Abstract
In view of the well-known phenomenon of trophoblast immune privilege, trophoblast stem cells (TSCs) might be expected to be immune privileged, which could be of interest for cell or gene therapies. Yet in the ectopic sites tested so far, TSC transplants fail to show noticeable immune privilege and seem to lack physiological support. However, we show here that after portal venous injection, green fluorescent protein (GFP)-labeled TSCs survive for several months in the livers of allogeneic female but not male mice. Gonadectomy experiments revealed that this survival does not require the presence of ovarian hormones but does require the absence of testicular factors. By contrast, GFP-labeled allogeneic embryonic stem cells (ESCs) are reliably rejected; however, these same ESCs survive when mixed with unlabeled TSCs. The protective effect does not require immunological compatibility between ESCs and TSCs. Tumors were not observed in animals with either successfully engrafted TSCs or coinjected ESCs. We conclude that in a suitable hormonal context and location, ectopic TSCs can exhibit and confer immune privilege. These findings suggest applications in cell and gene therapy as well as a new model for studying trophoblast immunology and physiology.
Keywords: Trophoblast, Stem cells, Cell therapy, Transplantation, Immune privilege, Mice
INTRODUCTION
The isolation of mouse trophoblast stem cells (TSCs) (30) and the in vitro differentiation of human embryonic stem cells (ESCs) into trophoblast cells (32) seemed to open fascinating prospects for using the phenomenon of trophoblast immune privilege (18,25,31) in heterologous transplantation and cell therapy (29). However, attempts to transplant wild-type TSCs or their in vitro-differentiated derivatives have not been promising. When injected subcutaneously into nonpregnant females, syngeneic TSCs differentiated within 5–10 days into giant trophoblast cells that rapidly degenerated thereafter (10). Following injection into the tail veins of nonpregnant females, allogeneic TSCs and trophoblast cells were cleared from host lungs by natural killer cells within a day (10). TSCs subcutaneously injected at high dosage into male nude mice formed giant trophoblast and spongiotrophoblast cells that were resorbed within 2 weeks (20). Thus, in the ectopic sites tested so far, TSCs/trophoblast cell transplants fail.
Research in sheep has shown that the uterus becomes moderately immune privileged under the influence of progesterone (17); hence, the previous failure to achieve TSC engraftment may indicate that synergistic interaction of trophoblastic and uterine immune mechanisms is required for the survival of the trophoblast and hence the fetus. Based on this notion, we surmised that a partially immune-privileged site other than the uterus might also allow TSCs to survive and to exert a protective action upon other nearby cells, enabling the latter to survive in locations where they normally could not. Furthermore, because trophoblast cells normally occur only in females, we also wondered whether a female hormonal environment is one of the physiological requirements for ectopic TSC survival. Here, we show that (i) the liver provides a physiological and immunological environment conducive for prolonged TSC survival, which allowed us to further demonstrate (ii) a surprising lack of dependence of TSC survival on systemically provided ovarian hormones, (iii) a striking susceptibility of transplanted TSCs to testicular factors, and (iv) a strong immunoprotective effect of injected TSCs on coinjected ESCs. Together, these findings provide a framework for using transplanted TSCs and their derivatives in cell and gene therapy and as a model to study trophoblast immunology and physiology.
MATERIALS AND METHODS
Cell Lines
We used three ESC lines: a strain 129 line expressing GFP under control of the Hprt promoter (19); a C57Bl/6 line purchased from the ATCC (SCRC-1002); and a CBA line (21). The C57Bl/6 and CBA ES cells were labeled with GFP as described further below. The ESCs were maintained on mitomycin-treated primary mouse embryo fibroblasts in the presence of 1,000 U/ml LIF (ESGRO). We also utilized two TSC lines: an outbred (ICR) GFP-transgenic line (30) and a hybrid (C57Bl/6xBalb/c F1) line that was derived in our laboratory as described (30), except that we doubled the amount of FGF and added TGFb (11) during derivation and maintenance. In addition, the “rat ESC-like cells” (line B10) were cultured as described previously (13).
Labeling of Cells
Virus suspensions were produced using the GFP expressing lentiviral vector pFUGW (22) and tittered after serial dilution by counting the number of GFP-positive 293T cells. The ESCs or TSCs were seeded at moderate density in 60-mm plates and incubated over-night. Two hours before transduction, the medium was changed, and transductions were carried out for 24 h at a multiplicity of infection of ~1 in the presence of 8 µg/ml Polybrene (Sigma). The transduced cells where passaged at low density, and after 7–14 days the fluorescent colonies were picked, pooled, and further expanded for the injections.
