Abstract
The key to improving the sensitivity of in vivo molecular imaging is to increase the target-to-background signal ratio (TBR). Optical imaging has a distinct advantage over other molecular imaging methods in that the fluorescent signal can be activated at the target thus reducing background signal. Previously, we found that H-dimer formation quenches fluorescence of xanthene fluorophores, and among these, TAMRA had the highest quenching ratio. Another approach to lowering background signal is to employ pH activation based on the photon-induced electron transfer (PeT) theory. We hypothesized that combining these two strategies could lead to greater quenching capacity than was possible with either probe alone. A pH-sensitive fluorophore, pHrodo or TAMRA was conjugated to the cancer targeting molecules, avidin (Av) and trastuzumab (Tra). As expected, both pHrodo and TAMRA formed H-dimers when conjugated to avidin or antibody and the dimerization resulted in efficient fluorescence quenching. In addition, pHrodo conjugated probes showed pH-dependent fluorescence activation. When the probes were used in an in vivo animal model, fluorescence endoscopy with Av-pHrodo depicted tumors with high TBR 1 h and 2 h after injection. Av-TAMRA also visualized tumors 1 h and 2 h after the injection, however, TBR was lower due to the background signal from non-specific binding 1 h after the injection as well as background fluorescence from the unbound agent. Thus, we demonstrate that a dual-controlled activatable optical probe based on the combination of H-dimer formation and pH activation can achieve high TBR at early time points during in vivo molecular imaging.
Introduction
Optical molecular imaging is being investigated as a useful tool for cancer detection and diagnosis both in the clinic and in the basic sciences.1,2 Optical imaging has several advantages over other molecular imaging methods because it is relatively easy-to-use, inexpensive, allows real-time multicolor imaging and requires no exposure to radiation. Moreover, if the fluorescent emission is in the visible light range, multicolor fluorescence signals can be distinguished with the naked eye.3–5 However, optical imaging has difficulty in detecting objects deep within tissue because of the poor penetration of light. However, a fiber-optic endoscopic imaging system can enable direct visualization of emitted light even deep within the body. Endoscopy is used in the diagnosis of gastrointestinal cancers, peritoneal metastasis, lung cancers, etc. However, endoscopy would be improved by applying fluorescent probes that increase the target-to-background signal ratio (TBR). An inherent advantage of optical imaging is that the fluorescent probes can be activated at the target, thus reducing non-specific background signals. Activatable probes, which yield fluorescence signals only within the target cells, therefore result in favorable TBRs.
Recently, we reported that one of the popular xanthene fluorophores, TAMRA (carboxytetramethylrhodamine), can readily form H-type dimers on proteins resulting in an efficient fluorescence quench (self-quench).6 When TAMRA is conjugated to a cancer targeting molecule, the H-dimer formation resulted in minimal signal due to fluorescence quenching prior to binding and internalization into the target cell while the signal was activated only after internalization. For instance, using the d-galactose receptor as a target, avidin-TAMRA enabled visualization of d-galactose receptor positive tumors with high TBR in fluorescence endoscopy 2 h after the probe was injected. However, in such FRET-based self-quench systems, a slight fluorescence remains even in the quenched state.
Another approach for activatable optical probes employs the intramolecular pH activation system based on the photon-induced electron transfer (PeT) theory. That is, the fluorescence intensity changes depending on the environmental pH, for example, sensing low pH in the lysosome of target cells. Previously, we successfully synthesized pH activatable probes with various pH thresholds using the BODIPY-core fluorophore platform, and the probe enhanced the visualization of tumors in vivo with high TBR.7
We hypothesized that these two independent activation methods, H-dimer formation and pH activation, could be combined to gain a synergistic effect that could lead to greater quenching capacity than either probe alone. Since the chemical structure of a fluorophore is critical to the efficient formation of the H-dimer, we employed a pH-sensitive fluorophore, pHrodo™ dye, which is supposed to have a chemical structure similar to TAMRA (Fig. 1) and which has an identical excitation and emission spectra to TAMRA. In order to demonstrate this synergistic effect, pHrodo or TAMRA were conjugated to the cancer targeting molecules avidin or trastuzumab, which target the d-galactose receptor and HER2/neu antigen, respectively. Subsequently, their ability to detect tumors in vivo was investigated with fluorescence endoscopy using a mouse model of ovarian cancer with peritoneal metastases.
