Abstract
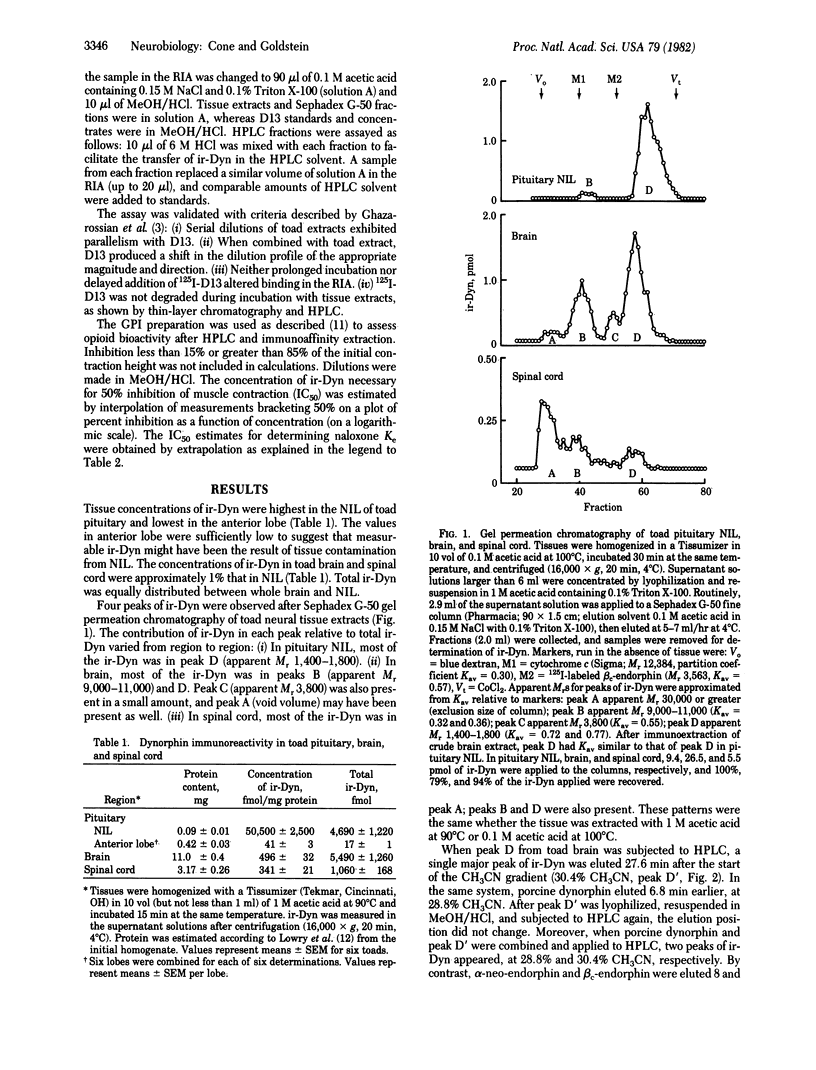
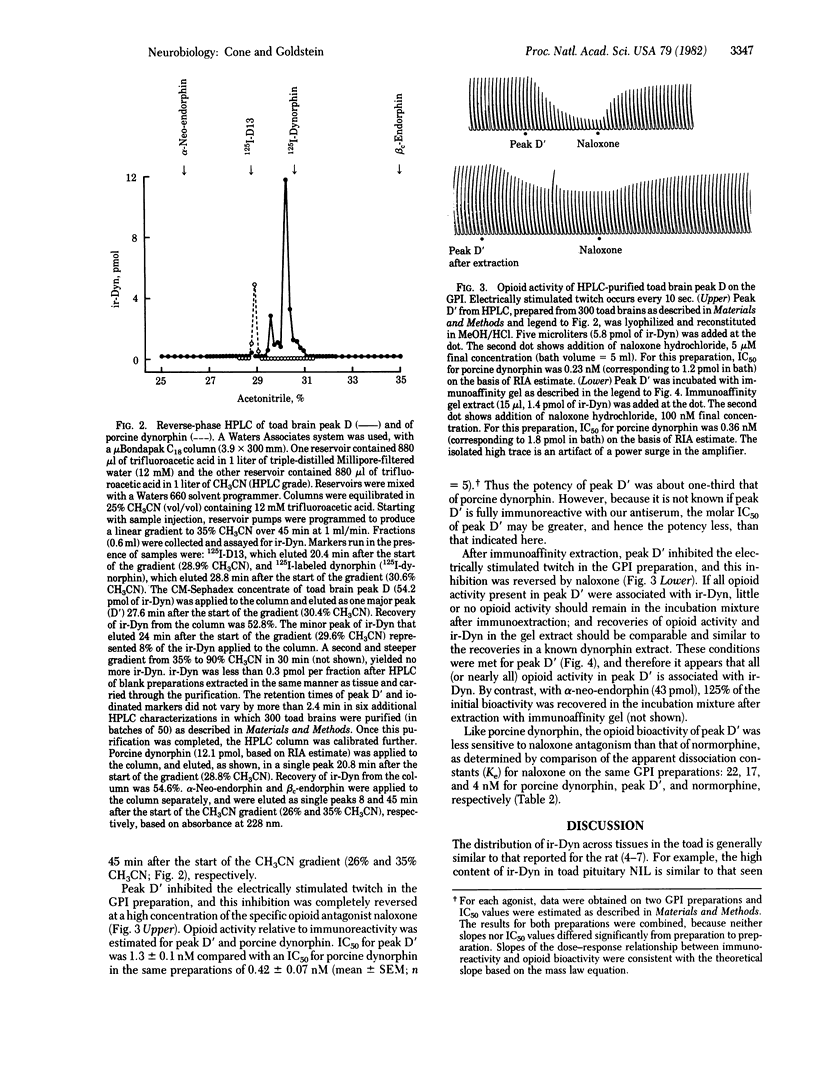
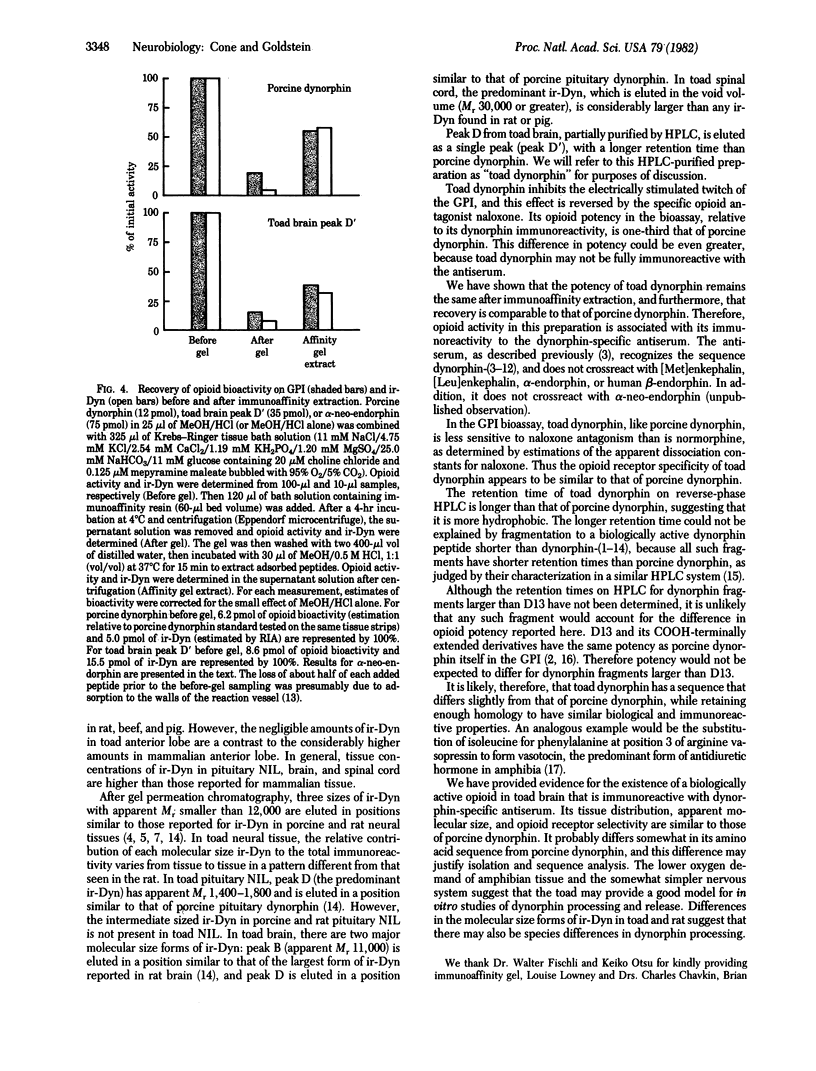
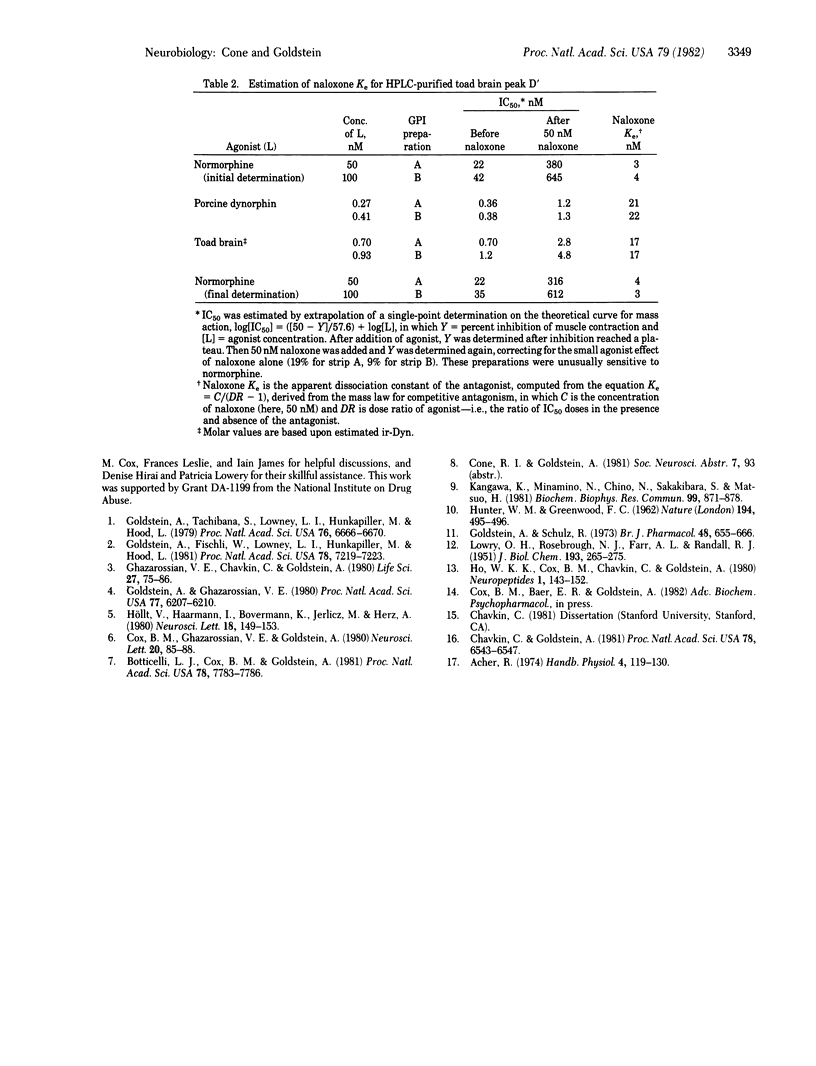
We have provided evidence for the existence of a biologically active opioid in toad (Bufo marinus) brain that is immunoreactive with antiserum raised against dynorphin (1-13). Compared with porcine dynorphin, this opioid is similar in apparent molecular weight on the basis of gel permeation chromatography and is more hydrophobic on the basis of high-performance liquid chromatography. After purification, its opioid biological activity was demonstrated on the guinea pig ileum myenteric plexus-longitudinal muscle preparation. It was found to be less potent, and to have a similar sensitivity to antagonism by naloxone, in comparison with porcine dynorphin. Because it is immunoreactive with antiserum specific for porcine dynorphin, it probably has considerable sequence homology. Generally, the tissue distribution of immunoreactive dynorphin in the toad is similar to that in the rat, with highest concentrations in the neurointermediate lobe of the pituitary. However, the anterior lobe of the toad pituitary contains considerably