Abstract
Food restriction and hypoinsulinemia can affect the synthesis, turnover and receptor function of serotonin (5-HT) in brain. This study explored the effects of food restriction and streptozotocin treatment on behavioral effects related to 5-HT1A (8-OH-DPAT) and 5-HT2A (DOI) receptor activation. Lower lip retraction and flat body posture (8-OH-DPAT) and head twitching (DOI) were measured in rats during free feeding, food restriction, after treatment with streptozotocin, and finally after insulin replacement. 8-OH-DPAT induced lower lip retraction and flat body posture whereas DOI induced head twitching. One week of food restriction (10 g/day) decreased 8-OH-DPAT induced lower lip retraction, 8-OH-DPAT induced flat body posture, and DOI-induced head twitching. Subsequently, one week of free access to food restored sensitivity to 8-OH-DPAT and DOI-induced behavioral effects. Finally, one week after streptozotocin, 8-OH-DPAT induced flat body posture and DOI-induced head twitching were markedly reduced whereas 8-OH-DPAT induced lower lip retraction was unchanged. One week of insulin replacement restored sensitivity to 8-OH-DPAT and DOI-induced behavioral effects. These results show that modest food restriction or experimentally-induced diabetes can profoundly affect sensitivity to drugs acting at 5-HT1A or 5-HT2A receptors; these results could be relevant to understanding the comorbidity of depression and diabetes.
Introduction
Serotonin (5-HT) plays a major role in regulating a wide variety of physiologic and behavioral processes including food intake; conversely, nutritional status can regulate the activity of 5-HT systems. For example, 5-HT neurons in the median raphe nucleus modulate ingestive behavior (Wirtshafter, 2001); in normal animals stimulation of 5-HT2C or 5-HT1B receptors can cause hypophagia whereas stimulation of 5-HT1A and 5-HT2B receptors can cause hyperphagia (De Vry and Schreiber, 2000). On the other hand, food restriction can decrease 5-HT concentration and rate of synthesis in brain (Haleem and Haider, 1996; Haider and Haleem, 2000), down-regulate the density of 5-HT transporters in frontal cortex (Huether et al., 1997), and decrease the expression of 5-HT1B receptors in hypothalamus (Gur et al., 2003). The functional consequences of these changes in 5-HT neurotransmission are not fully understood, although diet-induced changes in sensitivity of 5-HT systems in brain could have important implications for the therapeutic use of many drugs.
Diabetics have a higher prevalence of major depression and depressive symptoms, as compared to non-diabetics (Anderson et al., 2001), and experimentally-induced diabetes can dramatically alter sensitivity to drugs acting on certain monoamine transporters and receptors (e.g., dopamine [Sevak et al., 2007]). Indeed, the comorbidity of depression and diabetes could be related to the same underlying changes in dopaminergic and other neurotransmitter systems. Given the importance of 5-HT in the pathogenesis and treatment of various psychiatric disorders, it might also be the case that sensitivity to drugs acting on 5-HT systems would be similarly altered by diabetes. While in rats streptozotocin-induced diabetes increases cortical 5-HT2A receptor density (Sumiyoshi et al., 1997), sensitivity to DOI (a 5-HT2A/2C receptor agonist) induced wet-dog shakes is not changed (Amano et al., 2007). On the other hand, experimentally-induced diabetes reportedly attenuates the antidepressant-like effects of the 5-HT1A receptor agonist 8-OH-DPAT in the mouse tail suspension test (Miyata et al., 2004a). To further study the relationship among food intake, insulin status, and sensitivity of monoaminergic systems, this experiment examined sensitivity to the behavioral effects of direct-acting 5-HT receptor agonists in rats under four conditions: free-feeding, food restricted, diabetic, and diabetic with insulin replacement.
Methods
Subjects
Eleven adult male Sprague-Dawley rats (Harlan, Indianapolis, Indiana, USA) were housed individually on a 12/12-h light/dark cycle (experiments were conducted during the light period) with free access to water in the home cage. Except for one week of limited access (10 g/day) to food, all animals had free access to food in the home cage. All animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC, USA).