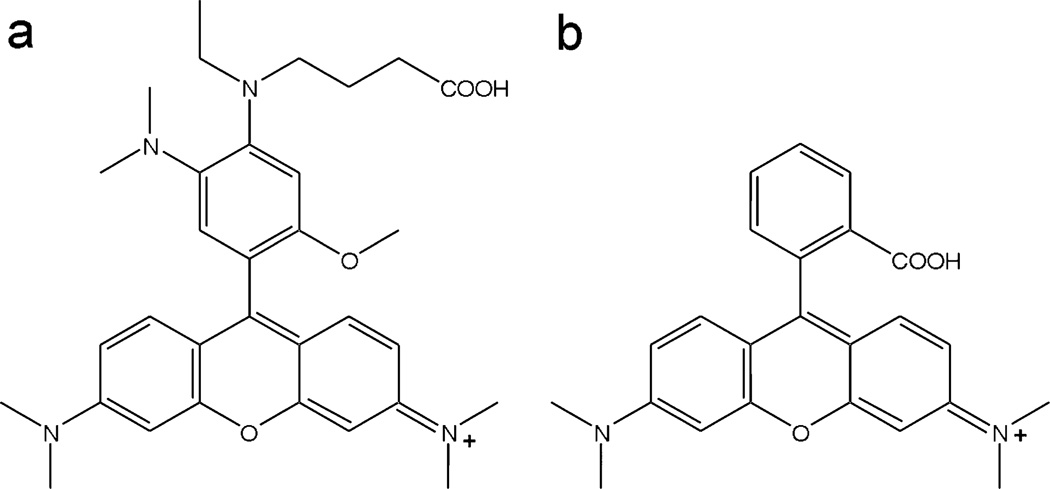
Fig. 1.
The proposed structure of pHrodo (a) and the structure of TAMRA (b).
Results
H-dimer formation and quenching efficiency of pHrodo and TAMRA conjugates in vitro
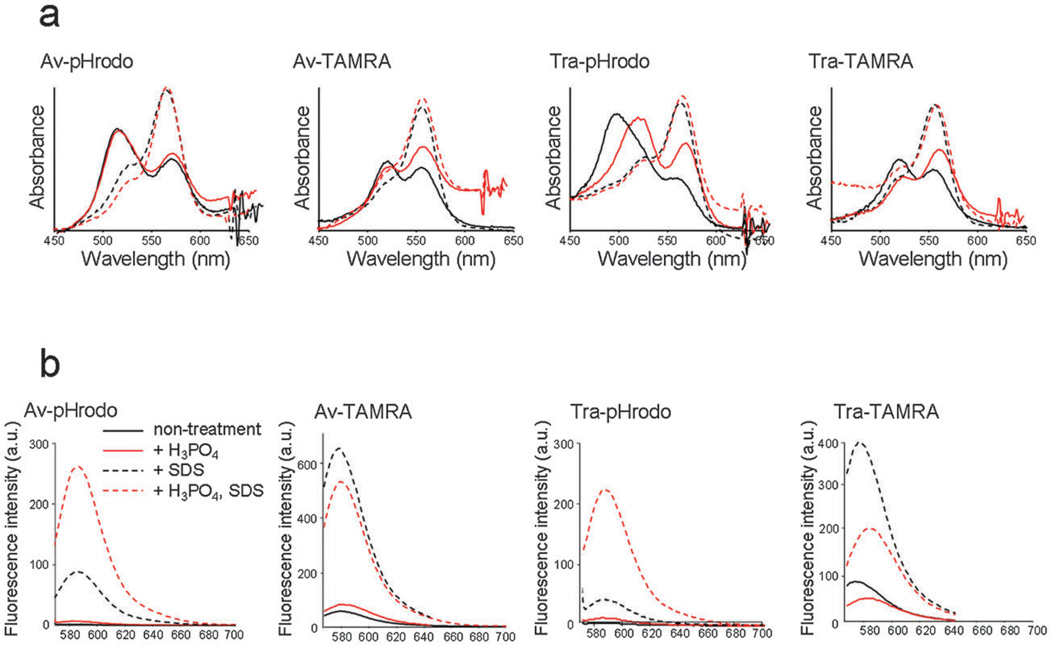
Fig. 2(a) shows the absorbance spectra under each of the conditions, and H-dimer formation ratio was expressed as follows:
Here, diAbs and moAbs represent the absorbance at the H-dimer peak and at the monomer peak, respectively. The H-dimer ratio values at pH 7.4 are 3.63, 5.91, 2.74 and 2.79 for Av-pHrodo, Tra-pHrodo, Av-TAMRA and Tra-TAMRA, respectively. The H3PO4 addition did not affect the absorbance spectrum for Av-pHrodo and the H-dimer ratio was 3.62, however, for Tra-pHrodo, the H-dimer ratio decreased to 2.79. In addition, the dimer absorbance peak was red-shifted.