lower concentrations than are found in the rat anterior lobe. There appear to be three size classes of immunoreactive dynorphin in toad neural tissue, each with apparent molecular weight below 12,000, similar to the size classes of immunoreactive dynorphin found in pig and rat. However, in toad spinal cord (and possibly in brain) there is immunoreactive dynorphin of greater apparent molecular weight, which has not been reported in mammalian tissue. The contribution of each molecular size to the total immunoreactivity varies from tissue to tissue and is different from that observed in the rat.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botticelli L. J., Cox B. M., Goldstein A. Immunoreactive dynorphin in mammalian spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7783–7786. doi: 10.1073/pnas.78.12.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B. M., Ghazarossian V. E., Goldstein A. Levels of immunoreactive dynorphin in brain and pituitary of Brattleboro rats. Neurosci Lett. 1980 Oct 20;20(1):85–88. doi: 10.1016/0304-3940(80)90238-4. [DOI] [PubMed] [Google Scholar]
- Ghazarossian V. E., Chavkin C., Goldstein A. A specific radioimmunoassay for the novel opioid peptide dynorphin. Life Sci. 1980 Jul 7;27(1):75–86. doi: 10.1016/0024-3205(80)90021-1. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Fischli W., Lowney L. I., Hunkapiller M., Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Ghazarossian V. E. Immunoreactive dynorphin in pituitary and brain. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6207–6210. doi: 10.1073/pnas.77.10.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Schulz R. Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol. 1973 Aug;48(4):655–666. doi: 10.1111/j.1476-5381.1973.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Tachibana S., Lowney L. I., Hunkapiller M., Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Höllt V., Haarmann I., Bovermann K., Jerlicz M., Herz A. Dynorphin-related immunoreactive peptides in rat brain and pituitary. Neurosci Lett. 1980 Jun;18(2):149–153. doi: 10.1016/0304-3940(80)90318-3. [DOI] [PubMed] [Google Scholar]
- Kangawa K., Minamino N., Chino N., Sakakibara S., Matsuo H. The complete amino acid sequence of alpha-neo-endorphin. Biochem Biophys Res Commun. 1981 Apr 15;99(3):871–878. doi: 10.1016/0006-291x(81)91244-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]