Experimental design
Activation of certain subtypes (e.g., 5-HT1A) of 5-HT receptors can produce a characteristic behavioral response comprising lower lip retraction, flat body posture, and forepaw treading; the current study examined lower lip retraction and flat body posture after administration of the 5-HT1A receptor agonist 8-OH-DPAT. Activation of another 5-HT receptor subtype (5-HT2A) can increase head twitching; the current study also examined head twitching after the administration of the 5-HT2A receptor agonist DOI. Tests were conducted in the home cage and all rats were tested under all conditions. For 8-OH-DPAT a cumulative dosing procedure was used with rats receiving vehicle (i.p.) prior to the first 10-min cycle followed by increasing doses of drug (i.p.) every 10 min, with the cumulative dose increasing by 0.5 log unit per injection. Lower lip retraction (i.e., when the lower incisors were visible [Berendsen et al., 1989]) and flat body posture (when the entire ventral surface of the body was in contact with the cage floor [Colpaert et al., 1989]) were monitored and recorded as present or absent during the last min of each 10-min cycle. Three doses of DOI (0.32, 1.0, and 3.2 mg/kg) were studied on three consecutive days with the order of testing randomized among animals. For a test, rats received vehicle (i.p.) and head twitching was counted for 20 min; rats then received an injection of DOI and head twitching was counted for another 20 min. A head twitch was defined as spontaneous (not stimulated), irregularly occurring horizontal head movement, resembling a strong pinna reflex and involving the whole head (Corne et al., 1963).
After these effects of 8-OH-DPAT (day 1) and DOI (days 2–4) were measured, access to food was restricted to 10 g/day for 7 days; on days 12–15 the same tests were repeated with the same drugs in all food-restricted rats. To confirm that any changes in sensitivity to 8-OH-DPAT and DOI were due to food restriction, free-feeding was resumed and after 7 days the same tests were repeated with 8-OH-DPAT and DOI.
Next all rats were rendered diabetic by an i.p. injection of 50 mg/kg of streptozotocin. One week after the administration of streptozotocin (day 8–11), the same tests were conduced with 8-OH-DPAT and DOI. The next day (12 days after the administration of streptozotocin), two insulin pellets (i.e., Linplant) were surgically implanted (s.c.) under ketamine (36 mg/kg i.m.) and xylazine (4.8 mg/kg i.m.) anesthesia. Beginning 10 days after the insertion of Linplant pellets (i.e., 22 days after streptozotocin), the same drugs were tested a final time over four consecutive days. Body weights were measured daily throughout the study.
Drugs
(+)- 8-Hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT) and streptozotocin were purchased from Sigma-Aldrich (St. Louis, MO). (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride (DOI) was provided by the Research Technology Branch, National Institute of Drug Abuse (Rockville, MD). Linplant pellets (i.e., sustained-release insulin implants) were purchased from LinShin Canada, Inc (Scarborough, Ontario). According to the manufacturer, each Linplant releases 2 U of insulin over 24 h for at least 40 days (Wang, 1991). Except for ketamine hydrochloride and xylazine hydrochloride, both of which were purchased as commercially-available solutions (Vetus Animal Health, Burns Veterinary Supply Inc., Westbury, NY), drugs were dissolved in sterile 0.9% saline. Streptozotocin solutions were prepared immediately before administration. Injection volumes were 0.2–1.0 ml.
Data analyses
For studies with 8-OH-DPAT, dose-response functions were determined for the percentage of rats showing lower-lip retraction or flat body posture after each dose. ED50 values were estimated using liner regression and 95% confidence limits were calculated. The data were further analyzed by fitting straight lines to the individual dose-response data by means of GraphPad Prism version 5.0 for Windows (GraphPad Software, Inc., San Diego, CA), using the following equation: effect = slope × log(dose) + intercept. For these analyses, only the linear portion of the dose-effect curve was used, defined by doses producing 25 to 75% of the maximum possible effect, including not more than one dose producing less than 25% and not more than one dose producing more than 75% of the maximum possible effect. Other doses were excluded from the analyses. The slopes and intercepts of dose-response functions for each condition were compared with an F-ratio test using GraphPad; two conditions were considered to be significantly different when the data sets could not be described with a single line.
