Abstract
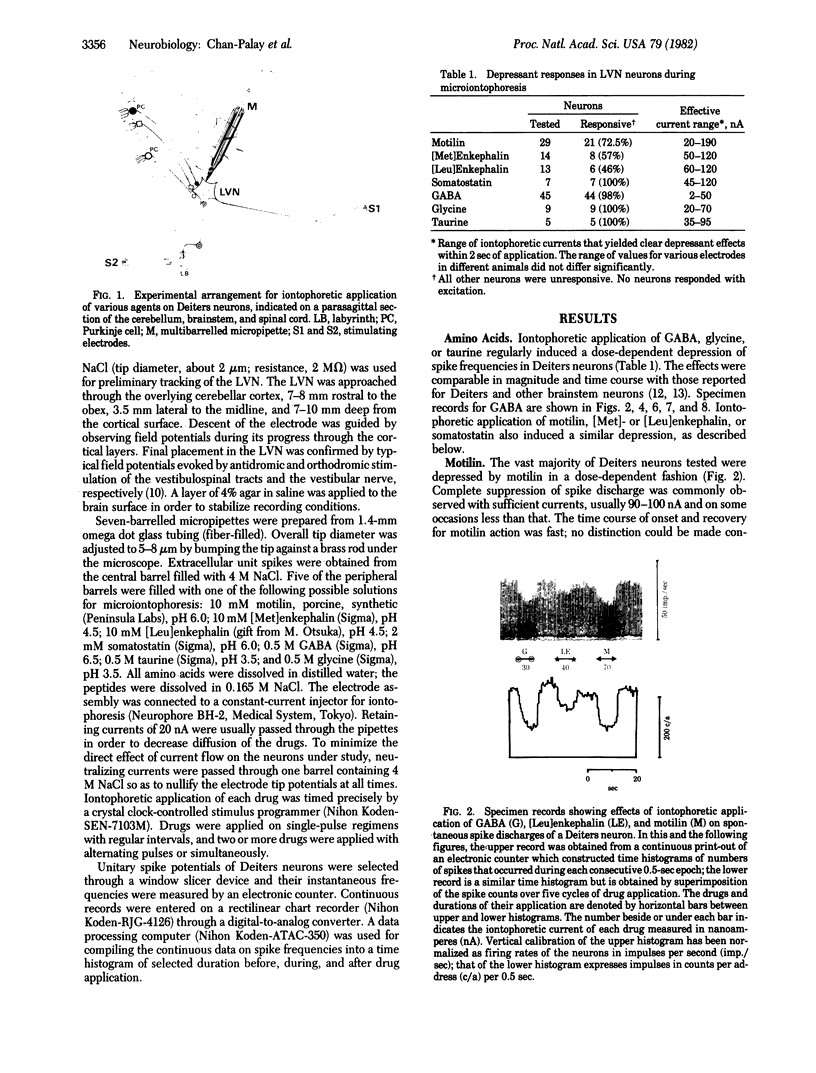
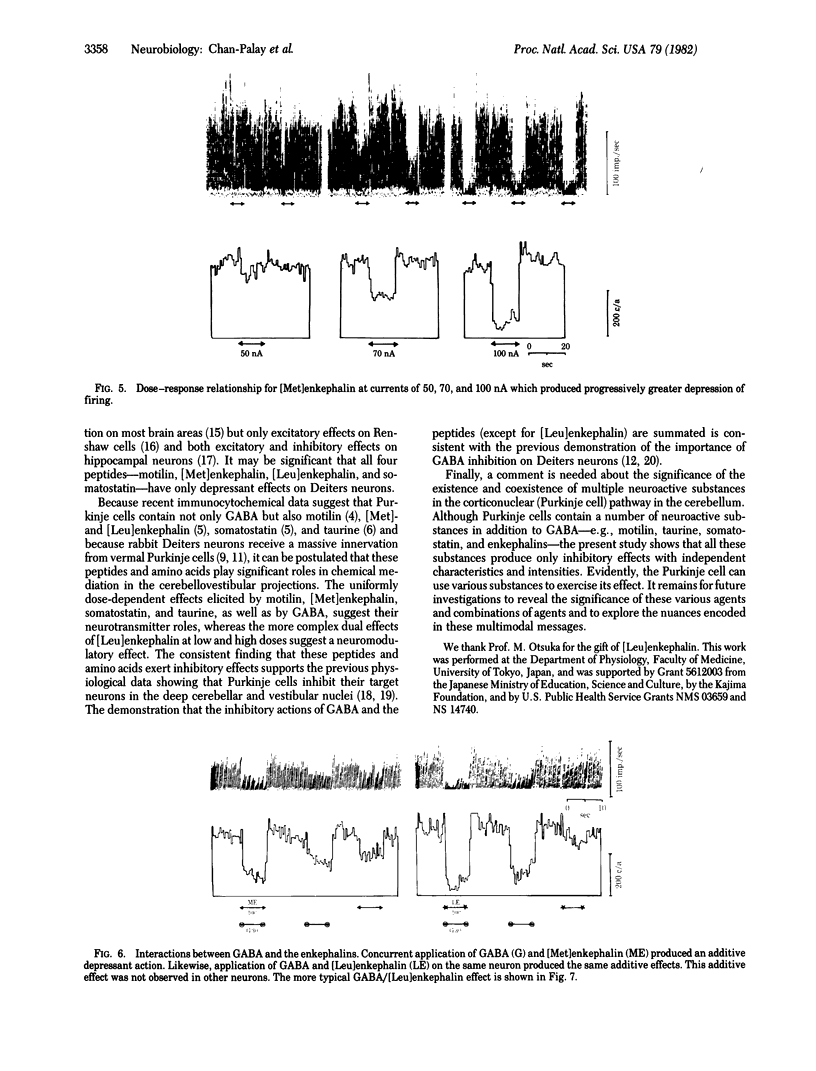
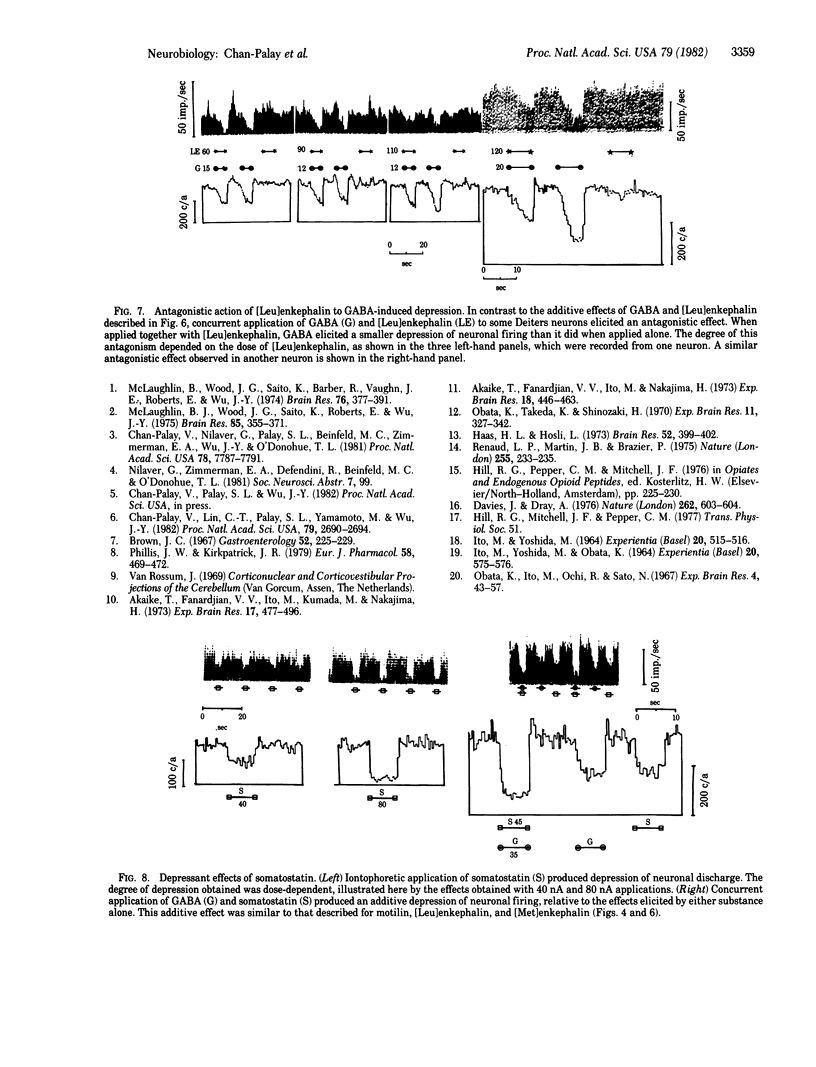
Motilin, [Met]enkephalin, [Leu]enkephalin, somatostatin, taurine, γ-aminobutyric acid (GABA), and glycine were tested for their effects on Deiters neurons of the lateral vestibular nucleus in rabbits. Iontophoresis was carried out with multibarrelled micropipettes. All four peptides and three amino acids produced depression of neuron firing. No facilitatory responses were observed. The depressant action of each peptide when iontophoresed alone was dose-dependent and was rapid in onset and recovery. Their characteristic actions suggest the possibility of their independent roles as strong inhibitors, although the experimental paradigm does not allow conclusions about the individual potency of each peptide. When GABA was administered together with motilin, [Met]enkephalin, or somatostatin, the effects of the peptide and GABA were additive, producing depression greater than that with application of either substance alone. When GABA was applied in conjunction with [Leu]enkephalin, more complex interactions were observed. At low iontophoretic currents, [Leu]enkephalin antagonized the action of GABA, producing a depression less than that of GABA alone and of considerably slower onset, suggesting an additional modulatory effect. These observations support the conclusion that all substances tested are chemical mediators in the lateral vestibular nucleus and [Leu]enkephalin may be a neuromodulator as well. Because recent immunocytochemical studies indicate that Purkinje cells in the cerebellar cortex are chemically heterogeneous