptors, such as IGFR1, MET, PDGFRα/β and uPAR, have been reported to be altered in GBM, and their ability to compensate for EGFR has been implicated in the persistent activation of downstream survival signaling, even in the presence of EGFR inhibitors [93, 94, 95, 96]. For example, treatment with either erlotinib or the MET inhibitor SU11274 alone had no discernible effect on inhibiting the activation of the PI3K/AKT pathway in U87MGΔEGFR cells. However, when used in combination, downstream signaling as measured by activated Akt and S6 ribosomal protein was significantly inhibited and anchorage-independent growth of these cells was also reduced [93, 97] Similarly, activation of c-Met expression in response to EGFR inhibition led to the survival of GBM tumor cells in a mouse model of glioblastoma and inhibiting MET reversed this phenotype [98]. These results indicate that co-activation of RTKs within the same tumor may confer resistance to EGFR TKIs. Persistent activation of the PI3K/Akt pathway may also be due to mutations in the tumor suppressor gene PTEN, which is a negative regulator of this pathway. The frequency of PTEN deletion or mutation in GBM is around 40–50%, indicating that there is a high probability that the antitumor activity of many EGFR inhibitors will ultimately be negated due to defects in components of the targeted pathway [93]. Co-expression of ΔEGFR and wild type PTEN has been shown to correlate with tumor sensitivity to EGFR TKIs; however, as mentioned before, there is considerable variability in patient response despite the presence of this genetic signature [70]. This suggests that multiple inputs to PI3K signaling may confer insensitivity to these therapies. More recently, PTEN-deficient glioblastoma cell lines were shown to employ the autophagic process as a survival pathway of escape [99, 100, 101]. Specifically, overexpression and accumulation of αB-crystallin, a heat shock protein that can impair caspase activation, following erlotinib treatment was observed in DBTRG-05 and U87 glioma cells, respectively [99]. Likewise, inhibition of autophagy or autophagosome maturation was shown to increase the death-inducing activity of both erlotinib and an mTOR inhibitor, PI-103, respectively [99, 100]. Thus, cooperation between TKIs and inhibitors of predictive compensatory mechanisms may be required to achieve a more robust anti-tumor effect against GBM.
Cooperative tumor cell-tumor cell interaction has also been linked to the maintenance of intratumoral heterogeneity in GBM [84]. Inda et al., demonstrated that ΔEGFR-expressing cells release interluekin-6 (IL-6) in order to activate wtEGFR expressing cells through a paracrine cytokine signaling circuit [84]. The secretion of IL-6 was also correlated with enhanced tumorigenic growth of U87 glioma cells [84]. Prior to these studies, IL-6 had been implicated in drug resistance and survival signaling through both the BclxL and STAT3 pathways in prostate cancer [102]. Interestingly, these effects were significantly attenuated by transfection with anti-sense Bcl-xL olignonucleotides, further supporting the notion that RNA-based therapies should be more closely considered as a therapeutic option in GBM therapy [102]. More recently, IL-6, as part of the EGFR-ID3-IL-6 signaling axis, was shown to also promote tumor cell heterogeneity in GSC populations [103]. Taken together, it is clear that GBM tumors possess a number of intrinsic variables that would render single-based therapies inept, and understanding this variability will be important to the optimization of these agents for clinical use.