For studies with DOI, data were analyzed with separate two-way analyses of variance (ANOVA) for repeated measures with one factor comprising treatment (control, food restriction, and food replacement, streptozotocin treatment, and insulin replacement) and a second factor comprising dose (p < 0.05). A post-hoc Tukey-Kramer test was used to examine significant differences among treatments (p < 0.05). Body weights were analyzed using a one-factor (days after streptozotocin) ANOVA, followed by Dunnett’s 2-sided tests.
Results
Effects of food restriction on 8-OH-DPAT and DOI-induced behavioral effects
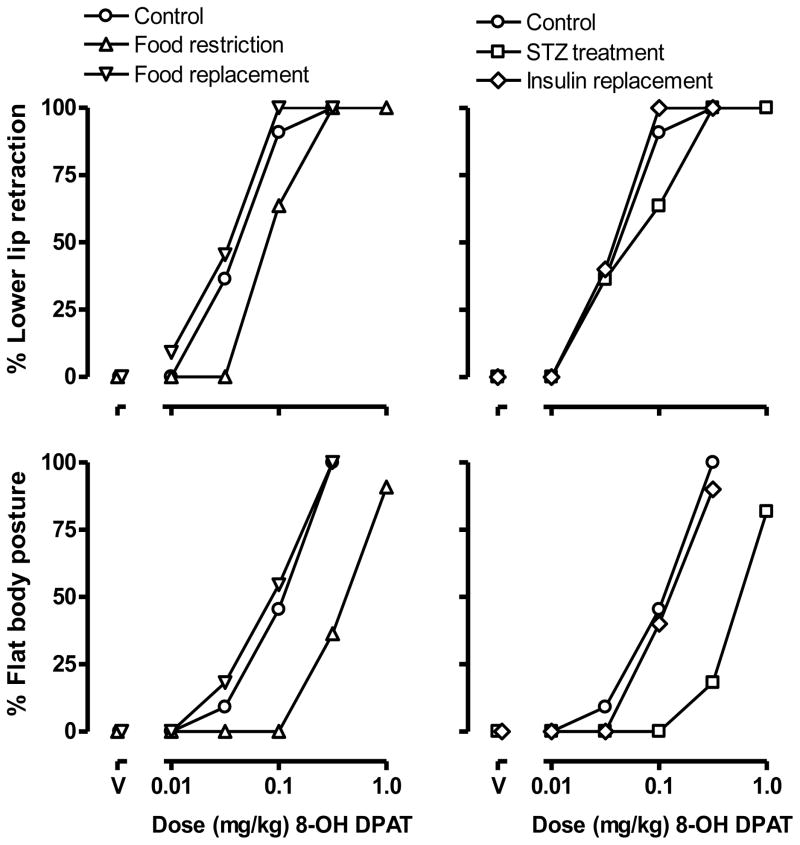
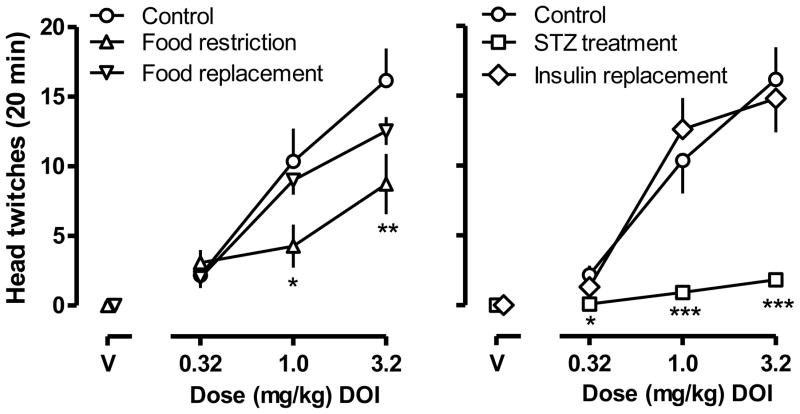
8-OH-DPAT dose-dependently increased the incidence of lower-lip retraction with all 11 rats showing lower lip retraction after receiving a dose of 0.32 mg/kg (ED50 [95% CL] = 0.053 mg/kg [0.027, 0.081]; circles, upper panels, Fig. 1). 8-OH-DPAT also dose-dependently increased the percentage of rats showing flat body posture (ED50 = 0.120 mg/kg [0.077, 0.162]; circles, lower panels, Fig. 1). DOI dose-dependently increased the frequency of head twitching with doses of 0.32, 1.0 and 3.2 mg/kg producing averages of 2.2 ± 0.6, 10.4 ± 2.4, and 16.2 ± 2.3 twitches, respectively, in a 20-min observation period (circles, Fig. 2).
