Abstract
Background
In most mammals, the testes provide a stable environment for spermatogenesis, which depends on a lower temperature than the core body temperature. It has been reported that mild testicular heating safely and reversibly suppresses spermatogenesis, and is under consideration for its potential application as a male contraceptive. Previously, we focused on the molecular mechanism of germ cell apoptosis and anti-apoptotic factors induced by heat treatment in humans and mice. However, the recovery process remains under investigation.
Results
In this study, we found that lipid droplets in mouse testes are dramatically increased after a brief period of scrotal hyperthermia, and gradually dissipate following temperature normalization. Analysis of the human testis proteome revealed nine proteins associated with lipid droplets. Two of them, ADFP (also known as ADRP and PLIN2) and TIP47 (also known as PLIN3) may participate in acute lipid droplet formation in mammalian testes. We show that Adfp expression is upregulated after scrotal heat treatment in mice. Surprisingly, we find Adfp lacking its 5′-UTR is observed in Adfp Δ1/Δ1 mouse testes, but is not detectable in liver.
Conclusions
These results reveal testis Adfp transcriptional regulation is tissue-specific, and is associated with lipid droplet accumulation induced by heat. The results also indicate that the testes could retain functional proteins through testes-specific transcriptional regulation.
Introduction
Spermatogenesis in mammals takes place in the testes [1]. The adult testes of most mammalian species, including humans, are located extra-abdominally and function at a temperature that is 2°C to 4°C lower than the core body temperature [2]. Testicular heating, which can be caused by saunas, hot baths or by wearing close-fitting underwear, could inhibit spermatogenesis [3], [4]. It has been reported that mild testicular heating safely and reversibly suppresses spermatogenesis in several mammalian species, including humans [5], [6], mice [7], [8], rats [9], [10], monkeys [11], bulls [12] and sheep [13]. Morphological changes in the testes after transient exposure of the scrota to heat are marked by germ cell loss in humans [14], rats [10], mice [7] and monkeys [11] via stage- and germ cell-specific apoptotic pathways. In recent studies, we examined the suppressive effect of testicular heat treatment on spermatogenesis in humans [14] and mice [15]. We have also studied the molecular mechanism of the effect of heat on spermatogenesis, primarily in germ cells [15], [16].
In the present study, we found an abnormality in the testis and epididymis after a temporary heat stress. This result could not be explained by the effect of heat on germ cells alone. Indeed, morphological abnormalities of the Sertoli cells could also affect spermatogenesis [17]–[19]. In mammalian testes, lipid droplets are mainly located in Leydig cells and Sertoli cells, although some lipid droplets have been reported in germ cells, specifically in the residual body of elongated spermatids [20], [21]. It is known that lipid droplets accumulate in severe testicular injury, such as cryptorchidism [22], irradiation [23] and vitamin E deficiency [24].
Recently, transient lipid droplet accumulation following mild testicular hyperthermia has been reported in rats [25], [26]. Lipid droplets contain a core of neutral lipid surrounded by a phospholipid monolayer and are coated by specific proteins [27]. In the present study, we used a mouse model for scrotal transient heat stress as previously described [16], [28], [29]. We determined that testicular lipid droplets dramatically increased following a brief period of scrotal hyperthermia, and that they gradually regressed to normal levels after a week of recovery. Following analysis of the human testis proteome (data not published), we focused on adipose differentiation-related protein (ADFP, also known as ADRP and PLIN2) and tail-interacting protein of 47 kDa (TIP47, also known as PLIN3), which are members of the perilipin (PLIN) family of lipid droplet associated proteins. ADFP binding to lipid droplets is generally limited to adipocytes, steroidogenic cells and other tissues which accumulate lipids [27]. It has been demonstrated that absence of ADFP expression reduced lipid droplet formation and can protect against fatty liver [30]. TIP47 expression occurs in the same cell types as ADFP [27] and can functionally compensate for it [31], [32].
Both ADFP and TIP47 may participate in acute lipid droplet formation in mammalian testes. In this study, we prove that Adfp expression is up-regulated in the testes after heat treatment in mice. Surprisingly, we find testicular Adfp expression was not affected in Adfp Δ1/Δ1 gene trapping mouse, but hepatic Adfp expression is ablated. These results reveal that Adfp transcription is specifically regulated in the testes, and associated with lipid droplet accumulation induced by heat treatment.