Preparation of Cells for Injection
Through trypsinization and pipetting, the cells were thoroughly disaggregated into a mixture of predominantly single cells and some small aggregates (poorly disaggregated samples caused instantaneous death). The cells were counted with a hemocytometer, aliquoted into portions of ~1 million cells per 0.2 ml of PBS, and kept on ice until injection; under these conditions they survived for at least 4 h (by trypan blue exclusion), a time that was never exceeded. Immediately before injection, the cells were resuspended by gently flicking the tube.
Surgeries
All animal experiments were approved by the University Laboratory Animal Care Commission. The mice were purchased from the Jackson Laboratory and maintained under specified pathogen-free conditions with free access to food and water in a 0600 to 1800 h light cycle. The surgeries were performed under isofluorene anesthesia. For portal vein injections, the abdominal cavity was opened, cells were slowly infused using a 30-gauge syringe, and bleeding was prevented by briefly applying digital pressure. Ovarectomies and orchidectomies were performed after exteriorizing the gonads through small incisions and ligating the oviduct and vas deferens as well as prominent ovarian and testicular blood vessels.
Histology and Microscopy
Cryosections
The liver pieces were fixed in 4% paraformaldehyde (24 h, 4°C), soaked in 30% sucrose (48–72 h, 4°C), and then placed in OTC and frozen at −180°C for at least 24 h. Serial 10-µm sections were cut from calculated locations within each liver piece with a Tissue Tek II cryotome (Miles, Inc.). The sections were viewed with an Olympus IX 71 microscope outfitted with a wide GFP fluorescence filter (U-N31054).
Hematoxylin/Eosin (H/E) Staining
In order to preserve the integrity of the cryosectioned tissue throughout staining, a thin coating of 1% celloidin was applied for 5 min and allowed to dry, followed by standard H/E staining. All sections that are compared with each other were photographed at the same original microscopic magnification and are presented at the same final magnification.
Definition of Transplant Survival and Survival Index
The liver of each animal was cut into four quarters. Initially, one quarter was cryosectioned, resulting in about 50 cryosections separated by approximately even distances. If at least one of those cryosections showed an unambiguous patch of intact fluorescent cells, the transplant was scored as a survival and the remaining quarters were not studied. If, however, the first quarter did not reveal transplant survival, a further quarter was analyzed and so on. As a semiquantitative assessment of the survival, we determined a “survival index” as the percentage of slides containing one or more unambiguous patches of fluorescent cells.
RT-PCR
Total RNA was isolated with the TRIZOL (Invitrogen) procedure. After treatment with DNAse I, 2 µg of RNA were reverse-transcribed with random hexamers using the first-strand cDNA synthesis Superscript II from Invitrogen; control reactions excluded reverse transcriptase. Aliquots of the cDNA samples were subjected to PCR, using an annealing temperature of 60°C. The primers used and the resulting product sizes (in bp) are: Oct4 (gagggatggcatactgtggac, ggtgtaccccaaggtgatcc; 272); Nanog (tatcccagcatccattgcag, gtcctccccgaagttatggag; 252); Gata6 (gccgggagcaccagtaca, gtgacagttggcacaggacag; 419); Eomesodermin (cggcaaagcggacaataac, gttgtcccggaagcctttg; 361); placental lactogen (ctgcttccatccatactccaga, gacaactcggcacctcaaga; 410); Errbeta (ctccagcatctccaggaagag, cacttggggaccagatgagc; 464); Hprt (cagtcccagcgtcgtgattag, atccagcaggtcagcaaagaac; 229); Cdx2 (ctctcggagagcccaagtgtg, gcagtccctaggaagccaagtga; 162); FAS ligand (aaccccagtacaccctctgaaa, ggttccatatgtgtcttcccattc, 108).
RESULTS
Green Fluorescent Protein (GFP)-Labeled Allogeneic TSCs Survive Portal Vein (PV) Injection
In preliminary experiments, we first tested a randomly chosen, outbred TSC line derived from GFP-transgenic ICR mice (30) for its ability to survive PV injection into Balb/c mice (MHC haplotype H2k-d). When inspected ~1 month after injection, the livers of the TSC-injected mice all showed large patches of fluorescent cells (Table 1, group 1). Use of outbred cells, however, complicates the analysis of strain combinations. Therefore, in order to repeat these experiments with genetically defined TSCs, we derived a new TSC line (F1 TSCs) from C57Bl/6[H2k-b]xBalb/c F1 mice and labeled it with GFP by lentiviral transduction. Based on well-known specific features of mouse TSCs (30) and in direct comparison with ICR TSCs and other embryo stem cell lines, the identity of the new TSC line was verified through its morphology (Fig. 1A), molecular signature (Cdx2+Eomes+Errbeta+Oct4−Nanog−Gata6−), and the downregulation of TSC markers (Cdx2, Eomes, Errbeta) upon removal of FGF4 (Fig. 1B). In addition, the giant trophoblast differentiation marker placental lactogen was found both in the presence and absence of FGF, indicating background differentiation [comp. ref. (30)].
Table 1.