Cancer Stem Cells
Although the genetic pathways that are involved in the development of malignant gliomas are now reasonably well-characterized [2, 3], the cellular origins of these tumors are poorly understood [18]. There is growing evidence that glioma stem cells are major contributors to resistance to standard treatments, and several studies have demonstrated that malignant brain tumors that were enriched with cancer stem cells (CSCs) were more resistant to radiotherapy and chemotherapy than other tumors due to their ability to alter both checkpoint and DNA repair pathways [85, 104]. Many conventional therapies specifically target rapidly proliferating cells, while sparing the quiescent, tumor cell compartment. This arises from the notion that proliferation is the main problem for cancer treatment [105]. Indeed, rapidly proliferating cells make up the bulk of the tumor; however, if the CSCs that are left behind repopulate the tumor, then the question that inevitably arises is: are current anti-cancer therapies targeting the right population of cells? CSCs are thought to be the source of tumor relapse due to increased genetic stability, decreased oxidative stress, or the presence of multiple drug resistant transporters [106]. In the latter case, activation of these transporters could lead to increased efflux of anti-EGFR agents from the tumor, resulting in decreased intracellular concentrations of drug. Additionally, the genetic stability of this population would render them unperturbed by the pro-apoptotic signals generated by EGFR TKIs. The challenge would therefore remain in designing novel therapies that specifically target the CSC population or CSC-specific multidrug resistance transporters to eradicate these sources of tumor maintenance. More importantly, as a role for neural stem/progenitor cells (NSCs) in learning and memory is becoming accepted, understanding the biological differences between normal and cancer stem cells is required to develop such selective therapies in order to spare normal brain cells. In fact, a recent study testing the efficacy of bortezomib, a protease inhibitor, on treating multiple low- and high-grade glioma stem-like cell (GSC) cultures revealed that bortezomib reduces GSC populations by 80% with minimal effects on NSC populations [107]. Additionally, erlotinib was shown to produce similar results as bortezomib, while TMZ and cisplatin were shown to be more toxic to NSCs and less effective against GSCs [107]. Thus, it appears that combining newer, promising agents with TKIs, rather than with older chemotherapeutic agents, could result in more durable responses in GBM patients. The nonreceptor tyrosine kinase BMX has also been implicated in maintaining self-renewal and tumorigenic potential of GSC populations by activating STAT3 [108]. Furthermore, BMX knockdown potently inhibited STAT3 activation and growth of GSC-derived intracranial tumors, suggesting that BMX represents a GSC therapeutic target [108]. Thus, identifying more GSC-specific targets and testing the efficacy of additional selective drugs are clearly warranted in an effort to overcome the contribution of cancer stem cells to therapy resistance.
Tumor Microenvironment
Malignant glioma patients are known to be profoundly immunosuppressed, and even if systemic immune responses are generated by EGFR-specific monoclonal antibodies or peptide vaccines, they could be negated in the tumor microenvironment by a variety of immunosuppressive growth factors and cytokines [109]. In contrast to EGFR TKIs, the mechanisms of resistance to these therapies in any cancer are poorly defined [91]. Growth factors, such as TGFβ, have been shown to promote tumor escape from immunosurveillance, and high plasma levels of TGFβ correlate with a negative prognosis in a variety of cancers [45]. Additionally, the production of TGFβ can lead to the accumulation of CD3+, CD4+, CD25+, FOXP3+ TReg cells. Regulatory T cells (TRegs) are a specialized subpopulation of T cells that function to actively suppress the immune system in order to maintain system homeostasis and prevent pathological self-reactivity. In essence, immunosuppressive cell populations, such as TRegs, could be attributed to the attenuated efficacy of EGFR-specific immunotherapy agents by way of inhibiting antitumor immunity. In GBM, increased numbers of TReg cells have been found in the peripheral blood and also in the tumor microenvironment, and though a prognostic role of TRegs present in glioma has not been thoroughly evaluated, TRegs have been shown to be associated with poor clinical outcome in other systemic tumors [109]. In a syngeneic murine model of glioma, depletion of TRegs resulted in complete tumor rejection and enhanced survival [110]. Given that STAT3 is required for TGFβ production, inhibitors of the JAK/STAT pathway have been explored as a potential strategy to overcome TReg-mediated immunosuppression [109]. Additionally, STAT3 is overexpressed in glioma and the STAT3 inhibitor WP1066, was shown to be effective in inducing apoptosis and inhibiting the in vivo growth of U87 glioma cells [111]. Currently, GC1008, a TGFβ-specific antibody, is in the initial stages of clinical development for metastatic kidney cancer and malignant melanoma, and given the role of TGFβ in immunosuppression, GC1008 or WP1066 in combination with EGFR-specific immunotherapy agents could potentially be more efficacious in the treatment of malignant glioma.
Finally, aside from a possible role for IL-6 in intrinsic resistance, other studies have shown that secretion of IL-6 by stromal cells into the tumor microenvironment may also promote tumor survival and block apoptosis, thereby resulting in chemotherapeutic resistance [112]. These effects were also shown to be via the JAK/STAT and Bcl-xL pathways [112]. More recently, TGFβ-dependent IL-6 secretion was shown to be responsible for the acquired resistance of lung tumor cells to the EGFR TKI erlotinib [113]. Thus, as an alternative approach, blocking IL-6-mediated signaling may enhance the efficacy of EGFR-targeted therapies.
