Abstract
Background
Maternal immunization has gained traction as a strategy to diminish maternal and young infant mortality attributable to infectious diseases. Background rates of adverse pregnancy outcomes are crucial to interpret results of clinical trials in Sub-Saharan Africa.
Methods
We developed a mathematical model that calculates a clinical trial's expected number of neonatal and maternal deaths at an interim safety assessment based on the person-time observed during different risk windows. This model was compared to crude multiplication of the maternal mortality ratio and neonatal mortality rate by the number of live births. Systematic reviews of severe acute maternal morbidity (SAMM), low birth weight (LBW), prematurity, and major congenital malformations (MCM) in Sub-Saharan African countries were also performed.
Findings
Accounting for the person-time observed during different risk periods yields lower, more conservative estimates of expected maternal and neonatal deaths, particularly at an interim safety evaluation soon after a large number of deliveries. Median incidence of SAMM in 16 reports was 40.7 (IQR: 10.6–73.3) per 1,000 total births, and the most common causes were hemorrhage (34%), dystocia (22%), and severe hypertensive disorders of pregnancy (22%). Proportions of liveborn infants who were LBW (median 13.3%, IQR: 9.9–16.4) or premature (median 15.4%, IQR: 10.6–19.1) were similar across geographic region, study design, and institutional setting. The median incidence of MCM per 1,000 live births was 14.4 (IQR: 5.5–17.6), with the musculoskeletal system comprising 30%.
Interpretation
Some clinical trials assessing whether maternal immunization can improve pregnancy and young infant outcomes in the developing world have made ethics-based decisions not to use a pure placebo control. Consequently, reliable background rates of adverse pregnancy outcomes are necessary to distinguish between vaccine benefits and safety concerns. Local studies that quantify population-based background rates of adverse pregnancy outcomes will improve safety assessment of interventions during pregnancy.
Introduction
Maternal and neonatal mortality in Sub-Saharan Africa present a major barrier to achievement of Millennium Development Goals 4 and 5. Sub-Saharan Africa accounts for 52-57% of all maternal deaths worldwide and contains 23 of the 27 countries with neonatal morality rates greater than 30 per 1000 live births [1], [2], [3]. Maternal immunization has gained traction as one strategy to diminish maternal and infant mortality attributable to infectious diseases [4], [5], [6], [7], [8], and may also reduce prematurity and low birth weight [9], [10]. Vaccines protect pregnant women who are more susceptible to severe disease [4], [11], mitigate harms of infection on the developing fetus [8], [9], [12], [13], and passively immunize infants too young to be successfully vaccinated [14], [15]. Maternal immunization may protect young infants directly through placental transport of IgG antibodies (with higher antibody titers found in infants of increasing gestational age) and indirectly through herd immunity [16], [17], [18].
Vaccination to prevent tetanus, influenza, hepatitis B, and invasive meningococcal disease is currently recommended for pregnant women in high-income countries [19]. Maternal immunization with tetanus toxoid to prevent both maternal and neonatal tetanus is already widespread in developing countries, and concerted efforts are exploring the feasibility of administering additional vaccines (e.g., influenza, meningococcal, yellow fever). Before maternal immunization can become a mainstream public health intervention, convincing data must document each additional vaccine's safety for the pregnant woman and her developing fetus and newborn. Several randomized controlled trials of maternal immunization with influenza vaccine are underway in developing countries, including in Sub-Saharan Africa. To provide a benefit to all study participants, such trials often administer to controls a licensed vaccine unrelated to the outcome of interest rather than a placebo. In this situation, it may be difficult to distinguish whether a difference in outcomes between trial arms represents benefit from the interventions with superior results or harms of interventions with poorer outcomes. For example, if a two-arm trial demonstrates a significant decrease in the rate of small for gestational age (SGA) in vaccine group A compared to group B, does this indicate that vaccine A has reduced the incidence of SGA or that vaccine B has increased the incidence of SGA [15]? Consequently, knowledge of the background rates of adverse pregnancy outcomes is crucial to interpret the results of clinical trials in Sub-Saharan Africa. Further adaptations of these data to yield the number of expected adverse events based on the amount of person-time observed before and after delivery can provide an important context to inform interim decisions made by independent Data Safety Monitoring Boards (DSMB) and researchers involved with clinical trials in such vulnerable populations.
This review seeks to assemble and synthesize available data on pregnancy outcomes in Sub-Saharan Africa to provide a context for assessing the safety of maternal immunization at interim stages during a clinical trial as well as after follow-up is complete. We present expected rates of maternal mortality, severe acute maternal morbidity (SAMM), neonatal mortality, low birth weight (LBW), and major congenital malformations (MCM) in a hypothetical cohort of pregnant women enrolled in a clinical trial in Sub-Saharan Africa.
Methods
Maternal Mortality, Stillbirths, and Neonatal Mortality
We used a mathematical model to develop interim estimates of maternal and neonatal deaths over the course of a clinical trial. Maternal mortality was defined as death from direct or indirect obstetric causes during pregnancy or <42 days after pregnancy termination [2]. Stillbirths, early neonatal deaths, and late neonatal deaths were defined as death at birth, death of a liveborn infant within the 1st week of life, and death of a liveborn infant between 1 and 4 weeks of life, respectively [20].