Summary of TSC and ESC Injection Experiments
| Group | Donor Cell Type |
Strains (Donor/Host) |
Host Gender |
Transplants Surviving |
|---|---|---|---|---|
| 1 | TSCs* | ICR*/Bc | F | 3/3 |
| 2 | TSCs* | B6xBc*/Bc | F | 5/5 |
| 3 | TSCs* | B6xBc*/CBA | F | 1/1 |
| 4 | TSCs* | B6xBc*/B6xBc | F | 3/3 |
| 5 | TSCs* | B6xBc*/B6xBc | M | 0/3† |
| 6 | TSCs* | B6xBc*/Bc | M | 0/4 |
| 7 | TSCs* | B6Bc*/Bc | F‡ | 4/4 |
| 8 | TSCs* | B6xBx*/Bc | M§ | 5/5 |
| 9 | ESCs* | 129*/Bc | F | 0/2 |
| 10 | ESCs* | B6*/Bc | F | 0/6¶ |
| 11 | ESCs* | CBA*/Bc | F | 0/5 |
| 12 | ESCs* | B6*/B6 | F | 3/3# |
| 13 | TSCs+ESCs* | B6xBc +B6*/Bc | F | 5/5† |
| 14 | TSCs+ESCs* | B6xBc+CBA*/Bc | F | 4/4† |
TSCs, ESC, or their 1:1 mixture (always a total of 106 cells) were PV injected, and ~1 month later the host livers were cryosectioned and viewed and photographed under epifluorescence. H2 haplotypes of mouse strains: strain C57B1/6 (abbreviated as B6), H2b; strain Balb/c (abbreviated as Bc), H2d; strain CBA, H2k; strain 129, H2b′; strain ICR is outbred.
Injected cells carrying the GFP label.
One slide (out of 20) in one animal contained one trace of fluorescence labeling.
Ovarectomized.
Castrated.
Excludes one animal with externally visible (fluorescent) liver tumor.
Two of three animals had an externally visible liver tumor.
Figure 1.
Characteristics of F1 TSCs and comparison with previously published “rat ESC-like cells” (13). (A) Bright field photo (upper) and fluorescent image (lower) of a colony of GFP-labeled TSCs (C57Bl/6xBalb/c F1). Original magnification: 4×. (B) Expression of embryonic lineage markers by F1 TSCs in comparison with ICR TSCs, TSCs induced to differentiate (removal of FGF and feeder factors for 4 days), mouse ESCs (line D3), mouse extraembryonic endoderm stem cells (XEN, line CX4), and “rat ESC-like cells” (“RESC-like,” line B10). The lineage markers are explained in the main text.
In our cell line comparisons, we also included a rat blastocyst-derived cell line of uncertain identity that we have previously found to be immune privileged after PV injection (13); these rat cells had first been presumed to be ESC-like but this identity was later put into question (5). We now find, in agreement with Buehr et al. (5), that our rat cells lack the key ESC marker Oct4 but express the trophectoderm/trophoblast lineage markers Cdx2, Eomesodermin, and Placental Lactogen (Fig. 1B). Also, the TSCs and the trophectoderm-like rat cell cultures, but not the mouse ESCs, express an mRNA encoding FAS ligand (Fig. 1B), although we did not determine whether FAS ligand was present on undifferentiated or only differentiated cells. FAS ligand is not a lineage marker, but is of interest as an immunomodulatory molecule that we have previously implicated in the allogeneic survival of the rat cells (13).