PERSPECTIVES
The successful application of EGFR-targeted therapy for the treatment of glioblastoma has been proven to be very challenging. A deeper understanding of the intricate inter-relationships that underlie the pathobiology of this disease will be required to achieve stable therapeutic responses to targeted agents. Malignant brain tumors require a very complex signaling network that is not only driven by EGFR, and this complexity dictates tumor sensitivity to EGFR-targeted therapies. Therefore, blocking EGFR alone may not sufficiently translate into a clinical benefit for GBM patients. In essence, a departure from the status quo of single-based EGFR targeted therapy should be considered, which holds promise that substantial increases in long-term disease control can be achieved not only for glioma patients, but also for individuals afflicted with other cancers that are driven by these genetic lesions. With the development of novel combinatorial therapies, improvement in patient quality of life may be achieved through tailored, personalized choices of appropriate therapeutic modalities.
Acknowledgments
Funding sources for this manuscript include support from the Goldhirsh Foundation (to F.B.F.), the NIH (P01-CA95616 to W.K.C. and F.B.F.), the NIH (1 F31 NS076343-01 to T.E.T.) and W.K.C. is a fellow of the National Foundation for Cancer Research.
List of Abbreviations
- GBM
Glioblastoma multiforme
- WHO
World Health Organization
- EGFR/ERRB1/HER1
Epidermal growth factor receptor
- ERBB2
human epidermal growth factor receptor 2
- ERBB3
v-erb-b2 erythroblastic leukemia viral oncogene homolog 3
- ERBB4
v-erb-a erythroblastic leukemia viral oncogene homolog 4
- TKI
tyrosine kinase inhibitor
- RTKs
receptor tyrosine kinases
- PI3K
phosphatidylinositol 3-kinase
- Akt
protein kinase B
- MAPK
mitogen-activated protein kinase
- Cbl
Casitas B-lineage
- NSCLC
non small cell lung carcinoma
- IDH1
isocitrate dehydrogenase 1
- IDH2
isocitrate dehydrogenase 2
- PTEN
phosphatase and tensin homolog
- LOH
loss of heterozygosity
- PDGF
platelet-derived growth factor
- PDGFR-α/β
platelet-derived growth factor receptor alpha/beta
- ERK
extracellular-signal-regulated kinases
- MMPs
matrix metalloproteinases
- mAb
monoclonal antibody
- BCNU
carmustine
- CCNU
lomustine
- TMZ
temozolomide
- VEGF
vascular endothelial growth factor
- RNA
ribonucleic acid
- BBB
blood-brain barrier
- GERT
cetuximab, radiotherapy and temozolomide
- CTD
carboxyl-terminus domain
- DCs
dendritic cells
- mRNA
messenger ribonucleic acid
- APC
antigen-presenting complex
- CTLs
cytotoxic T-lymphocytes
- ATP
adenosine triphosphate
- RNAi
ribonucleic acid interference
- siRNAs
small interfering ribonucleic acids
- DexAMs
cyclodextrin-modified dendritic polyamine complexes
- DNA
deoxyribonucleic acid
- Bcl-xL
B-cell lymphoma-extra large
- IGFR
insulin growth factor receptor
- MET
hepatocyte growth factor receptor
- uPAR
urokinase plasminogen activator receptor
- mTOR
mammalian target of rapamycin
- IL-6
interleukin-6
- STAT3
Signal transducer and activator of transcription 3
- ID3
inhibitor of differentiation 3
- CSCs
cancer stem cells
- NSCs
neural stem/progenitor cells
- GSCs
glioma stem-like cells
- BMX
bone marrow X-linked nonreceptor tyrosine kinase
- TGFβ
transforming growth factor beta
- CD
cluster of differentiation
- FOXP3
forkhead box P3
- TRegs
regulatory T cells
- JAK
janus kinase
REFERENCES
- [1].Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- [2].Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr., Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- [5].Karpel-Massler G, Schmidt U, Unterberg A, Halatsch ME. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: where do we stand? Mol. Cancer Res. 2009;7(7):1000–1012. doi: 10.1158/1541-7786.MCR-08-0479. [DOI] [PubMed] [Google Scholar]
- [6].Hasselbalch B, Lassen U, Poulsen HS, Stockhausen MT. Cetuximab insufficiently inhibits glioma cell growth due to persistent EGFR downstream signaling. Cancer Invest. 2010;28(8):775–787. doi: 10.3109/07357907.2010.483506. [DOI] [PubMed] [Google Scholar]