In an interim analysis of vaccine safety by an independent DSMB during a clinical trial of maternal immunization, the expected number of maternal deaths depends on the person-time observed during different risk windows. We considered three periods: pregnancy, within 24 hours of delivery, and the first 42 days post-partum. Large prospective cohort studies in Sub-Saharan Africa were combined by random-effects meta-analysis to determine the proportions of maternal deaths occurring during each interval [21], [22], [23], [24], [25], [26], [27]. The expected number of maternal deaths was thereby calculated using an observation-time model:
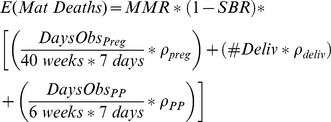 |
(1) |
Where E(Mat Deaths) is the expected number of maternal deaths at a particular stage of recruitment; MMR is the maternal mortality ratio; SBR is the stillbirth rate; DaysObsPreg, #Deliv, and DaysObsPP are the total number of days of observation during pregnancy, the number of deliveries, and the days of observation during the post-partum period; ρpreg, ρdeliv, and ρPP represent the proportion of maternal deaths occurring before delivery, within 24 hours of delivery, and in the post-partum period, respectively.
This equation makes the following three assumptions, all of which bias towards underestimating the expected background number of deaths and hence create more stringent thresholds for evaluating vaccine safety.
The risk of maternal death before delivery is evenly distributed throughout pregnancy.
The risk of maternal death between 24 hours after delivery and 42 days post-partum is also uniformly distributed.
The maternal mortality ratio multiplied by 1 minus the stillbirth rate approximates the ratio of maternal deaths per 100,000 total births. We also assume that each birth contributes 46 weeks of maternal person-time, yielding an incidence of maternal deaths per 100,000 * 46 maternal weeks. The calculated incidence is lower than the true incidence of maternal deaths because women who die before finishing 6 weeks post-partum contribute fewer than 46 maternal weeks of person-time.
Similarly, the expected number of stillbirths and neonatal deaths at an interim analysis would depend on the number of deliveries and the amount of person-time observed:
| (2) |
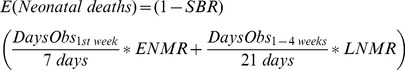 |
(3) |
Where E(SB) and E(Neonatal Deaths) are the expected number of stillbirths and neonatal deaths; TB is the sum of live-births and stillbirths that have occurred; DaysObs1st week and DaysObs1–4 weeks are the number of infant-days of observation during the first week of life and between 1 and 4 weeks of life, respectively.
Equation (3) assumes a uniform risk of death throughout the 1st week of life and a uniform risk of death between 1 and 4 weeks of life. We did not separate neonatal deaths in the 1st day of life from other early neonatal deaths as reliable country-specific early neonatal mortality rates have been described [1], but reliable proportions of deaths in the 1st day of life by country are not available.
We compared the observation-time model estimates of maternal and neonatal deaths to a crude calculation based on the number of live births:
| (4) |
| (5) |
Statistical calculations were performed in R Version 2.12.1, WinBUGS Version 1.4, and StatsDirect Version 2.7.8.
Guidelines for Systematic Reviews
Systematic reviews were conducted on the following pregnancy outcomes in Sub-Saharan Africa: severe acute maternal morbidity (SAMM), low birth weight (LBW), small for gestational age (SGA), prematurity, and major congenital malformations (MCM). For each systematic review, we searched the Medline electronic database for English, French, and Portuguese language publications in peer-reviewed journals. Prospective cohorts, retrospective cohorts, and cross-sectional studies with defined catchment populations were included. Studies in which all data were collected before 1991 were excluded except in the review of congenital malformations. Studies with <100 live births in Sub-Saharan Africa were excluded. Regions of Sub-Saharan Africa were defined by the Global Burden of Disease Study [28]. The reference lists of identified articles were reviewed to locate further eligible studies. All database searches were updated in March 2012.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [29]. All data were extracted independently by two authors, with disagreements mitigated by a third author. No research ethics board approval was obtained as data were extracted from published reports. No separate review protocol was developed beyond what is specified here. Bias was not assessed for individual studies once included.
Severe Acute Maternal Morbidity
Literature search terms were “severe maternal morbidity OR near miss” along with the name of each country in Sub-Saharan Africa. The principal outcome measure was the incidence of severe acute maternal morbidity (SAMM) or near-miss cases, defined as direct or indirect obstetric complications that threaten the woman's survival but do not lead to her death [30]. Studies were included if they contained a definition distinguishing severe maternal morbidity from all maternal morbidity [31] and provided a catchment population. Studies were excluded if they did not provide sufficient data to calculate the incidence of SAMM or if it was not possible to separate near-miss events from maternal deaths, as the latter are accounted for in the maternal mortality section. Where possible, the number of deliveries was used as the denominator. We considered 6 categories of SAMM: hemorrhage, hypertensive disease of pregnancy, dystocia, infections, anemia, and other.
Low birth weight and prematurity
Literature search terms were “low birth weight OR prematurity OR small for gestational age,” along with the name of each country in Sub-Saharan Africa. LBW was defined as birth weight <2.5 kg measured by the study team within 7 days of life or extracted from official birth records. Prematurity was defined as estimated gestational age at delivery <37 weeks as determined by ultrasound, last menstrual period, or validated exam within 7 days of life [32], [33], [34], [35]. Where possible, outcomes were extracted for singleton live-births.
Congenital Malformations
Literature search terms were “major congenital abnormalities OR congenital malformations [title] OR congenital anomalies [title]” along with the name of each country in Sub-Saharan Africa. The principal outcome measure was the incidence of MCM in liveborn infants, defined as structural defects of the body and/or the organs that affect viability or quality of life and require medical intervention [36]. Studies were excluded if the incidence of MCM could not be calculated or if malformations in stillborn infants could not be separated. Malformations were classified by organ system using International Classification of Diseases (ICD)-10 codes [37].