Fig. 2.
(a) Absorbance spectrum of each conjugate without SDS (solid line) and with SDS (dashed line), and at neutral pH (black) and acidic (red) conditions. The blue shifted peak without SDS represents H-dimer formation of fluorophores. Both pHrodo and TAMRA showed H-dimer formation. (b) Fluorescence spectra of each conjugate without SDS (solid line) and with SDS (dashed line). The fluorescence was increased by SDS addition (H-dimer dissociation) in both pHrodo and TAMRA conjugates. In addition, pHrodo probes showed fluorescence activation by lowering pH, and this effect resulted in the large fluorescence activation capacity for pHrodo conjugates.
The quenching effect of H-dimer formation was measured by dequenching with SDS to separate the H-dimers into monomers. The quenching effect in lower pH solutions was measured by adding H3PO4. The results of the fluorescence spectra are summarized in Fig. 2(b). The quenching efficiency of H-dimer formation was 54, 9.3, 10 and 7.3-fold for Av-pHrodo, Tra-pHrodo, Av-TAMRA and Tra-TAMRA, respectively. In addition, for Av-pHrodo and Tra-pHrodo, the fluorescence was activated 2.9 and 5.3-fold by the pH activation mechanism, and the overall quenching efficiency was 159 and 49-fold for Av-pHrodo and Tra-pHrodo, respectively.
In vitro cell fluorescence microscopic studies
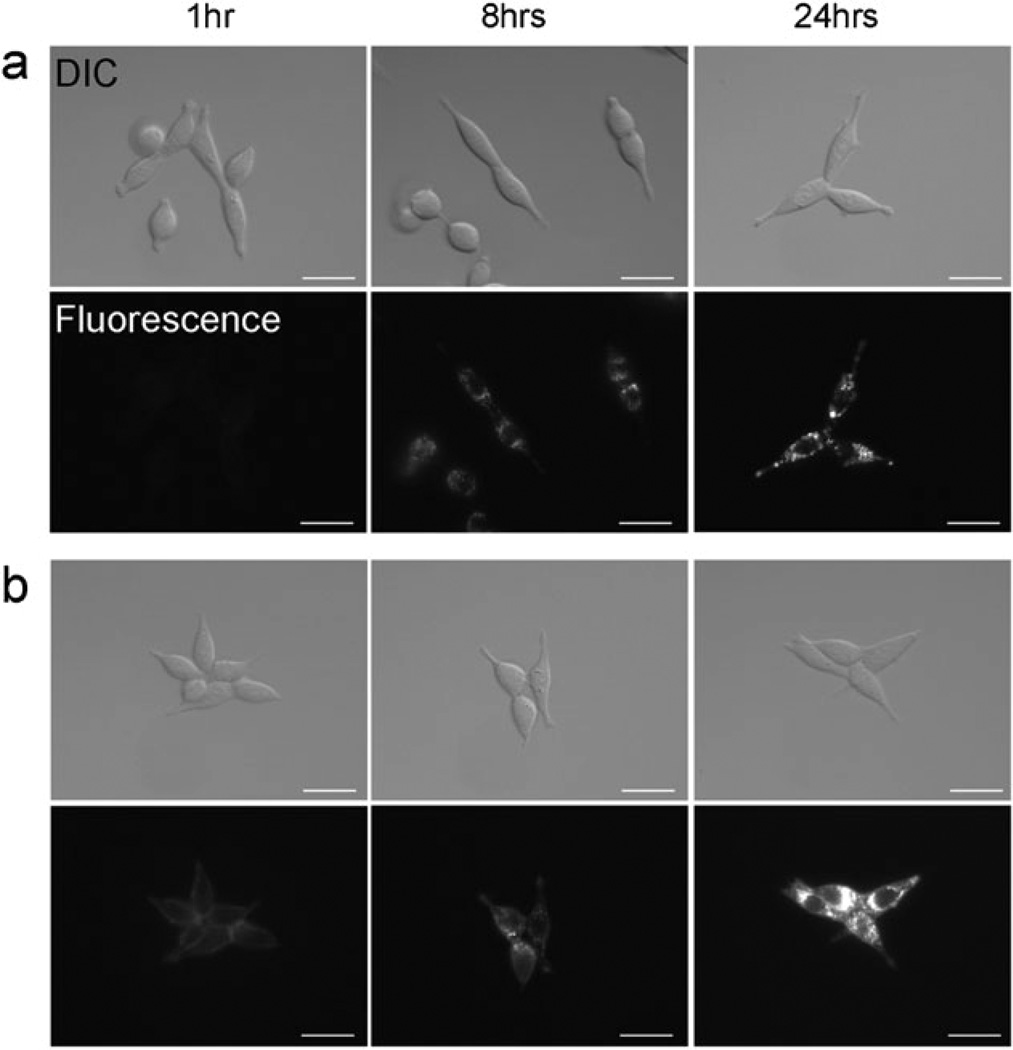