- [7].Wells A. EGF receptor. Int. J. Biochem. Cell Biol. 1999;31(6):637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- [8].Weinberg RA. The Biology of Cancer. Garland Science; New York: 2007. p. 796. [Google Scholar]
- [9].Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009;16(6):748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- [10].Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60(5):1383–1387. [PubMed] [Google Scholar]
- [11].Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl. Acad. Sci. U S A. 1994;91(16):7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc. Natl. Acad. Sci. U S A. 2003;100(11):6505–6510. doi: 10.1073/pnas.1031790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Biol. Chem. 1997;272(5):2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- [14].Nagane M, Lin H, Cavenee WK, Huang HJ. Aberrant receptor signaling in human malignant gliomas: mechanisms and therapeutic implications. Cancer Lett. 2001;162(Suppl):S17–S21. doi: 10.1016/s0304-3835(00)00648-0. [DOI] [PubMed] [Google Scholar]
- [15].Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, Yung WK, Gilbert MR, Aldape KA, Wen PY, Fine HA, Mehta M, Deangelis LM, Lieberman F, Cloughesy TF, Robins HI, Dancey J, Prados MD. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro. Oncol. 2010;12(1):95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rich JN, Rasheed BK, Yan H. EGFR mutations and sensitivity to gefitinib. N. Engl. J. Med. 2004;351(12):1260–1261. author reply 1260–1261. [PubMed] [Google Scholar]
- [17].Ji H, Sharpless NE, Wong KK. EGFR targeted therapy: view from biological standpoint. Cell Cycle. 2006;5(18):2072–2076. doi: 10.4161/cc.5.18.3277. [DOI] [PubMed] [Google Scholar]
- [18].Wen PY, Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- [19].Ladha J, Donakonda S, Agrawal S, Thota B, Srividya MR, Sridevi S, Arivazhagan A, Thennarasu K, Balasubramaniam A, Chandramouli BA, Hegde AS, Kondaiah P, Somasundaram K, Santosh V, Rao SM. Glioblastoma-specific protein interaction network identifies PP1A and CSK21 as connecting molecules between cell cycle-associated genes. Cancer Res. 2010;70(16):6437–6447. doi: 10.1158/0008-5472.CAN-10-0819. [DOI] [PubMed] [Google Scholar]
- [20].Scheithauer BW, Erdogan S, Rodriguez FJ, Burger PC, Woodruff JM, Kros JM, Gokden M, Spinner RJ. Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents: a clinicopathologic study of 17 cases. Am. J. Surg. Pathol. 2009;33(3):325–338. doi: 10.1097/PAS.0b013e31818d6470. [DOI] [PubMed] [Google Scholar]
- [21].Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- [22].Jones TS, Holland EC. Molecular pathogenesis of malignant glial tumors. Toxicol. Pathol. 2011;39(1):158–166. doi: 10.1177/0192623310387617. [DOI] [PubMed] [Google Scholar]
- [23].Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- [24].Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6(3):217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. discussion 223–214. [DOI] [PubMed] [Google Scholar]
- [25].Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tortora G, Bianco R, Daniele G, Ciardiello F, McCubrey JA, Ricciardi MR, Ciuffreda L, Cognetti F, Tafuri A, Milella M. Overcoming resistance to molecularly targeted anticancer therapies: Rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug. Resist. Updat. 2007;10(3):81–100. doi: 10.1016/j.drup.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- [28].Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56(21):5079–5086. [PubMed] [Google Scholar]
- [29].Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J. Biol. Chem. 1998;273(1):200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- [30].Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62(12):3335–3339. [PubMed] [Google Scholar]
- [31].Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, Ji XD, Suvarna P, Voland JR, Old LJ, Huang HJ, Cavenee WK. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61(14):5349–5354. [PubMed] [Google Scholar]
- [32].Silvani A, Gaviani P, Lamperti EA, Eoli M, Falcone C, Dimeco F, Milanesi IM, Erbetta A, Boiardi A, Fariselli L, Salmaggi A. Cisplatinum and BCNU chemotherapy in primary glioblastoma patients. J. Neurooncol. 2009;94(1):57–62. doi: 10.1007/s11060-009-9800-0. [DOI] [PubMed] [Google Scholar]