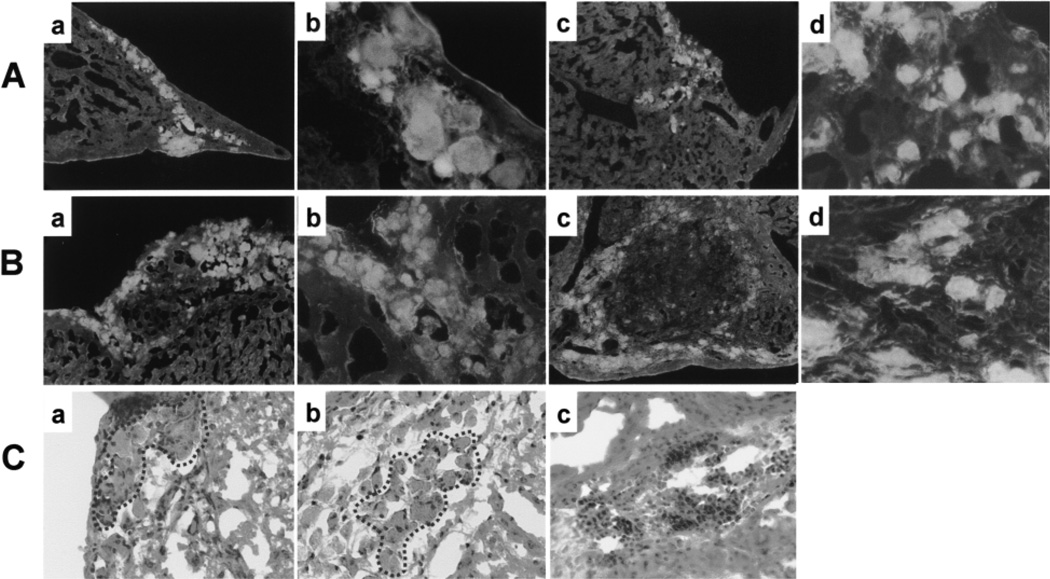
Having ascertained their identity, we injected the newly derived, genetically defined mouse TSCs into female Balb/c mice. Again, the injected TSCs survived in all cases (Table 1, group 2), appearing either as tight clusters (Fig. 2Aa and b) or loosely disseminated groups (Fig. 2Ac and d); occasionally these two arrangements, that occurred in roughly comparable proportions, transitioned into each other within the same tissue section. Both cluster types were found throughout the liver, with the tight clusters tending to be located in gaps or crevices, often subcapsular (Fig. 2Aa, b), while the disseminated cells clearly had entered the hepatic parenchyma (Fig. 2Ac, d). Interestingly, the TSC locations were easily identifiable on hematoxylin/eosin-stained parallel sections as patches of conspicuously brown-colored cells (Fig. 2Ca), but it was not practical to identify these locations without prior screening of the sections for fluorescent cells. We then tested whether the F1 TSCs survived injection into a CBA (H2k-k) mouse (Table 1, group 3) and into a rat (not shown), two genetic backgrounds that do not show an MHC haplotype overlap with the F1 TCS. Again the transplants survived. Overall, in experiments involving allogeneic TSCs from two genetic backgrounds, nine out of nine transplants survived (Table 1, groups 1–3). Finally, syngeneic injections (F1 TSCs into F1 mice) yielded similar results (Table 1, group 4), implying that the behavior of the surviving cells did not depend on the immunological constellation. It is noteworthy that even in the syngeneic context, the TSCs did not form tumors (unlike the rat trophectodermal cells; results not shown). This agrees with the previously observed lack of tumor formation after syngeneic TSC injection into other sites (10) and suggests that when ectopic TSCs survive in vivo, they undergo differentiation.
Figure 2.
Survival of allogeneic TSCs and TSC-mediated rescue of allogeneic ESCs. (A, B) Representative unstained frozen sections showing brightly fluorescing patches of stem cell descendents surrounded by weakly fluorescing liver tissue, 4 weeks after the injection of (A) GFP-labeled F1 TSCs or (B) a mixture of unlabeled F1 TSCs and GFP-labeled C57Bl/6 ESCs into female Balb/c mice. Each type of injection resulted in clusters of densely (a, b) or loosely (c, d) arranged fluorescent cells. Note that the two cluster types can transition into each other (Bc). Original magnifications: 10× (a, c) and 40× (b, d). (C) Four weeks after injection of TSCs (a) or TSCs + ESCs (b) and following H/E staining, clusters of conspicuous brown cells (circled by dotted lines) mark the transplant sites. Little lymphocyte infiltration is seen in these sites, whereas massive lymphocyte infiltration is seen in an ESC-injected mouse terminated 2 weeks after the injection (c). No ESC transplantation sites were ever identified 4 weeks after injection. Original magnification: 40×.
TSC Survival Requires a Nonmale Hormonal Environment
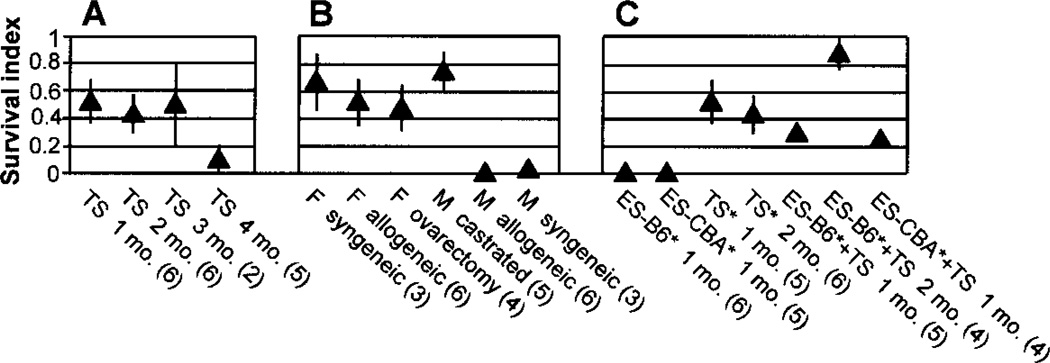
We next determined the significance of host gender. TSCs that were PV injected into females always survived in the host liver (Table 1, groups 1–4); by contrast, we did not observe a significant survival of TSCs injected into male hosts, regardless of syngeneic or not (Table 1, groups 5 and 6). This result was not unexpected because the natural environment of the conceptus suggests a requirement for female hormones, but formally it was also possible that rejection of the TSCs was caused by male hormones. In order to distinguish between these two possibilities, we performed the experiments with gonadectomized females and males. The results showed that, unexpectedly, ovarectomy had no effect (i.e., the livers of ovarectomized females provided a perfect environment for the TSCs) (Table 1, cp. groups 2 and 7; Fig. 3B). In striking contrast, castration completely abolished the gender difference (i.e., the livers of castrated males provided a perfect environment for the TSCs) (Table 1, cp. groups 2, 6, and 8; Fig. 3B). Each of these two results argues that (i) contrary to what might be expected, female hormones are not required and (ii) male hormones are toxic for the survival of PV-injected TSCs.