and exhibit immunoreactivity for motilin, taurine, the enkephalins, and somatostatin, as well as for the GABA-synthesizing enzyme glutamic acid decarboxylase, it is suggested that the Purkinje cell projections to vestibular and cerebellar nuclei are multimodal in their chemical coding. The uniformly depressant action of the peptides and amino acids reported here is consistent with earlier observations that Purkinje cells exert an inhibitory influence on the vestibular and central cerebellar nuclei.
Keywords: Purkinje cells, peptides, iontophoresis, cerebellum
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Fanardjian V. V., Ito M., Kumada M., Nakajima H. Electrophysiological analysis of the vestibulospinal reflex pathway of rabbit. I. Classification of tract cells. Exp Brain Res. 1973 Jul 30;17(5):477–496. doi: 10.1007/BF00234863. [DOI] [PubMed] [Google Scholar]
- Akaike T., Fanardjian V. V., Ito M., Nakajima H. Cerebella control of the vestibulospinal tract cells in rabbit. Exp Brain Res. 1973 Dec 20;18(5):446–463. doi: 10.1007/BF00234131. [DOI] [PubMed] [Google Scholar]
- Brown J. C. Presence of a gastric motor-stimulating property in duodenal extracts. Gastroenterology. 1967 Feb;52(2):225–229. [PubMed] [Google Scholar]
- Chan-Palay V., Nilaver G., Palay S. L., Beinfeld M. C., Zimmerman E. A., Wu J. Y., O'Donohue T. L. Chemical heterogeneity in cerebellar Purkinje cells: existence and coexistence of glutamic acid decarboxylase-like and motilin-like immunoreactivities. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7787–7791. doi: 10.1073/pnas.78.12.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Dray A. Effects of enkephalin and morphine on Renshaw cells in feline spinal cord. Nature. 