Fig. 1.
The percentage of rats (n=11) showing lower lip retraction (upper panels) and flat body posture (lower panels) after receiving 8-OH-DPAT, under five different sequential conditions: 1) control conditions (i.e., during free access to food and prior to food restriction; circles); 2) during food restriction (10 g/day; triangles); 3) after resumption of free access to food (inverted triangles); 4) after receiving 50 mg/kg of streptozotocin (squares); and 5) after insulin replacement (diamonds). Abscissae: dose in mg/kg body weight. “V” indicates vehicle.
Fig. 2.
DOI-induced head twitching in the same 11 rats (Fig. 1) under the same five conditions: 1) control conditions (i.e., during free access to food and prior to food restriction; circles); 2) during food restriction (10 g/day; triangles); 3) after resumption of free access to food (inverted triangles); 4) after receiving 50 mg/kg of streptozotocin (squares); and 5) after insulin replacement (diamonds). Ordinate: average number of twitches in a 20-min observation period ± SEM. Abscissae: dose in mg/kg body weight. “V” indicates vehicle. *, p<0.05, **, p<0.01, ***, p<0.001, all compared with vehicle control.
One week of food restriction significantly decreased the sensitivity of rats to 8-OH-DPAT induced lower lip retraction (ED50 = 0.097 mg/kg [0.059, 0.136]; triangles, upper left panel, Fig. 1). The linear portion of the dose response curves for 8-OH-DPAT induced lower lip retraction under control (i.e., free feeding) and food restriction conditions could be fitted with a common slope but different intercepts, indicating that food restriction changed this effect of 8-OH-DPAT. Food restriction also significantly decreased the potency of 8-OH-DPAT to induce flat body posture, shifting the dose-response curve 3.5-fold rightward (ED50 = 0.411 mg/kg [0.287, 0.535]; triangles, lower left panel, Fig. 1). The linear portion of the dose response curves for 8-OH-DPAT induced flat body posture under control and food restriction conditions could be fitted with a common slope but different intercepts, indicating that food restriction shifted the 8-OH-DPAT dose response curve significantly to the right. Food restriction also significantly decreased the frequency of head twitching, with 1.0 mg/kg DOI inducing 4.3 ± 1.5 twitches (F (2,20)=4.26, p<0.05) and 3.2 mg/kg inducing 8.7 ± 2.2 twitches (F (2,20)=6.83, p<0.01) within a 20 min observation period (triangles, left panel, Fig. 2). Two-way ANOVA revealed a significant main effect of treatment (F[2, 20] = 6.81, p<0.01), a significant main effect of dose (F[3, 30] = 46.09, p<0.0001), and a significant interaction between treatment and dose (F[6,60] = 3.97, p<0.005).
After one week of free feeding rats showed normal sensitivity to 8-OH-DPAT induced lower lip retraction (ED50 = 0.041 mg/kg [0.029, 0.053]; inverted triangles, upper left panel, Fig. 1) and 8-OH-DPAT induced flat body posture (ED50 = 0.094 mg/kg [0.051, 0.137]; inverted triangles, lower left panel, Fig. 1). In each case, the linear portion of the dose-response curves for 8-OH-DPAT induced lower lip retraction and flat body posture, under control and food replacement conditions, could be fitted with a common slope and a common intercept, indicating that the dose response curves were not significantly different. Sensitivity to DOI-induced head twitching also was restored after one week of free access to food (p>0.05; inverted triangles, left panel, Fig. 2).