Results
Maternal and Neonatal Mortality
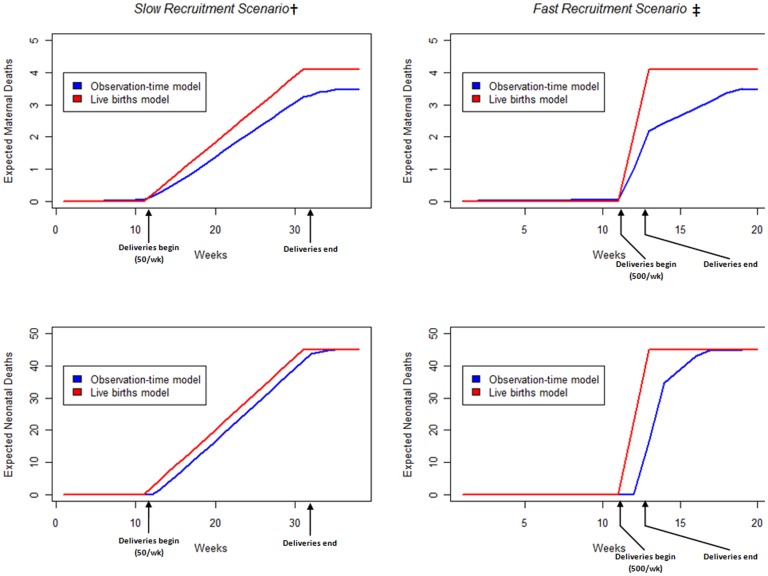
We compared the expected background rates of maternal and neonatal deaths over the course of a hypothetical clinical trial of 1000 pregnant women in Mali given by the observation-time model to a crude calculation based on live births (Figure 1). In the observation-time model, the proportions of maternal deaths occurring before, during, and after delivery were modeled as 18.6% (CI: 14–24), 44.9% (CI: 35–53), and 36.5% (CI: 27–47), respectively [21], [22], [23], [24], [25], [26], [27] (Table S1). Anticipated maternal and neonatal deaths were consistently lower in the observation-time model than the corresponding live births calculation. This effect was magnified immediately after a large number of deliveries. At the end of the trial, expected maternal deaths were also lower in the observation-time model because women were not observed for the entirety of pregnancy, and thus had already experienced part of the risk window for maternal death by the time of study inclusion.
Figure 1. Observation-time model vs. Live births model to calculate expected background maternal and neonatal deaths over the course of a clinical trial in 1000 pregnant women in Mali.
Assuming a maternal mortality ratio of 418.8 (327.5–519.8) per 100,000 live births, stillbirth rate of 23 (18–42) per 1,000 total births, early neonatal mortality rate of 33.5 (28.1–39.0) per 1,000 live births, and late neonatal mortality rate of 12.4 (9.9–15.5) per 1,000 live births estimated for Mali by recently published systematic analyses [3], [44], [45]. We assume women are recruited at 28 weeks gestation and deliver exactly 12 weeks later. † 50 pregnant women recruited each week over 20 weeks. ‡500 pregnant women recruited each week over 2 weeks.
Severe Acute Maternal Morbidity
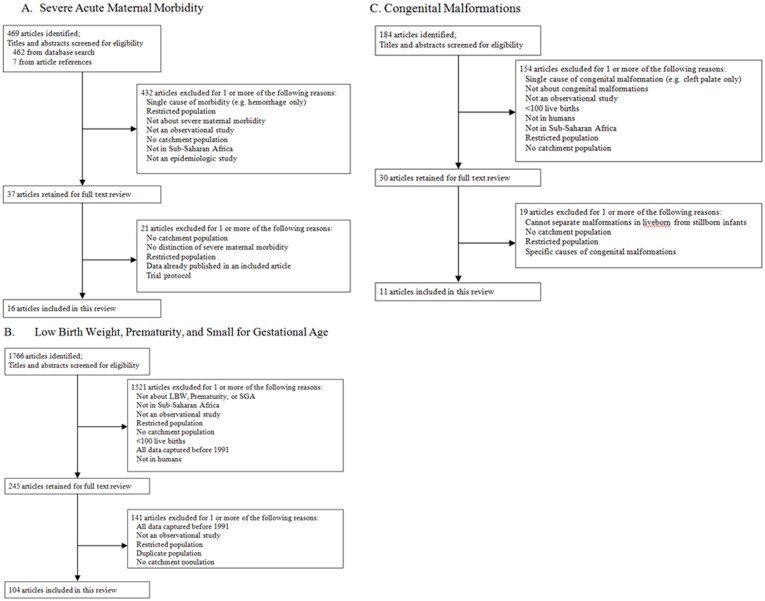
We screened 469 titles and abstracts and 37 full texts and included 16 studies of SAMM in Sub-Saharan Africa (Figure 2A). The median incidence of SAMM per 1,000 total births was 40.7 (IQR: 10.6–73.3) (Table 1). SAMM definitions were notably heterogeneous but were categorized as either organ failure/management-based [38] or disease-specific [39]. Among the 5 studies with organ failure/management-based definitions, the median incidence of SAMM was significantly lower than the 11 studies with disease-specific definitions (6.9 vs. 56.8 per 1,000 total births, p = .002), which may reflect more stringent requirements in the organ failure/management approach. Mortality indices of studies with organ failure/management-based criteria were substantially though not significantly higher (median 16.5% vs. 9.1%, p = .052). Studies conducted in West Africa had significantly higher SAMM incidences than those outside of West Africa (median 92.6 vs. 11.3, p = 0.001), even when restricting to studies with a disease-specific definition (median 92.6 vs. 31.5, p = 0.02). The most common causes of severe maternal morbidity were hemorrhage, dystocia, and hypertensive disorders, accounting for 34%, 22%, and 22% of all events, respectively.