Results
Heat shock of 42°C for 30 min reversibly suppresses spermatogenesis
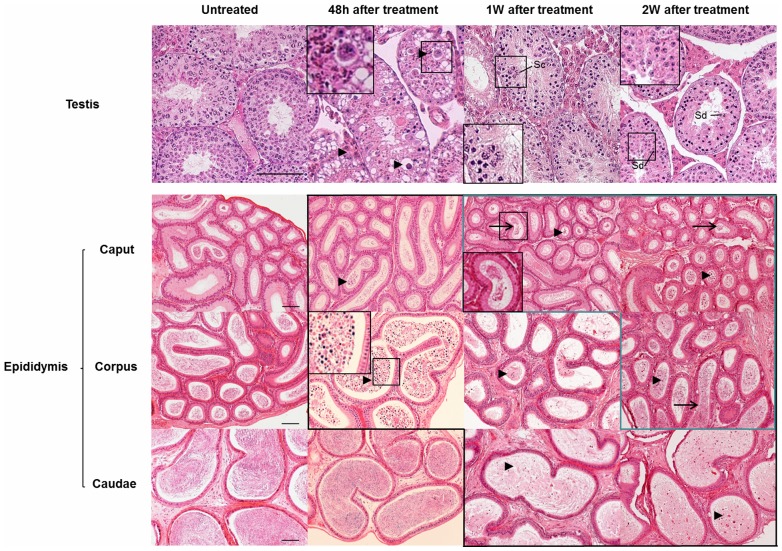
Testicular and epididymal tissues were harvested from four groups of mice: 12 hours, 48 hours, 1 week, 2 weeks and 6 weeks after a 42°C heat shock for 30 min, and untreated controls. Histological examination with hematoxylin and eosin (Figure 1) revealed acidophilic, coagulated germ cells in the seminiferous epithelium 48 hours after heat treatment. Most spermatocytes were affected, and the corpus and caput of the epididymis appeared full of abnormal cells, which were probably cast off from spermatogenic epithelium. After a 1-week recovery period, new spermatocytes appear in injured tubules which did not contain spermatid cells, and abnormal cells were still visible in the epididymis. Two weeks after heat treatment, most spermatogenic tubules recovered with the appearance of spermatid cells, and sperm was seen in the corpus and caput of the epididymis. After 6 weeks, the morphology of all seminiferous epithelium had completely recovered (Figure S1). We have previously reported that heat can cause germ cells to undergo apoptosis and agglutination [14]. In the present study, the TUNEL assay revealed that germ cells that agglutinate undergo apoptosis in the mouse (Figure S2). We further analyzed the testosterone and LH levels in two groups that were untreated and 48 hours after treatment. Serum testosterone and LH levels were significantly increased after treatment (Figure S3), which may have been caused by the loss of germ cells in the seminiferous tubule. This finding indicates that steroidogenesis is activated after heat injury.
Figure 1. Morphology of normal and heat treated testes was revealed by hematoxylin and eosin staining.
Microscopic examination reveals that degenerated and apoptotic germ cells (black triangles) are visible in testes harvested 48 hours after a 42°C heat shock treatment, and at different time points in the epididymis (picture with black frame). Sperm (black arrows) are detectable in the recovered epididymis (picture with blue frame). Scale bar = 50 µm, Sc: spermatocyte, Sd: spermatid. A high magnification image is shown in the black rectangle.
Lipid droplets are dramatically increased after brief scrotal hyperthermia and regress gradually
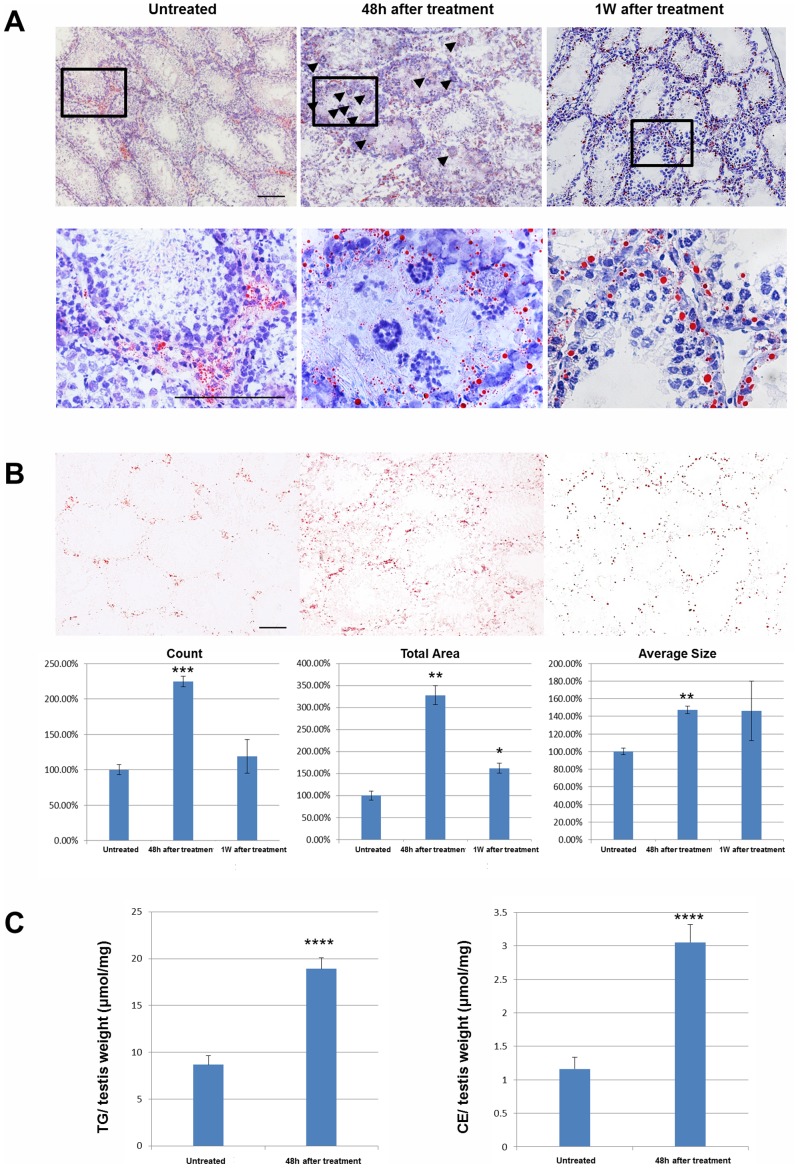
Lipid droplets were visualized by oil red O (ORO) histological staining. In normal mouse testes, lipid droplets in the seminiferous epithelium were generally small in size and number, with some variation was evident between the different spermatogenic stages. Forty-eight hours after heat treatment, there was a dramatic accumulation of lipid droplets. However, after 1 week of recovery, the number of lipid droplets had returned to the normal level, although a few large lipid droplets remained at the base of the seminiferous epithelium (Figure 2A). These observations were confirmed by the measurement of the ORO stained lipid droplet number, total area and average size using ImageJ software (Figure 2B). We further analyzed the lipid composition of the testes in untreated mice and those 48 hours after treatment. The cholesterol ester (TE) and triglyceride (TAG) levels were significantly increased after treatment (Figure 2C).