Effects of streptozotocin on 8-OH-DPAT and DOI-induced behavioral effects
One week after the administration of streptozotocin the sensitivity of rats to 8-OH-DPAT induced lower lip retraction was not changed (ED50 = 0.083 mg/kg [0.038, 0.129]; squares, upper right panel, Fig. 1). The linear portion of the dose-response curves of 8-OH-DPAT induced lower lip retraction under control and streptozotocin treatment conditions could be fitted with a common slope and a common intercept, indicating that streptozotocin did not change this effect of 8-OH-DPAT. However, the potency of 8-OH-DPAT to induce flat body posture decreased after streptozotocin treatment with the dose-response curve shifted 4-fold rightward (ED50 = 0.480 mg/kg [0.374, 0.585]; squares, lower right panel, Fig. 1). The linear portion of the dose response curves for 8-OH-DPAT induced flat body posture under control and streptozotocin treatment conditions could be fitted with a common slope but a different intercept, indicating that food restriction shifted the curve significantly to the right. Streptozotocin markedly decreased DOI-induce head twitching with a dose of 1.0 mg/kg inducing 0.91 ± 0.31 twitches (F (2,20)=11.03, p<0.001) and 3.2 mg/kg inducing 1.82 ± 0.54 twitches (F (2,20)=6.83, p<0.0001) within a 20 min observation period (squares, right panel, Fig. 2). Two-way ANOVA revealed a significant main effect of treatment (F[2, 20] = 27.72, p<0.0001), a significant main effect of dose (F[3, 30] = 51.39, p<0.0001), and a significant interaction between treatment and dose (F[6,60] = 9.58, p<0.0001).
Ten days after implantation of insulin pellets, rats showed normal sensitivity to 8-OH-DPAT induced lower lip retraction (ED50 = 0.041 mg/kg [0.029, 0.053]; diamonds, upper right panel, Fig. 1) and 8-OH-DPAT induced flat body posture (ED50 = 0.125 mg/kg [0.085, 0.164]; diamonds, lower right panel, Fig. 1). In each case, the linear portion of the dose-response curves for 8-OH-DPAT induced lower lip retraction and flat body posture, under control and insulin replacement conditions, could be fitted with a common slope and a common intercept, indicating that the dose response curves were not significantly different. Sensitivity of streptozotocin-treated rats to DOI-induced head twitching also was restored by insulin replacement (p>0.05; diamonds, right panel, Fig. 2).
Streptozotocin significantly decreased body weight over days (F[22,220] =3.17; p<0.0001; Fig. 3). After insulin replacement body weight increased progressively across days. For example, 8 days after insulin replacement (i.e., 20 days after streptozotocin), average body weight (378.3 ± 6.8 g) was not different from the average body weight before streptozotocin (i.e., day 0; 379.7 ± 4.2 g). Rats continued to gain body weight for the remainder of the study (Fig. 3).
Fig. 3.
Body weight (g) in the same 11 rats (Figs. 1 and 2) that received streptozotocin on day 0 and insulin replacement on day 12; rats had free access to food throughout this component of the study. Each data point represents the mean ± SEM for 11 rats. *, p<0.05 compared with day 0.
Discussion
Limited access to food or experimentally-induced diabetes can dramatically alter the behavioral and neurochemical effects of drugs acting on dopaminergic systems (e.g., Sevak et al., 2007) and the current study investigated whether food restriction or diabetes also modifies drugs acting on 5-HT systems. The major finding from this study is that results obtained with 5-HT receptor agonists are similar to those reported for dopamine receptor agonists insofar as both food restriction and experimentally-induced diabetes markedly decrease sensitivity to drugs acting selectively at either at 5-HT1A or 5-HT2A receptors. Free access to food and insulin replacement, respectively, restored normal sensitivity to both 5-HT receptor agonists, suggesting that nutrition and insulin status might be especially important in modulating the activity of 5-HT systems and, therefore, in modifying sensitivity to drugs acting on those systems.