1976 Aug 12;262(5569):603–604. doi: 10.1038/262603a0. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res. 1973 Mar 30;52:399–402. doi: 10.1016/0006-8993(73)90680-x. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced rom the cerebellar cortex. Experientia. 1964 Oct 15;20(10):575–576. doi: 10.1007/BF02150304. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M. The cerebellar-evoked monosynaptic inhibition of Deiters' neurones. Experientia. 1964 Sep 15;20(9):515–516. doi: 10.1007/BF02154085. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Barber R., Vaughn J. E., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res. 1974 Aug 23;76(3):377–391. doi: 10.1016/0006-8993(74)90815-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in developing axonal processes and presynaptic terminals of rodent cerebellum. Brain Res. 1975 Mar 7;85(3):355–371. doi: 10.1016/0006-8993(75)90813-6. [DOI] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Obata K., Takeda K., Shinozaki H. Further study on pharmacological properties of the cerebellar-induced inhibition of deiters neurones. Exp Brain Res. 1970 Nov 26;11(4):327–342. doi: 10.1007/BF00237907. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Kirkpatrick J. R. Motilin excites neurons in the cerebral cortex and spinal cord. Eur J Pharmacol. 1979 Oct 15;58(4):469–472. doi: 10.1016/0014-2999(79)90318-2. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B., Brazeau P. Depressant action of TRH, LH-RH and somatostatin on activity of central neurones. Nature. 1975 May 15;255(5505):233–235. doi: 10.1038/255233a0. [DOI] [PubMed] [Google Scholar]