- [33].Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin. Oncol. 2004;31(5):635–644. doi: 10.1053/j.seminoncol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [34].La Rocca RV, Mehdorn HM. Localized BCNU chemotherapy and the multimodal management of malignant glioma. Curr. Med. Res. Opin. 2009;25(1):149–160. doi: 10.1185/03007990802611935. [DOI] [PubMed] [Google Scholar]
- [35].Walker MD. The contemporary role of chemotherapy in the treatment of malignant brain tumor. Clin. Neurosurg. 1978;25:388–396. doi: 10.1093/neurosurgery/25.cn_suppl_1.388. [DOI] [PubMed] [Google Scholar]
- [36].Walker MD, Green SB, Byar DP, Alexander E., Jr., Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley M. S., Jr., Mealey J, Jr., Owens G, Ransohoff J, 2nd, Robertson JT, Shapiro WR, Smith K. R., Jr., Wilson CB, Strike TA. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 1980;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- [37].Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5(2):144–151. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- [38].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [39].Slamon D, Pegram M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin. Oncol. 2001;28(1 Suppl 3):13–19. doi: 10.1016/s0093-7754(01)90188-5. [DOI] [PubMed] [Google Scholar]
- [40].Norden AD, Drappatz J, Wen PY. Antiangiogenic therapy in malignant gliomas. Curr. Opin. Oncol. 2008;20(6):652–661. doi: 10.1097/CCO.0b013e32831186ba. [DOI] [PubMed] [Google Scholar]
- [41].Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152–1160. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- [42].Mischel PS, Cloughesy TF. Targeted molecular therapy of GBM. Brain Pathol. 2003;13(1):52–61. doi: 10.1111/j.1750-3639.2003.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN, McLendon RE, Moscatello D, Pegram CN, Reist CJ, Traweek ST, Wong AJ, Zalutsky MR, Bigner DD. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55(14):3140–3148. [PubMed] [Google Scholar]
- [44].Johnson LA, Sampson JH. Immunotherapy approaches for malignant glioma from 2007 to 2009. Curr. Neurol. Neurosci. Rep. 2010;10(4):259–266. doi: 10.1007/s11910-010-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ehrlich P. Collected Studies on Immunity. J. Wiley & Sons; New York: 1906. [Google Scholar]
- [47].Rivera F, Vega-Villegas ME, Lopez-Brea MF. Cetuximab, its clinical use and future perspectives. Anticancer Drugs. 2008;19(2):99–113. doi: 10.1097/CAD.0b013e3282f23287. [DOI] [PubMed] [Google Scholar]
- [48].Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56(1):155–162. doi: 10.1227/01.neu.0000145865.25689.55. discussion 162. [DOI] [PubMed] [Google Scholar]
- [49].Eller JL, Longo SL, Hicklin DJ, Canute GW. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery. 2002;51(4):1005–1013. doi: 10.1097/00006123-200210000-00028. discussion 1013-1004. [DOI] [PubMed] [Google Scholar]
- [50].Combs SE, Heeger S, Haselmann R, Edler L, Debus J, Schulz-Ertner D. Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)--phase I/II trial: study protocol. BMC Cancer. 2006;6:133. doi: 10.1186/1471-2407-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cho J, Pastorino S, Zeng Q, Xu X, Johnson W, Vandenberg S, Verhaak R, Cherniack AD, Watanabe H, Dutt A, Kwon J, Chao YS, Onofrio RC, Chiang D, Yuza Y, Kesari S, Meyerson M. Glioblastoma-Derived Epidermal Growth Factor Receptor Carboxyl-Terminal Deletion Mutants Are Transforming and Are Sensitive to EGFR-Directed Therapies. Cancer Res. 2011;71(24):1–10. doi: 10.1158/0008-5472.CAN-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Emrich JG, Brady LW, Quang TS, Class R, Miyamoto C, Black P, Rodeck U. Radioiodinated (I-125) monoclonal antibody 425 in the treatment of high grade glioma patients: ten-year synopsis of a novel treatment. Am. J. Clin. Oncol. 2002;25(6):541–546. doi: 10.1097/00000421-200212000-00001. [DOI] [PubMed] [Google Scholar]
- [53].Quang TS, Brady LW. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):972–975. doi: 10.1016/j.ijrobp.2003.09.096. [DOI] [PubMed] [Google Scholar]