Figure 2. Summary of literature search and study selection.
A. Severe Acute Maternal Morbidity. B. Low Birth Weight, Prematurity, and Small for Gestational Age. C. Congenital Malformations.
Table 1. The incidence of severe acute maternal morbidity in 16 studies in Sub-Saharan Africa published between 1995 and 2012.
| Study Author | Country | Years | SAMM Definition | MI (%) | Site of case detection | Denominator | Size | #SAMM per 1000 Births | SAMM by Cause (%) | |||||
| Hem | Dyst | HTN | Anem | Infect | Other | |||||||||
| Okong [46] | Uganda | 1999–2000 | Organ failure/management | 54 | Urban and rural hospitals | Hospital births | 55803 LB | 4.1 | 42 | 21 | 7.9 | 0 | 18 | 11 |
| Gandhi [47] | South Africa | — | Organ failure/management | — | Rural hospitals | Hospital and ancillary clinic births | 5728 TB | 5.4 | 19 | 6.5 | 52 | 0 | 9.7 | 13 |
| Cochet [48] | South Africa | 2000–2001 | Organ failure/management | 14 | Urban hospitals | Hospital births | 29832 TB | 6.9 | 40 | 0 | 15 | 0 | 12 | 34 |
| Van den Akker [49] | Malawi | 2007–2009 | Disease-Specific | 12 | Rural hospital | All facility deliveries in district | 33254 D | 10.2 | 32 | 11 | 20 | 0 | 32 | 5.1 |
| Mantel [38] | South Africa | 1996–1997 | Organ failure/management | 17 | Urban hospitals | All deliveries in region | 13429 D | 10.9 | 26 | 0 | 26 | 0 | 20 | 29 |
| Vandecruys [50] | South Africa | 1997–1999† | Organ failure/management | 16 | Urban hospitals | Hospital births | 26152 TB | 11.7 | 18 | 0 | 40 | 0 | 13 | 28 |
| Ali [51] | Sudan | 2008–2010 | Disease-Specific | 16 | Urban hospital | Hospital births | 9578 TB | 21.4 | 41 | 8 | 18 | 12 | 22 | 0 |
| Mayi-Tsonga [52] | Gabon | 2006 | Disease-Specific | — | Urban hospital | Hospital births | 4350 TB | 31.5 | 82 | 0 | 14 | 0 | 4.4 | 0 |
| Nyamtema [53] | Tanzania | 2008–2010 | Disease-Specific | 9.9 | Rural hospital | Hospital births | 6572 TB | 49.8 | 30 | 22 | 28 | 8.0 | 4.0 | 8.6 |
| Prual [54] | 6 countries (West Africa) | 1994–1996 | Disease-Specific | 3.7 | Predominantly urban communities | Pregnant women followed prospectively | 20326 D | 52.4 * | 50 | 34 | 10 | 0 | 1.0 | 4.0 |
| Prual [55] | Niger | — | Disease-Specific | 8.3 | Urban hospitals | Hospital and ancillary clinic births | 4081 TB | 56.8 | 13 | 56 | 18 | 0 | 3.4 | 8.6 |
| Gessessew [56] | Ethiopia | 1993–2003 | Disease-Specific | 6.9 | Urban hospital | Hospital births | 7150 D | 60.0 | 21 | 70 | 9.3 | 0 | 0 | 0 |
| Lori [57] | Liberia | 2008 | Disease-Specific | 19 | Rural hospital | Hospital births | 1386 TB | 86.6 | 42 | 5 | 11 | 21 | 14 | 4.0 |
| Filippi [39] | Benin, Côte d'Ivoire | 1999–2001 | Disease-Specific | 6.5 | Predominantly urban hospitals | Hospital births | 27620 TB | 98.6 | 34 | 15 | 26 | 19 | 5.4 | 0 |
| Oladapo [58] | Nigeria | 1999–2004 | Disease-Specific | 16 | Urban hospital | Hospital deliveries | 2577 D | 149.8 | 31 | 20 | 30 | 9.0 | 11 | 0 |
| Cham [59] | Gambia | 2006 | Disease-Specific | 3.6 | Urban and rural hospitals | Hospital births | 3280 TB | 242.7 | 20 | 26 | 26 | 17 | 1.4 | 10 |
| Weighted Mean | 46.4 | 34 | 22 | 22 | 10.5 | 7.0 | 5.0 | |||||||
SAMM: Severe Acute Maternal Morbidity; MI: Mortality Index (# of maternal deaths divided by the sum of near-miss cases and maternal deaths) TB: Total births; D: Deliveries; Hem: Hemorrhage; Dyst: Dystocia (includes uterine rupture); HTN: Hypertensive diseases of pregnancy (severe pre-eclampsia and eclampsia); Anem: Anemia; Infect: Infection;
Data from the year 2000 published by Vandecruys et al [50] were also published in Cochet et al [48]. In this table, those data were removed from Vandecruys et al [50] to avoid duplication.
In Prual et al [54], 109 Cesarean sections performed for scarred uterus, fetal distress, and premature rupture of membranes that did not meet criteria for severe dystocia were subtracted from the total number of severe maternal morbidities as these ostensibly did not directly threaten the life of the mother.