Figure 2. Morphological examination of lipid droplets was performed by oil red O staining (red) and counterstaining using hematoxylin (blue).
A high magnification image of regions in the black rectangle is shown in the lower panels. Black triangles point to degenerated and apoptotic germ cells (A). Oil red O staining was quantified by counting number, total area, and average size of staining from discontinuous slides, using three samples per group (B). Bars represent the mean ± SEM, Scale bar = 50 µm. (C) The lipid composition (cholesterol ester [CE] and triglyceride [TG]) in the droplets of testes from the untreated and the 48 hours after treatment groups. (*P<0.01, **P<0.001, ***P<0.0001, ****P<0.00001 compared to the untreated control).
Identification of lipid particle associated proteins in testis by bioinformatic analysis of the human testis proteome
To explore the molecular mechanism associated with lipid droplet accumulation, the identity of proteins associated with lipid particles was considered. We analyzed the cellular component of the human testis proteome which contained 7346 proteins (data not published) using Gene Ontology from the David bioinformatics database (http://david.abcc.ncifcrf.gov/) [33]. A list of human testicular proteins associated with lipid particles, and their mouse homologs are provided in Table 1. Two candidates from the PLIN family of lipid droplet associated proteins, ADFP and TIP47, were further researched.
Table 1. List of proteins associated with testicular lipid particles.
| Human EntrezGene ID | Mouse EntrezGene ID | Gene Name | Gene Description |
| 51099 | 67469 | ABHD5(CGI-58) | abhydrolase domain containing 5 |
| 857 | 12389 | CAV1 | caveolin 1, caveolae protein, 22 kDa |
| 858 | 12390 | CAV2 | caveolin 2 |
| 11104 | 23924 | KATNA1 | katanin p60 (ATPase containing) subunit A 1 |
| 123 | 11520 | PLIN2 (ADFP) | perilipin 2,adipose differentiation-related protein,Adipophilin |
| 729359 | 57435 | PLIN4 (S3–12) | perilipin 4, Plasma membrane associated protein, S3–12 |
| 10226 | 66905 | PLIN3 (TIP47) | perilipin 3,Cargo selection protein TIP47 |
| 27339 | 28000 | PRPF19 | PRP19/PSO4 pre-mRNA processing factor 19 homolog (S. cerevisiae) |
| 10280 | 18391 | SIGMAR1 | sigma non-opioid intracellular receptor 1 |
| 90627 | 243362 | STARD13 | StAR-related lipid transfer (START) domain containing 13 |
Adfp and Tip47 are located on the surface of lipid droplets in mouse testis after heat treatment
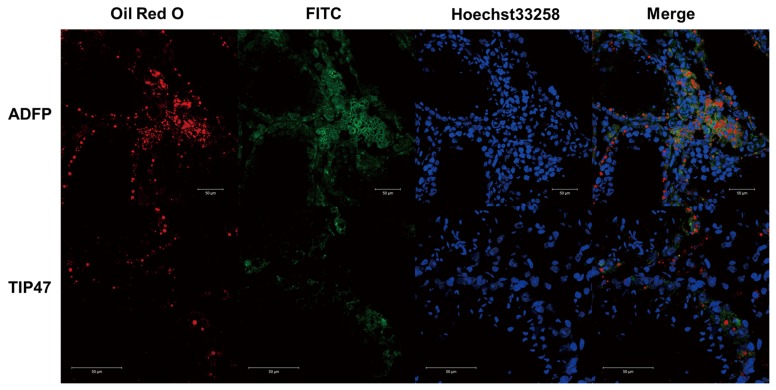
After 1 week of recovery, Adfp surrounded most lipid droplets, especially the large lipid droplets at the base of the seminiferous epithelium. Tip47 was not located at the surface of large lipid droplets, but was closely associated with a few small lipid droplets and dispersed through the cytoplasm in a variety of cell types in the seminiferous epithelium (Figure 3). In comparison with untreated controls (data not shown), Adfp was located on the surface of lipid droplets and Tip47 had more generalized expression in Leydig cells, Sertoli cells and germ cells in testes from different time points after heat treatment.