Figure 3.
Semiquantitative assessment of allograft survival. For each experimental group, the average survival index (see Materials and Methods for definition) and its SEM were determined. The numbers of injected mice studied per group are given in parentheses. (A) Time course of F1 TSC survival in female Balb/c mice (mo. × months). (B) Survival of F1 TSCs in male (M) or female (F) syngeneic or allogeneic (Balb/c) mice. Before injection, some of the allogeneic mice were castrated or ovarectomized as indicated, and all mice were killed 1 month after injection. (C) Survival of F1 TSCs and ESCs (C57Bl/6 [=B6] or CBA background, as indicated), alone or in combination, in female Balb/c mice; the GFP-labeled cell type is marked with an asterisk. The mice were killed 1 or 2 months after injection, as indicated. Note that the survival index in the coinjection groups is an underestimate because the number of labeled cells injected was only half compared to that in the separate injections.
Duration of TSC Survival
The experiments described so far were all terminated 1 month after injection. In order to determine the duration of TSC survival, we killed the injected mice at different time points and assessed the transplant survival with a semiquantitative approach. The results showed that there was no appreciable decrease until 3 months, but at 4 months they had disappeared in four out of five animals, with only a trace left in the fifth (Fig. 3A).
In summary, PV-injected TSCs survive for 3 months with little if any proliferation, regardless of their immunological compatibility but dependent on a nonmale hormonal context.
GFP-Labeled Allogeneic ESCs Are Rejected After PV Injection
Next, we injected GFP-labeled ESCs into Balb/c mice. In experiments involving allogeneic ESCs from three different genetic backgrounds (129, C57Bl/6, CBA), out of 13 transplants none survived until 4 weeks after injection (Table 1, groups 9, 10, and 11; Fig. 3C). By contrast, syngeneic ESCs (C57Bl/6 → C57Bl/6) always survived and tended to form tumors (Table 1, group 12). As expected, these tumors exhibited differentiation into cell types of all three germ layers (histology results not shown), attesting to the pluripotency of the cells. We did, however, observe modest patches of fluorescent cells until 2 weeks after injection, in line with the comparatively low immunogenicity of mouse ESCs (2). At the same time (2 weeks after injection), we observed a massive macrophage and lymphocyte infiltration of the ESC transplant, indicating that an immune reaction led to the disappearance of the ESCs (Fig. 2Cc). In summary, GFP-labeled allogeneic ESCs never survived longer than two weeks after PV injection.
PV-Injected Allogeneic TSCs Rescue the Survival of Coinjected GFP-Labeled ESCs
Having demonstrated that GFP-labeled TSCs but not ESCs survived allogeneic PV injection, we tested whether GFP-labeled ESCs could be rescued by coinjecting them with nonlabeled TSCs. We first mixed C57Bl/6xBalb/c F1 TSCs with C57Bl/6 ESCs. All livers from mice with mixed injections showed large patches of fluorescent cells (Fig. 2B, Table 1, group 13; Fig. 3C), indicating that the TSCs had rescued the ESCs. We then tested whether a haplotype overlap between the ESCs and TSCs was required for the rescue. To this end, we injected CBA (H2k-k) ESCs together with C57Bl/6(H2k-b)xBalb/c(H2k-d) TSCs. The results showed that the CBA ESCs survived in all injected mice (Table 1, group 14) to the same degree as observed before with the C57Bl/6 ESCs (Fig. 3C). Overall, in experiments involving allogeneic ESCs from two different genetic backgrounds, nine out of nine ESC transplants survived when coinjected with TSCs. No teratomas were observed in the mixed injections, but we did notice that compared to the standard 1-month time point (and unlike our observations with the TSC transplants), the survival index of the coinjected, GFP-labeled C57Bl/6 ESCs had increased further by 2 months after injection (Fig. 3C). Finally, because hematopoietic chimerism has been observed after PV injection of the rat “ESC-like cells” (13) and after (nonhepatic) injection of allogeneic mouse ESCs (2), we also determined whether GFP-expressing cells were present in the blood of ESC/TSC-injected mice. No fluorescent cells were seen at the time points studied (2 and 4 weeks after injection).