Low Birth Weight and Prematurity
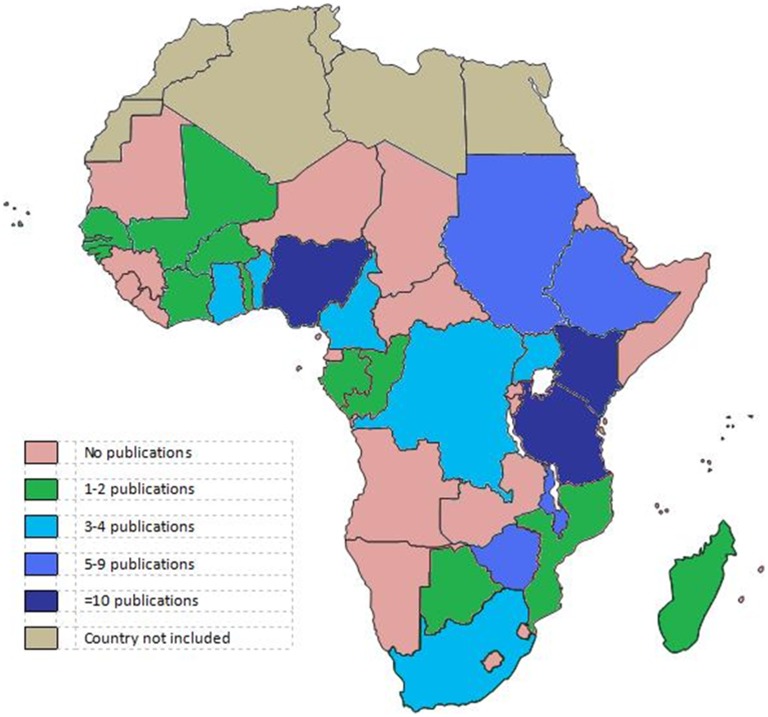
We screened 1766 titles and abstracts and 245 full texts and included 104 studies in our review of LBW, prematurity, and SGA (Figure 2B). Studies originated from 26 sub-Saharan African countries; Nigeria, Kenya, and Ethiopia each contributed ≥10 studies while 4 other countries contributed 5–9 studies (Figure 3). The median incidence of LBW in 97 studies was 13.3% (IQR: 9.9–16.4), the median incidence of prematurity in 30 studies was 15.4% (IQR: 10.6–19.1), and the median incidence of SGA in 14 studies was 10.5% (IQR: 6.5–18.8) (Table S2). There were 53 perinatal surveys, 30 record reviews, and 21 cohort studies. Fifty-eight studies were performed in urban or semi-urban hospitals, 19 in clinics, 15 in rural hospitals, and the remaining 12 were population-based. Estimates of the incidence of LBW and prematurity and the mean birth weight did not vary significantly by study design, setting, region of sub-Saharan Africa, or method of gestational age assessment. The median proportion of SGA in 4 studies that defined SGA as <10th percentile by standardized growth curve was significantly higher than 6 studies in which term-LBW was the only indicator of SGA (17.2% vs. 7.5%, p = 0.019).
Figure 3. Publications by country on low birth weight, prematurity, and small for gestational age in sub-Saharan Africa.
Major Congenital Malformations
We screened 184 titles and abstracts and 30 full texts and included 11 studies in our review of MCM among liveborn infants in Sub-Saharan Africa (Figure 2C). The median incidence per 1,000 live births was 14.4 (IQR: 5.5–17.6) (Table 2). Seven of the 11 studies were limited to physical examination findings at birth and did not have imaging support. The most common major malformations were found in the musculoskeletal (30%), central nervous (16%), and gastrointestinal systems (13%).
Table 2. The incidence of congenital malformations among liveborn infants in 11 studies in Sub-Saharan Africa published between 1966 and 2009.
| Author | Country | Years | Study Design | Method of CM Detection | # LB | # Expected MCM per 1000 LB | Distribution of Major Congenital Malformations (%): | |||||||||
| MSK | CNS | GI | GU | Chrom | CV | HEENT | Resp | Multip | Other | |||||||
| Ahuka [60] | Dem Rep Congo | 1993–2001 | Retrospective cohort, hospital based | Midwife exam at birth | 8824 | 4.1 | 31 | 47 | 17 | 2.8 | 0 | 0 | 0 | 0 | 0 | 2.8 |
| Sukhani [61] | Zambia | 1976 | Prospective cohort, hospital based | Physical exam at birth, imaging as indicated | 17030 | 5.3 | 13 | 19 | 16 | 9.7 | 13 | 9.7 | 0 | 4.3 | 8.6 | 6.5 |
| Embree [62] | Kenya | — | Prospective cohort, hospital based | Standardized exam at birth and 6 month intervals | 183 | 5.5 | — | — | — | — | — | — | — | — | — | — |
| Shija [63] | Zimbabwe | 1984 | Prospective cohort, hospital based | Physical exam at birth or pediatric surgery clinic | 18033* | 7.0 | 21 | 4.5 | 29 | 12 | 0 | 0 | 0 | 0 | 15 | 20 |
| Delport [64] | South Africa | 1986–1989 | Prospective cohort, hospital based | Physician exam at birth | 17351 | 11.9 | 18 | 19 | 9.2 | 7.8 | 14 | 15 | 1.0 | 0.5 | 3.9 | 11 |
| Abudu [65] | Nigeria | 1982–1983 | Prospective cohort, hospital based | Physician exam at birth, autopsy | 2912 | 14.4 | — | — | — | — | — | — | — | — | — | — |
| Venter [66] | South Africa | 1989–1992 | Prospective cohort, hospital based | Standardized exam by geneticist at birth, lab tests and imaging as indicated | 7617 | 15.2 | 23 | 28 | 5.2 | 8.6 | 18 | 0 | 0.9 | 0 | 7.8 | 7.8 |