Figure 3. Immunofluorescence of testicular ADFP and TIP47 in the group 1 week after treatment.
ADFP and TIP47 are labeled by FITC (green), lipid droplets are labeled by Oil red O (red), and nuclei are labeled by Hoechst 33258 (blue) in frozen sections of fresh testicular tissue. Scale bar = 50 µm.
Adfp expression was upregulated by heat treatment
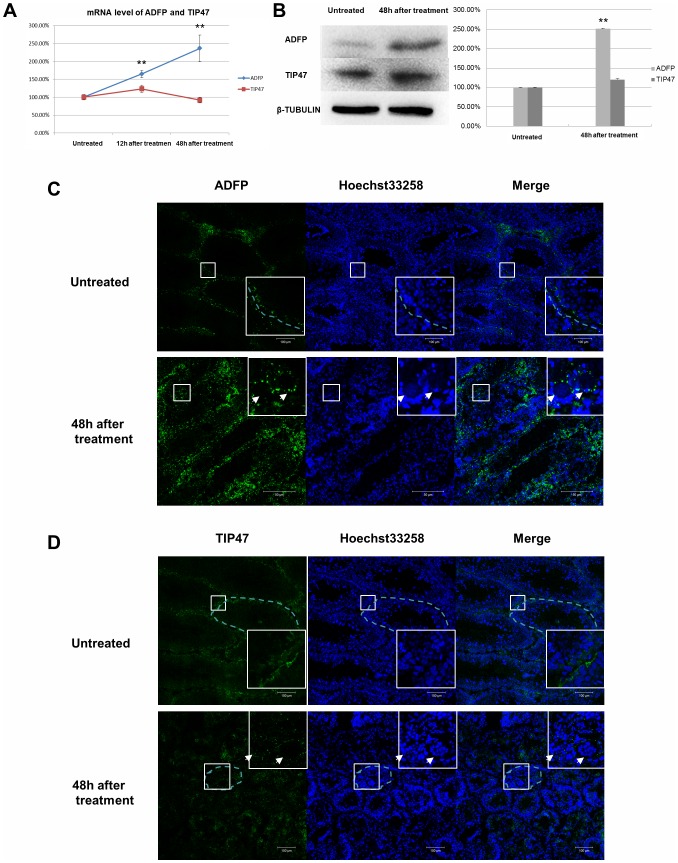
The level of Adfp mRNA was significantly increased after heat treatment, as determined by qRT-PCR (Figure 4A). Western blot analysis revealed that the quantity of Adfp protein was also upregulated (Figure 4B). Tip47 expression in the testes was not notably changed (Figure 4A, B). Indirect immunofluorescence revealed that Adfp staining was increased in seminiferous tubules, especially in the impaired tubules (Figure 4C). Interestingly, we observed that the Tip47 staining appeared as small round signals that surrounded acidophilic, coagulated germ cells, which was similar to the pattern of Adfp expression (Figure 4D).
Figure 4. The mRNA of Adfp and Tip47 level was compared using quantitative RT-PCR.
Adfp expression increased 12 hours and 48 hours after heat treatment (A). The protein expression level of Adfp and Tip47 was measured by Western blotting. Adfp is increased in the mouse testes 48 hours after heat treatment (B). Indirect immunofluorescence of Adfp (C) and Tip47 (D) reveals increasing intensity of Adfp 48 hours after heat treatment, especially in the seminiferous tubules associated with large numbers of degenerated and apoptotic germ cells (white arrow). **P<0.001, Scale bar = 100 µm.
