Abstract
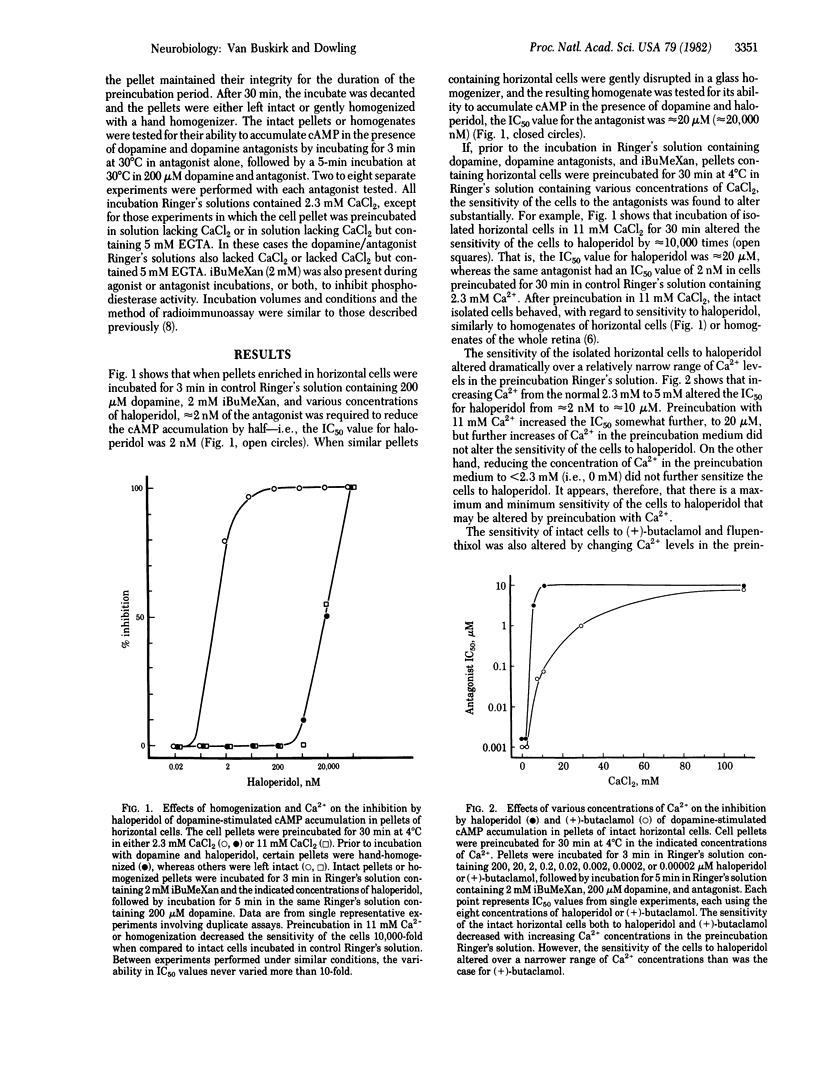
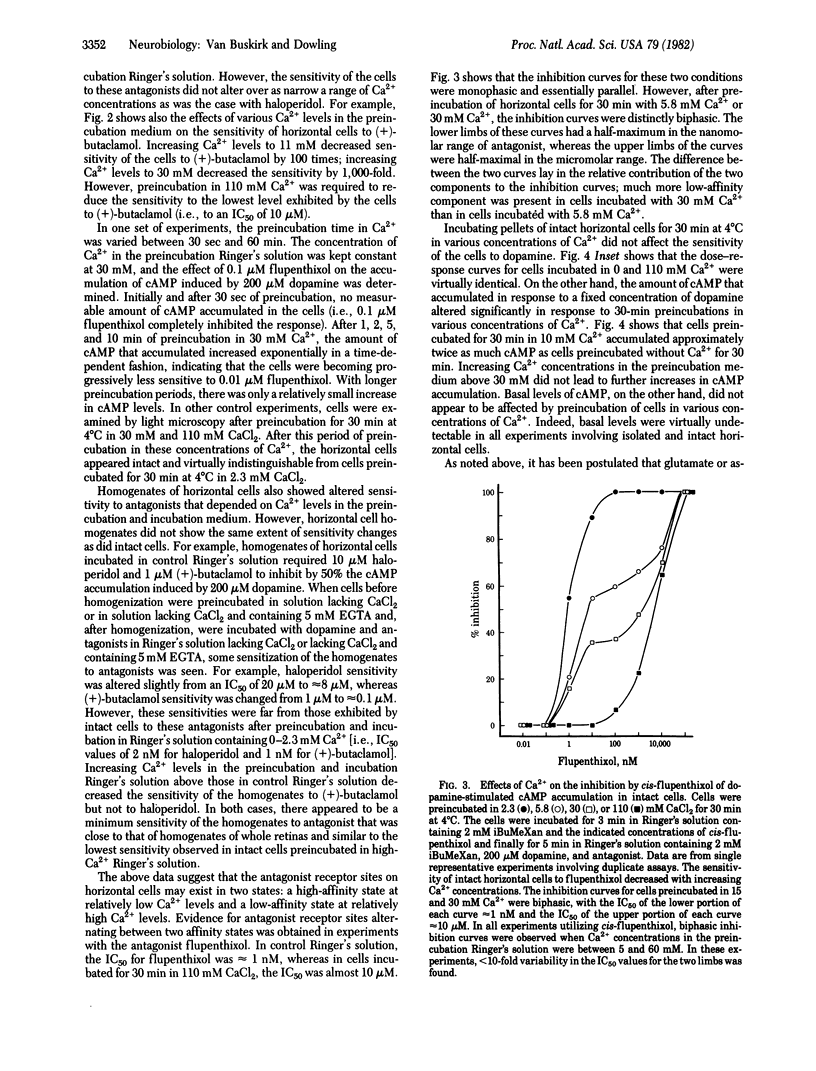
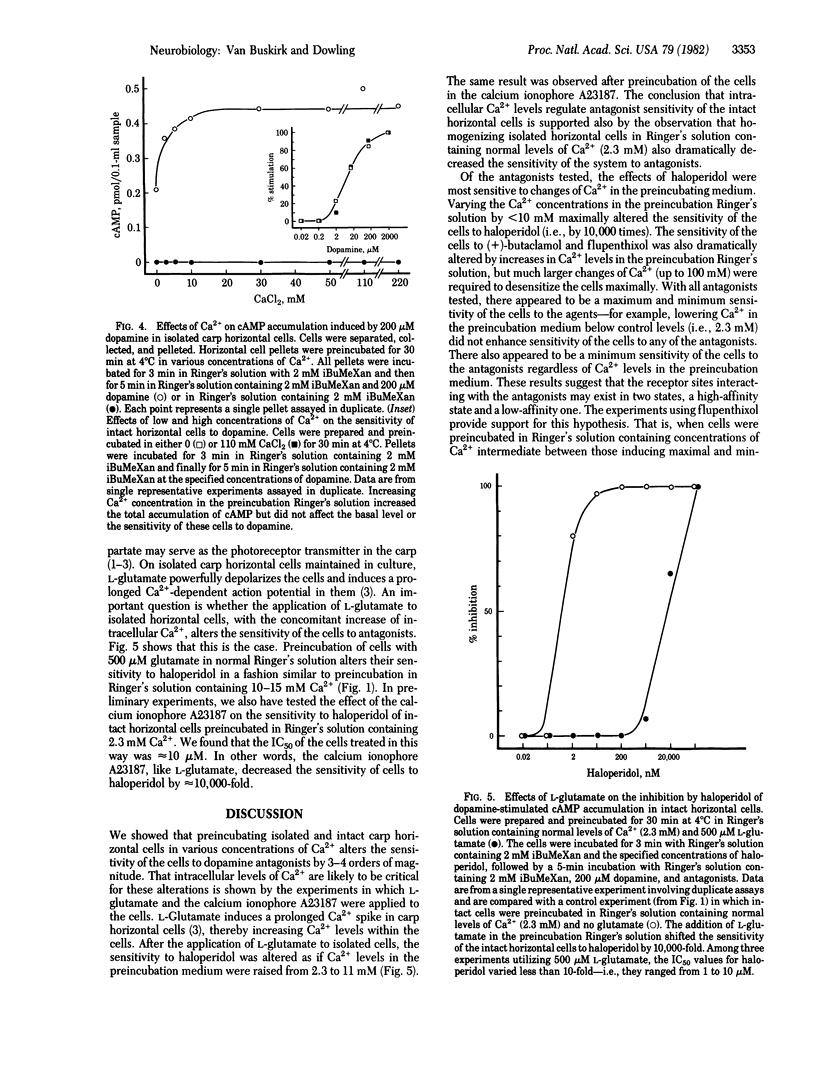
Horizontal cells of the carp retina possess dopamine receptors linked to adenylate cyclase. Isolated, intact horizontal cells respond to micromolar concentrations of dopamine, whereas nanomolar concentrations of haloperidol, (+)-butaclamol, and flupenthixol block the dopamine response. Preincubation in Ringer's solution containing increased levels of Ca2+ (5-110 mM) decreases the sensitivity of the cells to these antagonists by 1,000-10,000 times. Dopamine sensitivity of the cells is not affected by Ca2+ levels in the preincubation medium. Preincubation of the cells in Ringer's solution containing 500 microM L-glutamate, an agent that increases intracellular Ca2+ levels in intact horizontal cells, also decreases the sensitivity of the cells to haloperidol. These data suggest that antagonist sensitivity of intact horizontal cells may be regulated by intracellular Ca2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costa E., Gnegy M., Revuelta A., Uzunov P. Regulation of dopamine-dependent adenylate cyclase by a Ca++ binding protein stored in synaptic membranes. Adv Biochem Psychopharmacol. 1977;16:403–408. [PubMed] [Google Scholar]