To determine if internalization into target cells was required for activation of the probes, in vitro fluorescence microscopy studies were performed. After 8 and 24 h incubation, both Tra-TAMRA and Tra-pHrodo revealed bright fluorescent dots inside the cells (Fig. 3). Tra-TAMRA demonstrated fluorescence on the surface of 3T3/HER2+ cells at 1 h incubation, although the intensity was lower than after 8 h incubation. In contrast, Tra-pHrodo showed no fluorescence after 1 h incubation. Surface fluorescence was detected when the cells were incubated for 8 or 24 h with Tra-TAMRA. In the case of Tra-pHrodo, surface fluorescence was not seen at any time after incubation.
Fig. 3.
Fluorescence microscopy as well as differential interference contrast (DIC) images of Tra-pHrodo (a) or Tra-TAMRA (b) in 3T3/HER2+ tumor cells. The cell-surface fluorescence signal was not detected with Tra-pHrodo, but a weak signal was detected with Tra-TAMRA. Fluorescent dots were detected within the cytoplasm 8 h after incubation at 37 °C with both conjugates. Bars = 20 µm.
In vivo imaging studies in peritoneal tumor bearing mice
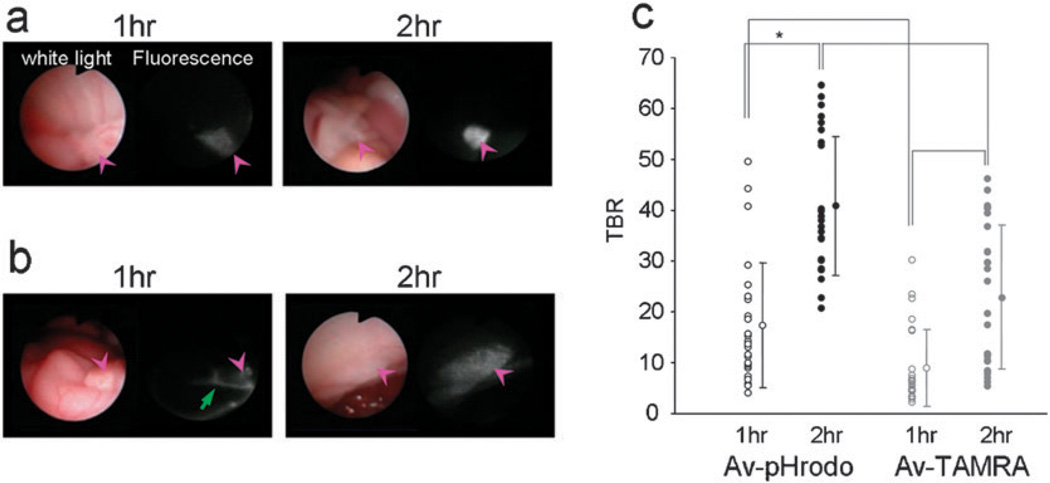
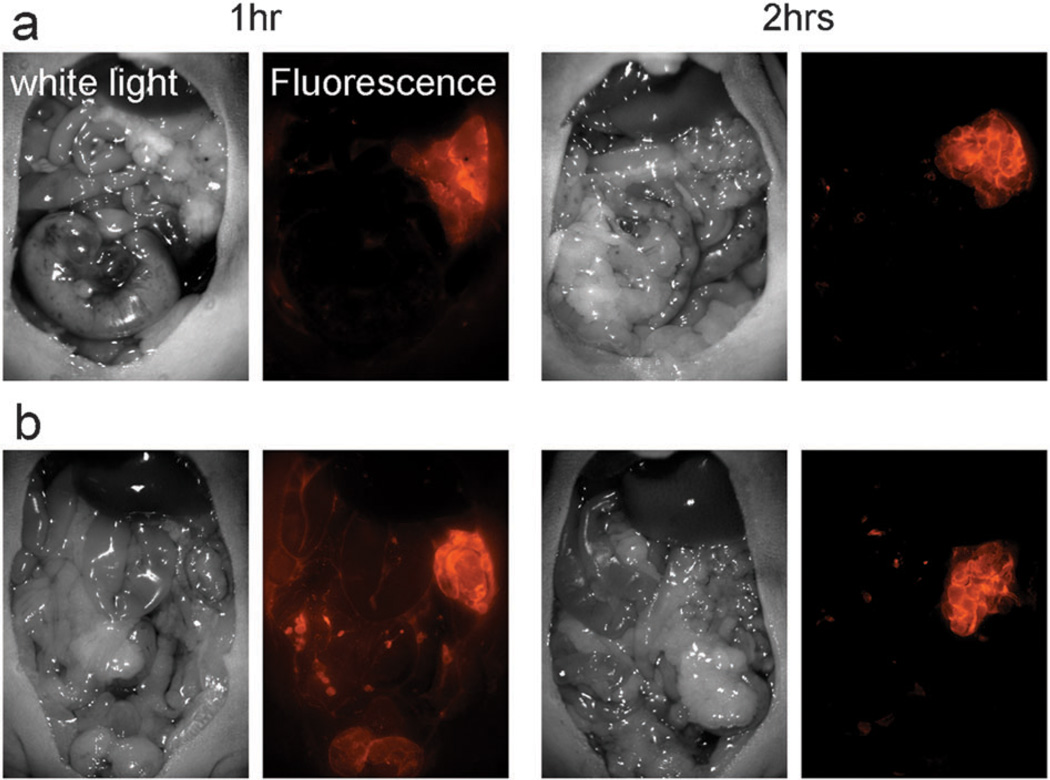