Testicular Adfp expression in the AdfpΔ1/Δ1 mouse
Plin2Gt(OST170322)Lex mice were generated by insertion of a trapping cassette in the intron between exons 1 and 2 (exon 2 contains the translation start site) of the Adfp gene (Figure 5A). Homozygote Plin2Gt(OST170322)Lex mice will subsequently be referred to as Adfp Δ1/Δ1 mice. RT-PCR data using primers targeted to exons 3–8 and exons 6–8 of murine Adfp shows that Adfp transcripts were present in the testes, but not the livers of Adfp Δ1/Δ1 mice. Additionally, data using primers targeted to exons 1–3 of mouse Adfp shows an absence of 5′-UTR mRNA from both the testes and liver from Adfp Δ1/Δ1 mice (Figure 5B).
Figure 5. The trapping cassette of Adfp Δ1/Δ1 mice is inserted in the intron between exons 1 and 2, before the Adfp translation start site.
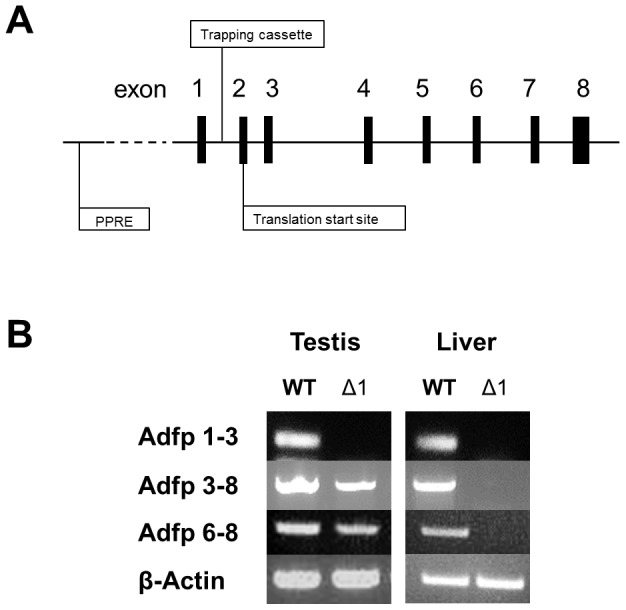
The peroxisome proliferator-activated receptor responsive element (PPRE) is located ∼2,000 bp upstream of the transcription start site (A). RT-PCR products are presented using primers targeted to exons 1–3, exons 3–8, and exons 6–8 of the Adfp transcripts of testes and liver from Adfp Δ1/Δ1 mice and wild type mice. β-actin was amplified as the control gene (B).
Discussion
Lipid droplets accumulation increasing in testis may supply the material for spermatogenic recovery
Lipid transport from Sertoli cells to germ cells is important for spermatogenesis [34], [35]. Recently, it has been demonstrated in vitro that lipid β-oxidation is a major pathway for the production of ATP, and that phagocytosis of apoptotic spermatogenic cells increases ATP production and lipid droplet accumulation in the Sertoli cells of mouse testes [36]. In cryptorchid rat testes, which could be considered as hyperthermic, the amount and concentration of triglycerides and cholesterol esters increased several fold [37]. In the current study, we found that transient scrotal hyperthermia induced lipid droplet accumulation in murine testes. It is known that distinct testicular injuries can induce lipid droplet accumulation in addition to hyperthermia, such as irradiation [23] and vitamin E deficiency [24]. We hypothesized that, after testicular injury, Sertoli cells could phagocytose and degrade apoptotic cells, storing the excess lipid component in lipid droplets. During recovery from injury, lipid droplets are then utilized to provide energy for metabolism and biosynthesis of germ cells.
Testicular proteins located in lipid droplets
In our list of testicular proteins associated with lipid droplets, Caveolin1 and Caveolin 2, which are transmembrane scaffolding proteins from the Caveolin protein family, may play a transport function of caveolae [38]. Another protein, ABHD5 (also known as CGI-58) channels fatty acids released from the hydrolysis of stored triacylglycerols into phospholipids [39]. Notably, ADFP, TIP47and S3–12 (also known as PLIN4), which are all members of the PLIN family of lipid droplet associated proteins, could bind the membrane of lipid droplets and may have a role in lipid droplet formation. The newly identified member of the PLIN family, PLIN4, which is structurally different with typical PLIN proteins [27] is not further discussed in this research.