| Stevenson [67] | South Africa | 1961–1964 | Prospective cohort, hospital based | Standardized exam at birth | 23675 | 15.6 | — | — | — | — | — | — | — | — | — | — |
| Khan [68] | Zambia | 1974–1975 | Prospective cohort, hospital based | Physical exam at birth | 8508 | 17.6 | 63 | 4.0 | 6.0 | 5.3 | 2.7 | 4.7 | 1.3 | 0 | 6.0 | 6.7 |
| Gupta [69] | Nigeria | 1964 | Prospective cohort, hospital based | Standardized exam at birth | 4054 | 26.9 | 39 | 14 | 19 | 5.5 | 0 | 10 | 7.3 | 0 | 0 | 5.5 |
| Bakare [70] | Nigeria | 2003–2004 | Prospective cohort, outpatient delivery wards | Physical exam at birth | 624 | 36.9 | 43 | 13 | 0 | 39 | 0 | 0 | 0 | 0 | 0 | 4.3 |
| Weighted Mean | 11.7 | 30 | 16 | 13 | 8.6 | 7.7 | 6.7 | 1.5 | 0.6 | 8.6 | 9.4 | |||||
CNS = Central Nervous System (ICD-10: Q00–Q07); Resp = Respiratory (ICD-10: Q30–Q34); CV = Cardiovascular (ICD-10: Q20–Q28); MSK = Musculoskeletal system (ICD-10: Q65–79); GI = Digestive system (ICD-10: Q35–Q45); GU = genital organs and urinary system (ICD-10: Q50–Q56, Q60–Q64); HEENT = Eye, ear, face, and neck (ICD-10: Q10–Q18); Chrom = Chromosomal abnormalities (ICD-10: Q90–Q99); Multip = Major congenital malformations in multiple systems.
Denominator given in total births.
Summary of Background Rates of Adverse Pregnancy Outcomes
Pregnancy outcomes including maternal deaths, SAMM, stillbirths, neonatal deaths, LBW, prematurity, and MCM are summarized for the 4 sub-regions of Sub-Saharan Africa (Table 3), and estimates of maternal deaths, stillbirths, neonatal deaths, LBW, and prematurity are presented for each country in sub-Saharan Africa (Table 4). In all categories except LBW and prematurity, anticipated adverse events were more frequent in West Africa than in the other 3 sub-regions. Variation was widest for severe maternal morbidity and major congenital malformations, the two categories for which the fewest data are available.
Table 3. Background rates of pregnancy outcomes by region of Sub-Saharan Africa.
| Region of Sub-Saharan Africa | Maternal deaths per 1000 Total Births ¥ | SAMM per 1000 Total Births † | Stillbirths per 1000 Total Births | Early NND per 1000 Total Births ¥ | Late NND per 1000 Total Births ¥ | % LBW | % <37 wks | % Liveborn infants with MCM † |
| Central | 4.5 (3.7–5.3) | 31.5 (—) | 24.6 (12.3–52.4) | 25.4 (22.6–28.3) | 8.8 (3.1–5.0) | 12.4 (10.3–34.2) | 17.9 (2.3–21.3) | 0.41 (—) |
| East | 4.0 (3.7–4.4) | 21.4 (10.2–59.4) | 24.8 (16.3–43.9) | 20.3 (19.1–21.5) | 6.9 (6.3–7.6) | 12.4 (6.3–37.1) | 11.6 (3.4–20.3) | 0.55 (0.53–1.76) |
| Southern | 1.7 (1.4–2.0) | 6.8 (5.2–11.0) | 20.1 (13.4–32.7) | 13.1 (12.2–14.3) | 4.2 (3.7–4.9) | 14.1 (6.0–20.3) | 18.7 (17.3–20.1) | 1.36 (0.70–1.56) |
| West | 4.6 (4.2–5.1) | 92.6 (56.8–242.7) | 33.3 (20.3–58.8) | 26.2 (24.1–28.4) | 9.7 (8.7–10.7) | 13.3 (5.5–29.0) | 13.4 (5.3–30.5) | 2.69 (1.44–3.69) |
SAMM = Severe Acute Maternal Morbidity; NND = Neonatal Deaths; LBW = Low Birth Weight; MCM = Major Congenital Malformations.
For Severe Maternal Morbidity, Low Birth Weight, Prematurity, and Major Congenital Malformations, the median and range of a systematic review is presented for the entire region. In Central Africa, only 1 data point was available for Severe Maternal Morbidity [52] and for Major Congenital Malformations [60], so no range was presented.
Maternal and neonatal deaths were expressed as a fraction of total births by multiplying the maternal mortality ratio (maternal deaths/live births) and the early and late neonatal mortality ratios (neonatal deaths/live births) by 1 minus the stillbirth rate [44].