- [54].Li L, Quang TS, Gracely EJ, Kim JH, Emrich JG, Yaeger TE, Jenrette JM, Cohen SC, Black P, Brady LW. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010;113(2):192–198. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- [55].Liu TF, Hall PD, Cohen KA, Willingham MC, Cai J, Thorburn A, Frankel AE. Interstitial diphtheria toxinepidermal growth factor fusion protein therapy produces regressions of subcutaneous human glioblastoma multiforme tumors in athymic nude mice. Clin. Cancer Res. 2005;11(1):329–334. [PubMed] [Google Scholar]
- [56].Gan HK, Lappas M, Cao DX, Cvrljevdic A, Scott AM, Johns TG. Targeting a unique EGFR epitope with monoclonal antibody 806 activates NF-kappaB and initiates tumour vascular normalization. J. Cell. Mol. Med. 2009;13(9B):3993–4001. doi: 10.1111/j.1582-4934.2009.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, Scott AM. Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2-7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res. 2001;61(14):5355–5361. [PubMed] [Google Scholar]
- [58].Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, Papenfuss AT, Poon AM, Hopkins W, Smyth FE, MacGregor D, Cher LM, Jungbluth AA, Ritter G, Brechbiel MW, Murphy R, Burgess AW, Hoffman EW, Johns TG, Old LJ. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl. Acad. Sci. U S A. 2007;104(10):4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57(8):1419–1424. [PubMed] [Google Scholar]
- [60].Kanaly CW, Ding D, Heimberger AB, Sampson JH. Clinical applications of a peptide-based vaccine for glioblastoma. Neurosurg. Clin. N. Am. 2010;21(1):95–109. doi: 10.1016/j.nec.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yamanaka R. Dendritic-cell- and peptide-based vaccination strategies for glioma. Neurosurg. Rev. 2009;32(3):265–273. doi: 10.1007/s10143-009-0189-1. discussion 273. [DOI] [PubMed] [Google Scholar]
- [62].Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert. Opin. Biol. Ther. 2009;9(8):1087–1098. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, Friedman HS, Bigner DD. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol. Cancer Ther. 2009;8(10):2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kohno M, Horibe T, Haramoto M, Yano Y, Ohara K, Nakajima O, Matsuzaki K, Kawakami K. A novel hybrid peptide targeting EGFR-expressing cancers. Eur. J. Cancer. 2011;47(5):773–783. doi: 10.1016/j.ejca.2010.10.021. [DOI] [PubMed] [Google Scholar]
- [65].Tada N, Horibe T, Haramoto M, Ohara K, Kohno M, Kawakami K. A single replacement of histidine to arginine in EGFR-lytic hybrid peptide demonstrates the improved anticancer activity. Biochem. Biophys. Res. Commun. 2011;407(2):383–388. doi: 10.1016/j.bbrc.2011.03.030. [DOI] [PubMed] [Google Scholar]
- [66].Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD. Immunologic Escape After Prolonged Progression-Free Survival With Epidermal Growth Factor ReceptorVariant III Peptide Vaccination in Patients With NewlyDiagnosed Glioblastoma. J. Clin. Oncol. 2010;28(31):4722–4279. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Halatsch ME, Gehrke EE, Vougioukas VI, Botefur IC;F,AB, Efferth T, Gebhart E, Domhof S, Schmidt U, Buchfelder M. Inverse correlation of epidermal growth factor receptor messenger RNA induction and suppression of anchorage-independent growth by OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in glioblastoma multiforme cell lines. J. Neurosurg. 2004;100(3):523–533. doi: 10.3171/jns.2004.100.3.0523. [DOI] [PubMed] [Google Scholar]
- [68].Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP, Neveu M, Pollack VA, Pustilnik LR, Reynolds MM, Sloan D, Theleman A, Miller P. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57(21):4838–4848. [PubMed] [Google Scholar]
- [69].Quatrale AE, Porcelli L, Silvestris N, Colucci G, Angelo A, Azzariti A. EGFR tyrosine kinases inhibitors in cancer treatment: in vitro and in vivo evidence. Front. Biosci. 2011;16:1962–1972. doi: 10.2741/3833. [DOI] [PubMed] [Google Scholar]