In vivo fluorescence endoscopy was performed in SHIN3 (d-galactose positive) peritoneal tumor bearing mice (Fig. 4). The tumors were detected by fluorescence endoscopy 1 h after the Av-pHrodo injection. Av-TAMRA also visualized tumors at 1 h post injection, but the background signal was higher than with Av-pHrodo and fluorescence within the injected fluid was also detected. Two hours after the imaging probe injection, the tumor nodules were clearly visualized by both Av-pHrodo and Av-TAMRA, although the background signal was lower for Av-pHrodo. The calculated tumor-to-background ratios (TBRs) are summarized in Fig. 4(c). The highest TBR was achieved with Av-pHrodo 2 h after the injection. Av-pHrodo showed relatively high TBR even at 1 h after injection, and there were no significant differences with Av-TAMRA, 2 h time point.
Fig. 4.
In vivo fluorescence endoscopic images in peritoneal tumor bearing mice with Av-pHrodo (a) or Av-TAMRA (b). The pink arrow heads show the tumor nodules. The tumors were visualized with both Av-pHrodo and Av-TAMRA 1 h after the injection, but fluorescence from excess injectate in the peritoneal cavity (green arrow) was also detected. Two hours after the imaging probe injection, the tumor nodules were clearly visualized by both Av-pHrodo and Av-TAMRA, although the background signal was lower for Av-pHrodo. The measured tumor-to-background ratios (TBRs) are summarized in (c). The highest TBR was accomplished by Av-pHrodo 2 h after the injection. (*p < 0.001, Mann-Whitney U test).