Table 4. Expected number of maternal and neonatal deaths and stillbirths for a hypothetical cohort of pregnant women corresponding to 1,000 births in Sub-Saharan Africa and the proportion of live-born infants expected to be low birth weight or premature.
| Maternal deaths per 1000 Total Births [1] ¥ | Stillbirths per 1000 Total Births [44] | Early neonatal deaths per 1000 Total Births [1] ¥ | Late neonatal deaths per 1000 Total Births [1] ¥ | % LBW† | % <37 wks† | |
| Central Africa | ||||||
| Angola | 3.3(2.1–4.5) | 25.1(12–54) | 28.0(24–33) | 10.2(8.0–13) | — | — |
| Central African Republic | 6.7(5.0–8.6) | 24.2(12–49) | 31.5(27–37) | 12.9(11–16) | — | — |
| Congo (Brazzaville) | 12.4 (—) | 16.7 (—) | ||||
| Congo (Dem Republic) | 4.7(3.6–5.9) | 25.6(14–55) | 24.4(21–28) | 8.2(6.7–9.7) | 20.0 (10.5–34.2) | 2.3 (—) |
| Equatorial Guinea | 2.1(1.3–3.2) | 16.9 (8–36) | 35.1(29–42) | 15.1(12–19) | — | — |
| Gabon | 4.2 (3.1–5.4) | 17.3 (9–38) | 22.4 (19–26) | 3.9 (3.1–5) | 10.5 (10.3–10.7) | 20.2 (19.1–21.3) |
| East Africa | ||||||
| Burundi | 8.7(6.1–11) | 27.7(15–61) | 19.1(16–22) | 9.3(7.6–11) | — | — |
| Comoros | 2.6(1.9–3.7) | 27.0(14–59) | 21.3(19–24) | 8.4(7.1–9.7) | — | — |
| Djibouti | 3.5(2.5–4.8) | 33.9(15–55) | 17.4(15–20) | 5.4(4.4–6.7) | — | — |
| Eritrea | 10.6(8.1–13) | 21.2(11–49) | 17.0(15–20) | 4.6(3.6–5.9) | — | — |
| Ethiopia | 5.2(3.8–6.6) | 25.6(15–52) | 24.3(21–28) | 8.5(6.9–10) | 9.7 (6.3–20.3) | 13.5 (11.6–15.3) |
| Kenya | 2.9(2.2–3.6) | 21.8(14–38) | 18.6(17–21) | 4.9(4.3–5.5) | 9.7 (7.9–18.0) | 11.3 (3.4–19.1) |
| Madagascar | 4.2(3.3–5.2) | 20.6(15–36) | 14.0(13–16) | 4.8(4.3–5.4) | — | 18.1 (—) |
| Malawi | 4.1(3.1–5.3) | 23.7(17–35) | 20.1(17–23) | 6.4(5.5–7.7) | 15.1 (13.3–18.3) | 17.5 (17.3–17.6) |
| Mozambique | 5.0(3.7–6.5) | 28.4(17–51) | 27.1(24–31) | 10.5(9.0–12) | 16.2 (—) | 15.4 (—) |
| Rwanda | 3.3(2.2–4.8) | 22.8(16–36) | 19.3(17–22) | 5.8(4.9–6.7) | — | — |
| Somalia | 4.6(3.2–6.3) | 30.1(15–64) | 16.5(14–19) | 9.5(7.8–12) | — | — |
| Sudan | 2.7(2.0–3.5) | 23.9(17–40) | 20.1(17–23) | 7.2(6.0–8.8) | 14.9 (8.3–18.0) | 5.7 (—) |
| Tanzania | 4.1(3.3–5.0) | 25.6(19–40) | 18.0(16–20) | 5.7(5.1–6.4) | 14.2 (8.6–22.4) | 8.3 (7.9–10.0) |
| Uganda | 2.7(2.0–3.4) | 24.8(19–36) | 20.6(18–23) | 5.9(5.1–6.8) | 9.6 (6.4–37.1) | 20.3 (—) |
| Zambia | 2.9(2.2–3.8) | 25.5(18–40) | 17.2(15–19) | 8.5(7.4–9.6) | — | — |
| Southern Africa | ||||||
| Botswana | 5.1(3.6–6.8) | 16.2 (9–37) | 14.8(12–18) | 3.4(2.4–4.6) | 13.0 (—) | 20.1 (—) |
| Lesotho | 2.3(1.7–3.2) | 25.2(14–54) | 20.4(27–34) | 8.1(6.7–9.7) | — | — |
| Namibia | 1.3(1.0–1.8) | 15.0(11–35) | 18.5(16–21) | 4.1(3.2–5.3) | — | — |
| South Africa | 0.9(0.7–1.2) | 20.4(14–31) | 10.6(10–12) | 3.4(3.1–3.8) | 14.7 (13.8–16.3) | — |
| Swaziland | 2.8(2.0–3.7) | 18.2(11–36) | 18.1(15–21) | 5.0(3.8–6.6) | — | — |
| Zimbabwe | 3.2(2.3–4.6) | 20.0(13–35) | 16.2(14–19) | 5.9(4.5–7.6) | 14.1 (6.0–20.3) | 17.3 (—) |
| West Africa | ||||||
| Benin | 3.2(2.5–4.1) | 24.3(17–41) | 22.8(20–26) | 5.8(4.8–6.9) | 15.7 (10.0–17.8) | — |
| Burkina Faso | 3.4(2.8–4.1) | 26.2(19–40) | 24.8(21–30) | 13.1(10–16.5) | 12.2 (—) | — |
| Cameroon* | 5.2(4.0–6.7) | 25.6(13–54) | 25.2(22–29) | 7.8(5.9–9.6) | 16.4 (9.6–20.3) | 20.3 (—) |
| Cape Verde | 1.3(0.9–1.7) | 15.6 (8–34) | 10.8(9.0–13) | 3.0(2.4–3.8) | 8.2 (—) | 13.4 (—) |
| Chad* | 5.9(4.8–7.1) | 29.2(14–64) | 30.8(27–36) | 13.7(11–16) | — | — |
| Côte d'Ivoire | 4.4(3.3–5.8) | 27.4(14–45) | 26.2(22–30) | 10.3(7.9–13) | 10.6 (—) | — |
| The Gambia | 2.7(1.9–3.5) | 26.0(14–53) | 22.4(19–26) | 7.3(5.6–9.4) | 18.6 (13.3–23.9) | 21.4 (12.3–30.5) |
| Ghana | 3.2(2.4–4.0) | 22.0(14–37) | 19.8(17–22) | 4.7(4.0–5.6) | 16.4 (13.3–20.3) | 14.1 (—) |
| Guinea | 6.5(5.1–7.9) | 23.8(16–48) | 28.5(25–33) | 10.3(8.4–13) | — | — |
| Guinea-Bissau | 8.2(6.3–10) | 29.6(16–62) | 30.9(26–36) | 13.8(11–17) | 13.3 (11.8–14.7) | — |
| Liberia | 8.8(7.1–10) | 26.9(14–56) | 23.5(21–26) | 7.6(6.5–9.0) | — | — |
| Mali | 4.1(3.2–5.1) | 23.2(18–42) | 32.7(27–38) | 12.1(9.7–15) | 18.6 (—) | 5.3 (—) |
| Mauritania | 5.4(4–7.0) | 27.4(17–51) | 24.7(21–29) | 6.3(4.8–8) | — | — |
| Niger | 5.1(4.1–6.2) | 22.9(17–41) | 20.0(17–24) | 11.6(9.6–14) | — | — |