Activation of 5-HT receptors, by various different mechanisms, can produce a characteristic behavioral response comprising lower lip retraction, flat body posture, and forepaw treading; these effects are used routinely to study drugs acting on 5-HT systems, including direct-acting receptor agonists. In particular, drugs with agonist activity at 5-HT1A receptors reliably produce lower lip retraction, which is thought to be mediated by presynaptic 5-HT1A receptors (Berendsen et al., 1989, 1994), and flat body posture, which is thought to be mediated by postsynaptic 5-HT1A receptors (Tricklebank et al., 1984). That food restriction appears to attenuate 8-OH-DPAT induced flat body posture more than 8-OH-DPAT induced lower lip retraction suggests that food restriction is more effective in modifying the sensitivity of rats to drugs acting at postsynaptic as compared to presynaptic 5-HT1A receptors. Although 8-OH-DPAT also has agonist activity at other (e.g., 5-HT7) receptors (Hedlund et al., 2004), there is no clear evidence to suggest that 8-OH-DPAT induced lower lip retraction or flat body posture are mediated by activation of those receptors. In an earlier study, 24 of hr food deprivation did not affect either binding to or sensitivity of presynaptic 5-HT1A receptors (Chaouloff et al., 1997). Thus, diet-induced changes in 5-HT receptor sensitivity might occur selectively for different subtypes of 5-HT receptors. It is clear from this study that just one week of food restriction markedly decreases the sensitivity of rats to behavioral effects that are presumed to be mediated by 5-HT1A receptors, although it is not clear whether these diet-induced changes are due to altered receptor number, sensitivity, second-message signaling, or to other factors.
Activation of another subtype (5-HT2A) of 5-HT receptor can increase head twitching and this behavioral effect also is used widely to study the actions of drugs acting at 5-HT receptors. DOI reliably increased head twitching in normal rats and this effect was markedly decreased by food restriction and nearly eliminated by the administration of streptozotocin. Sensitivity to DOI-induced head twitching was restored by free access to food and by insulin replacement, respectively. These results provide still another example of the rapid and marked changes in sensitivity to drug-induced behavioral effects that can occur after modest food restriction or after the experimental induction of diabetes. To the extent that therapeutic or abuse-related effects of drugs are mediated by 5-HT2A receptors, these data suggest that nutritional factors could play an important role in determining sensitivity to and effectiveness of some drugs.
Streptozotocin has been used extensively to induce diabetes and in some studies to examine the changes that occur in sensitivity to drugs acting at specific neurotransmitter receptors or transporters. A single injection of 50 mg/kg streptozotocin reliably eliminates insulin-secreting pancreatic β-islet cells, thereby increasing blood glucose, decreasing body weight, and modifying the behavioral and neurochemical effects of drugs acting on dopamine systems (Galici et al., 2003; Sevak et al., 2007). Similarly in the current study, streptozotocin markedly decreased body weight that recovered rapidly after insulin replacement. In addition to well-documented changes in dopamine systems that occur after streptozotocin treatment, experimentally-induced diabetes also can lead to long-lasting changes in the activity of 5-HT systems. For example, 5-HT1A receptor agonists have antidepressant-like effects in several animal models (Cervo and Samanin, 1987; McGrath and Norman, 1999); however, in mice, the antidepressant-like effects of 8-OH-DPAT are markedly attenuated by streptozotocin (Miyata et al., 2004a). Moreover, DOI is less effective in producing head twitching in mice that are treated with streptozotocin (Miyata et al., 2004b). Interestingly, the density and affinity of 5-HT2A receptors are decreased in diabetic mice and can be restored by insulin replacement (Sumiyoshi et al., 1997; Jackson and Paulose, 1999). In the current study, both 8-OH-DPAT induced flat body posture and DOI induced head twitching were markedly reduced after streptozotocin treatment. One determinant of brain tryptophan content is the concentration of circulating insulin, with increasing concentrations of insulin decreasing plasma concentrations of large neutral amino acids that normally compete with tryptophan for uptake into the brain (Curzon and Fernando, 1977). Like food restriction, streptozotocin can decrease the threshold for lateral hypothalamic self administration (Carr, 1996) and it has been proposed that hypoinsulinemia (food restriction or experimentally-induced diabetes) can modulate monoamine transporter gene expression (Carr et al., 2000). For example, food-restricted rats are less sensitive than control rats to insulin (Alonso et al., 2005). Food restriction also decreases insulin secretion (Vuguin et al., 2001) and reduces plasma insulin-like growth factor-I (IGF-I) in rats (Monaco and Donovan, 1997; Takenaka et al., 2000). Thus, while it is not clear what mechanism(s) mediates changes in 5-HT function under different nutritional conditions, it is clear that at least some of these changes occur rapidly, are very robust, and are reversible. Given that one behavioral effect of 8-OH-DPAT was decreased (flat body posture) by streptozotocin while another effect remained essentially unchanged (lower lip retraction), it appears unlikely that pharmacokinetic factors play a significant role in these results. It also appears unlikely that changes in general arousal contribute to these effects since experimentally-induced diabetes often decreases whereas food restriction can increase or have no effect on locomotion (e.g., Sevak et al., 2007, in press).