The results of fluorescence spectral imaging are shown in Fig. 5. The results were concordant with endoscopy, and tumors were clearly visualized with both Av-pHrodo and Av-TAMRA at 2 h after the injection. A certain level of background signal was detected for Av-TAMRA, 1 h after the injection.
Fig. 5.
Fluorescence spectral images of the peritoneal cavity for Av-pHrodo (a) and Av-TAMRA (b). The results were consistent with endoscopic images. Tumors were detected with a low background signal for Av-pHrodo, but the injectate fluorescence was detected with Av-TAMRA at 1 h after the injection.
Discussion
It has been previously reported that H-dimer formation results in quenching of fluorescence when using xanthene fluorophores.6 Among the fluorophores tested, TAMRA had the most favorable properties as an activatable probe for in vivo molecular imaging.6,8 In the current study, pHrodo, which has a structure similar to TAMRA, also formed H-dimers when conjugated to avidin or an antibody, and the dimerization resulted in efficient fluorescence quenching. In addition, pHrodo conjugated probes showed pH-dependent fluorescence activation, and the fluorescence intensity was activated 3–5 fold by adding H3PO4. In contrast, the fluorescence signals of TAMRA conjugates were similar or even decreased at the acidic pH compared with those at neutral pH. Acidification did not materially affect the absorbance spectra of Av-pHrodo, however, in Tra-pHrodo, a portion of the H-dimers was dissociated at low pH without SDS. In addition, the peak wavelength of the dimer was red shifted at low pH. We have not elucidated the explanation for this phenomenon yet, but there may be additional mechanisms, such as protein dye interactions which stabilize the H-dimers. The positive charge on the phenyl ring under acidic conditions might weaken the interaction and destabilize the dimer causing dissociation and a change in the absorbance peak. Consequently, pHrodo conjugated probes achieved larger quenching capacities than TAMRA probes. That is, fairly low baseline fluorescence was measured for pHrodo probes, although a slight fluorescent signal remained for TAMRA probes at the baseline.
Both avidin (binds to d-galactose receptor) and trastuzumab (binds to human epidermal growth factor receptor type 2 (HER2)) are internalized within the cell after binding their respective targets. After internalization, the avidin tetramer is broken down into monomers, and trastuzumab is degraded within the lysosome; as a result, each fluorophore is separated from each other thus deconstructing the H-dimer leading to dequenching. In addition, since pH in the lysosome is acidic, pHrodo fluorescence can be additionally activated in the lysosome. As shown in Fig. 3, both Tra-TAMRA and Tra-pHrodo showed strong fluorescent dots inside the target cells using in vitro microscopy. Tra-TAMRA showed a weak fluorescence on the surface of the cell, but Tra-pHrodo showed fluorescence only inside of the cells. This reflects the strong quenching capacity of pHrodo at neutral conditions, outside the cell. The fluorescence accumulation was blocked by adding excess unlabeled trastuzumab treatments in both conjugates. In the target negative parental cell line, the fluorescence signal was not detected (Supplemental Fig. 1a,b†). These results showed the target specific internalization and activation of the conjugates. In addition, the fluorescence of rhodamine derivative-conjugates was stable in cells for 48 h incubation in both conjugates (Supplemental Fig. 1c,d†).
When the probes were used in animal models in conjunction with in vivo fluorescence endoscopy, Av-pHrodo clearly depicted tumors 1 h and 2 h after the injection (Fig. 4(a)). In contrast, while Av-TAMRA visualized tumors 1 h and 2 h after the injection, the TBR was somewhat low due to the background signal from non-specific binding 1 h after the injection (Fig. 4(b)). In addition, fluorescence from the remaining injected solution was detected. Since TAMRA is fluorescent at neutral pH and self-quenching is incomplete, Av-TAMRA has a weak fluorescence prior to activation. This likely caused the background signal at 1 h post injection. In contrast, since Av-pHrodo has a larger quenching capacity because it has both pH-dependent activation and H-dimer formation, the signal was detected only in the cancer tissues. Even so, the results of TAMRA were superior to the non-activatable (always-ON) probe, Av-Alexa488 as reported previously. In the case of Av-Alexa488, fluorescence from fluid was strongly detected, and TBR was low even 2 h after the injection.6
Thus, we demonstrate that an activatable optical probe based on the dual mechanisms of H-dimer formation and pH activation can achieve high TBR at early time points during in vivo molecular imaging. These characteristics are of importance during fluorescence endoscopy, because background signal is reduced and therefore it is easier to see the target. pHrodo conjugated molecular targeting probes are a promising tool for cancer detection. In addition, by expanding this strategy to the near-infrared (NIR) fluorophores, which has better tissue penetration than visible range fluorescence, deeper-tissue detection and non-invasive imaging should be possible. Self-quenched NIR fluorophores conjugated to antibodies or peptides have been shown to be useful for in vivo molecular imaging.9,10 The improvement of TBR by the synergic effect with the pH-activation strategy should accomplish more sensitive and specific cancer detection and characterization.