Modified testicular ADFP expression is an independent mechanism of lipid regulation from liver
An evolutionarily conserved peroxisome proliferator-activated receptor (PPAR)-response element is located ∼2,000 bp upstream of the transcription start site in mouse and human ADFP, and ADFP is known to be transcriptionally regulated by PPAR-alpha (PPARα) in mouse liver [40]. In this study, we detected an Adfp transcript in the testes of Adfp Δ1/Δ1 mice. Another Adfp mutant mouse model, which lacks exons 2 and 3, is a complete knock-out, with no Adfp transcripts detectable in the testes [41]. Furthermore, there was no Adfp transcript detectable in the liver of these mice, or in the liver of Adfp Δ1/Δ1 mice. These results suggest that the transcriptional regulatory region for Adfp in testicular tissue is near exon 2, which interestingly contains the translation start site for this gene. The transcriptional control of testicular Adfp expression may not be as sensitive as hepatic Adfp to the multiple ligands for PPARα from blood. It is still not clear why 5′UTR is missing in Adfp transcripts in the testis of Adfp Δ1/Δ1 mice. Our results indicate that the mechanism of lipid regulation in the testis is independent from that in the liver. This phenomenon might contribute to the maintainance of local lipid homeostasis in the testis.
Materials and Methods
Animals
This mouse study was approved by the ethics committees of Nanjing Medical University and was in accordance with the national and international guidelines. Plin2Gt(OST170322)Lex mice were bred at the animal center of Nanjing Medical University (Nanjing, China), and were originally transferred from the Mutant Mouse Regional Resource Center (MMRRC). The strain information can be found at the following website: http://www.mmrrc.org/strains/11683/011683.html. Mice were housed under specific pathogen-free conditions with unlimited access to food and water. Genotyping was performed using the provided MMRRC protocol (http://www.mmrrc.org/strains/11683/di_protocol.pdf).
Induction of transient heat stress
Male, wild-type mice, aged 8 to 9 wk were subjected to a single heat stress of 42°C for 30 min. Each animal was anesthetized, and the lower third of the body, including the hind legs, tail, and scrotum, were submerged in a 42°C water bath. Control animals were anesthetized and left at room temperature. After 30 min, each animal was dried, and returned to its cage.
RNA extraction, RT-PCR and quantitative RT-PCR
Total RNA was extracted from adult testes from each control and heated group using the RNeasy Plus Micro Kit with on-column DNase digestion (Qiagen Ltd., Crawley, West Sussex, UK). Random primed cDNA was prepared using the PrimeScript™ RT Master Mix (TaKaRa Bio Inc., Otsu, Japan). Primer sequences are presented in the Supporting Information (Table S1). The various cDNAs were PCR-amplified with specific primers in 20 µl of GoTaq Green Master Mix (Promega Corporation, CA, USA). The amplification conditions consisted of initial denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec. The final extension was carried out at 72°C for 10 min. The PCR products were analyzed by 1.5% w/v agarose gel electrophoresis, using mouse β-actin as the control gene. Quantitative RT-PCR was performed using the ABI PRISM 7300 sequence detection system (Applied Biosystems) using the following thermal cycling conditions: 30 sec at 95°C; followed by 40 cycles of 5 sec at 95°C, 31 sec at 55°C, and 30 sec at 72°C. Beta-actin was amplified in parallel as a loading control.
Western blot analysis
Samples that contained 100 µg of protein from adult mouse testes were electrophoresed and transferred to a nitrocellulose membrane (Amersham Biosciences, Uppsala, Sweden). The membranes were blocked and then incubated overnight with primary antibodies against Adfp (Abcam, Cambridge, UK) and Tip47 (Abcam) at a dilution of 1∶200. Membranes were then washed and incubated for 1 h with a horseradish peroxidase conjugated anti-rabbit IgG secondary antibody at a dilution of 1∶2,000 (Beijing Zhong-Shan Biotechnology Co.). Proteins were detected using an ECL kit and AlphaImager (Amersham).
Oil red O staining
ORO (0.5%) was dissolved in 70% ethanol and filtered to remove undissolved particles. For lipid staining, cells were fixed in 4% paraformaldehyde in PBS for 15 min, washed with PBS, then incubated with ORO for 10 min, and finally washed in PBS to remove unincorporated staining solution. Staining was assessed by bright-field microscopy and quantified by ImageJ 1.43u software (National Institutes of Health, Bethesda, Maryland, USA) after appropriate thresholding. There were four samples in each group (untreated, 48 hours after treatment and 1 week after treatment). Three discontinuous slides of each mouse from each group were used for the analysis.
Lipid profile measurements
The testes from four mice in each group (untreated and 48 hours after treatment) were collected and the lipid profiles were determined with an enzymatic analysis using a tissue triglyceride assay kit and a cholesterol ester assay kit (Applygen, Beijing, China).
Serum testosterone and LH assessment
Serum samples from four mice in each group (untreated and 48 hours after treatment) were collected and determined with a RD Mouse Testosterone(T) Elisa kit and a RD Mouse Luteinizing Hormone Elisa kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
TUNEL assay
DNA fragmentation as an index of apoptosis was determined by the TUNEL assay (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's specifications using paraffin sections of the mouse testis.