- [70].Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- [71].Knight LA, Di Nicolantonio F, Whitehouse P, Mercer S, Sharma S, Glaysher S, Johnson P, Cree IA. The in vitro effect of gefitinib ('Iressa') alone and in combination with cytotoxic chemotherapy on human solid tumours. BMC Cancer. 2004;4:83. doi: 10.1186/1471-2407-4-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hegi ME, Diserens AC, Bady P, Kamoshima Y, Kouwenhoven MC, Delorenzi M, Lambiv WL, Hamou MF, Matter MS, Koch A, Heppner FL, Yonekawa Y, Merlo A, Frei K, Mariani L, Hofer S. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib--a phase II trial. Mol. Cancer Ther. 2011;10(6):1102–1112. doi: 10.1158/1535-7163.MCT-11-0048. [DOI] [PubMed] [Google Scholar]
- [73].Learn CA, Hartzell TL, Wikstrand CJ, Archer GE, Rich JN, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Resistance to tyrosine kinase inhibition by mutant epidermal growth factor receptor variant III contributes to the neoplastic phenotype of glioblastoma multiforme. Clin. Cancer Res. 2004;10(9):3216–3224. doi: 10.1158/1078-0432.ccr-03-0521. [DOI] [PubMed] [Google Scholar]
- [74].Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat. Rev. Drug Discov. 2004;3(5):430–446. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- [75].Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, Galanis E, Giannini C, Wu W, Dinca EB, James CD. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol. Cancer Ther. 2007;6(3):1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- [76].Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sowery RD, Hadaschik BA, So AI, Zoubeidi A, Fazli L, Hurtado-Coll A, Gleave ME. Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int. 2008;102(3):389–397. doi: 10.1111/j.1464-410X.2008.07618.x. [DOI] [PubMed] [Google Scholar]
- [78].Shir A, Levitzki A. Inhibition of glioma growth by tumor-specific activation of double-stranded RNA-dependent protein kinase PKR. Nat. Biotechnol. 2002;20(9):895–900. doi: 10.1038/nbt730. [DOI] [PubMed] [Google Scholar]
- [79].Kang CS, Zhang ZY, Jia ZF, Wang GX, Qiu MZ, Zhou HX, Yu SZ, Chang J, Jiang H, Pu PY. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13(5):530–538. doi: 10.1038/sj.cgt.7700932. [DOI] [PubMed] [Google Scholar]
- [80].Kim C, Shah BP, Subramaniam P, Lee KB. Synergistic induction of apoptosis in brain cancer cells by targeted codelivery of siRNA and anticancer drugs. Mol. Pharm. 2011;8(5):1955–1961. doi: 10.1021/mp100460h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr. Opin. Genet. Dev. 2008;18(1):73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [82].Wykosky J, Mukasa A, Furnari F, Cavenee WK. Escape from targeted inhibition: the dark side of kinase inhibitor therapy. Cell Cycle. 2010;9(9):1661–1662. doi: 10.4161/cc.9.9.11592. [DOI] [PubMed] [Google Scholar]
- [83].Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin. Cancer Res. 2008;14(10):2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- [84].Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, Brennan C, Johns TG, Bachoo R, Hadwiger P, Tan P, Depinho RA, Cavenee W, Furnari F. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24(16):1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol. Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc. Natl. Acad. Sci. U S A. 1998;95(10):5724–5729. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mukasa A, Wykosky J, Ligon KL, Chin L, Cavenee WK, Furnari F. Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence. Proc. Natl. Acad. Sci. U S A. 2010;107(6):2616–2621. doi: 10.1073/pnas.0914356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, Hung MC. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13(5):385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23(16):2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- [90].Jiang Z, Zheng X, Rich KM. Down-regulation of Bcl-2 and Bcl-xL expression with bispecific antisense treatment in glioblastoma cell lines induce cell death. J. Neurochem. 2003;84(2):273–281. doi: 10.1046/j.1471-4159.2003.01522.x. [DOI] [PubMed] [Google Scholar]