Experimental
Reagents
The NHS esters of pHrodo and TAMRA were purchased from Invitrogen Corporation (Carlsbad, CA). Avidin was purchased from Pierce Biochemical, Inc. (Milwaukee, WI). Trastuzumab, an FDA-approved humanized anti-HER-2 antibody, was purchased from Genentech Inc. (South San Francisco, CA). All other chemicals used were of reagent grade.
Determination of pHrodo chemical structure
The structure of pHrodo has been determined with mass spectroscopic analysis and NMR. The NHS ester of pHrodo was used for this purpose. Briefly, mass spectroscopic analysis was performed with LCMS-IT-TOF systems (Shimadzu America Co., Columbia, MD). 1H- and 13C-NMR analyses were performed with a Gemini or Mercury 300 MHz spectrometer (Varian, Palo Alto, CA). By summarizing both results with reference to the patent document,11 the structure of pHrodo was suggested as shown in Fig. 1(a).
NMR 1H (300 MHz, DMSO) δ: 7.35 (1H, d, J 1Hz, Bz –CH–). 7.32 (1H, d, J 1Hz, Bz –CH–). 7.18 (1H, d, J 1Hz, Ar –CH–). 7.14 (1H, d, J 1Hz, Ar –CH–). 6.93 (2H, d, J 1Hz, 2Ar –CH–). 6.82 (2H, d, J 1Hz, 2Ar –CH–). 3.65 (1H, s –CH3), 3.41 (2H, q, –N–CH2–CH3), 3.30 (6H, s, Bz–N–(CH3)2), 3.28 (12H, s, 2Ar–N–(CH3)2), 2.82 (2H, s, –CH3), 2.75 (2H, t, –N–CH2CH2–), 2.65 (4H, s, suc –CH2CH2–), 2.27 (2H, t, sucO2C–CH2CH2–), 1.89 (2H, m, –CH2CH2CH2–), 1.09 (3H, t, –N–CH2–CH3). LCMS-IT-TOF (m/z) 642.33.
Synthesis of fluorophore conjugated avidin and trastuzumab
Avidin (14 nmol) was incubated with the NHS ester of pHrodo or TAMRA (70 nmol) in 0.1 M Na2HPO4 (pH 8.5) at room temperature for 30 min. Each mixture was purified with a Sephadex G50 column (PD-10; GE Healthcare, Piscataway, NJ). Trastuzumab (6.5 nmol) was treated with NHS esters (65 nmol) and purified in the same manner as above. The number of fluorophore molecules per avidin or trastuzumab was ~2–3.
Determination of fluorescence activation capacities in vitro
The quenching–dequenching abilities of each conjugate were investigated by treating the conjugates with 5% SDS to disassociate molecular interactions between fluorophores. For pHrodo probes, H3PO4 was added to the solution (430 mM) to make it acidic (pH ~ 4). For antibody conjugates, 2-mercaptoethanol (2-ME) was also added to uncouple S–S bonds between the light and heavy chains and the mixture was heated at 95 °C for 2 min. The fluorescence signal intensity of each sample was measured with a fluorescence spectrometer (Perkin-Elmer LS55, Perkin-Elmer, Shelton, CT, USA). The absorption spectrum was measured with a UV-Vis system (8453 Value UV-Visible Value System; Agilent Technologies, Santa Clara, CA).
Cell culture
A d-galactose receptor positive ovarian cancer cell line, SHIN3, and a HER2 gene transfected cell line, NIH3T3/HER2+ were used. The cells were grown in RPMI 1640 (Invitrogen Corporation, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen Corporation, Carlsbad, CA), 0.03% l-glutamine, 100 units mL−1 penicillin, and 100 µg mL−1 streptomycin in 5% CO2 at 37 °C.