- De Wied D., Kovács G. L., Bohus B., Van Ree J. M., Greven H. M. Neuroleptic activity of the neuropeptide beta-LPH62-77 ([Des-Tyr1]gamma-endorphin; DT gamma E). Eur J Pharmacol. 1978 Jun 15;49(4):427–436. doi: 10.1016/0014-2999(78)90317-5. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Watling K. J. Dopaminergic mechanisms in the teleost retina. II. Factors affecting the accumulation of cyclic AMP in pieces of intact carp retina. J Neurochem. 1981 Feb;36(2):569–579. doi: 10.1111/j.1471-4159.1981.tb01629.x. [DOI] [PubMed] [Google Scholar]
- Gnegy M., Treisman G. Effect of calmodulin on dopamine-sensitive adenylate cyclase activity in rat striatal membranes. Mol Pharmacol. 1981 Mar;19(2):256–263. [PubMed] [Google Scholar]
- Gnegy M., Uzunov P., Costa E. Participation of an endogenous Ca++-blinding protein activator in the development of drug-induced supersensitivity of striatal dopamine receptors. J Pharmacol Exp Ther. 1977 Sep;202(3):558–564. [PubMed] [Google Scholar]
- Hedden W. L., Jr, Dowling J. E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen J., Laduron P. Differential distribution of opiate and neuroleptic receptors and dopamine-sensitive adenylate cyclase in rat brain. Life Sci. 1977 Jan 15;20(2):281–288. doi: 10.1016/0024-3205(77)90323-x. [DOI] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo N. W., Schallert T., Overstreet D. H., Ling N. C., Ragan P., Reisine T. D., Yamamura H. I. Inhibition of in vivo 3H-spiperone binding by the proposed antipsychotic Des-Tyr1-gamma-endorphin. Eur J Pharmacol. 1979 Dec 20;60(4):359–364. doi: 10.1016/0014-2999(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Titeler M., Seeman P. Selective labelling of different dopamine receptors by a new agonist 3H-ligand: 3H-N-propylnorapomorphine. Eur J Pharmacol. 1979 Jun 15;56(3):291–292. doi: 10.1016/0014-2999(79)90187-0. [DOI] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling K. J., Dowling J. E. Dopaminergic mechanisms in the teleost retina. I. Dopamine-sensitive adenylate cyclase in homogenates of carp retina; effects of agonists, antagonists, and ergots. J Neurochem. 1981 Feb;36(2):559–568. doi: 10.1111/j.1471-4159.1981.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Wu S. M., Dowling J. E. L-aspartate: evidence for a role in cone photoreceptor synaptic transmission in the carp retina. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5205–5209. doi: 10.1073/pnas.75.10.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]