- [91].Johns TG, Perera RM, Vernes SC, Vitali AA, Cao DX, Cavenee WK, Scott AM, Furnari FB. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin. Cancer Res. 2007;13(6):1911–1925. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- [92].Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Janne PA. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 2011;3(99):99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, Chin L, DePinho RA. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- [94].Hu J, Jo M, Cavenee WK, Furnari F, VandenBerg SR, Gonias SL. Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells. Proc. Natl. Acad. Sci. U S A. 2011;108(38):15984–15989. doi: 10.1073/pnas.1113416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD, Betensky RA, Louis DN, Iafrate AJ. Mosaic Amplification of Multiple Receptor Tyrosine Kinase Genes in Glioblastoma. Cancer Cell. 2011;20(6):810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- [97].Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, Furnari FB, White FM. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc. Natl. Acad. Sci. U S A. 2007;104(31):12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jun HJ, Acquaviva J, Chi D, Lessard J, Zhu H, Woolfenden S, Bronson RT, Pfannl R, White F, Housman DE, Iyer L, Whittaker CA, Boskovitz A, Raval A, Charest A. Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene. 2011:1–12. doi: 10.1038/onc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Eimer S, Belaud-Rotureau MA, Airiau K, Jeanneteau M, Laharanne E, Veron N, Vital A, Loiseau H, Merlio JP, Belloc F. Autophagy inhibition cooperates with erlotinib to induce glioblastoma cell death. Cancer Biol. Ther. 2011;11(12):1017–1027. doi: 10.4161/cbt.11.12.15693. [DOI] [PubMed] [Google Scholar]
- [100].Fan QW, Weiss WA. Autophagy and Akt promote survival in glioma. Autophagy. 2011;7(5):536–538. doi: 10.4161/auto.7.5.14779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, Haas-Kogan D, James CD, Oakes SA, Debnath J, Shokat KM, Weiss WA. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci. Signal. 2010;3(147):ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pu YS, Hour TC, Chuang SE, Cheng AL, Lai MK, Kuo ML. Interleukin-6 is responsible for drug resistance and anti-apoptotic effects in prostatic cancer cells. Prostate. 2004;60(2):120–129. doi: 10.1002/pros.20057. [DOI] [PubMed] [Google Scholar]
- [103].Jin X, Yin J, Kim SH, Sohn YW, Beck S, Lim YC, Nam DH, Choi YJ, Kim H. EGFR-AKT-Smad Signaling Promotes Formation of Glioma Stem-like Cells and Tumor Angiogenesis by ID3-Driven Cytokine Induction. Cancer Res. 2011;71(22):7125–7134. doi: 10.1158/0008-5472.CAN-11-1330. [DOI] [PubMed] [Google Scholar]
- [104].Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- [105].Sanchez-Garcia I, Vicente-Duenas C, Cobaleda C. The theoretical basis of cancer-stem-cell-based therapeutics of cancer: can it be put into practice? Bioessays. 2007;29(12):1269–1280. doi: 10.1002/bies.20679. [DOI] [PubMed] [Google Scholar]
- [106].Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143(3):355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gong X, Schwartz PH, Linskey ME, Bota DA. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 76(13):1126–1134. doi: 10.1212/WNL.0b013e318212a89f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, Shou W, Hambardzumyan D, Lee J, Hjelmeland AB, Sloan AE, Bredel M, Stark GR, Rich JN, Bao S. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19(4):498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Heimberger AB, Kong LY, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Wei J, Qiao W, Schmittling RJ, Archer GE, Sampson JH, Hiraoka N, Priebe W, Fuller GN, Sawaya R. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin. Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- [110].Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- [111].Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26(17):2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- [112].Dalton WS. The tumor microenvironment as a determinant of drug response and resistance. Drug. Resist. Updat. 1999;2(5):285–288. doi: 10.1054/drup.1999.0097. [DOI] [PubMed] [Google Scholar]
- [113].Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, Kenner L, Sordella R. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc. Natl. Acad. Sci. U S A. 2010;107(35):15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]