DISCUSSION
The primary finding of this report is that allogeneic TSCs, which normally reside inside the uterus and did not survive for any duration in previously tested ectopic locations, can survive for 3 months in the liver. Thus, in our model, the liver seems to play a uterus-like role both physiologically and immunologically, the latter role being in line with the well-known fact that the liver environment can provide a certain degree of immune privilege (3). On the other hand, immune-privileged features of the trophoblast lineage (18,25,31) are also important contributors to survival of the GFP-labeled TSCs because the liver is incapable of protecting GFP-labeled ESCs [(12), present study]. Note in this respect that although rejection of PV-injected ESCs from different genetic backgrounds was highly reproducible in our setup, this has not been a universal observation (23); factors causing variability appear to include the immunogenicity of GFP, cell dosage, recipient age/strain/health, and the time point studied (1,4,15,27,28).
Within the accuracy of our semiquantitative method, the number of surviving TSC-derived cells was unchanged during the first 3 months after transplantation, but clearly declined dramatically in the fourth month. We cannot exclude that this decline was due to a late-onset immune reaction, but a nonimmunological, transplant-intrinsic mechanism appears to explain the observed time pattern more easily. Indeed, because the transplanted TSCs did not seem to proliferate, they must have differentiated rapidly into trophoblast cells [comp. ref. (10)], a cell type whose natural life span is rather short (that the TSC transplants nevertheless lasted longer than a normal pregnancy may reflect a reserve ensuring that trophoblast life span does not limit pregnancy). The assumption that TSC differentiation lead to transplant rejection is less plausible in view of the known immune privilege of the differentiated trophoblast (18,25,31); it is also hard to imagine that a dramatic immune response starts only 3 months after TSC transplantation. Future experiments will discriminate immunological from non-immunological mechanisms of TSC transplant demise by comparing long-term (>3 months) transplant survival in syngeneic, allogeneic, and immune-suppressed allogeneic hosts.
The second finding of this report is that the survival of the transplanted TSCs is not due to their requiring ovarian hormones, but does require absence of products of the male gonads. Such long survival of trophoblast cells in the absence of ovarian hormones has not previously been observed. Future work will determine whether the opposite treatment, namely addition of a pregnant hormonal context, exerts any effect in our model. Also, we are not aware of any report that testicular factors have a detrimental influence on trophoblast survival in vivo. However, it has been reported that in the presence of low-density lipoproteins, supraphysiological doses of testosterone can exert a toxic effect on freshly isolated term human trophoblast cells (33); furthermore, male hormones have shown proinflammatory effects (14) that may play a role in abortion (7). Thus, our model provides a starting point for systematically assessing the hormonal and other physiological requirements for trophoblast cells in vivo.
The third finding of this report is the ability of PV-injected TSCs to protect GFP-labeled ESCs that are allogeneic with respect to the host. This suggests that the ESCs benefit as bystander cells from the well-known ability of trophoblast cells to exert various forms of local immune suppression (18,25,31). However, in view of the known tendency of trophoblast cells to fuse with each other (24), we cannot exclude the possibility that the ESCs fused with the TSCs, and the fused cells maintained the immune privilege of the TSCs. However, that possibility is made less likely when we consider the extremely high efficiency of the observed ESC rescue. It is important to note that the protected ESCs did not have to be of the same genetic background as the TSCs, which is in line with the largely nonspecific nature of at least some of the mechanisms of local trophoblast immune suppression, and is of interest with respect to potential practical applications. Clearly, it will be of interest to determine whether PV-injected TSCs or trophoblast cells can also protect cells other than GFP-labeled ESCs.
We did not determine the upper limit of ESC survival, but we noticed that the number of ESC descendents appeared to have increased between 1 and 2 months posttransplantation (Fig. 3C). Whether this increase will eventually lead to the formation of teratomas, whether the ESCs or their descendents outlive the TSCs, whether and how the ESCs differentiate, and whether (upon differentiation) immunological tolerance develops, are all fascinating subjects for future research.
Even without an ability to protect coinjected cells, however, TSCs may be of therapeutic interest. The ability to survive—without tumor formation—in the allogeneic liver, in conjunction with an unlimited capacity to proliferate in vitro, makes the TSCs (or trophoblast cells obtained from ESCs) (32) a promising therapeutic vehicle. Indeed, if not already the wild-type TSCs, then genetically modified TSCs could act as an independent therapy or as adjunctive support for coinjected therapeutic cells. The unlimited in vitro proliferative capacity of TSCs (or of ESCs used to produce trophoblast cells) not only ensures unlimited supply, but also significantly facilitates genetic manipulation, thus providing a convenient starting point for developing off-the-shelf cytokine delivery systems. By contrast, genetic manipulation of primary cells—usually by viral transduction (6,26)—must be done repeatedly, is less reproducible, and attempts to increase transduction efficiency tend to reduce viability (6). The long, but not indefinite, duration of intrahepatic TSC survival (which exceeds normal trophoblast life span more than threefold) makes these cells particularly interesting as conditioning reagents, especially because the hepatic environment is known to facilitate tolerance induction (3).