In summary, restricted access to food or experimentally-induced diabetes can attenuate the sensitivity of rats to the behavioral effects of drugs acting (agonists) directly at 5-HT1A or 5-HT2A receptors. Together with previous data showing similar decreased sensitivity to drugs acting at dopamine receptors, these results suggest that relatively modest changes in nutrition or insulin status could have profound effects on the effectiveness of drugs acting at monoaminergic receptors as well as other sites (e.g., transporters). Given the high co-morbidity of diabetes and depression (de Groot et al., 2001; Knol et al., 2006; Barnard et al., 2006; Ali, et al., 2006) and the apparent functional impact of food restriction on antidepressant effects of drugs in animals (Soubrié et al., 1989), it will be important to better characterize how underlying changes in neurotransmission, in general, and receptor sensitivity, in particular, might contribute to the activity of antidepressants and other psychotherapeutics.
Acknowledgments
The authors thank W. Koek for helpful suggestions and C. Cruz, D. Mojica and O. Dominguez for expert technical assistance.
Sponsorship: This work was supported, in part, by a USPHS Senior Scientist Award (K05 DA17918) to CPF
References
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Alonso A, Fernandez Y, Fernandez R, Ordonez P, Moreno M, Diaz F, Patterson AM, Gonzalez C. Effect of food restriction on the insulin signalling pathway in rat skeletal muscle and adipose tissue. J Nutr Biochem. 2005;16:602–609. doi: 10.1016/j.jnutbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Amano M, Suemaru K, Cui R, Umeda Y, Li B, Gomita Y, Kawasaki H, Araki H. Effects of Physical and Psychological Stress on 5-HT2A Receptor-mediated Wet- dog Shake Responses in Streptozotocin-induced Diabetic Rats. Acta Med Okayama. 2007;61:205–212. doi: 10.18926/AMO/32870. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med. 2006;23:445–448. doi: 10.1111/j.1464-5491.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Jenck F, Broekkamp CL. Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Bourgondien FG, Broekkamp CL. Role of dorsal and median raphe nuclei in lower lip retraction in rats. Eur J Pharmacol. 1994;263:315–318. doi: 10.1016/0014-2999(94)90728-5. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim G, Cabeza de Vaca S. Hypoinsulinemia may mediate the lowering of self-stimulation thresholds by food restriction and streptozotocin-induced diabetes. Brain Res. 2000;863:160–168. doi: 10.1016/s0006-8993(00)02143-0. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Potential antidepressant properties of 8-hydroxy-2-(di-n- propylamino)tetralin, a selective serotonin1A receptor agonist. Eur J Pharmacol. 1987;144:223–229. doi: 10.1016/0014-2999(87)90523-1. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Aquerre S, Hay M, Mormede P. Effects of food deprivation on midbrain 5-HT1A autoreceptors in Lewis and SHR rats. Neuropharmacology. 1997;36:483–488. doi: 10.1016/s0028-3908(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Koek W, Lategan A. 5-Hydroxytryptophan-induced flat body posture in the rat: antagonism by ritanserin and potentiation after 5,7-dihydroxytryptamine. Eur J Pharmacol. 1989;169:175–178. doi: 10.1016/0014-2999(89)90830-3. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G, Fernando JC. Drugs altering insulin secretion: effects on plasma and brain concentrations of aromatic amino acids and on brain 5-hydroxytryptamine turnover. Br J Pharmacol. 1977;60:401–408. doi: 10.1111/j.1476-5381.1977.tb07515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24:341–353. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Galici R, Galli A, Jones DJ, Sanchez TA, Saunders C, Frazer A, Gould GG, Lin RZ, France CP. Selective decreases in amphetamine self-administration and regulation of dopamine transporter function in diabetic rats. Neuroendocrinology. 2003;77:132–140. doi: 10.1159/000068650. [DOI] [PubMed] [Google Scholar]