Indirect immunofluorescence and combined oil red O and immunofluorescence staining
Frozen sections of fresh testicular tissue were fixed with 4% paraformaldehyde in PBS for 40 min and permeabilized with 0.2% Triton X-100 in PBS for 20 min at 37°C. After blocking in PBS containing calf serum for 2 h, samples were incubated overnight with a 1∶200 dilution of antibodies against Adfp (Abcam) and Tip47 (Abcam) at 4°C. Samples were then incubated with an anti-rabbit IgG secondary antibody labeled with FITC (Beijing Zhong-Shan Biotechnology Co.) at a 1∶100 dilution for 1 h at room temperature. Combined ORO and immunofluorescence staining was performed as previously described, with a slight modification [42]. Briefly, after ORO staining was performed, the above immunofluoresence protocol was followed, continuing from the blocking step. ORO can be excited at wavelengths between 540 and 580 nm, similar to Texas red. Slides were viewed with an LSM710 confocal microscope (Zeiss, Germany) driven by ZEN2009 software (Zeiss).
Statistical analysis
All experiments were repeated at least three times. The differences between treatment and control groups were analyzed using one-way ANOVA. Values of P<0.05were considered to be statistically significant. All data represent the mean plus or minus the standard error of the mean.
Supporting Information
The List of Primers.
(XLS)
The morphology of seminiferous epithelium after treated 6 weeks.
(TIF)
The TUNEL assay of seminiferous epithelium after treated 48 hours.
(TIF)
The serum testosterone and LH levels in two groups (untreated and 48 hours after treatment).
(TIF)
Funding Statement
This work was supported by China National 973 Program Project (2011CB944304). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bonde JP (2010) Male reproductive organs are at risk from environmental hazards. Asian J Androl 12: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ivell R (2007) Lifestyle impact and the biology of the human scrotum. Reprod Biol Endocrinol 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch R, Lewis-Jones DI, Machin DG, Desmond AD (1986) Improved seminal characteristics in infertile men after a conservative treatment regimen based on the avoidance of testicular hyperthermia. Fertil Steril 46: 476–479. [DOI] [PubMed] [Google Scholar]
- 4. Mieusset R, Bujan L (1995) Testicular heating and its possible contributions to male infertility: a review. Int J Androl 18: 169–184. [DOI] [PubMed] [Google Scholar]
- 5. Mieusset R, Bujan L (1994) The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int J Androl 17: 186–191. [DOI] [PubMed] [Google Scholar]
- 6. Kandeel FR, Swerdloff RS (1988) Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril 49: 1–23. [DOI] [PubMed] [Google Scholar]
- 7. Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, et al. (2001) Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod 65: 229–239. [DOI] [PubMed] [Google Scholar]
- 8. Hand JW, Walker H, Hornsey S, Field SB (1979) Effects of hyperthermia on the mouse testis and its response to X-rays, as assayed by weight loss. Int J Radiat Biol Relat Stud Phys Chem Med 35: 521–528. [DOI] [PubMed] [Google Scholar]
- 9. Chowdhury AK, Steinberger E (1964) A Quantitative Study of the Effect of Heat on Germinal Epithelium of Rat Testes. Am J Anat 115: 509–524. [DOI] [PubMed] [Google Scholar]
- 10. Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, et al. (1999) Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology 140: 1709–1717. [DOI] [PubMed] [Google Scholar]
- 11. Lue Y, Wang C, Liu YX, Hikim AP, Zhang XS, et al. (2006) Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis). J Clin Endocrinol Metab 91: 539–545. [DOI] [PubMed] [Google Scholar]
- 12. Barth AD, Bowman PA (1994) The sequential appearance of sperm abnormalities after scrotal insulation or dexamethasone treatment in bulls. Can Vet J 35: 93–102. [PMC free article] [PubMed] [Google Scholar]
- 13. Mieusset R, Quintana Casares P, Sanchez Partida LG, Sowerbutts SF, Zupp JL, et al. (1992) Effects of heating the testes and epididymides of rams by scrotal insulation on fertility and embryonic mortality in ewes inseminated with frozen semen. J Reprod Fertil 94: 337–343. [DOI] [PubMed] [Google Scholar]
- 14. Wang C, Cui YG, Wang XH, Jia Y, Sinha Hikim A, et al. (2007) transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab 92: 3292–3304. [DOI] [PubMed] [Google Scholar]
- 15. Zhu YF, Cui YG, Guo XJ, Wang L, Bi Y, et al. (2006) Proteomic analysis of effect of hyperthermia on spermatogenesis in adult male mice. J Proteome Res 5: 2217–2225. [DOI] [PubMed] [Google Scholar]
- 16. Zhu H, Cui Y, Xie J, Chen L, Chen X, et al. (2010) Proteomic analysis of testis biopsies in men treated with transient scrotal hyperthermia reveals the potential targets for contraceptive development. Proteomics 10: 3480–3493. [DOI] [PubMed] [Google Scholar]
- 17. Grinspon RP, Rey RA (2010) Anti-mullerian hormone and sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr 73: 81–92. [DOI] [PubMed] [Google Scholar]