Fluorescence microscopy studies
3T3/HER2+ cells (1 × 104) were plated on a cover glass-bottomed culture well and incubated for 16 h. Then, trastuzumab-TAMRA or trastuzumab-pHrodo were added to the medium (30 µg mL−1), and the cells were incubated for either 1, 8 or 24 h. Cells were washed once with PBS, and fluorescence microscopy was performed using an Olympus BX51 microscope (Olympus America, Inc., Melville, NY) equipped with the following filters: excitation wavelength 530 to 570 nm, emission wavelength 590 nm long pass. Transmitted light differential interference contrast images were also acquired.
In vivo fluorescence imaging in peritoneal tumor bearing mice
All procedures were carried out in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), National Research Council, and approved by the National Cancer Institute Animal Care and Use Committee. Intraperitoneal (i.p.) tumor implants were introduced by an i.p. injection of 2 × 106 SHIN3 (d-galactose receptor positive ovarian cancer cells) in female nude mice (National Cancer Institute Animal Production Facility, Frederick, MD). The imaging studies were performed 3 weeks after the injection of tumor cells.
d-Galactose targeting probe, pHrodo conjugated avidin (Av-pHrodo) or TAMRA conjugated avidin (Av-TM) was injected into tumor bearing nude mice (50 µg, i.p., n = 4 for each group). The mice were anesthetized with pentobarbital (30 mg kg−1 i.v., with 0.1% scopolamine butyl bromide) 1 or 2 h after the probe injection, and fluorescence endoscopy was performed using the Olympus EVIS ExERA-II CLV-180 system (Olympus Corp., Tokyo, Japan). The excitation wavelength used was 543/25 nm and the emission filter was 597.5/55 nm. The fluorescence intensities of tumor and background were measured on the images (n = 20) using Image-J software (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) and the tumor to background ratio was calculated. After the endoscopy, the mice were sacrificed with carbon dioxide and ex vivo imaging of the peritoneal implants was performed on a Maestro In-Vivo Imaging System (CRi, Inc., Woburn, MA). A band-pass excitation filter from 503 to 555 nm and a long-pass emission filter over 580 nm were used. The tunable emission filter was automatically stepped in 10 nm increments from 500 to 800 nm while the camera captured images at each wavelength interval with constant exposure. The spectral fluorescence images consisting of auto-fluorescence spectra and the spectra from pHrodo or TAMRA were obtained and then unmixed based on their spectral patterns.
Conclusion
We demonstrate that a dual activatable optical probe based on the combination of H-dimer formation and pH activation can achieve high TBR at early time points during in vivo molecular imaging.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Drs Masatoshi Takahashi and Masayuki Nishimura (Shimadzu Scientific Instruments) for great assistance with the mass spectroscopic analyses of the fluorescence dyes.
Footnotes
Electronic supplementary information (ESI) available: Supplemental Fig. 1; real-time fluorescence and white light endoscopy videos. See DOI: 10.1039/b917876g
References
- 1.Weissleder R, Pittet MJ. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce MC, Javier DJ, Richards-Kortum R. Int. J. Cancer. 2008;123:1979–1990. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosaka N, Ogawa M, Sato N, Choyke PL, Kobayashi H. J. Invest. Dermatol. 2009;129:2818–2822. doi: 10.1038/jid.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi H, Ogawa M, Kosaka N, Choyke PL, Urano Y. Nanomedicine. 2009;4:411–419. doi: 10.2217/nnm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longmire M, Kosaka N, Ogawa M, Choyke PL, Kobayashi H. Cancer Sci. 2009;100:1099–1104. doi: 10.1111/j.1349-7006.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. ACS Chem. Biol. 2009;4:535–546. doi: 10.1021/cb900089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Nat. Med. 2009;15:104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longmire MR, Ogawa M, Hama Y, Kosaka N, Regino CA, Choyke PL, Kobayashi H. Bioconjugate Chem. 2008;19:1735–1742. doi: 10.1021/bc800140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr Nat. Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa M, Regino CA, Choyke PL, Kobayashi H. Mol. Cancer Ther. 2009;8:232–239. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US 2008/0274907 A1. United States Pat. 2008
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.