- Gur E, Newman ME, Avraham Y, Dremencov E, Berry EM. The differential effects of food restriction on 5-HT1A and 5-HT1B receptor mediated control of serotonergic transmission in the hippocampus and hypothalamus of rats. Nutr Neurosci. 2003;6:169–175. doi: 10.1080/1028415031000115936. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Haider S. Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport. 1996;7:1153–1156. doi: 10.1097/00001756-199604260-00011. [DOI] [PubMed] [Google Scholar]
- Haider S, Haleem DJ. Decreases of brain serotonin following a food restriction schedule of 4 weeks in male and female rats. Med Sci Monit. 2000;6:1061–1067. [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH- DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Huether G, Zhou D, Schmidt S, Wiltfang J, Ruther E. Long-term food restriction down-regulates the density of serotonin transporters in the rat frontal cortex. Biol Psychiatry. 1997;41:1174–1180. doi: 10.1016/s0006-3223(96)00265-x. [DOI] [PubMed] [Google Scholar]
- Jackson J, Paulose CS. Enhancement of [m-methoxy 3H]MDL100907 binding to 5HT2A receptors in cerebral cortex and brain stem of streptozotocin induced diabetic rats. Mol Cell Biochem. 1999;199:81–85. doi: 10.1023/a:1006938713276. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- McGrath C, Norman TR. (+)-S-20499 -- a potential antidepressant? A behavioural and neurochemical investigation in the olfactory bulbectomised rat. Eur Neuropsychopharmacol. 1999;9:21–27. doi: 10.1016/s0924-977x(97)00103-x. [DOI] [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J. Diabetes attenuates the antidepressant-like effect mediated by the activation of 5-HT1A receptor in the mouse tail suspension test. Neuropsychopharmacology. 2004a;29:461–469. doi: 10.1038/sj.npp.1300354. [DOI] [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J. Diabetes inhibits the DOI-induced head-twitch response in mice. Psychopharmacology (Berl) 2004b;177:224–229. doi: 10.1007/s00213-004-1942-3. [DOI] [PubMed] [Google Scholar]
- Monaco MH, Donovan SM. Moderate food restriction reduces serum IGF-I and alters circulating IGF-binding protein profiles in lactating rats. J Endocrinol. 1997;152:303–316. doi: 10.1677/joe.0.1520303. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Galli A, France CP. Insulin replacement restores the behavioral effects of quinpirole and raclopride in streptozotocin-treated rats. J Pharmacol Exp Ther. 2007;320:1216–1223. doi: 10.1124/jpet.106.115600. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs. Eur J Pharmacol. doi: 10.1016/j.ejphar.2008.07.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrié P, Martin P, Massol J, Gaudel G. Attenuation of response to antidepressants in animals induced by reduction in food intake. Psych Res. 1989;27:149–159. doi: 10.1016/0165-1781(89)90130-3. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Ichikawa J, Meltzer HY. The effect of streptozotocin-induced diabetes on dopamine2, serotonin1A and serotonin2A receptors in the rat brain. Neuropsychopharmacology. 1997;16:183–190. doi: 10.1016/S0893-133X(96)00185-6. [DOI] [PubMed] [Google Scholar]
- Takenaka A, Oki N, Takahashi SI, Noguchi T. Dietary restriction of single essential amino acids reduces plasma insulin-like growth factor-I (IGF-I) but does not affect plasma IGF-binding protein-1 in rats. J Nutr. 2000;130:2910–2914. doi: 10.1093/jn/130.12.2910. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- Vuguin P, Ma X, Yang X, Surana M, Liu B, Barzilai N. Food deprivation limits insulin secretory capacity in postpubertal rats. Pediatr Res. 2001;49:468–473. doi: 10.1203/00006450-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Wang PY. Sustained-release implants for insulin delivery. In: Pickup JC, editor. Biotechnology of Insulin Therapy. Blackwell Scientific; London, UK: 1991. pp. 42–74. [Google Scholar]
- Wirtshafter D. The control of ingestive behavior by the median raphe nucleus. Appetite. 2001;36:99–105. doi: 10.1006/appe.2000.0373. [DOI] [PubMed] [Google Scholar]