Finally, we note that the present results confirm the suspicion, caused by the findings of Buehr et al. (5), that rat blastocyst-derived “ESC-like cells” previously shown by us to survive allogeneic PV injection (13) are not bona fide ESCs but exhibit trophoblastic features. However, these rat cells are not TSCs either, because they can give rise to blood chimerism (13), have different growth factor requirements (5), and can form syngeneic tumors (unpublished). Nevertheless, their trophoblastic features raise the possibility that the rat “ESC-like cells” share with mouse TSCs some mechanisms of immune privilege.
In conclusion, our results suggest that it is worth-while to explore a new “stem cell-based trophoblastic approach” to therapeutic cell transplantation. It will be of interest to compare this approach with the Sertoli cell-based approach being explored by other laboratories (8,9,16).
ACKNOWLEDGMENTS
We thank the Roche Organ Transplantation Research Foundation (ROTRF) for financial support, Dr. J. McWhir (Roslin Institute Edinburgh) for the CBA ESCs, Dr. J. Rossant (University of Toronto) for the ICR TSCs, Dr. D. Baltimore (Caltech) for vector pFUGW, Dr. Y. Tian (TAMU) for access to his fluorescence microscope, and Drs. N. Maeda (UNC), D. Kraemer, and I. Tizard (TAMU) for encouragement and discussions. We also thank J. Hagaman (UNC) and the TAMU Comparative Medicine Program for assistance with surgical techniques, the VTPB Histology Laboratory for use of equipment, and Dr. A. Ambrus for help with the evaluation of histological sections. Finally, we thank the reviewers for useful comments.
REFERENCES
- 1.Andersson G, Denaro M, Johnson K, Morgan P, Sullivan A, Houser S, Patience C, White-Scharf ME, Down JD. Engraftment of retroviral EGFP-trans-duced bone marrow in mice prevents rejection of EGFP transgenic skin grafts. Mol. Ther. 2003;8(3):385–391. doi: 10.1016/s1525-0016(03)00210-7. [DOI] [PubMed] [Google Scholar]
- 2.Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24(10):2192–2201. doi: 10.1634/stemcells.2006-0022. [DOI] [PubMed] [Google Scholar]
- 3.Bowen DG, McCaughan GW, Bertolino P. Intrahe-patic immunity: A tale of two sites? Trends Immunol. 2005;26(10):512–517. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Bubnic SJ, Nagy A, Keating A. Donor hematopoietic cells from transgenic mice that express GFP are immunogenic in immunocompetent recipients. Hematology. 2005;10(4):289–295. doi: 10.1080/10245330500093468. [DOI] [PubMed] [Google Scholar]
- 5.Buehr M, Nichols J, Stenhouse F, Mountford P, Greenhalgh CJ, Kantachuvesiri S, Brooker G, Mullins J, Smith AG. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol. Reprod. 2003;68(1):222–229. doi: 10.1095/biolreprod.102.006197. [DOI] [PubMed] [Google Scholar]
- 6.Callewaert H, Gysemans C, Cardozo AK, Elsner M, Tiedge M, Eizirik DL, Mathieu C. Cell loss during pseudoislet formation hampers profound improvements in islet lentiviral transduction efficacy for transplantation purposes. Cell Transplant. 2007;16(5):527–537. doi: 10.3727/000000007783464948. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Sem. Fetal Neonatal Med. 2006;11(5):302–308. doi: 10.1016/j.siny.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Dufour JM, Gores P, Hemendinger R, Emerich DF, Halberstadt CR. Transgenic Sertoli cells as a vehicle for gene therapy. Cell Transplant. 2004;13(1):1–6. doi: 10.3727/000000004772664833. [DOI] [PubMed] [Google Scholar]
- 9.Dufour JM, Lord SJ, Kin T, Rayat GR, Dixon DE, Bleackley RC, Korbutt GS, Rajotte RV. Comparison of successful and unsuccessful islet/Sertoli cell cotransplant grafts in streptozotocin-induced diabetic mice. Cell Transplant. 2008;16(10):1029–1038. [PubMed] [Google Scholar]
- 10.Erlebacher A, Lukens AK, Glimcher LH. Intrinsic susceptibility of mouse trophoblasts to natural killer cell-mediated attack in vivo. Proc. Natl. Acad. Sci. USA. 2002;99(26):16940–16945. doi: 10.1073/pnas.222652199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlebacher A, Price KA, Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev. Biol. 2004;275(1):158–169. doi: 10.1016/j.ydbio.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Fair JH, Cairns BA, Lapaglia MA, Caballero M, Pleasant WA, Hatada S, Kim HS, Gui T, Pevny L, Meyer AA, Stafford DW, Smithies O, Frelinger JA. Correction of factor IX deficiency in mice by embryonic stem cells differentiated in vitro. Proc. Natl. Acad. Sci. USA. 2005;102(8):2958–2963. doi: 10.1073/pnas.0409840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fandrich F, Lin X, Chai GX, Schulze M, Ganten D, Bader M, Holle J, Huang DS, Parwaresch R, Zavazava N, Binas B. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat. Med. 2002;8(2):171–178. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- 14.Fimmel S, Zouboulis CC. Influence of physiological androgen levels on wound healing and immune status in men. Aging Male. 2005;8(3):166–174. doi: 10.1080/13685530500233847. [DOI] [PubMed] [Google Scholar]