- 18. Kyronlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, et al. (2011) GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol 333: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mark M, Yoshida-Komiya H, Gehin M, Liao L, Tsai MJ, et al. (2004) Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci U S A 101: 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kerr JB, De Kretser DM (1975) Cyclic variations in Sertoli cell lipid content throughout the spermatogenic cycle in the rat. J Reprod Fertil 43: 1–8. [DOI] [PubMed] [Google Scholar]
- 21. Paniagua R, Rodriguez MC, Nistal M, Fraile B, Amat P (1987) Changes in the lipid inclusion/Sertoli cell cytoplasm area ratio during the cycle of the human seminiferous epithelium. J Reprod Fertil 80: 335–341. [DOI] [PubMed] [Google Scholar]
- 22. Nistal M, Regadera J, Winitzky P, Tejerina E, Chemes H (2005) Granular changes in Sertoli cells in children and pubertal patients. Fertil Steril 83: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 23. Abreu I, David-Ferreira JF (1982) Fine structure of seminiferous tubules from prenatally irradiated rats. Cell Tissue Res 222: 143–152. [DOI] [PubMed] [Google Scholar]
- 24. Bensoussan K, Morales CR, Hermo L (1998) Vitamin E deficiency causes incomplete spermatogenesis and affects the structural differentiation of epithelial cells of the epididymis in the rat. J Androl 19: 266–288. [PubMed] [Google Scholar]
- 25. Furland NE, Luquez JM, Oresti GM, Aveldano MI (2011) Mild testicular hyperthermia transiently increases lipid droplet accumulation and modifies sphingolipid and glycerophospholipid acyl chains in the rat testis. Lipids 46: 443–454. [DOI] [PubMed] [Google Scholar]
- 26. Aktas C, Kanter M (2009) A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term. J Mol Histol 40: 31–39. [DOI] [PubMed] [Google Scholar]
- 27. Bickel PE, Tansey JT, Welte MA (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791: 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui Y, Zhu H, Zhu Y, Guo X, Huo R, et al. (2008) Proteomic analysis of testis biopsies in men treated with injectable testosterone undecanoate alone or in combination with oral levonorgestrel as potential male contraceptive. J Proteome Res 7: 3984–3993. [DOI] [PubMed] [Google Scholar]
- 29. Paul C, Teng S, Saunders PT (2009) A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod 80: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, et al. (2006) Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol 26: 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sztalryd C, Bell M, Lu X, Mertz P, Hickenbottom S, et al. (2006) Functional compensation for adipose differentiation-related protein (ADFP) by Tip47 in an ADFP null embryonic cell line. J Biol Chem 281: 34341–34348. [DOI] [PubMed] [Google Scholar]
- 32. Chang BH, Li L, Saha P, Chan L (2010) Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J Lipid Res 51: 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, et al. (2003) DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3. [PubMed] [Google Scholar]
- 34. Furland NE, Maldonado EN, Aveldano MI (2003) Very long chain PUFA in murine testicular triglycerides and cholesterol esters. Lipids 38: 73–80. [DOI] [PubMed] [Google Scholar]
- 35. Yildiz Y, Matern H, Thompson B, Allegood JC, Warren RL, et al. (2006) Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J Clin Invest 116: 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiong W, Wang H, Wu H, Chen Y, Han D (2009) Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 137: 469–479. [DOI] [PubMed] [Google Scholar]
- 37. Furland NE, Maldonado EN, Aresti PA, Aveldano MI (2007) Changes in lipids containing long- and very long-chain polyunsaturated fatty acids in cryptorchid rat testes. Biol Reprod 77: 181–188. [DOI] [PubMed] [Google Scholar]
- 38. Stern CM, Mermelstein PG (2010) Caveolin regulation of neuronal intracellular signaling. Cell Mol Life Sci 67: 3785–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, et al. (2010) CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J Lipid Res 51: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI (2006) PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res 47: 931–943. [DOI] [PubMed] [Google Scholar]
- 41. Imanishi Y, Sun W, Maeda T, Maeda A, Palczewski K (2008) Retinyl ester homeostasis in the adipose differentiation-related protein-deficient retina. J Biol Chem 283: 25091–25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koopman R, Schaart G, Hesselink MK (2001) Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol 116: 63–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The List of Primers.
(XLS)
The morphology of seminiferous epithelium after treated 6 weeks.
(TIF)
The TUNEL assay of seminiferous epithelium after treated 48 hours.
(TIF)
The serum testosterone and LH levels in two groups (untreated and 48 hours after treatment).
(TIF)