- 15.Gambotto A, Dworacki G, Cicinnati V, Kenniston T, Steitz J, Tuting T, Robbins PD, DeLeo AB. Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: Identification of an H2-Kd-restricted CTL epitope. Gene Ther. 2000;7(23):2036–2040. doi: 10.1038/sj.gt.3301335. [DOI] [PubMed] [Google Scholar]
- 16.Halberstadt C, Emerich DF, Gores P. Use of Sertoli cell transplants to provide local immunoprotection for tissue grafts. Expert Opin. Biol. Ther. 2004;4(6):813–825. doi: 10.1517/14712598.4.6.813. [DOI] [PubMed] [Google Scholar]
- 17.Hansen PJ. Regulation of uterine immune function by progesterone--lessons from the sheep. J. Reprod. Immunol. 1998;40(1):63–79. doi: 10.1016/s0165-0378(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 18.Hunt JS. Stranger in a strange land. Immunol. Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakoki M, Tsai YS, Kim HS, Hatada S, Ciavatta DJ, Takahashi N, Arnold LW, Maeda N, Smithies O. Altering the expression in mice of genes by modifying their 3′ regions. Dev. Cell. 2004;6(4):597–606. doi: 10.1016/s1534-5807(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 20.Kibschull M, Nassiry M, Dunk C, Gellhaus A, Quinn JA, Rossant J, Lye SJ, Winterhager E. Connexin31-deficient trophoblast stem cells: A model to analyze the role of gap junction communication in mouse placental development. Dev. Biol. 2004;273(1):63–75. doi: 10.1016/j.ydbio.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Lodge P, McWhir J, Gallagher E, Sang H. Increased gp130 signaling in combination with inhibition of the MEK/ERK pathway facilitates embryonic stem cell isolation from normally refractory murine CBA blastocysts. Cloning Stem Cells. 2005;7(1):2–7. doi: 10.1089/clo.2005.7.2. [DOI] [PubMed] [Google Scholar]
- 22.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 23.Magliocca JF, Held IK, Odorico JS. Undifferentiated murine embryonic stem cells cannot induce portal tolerance but may possess immune privilege secondary to reduced major histocompatibility complex antigen expression. Stem Cells Dev. 2006;15(5):707–717. doi: 10.1089/scd.2006.15.707. [DOI] [PubMed] [Google Scholar]
- 24.Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update. 2003;9(6):531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- 25.Petroff MG. Immune interactions at the maternal-fetal interface. J. Reprod. Immunol. 2005;68(1–2):1–13. doi: 10.1016/j.jri.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Sabek OM, Fraga DW, Henry J, Gaber LW, Kotb M, Gaber AO. Expression of transforming growth factor-beta by human islets: Impact on islet viability and function. Cell Transplant. 2007;16(8):775–785. doi: 10.3727/000000007783465217. [DOI] [PubMed] [Google Scholar]
- 27.Skelton D, Satake N, Kohn DB. The enhanced green fluorescent protein (eGFP) is minimally immunogenic in C57BL/6 mice. Gene Ther. 2001;8(23):1813–1814. doi: 10.1038/sj.gt.3301586. [DOI] [PubMed] [Google Scholar]
- 28.Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6(7):1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Ueda H, Yokono K, Taniguchi H. Normalization of blood glucose after islet cografting with placental tissues in diabetic mice. Cell Transplant. 2002;11(5):455–457. [PubMed] [Google Scholar]
- 30.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282(5396):2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 31.Trowsdale J, Betz AG. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006;7(3):241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 32.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20(12):1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 33.Zhu XD, Bonet B, Knopp RH. 17beta-Estradiol, progesterone, and testosterone inversely modulate low-density lipoprotein oxidation and cytotoxicity in cultured placental trophoblast and macrophages. Am. J. Obstet. Gynecol. 1997;177(1):196–209. doi: 10.1016/s0002-9378(97)70462-9. [DOI] [PubMed] [Google Scholar]