| Nigeria | 4.7(3.8–5.6) | 41.7(25–72) | 27.5(24–32) | 9.9(8.3–11) | 12.2 (5.5–29) | 13.5 (10.6–19.4) |
| Sao Tome and Principe | 2.6(3.3–3.3) | 21.0(11–48) | 16.7(15–19) | 4.1(3.5–4.7) | — | — |
| Senegal | 3.6(2.7–4.5) | 33.8(27–50) | 18.8(16–22) | 6.6(5.3–7.9) | 10.3 (9.5–18.8) | — |
| Sierra Leone | 6.0(4.7–7.4) | 30.0(16–66) | 26.3(23–30) | 9.0(7.5–11) | — | — |
| Togo | 3.9(2.6–5.5) | 25.0(13–54) | 26.7(23–31) | 6.9(5.6–8.7) | — | 11.1 (—) |
Maternal and neonatal deaths were expressed as a fraction of total births by multiplying the maternal mortality ratio (maternal deaths/live births) and the neonatal mortality ratio (neonatal deaths/live birth) by 1 minus the stillbirth rate [44].
For % LBW and % <37 wks, the median and range for all studies performed in the specified country are presented.
Note –UN Data classifies Cameroon and Chad as falling within the Middle Africa sub-region rather than the West Africa sub-region. However, in this table these countries are kept within the West Africa sub-region to maintain congruity with global burden of disease publications.
Discussion
We present a comprehensive review of pregnancy outcomes in Sub-Saharan Africa intended to inform safety assessments of current and future clinical trials conducted in pregnant women or neonates. The observation-time model of maternal and neonatal mortality accounts for the logistical realities of variation in gestational age at recruitment, time to delivery, and follow-up time. This model yields lower mortality estimates than multiplying mortality rates by cumulative live births, especially if a safety evaluation is conducted immediately after a cluster of deliveries. Overall, our review noted that the incidences of maternal and neonatal mortality, severe acute maternal morbidity, stillbirths, and major congenital malformations are highest in West Africa, the least developed sub-region of the continent. However, relatively few detailed published studies of severe acute maternal morbidity and congenital malformations in Sub-Saharan Africa exist, especially in the community setting.
Of all adverse pregnancy outcomes, the incidences of SAMM and MCM were the most variable across sub-regions. Studies with more stringent organ failure/management-based definitions found lower incidences of SAMM. Many studies of major congenital malformations were not included because malformations among live- and stillborn infants could not be separated. The limited time frame and diagnostic tools to detect congenital malformations in many studies may have led to systematic underreporting of specific types of malformations not readily apparent by physical examination at birth, such as many cardiovascular anomalies.
This compilation of pregnancy outcomes relevant to a maternal immunization clinical trial is consistent with the few other published summaries of pregnancy outcomes in Sub-Saharan Africa. Kaye et al [40] present a novel and pioneering review of causes of SAMM and case-fatality ratios. Although our review provides corroborating results, it also advances the field by providing several notable enhancements as we update the literature search, focus more explicitly on the background incidence of SAMM, and apply more stringent requirements for the catchment population. Careful interpretation is necessary to compare background rates of SAMM with those observed in a trial, as superior antenatal surveillance may decrease SAMM but better management of emergencies could prevent deaths, thereby elevating the rate of near-miss cases.
Our systematic review of LBW and prematurity does not yield nationally representative populations as are sought out in Demographic and Health Surveys and Multiple Indicator Cluster Surveys [41], [42]. Nevertheless, it allows designers of clinical trials to tailor expected background rates to recruitment sites by geographic and institutional setting. Despite this different approach, importantly, our overall estimates of the incidence of LBW are comparable to UNICEF values for sub-Saharan Africa overall (14%), Eastern and Southern Africa (14%), and West and Central Africa (13%) [43].
We recognize that our study has several limitations. First, we do not address differences in neonatal and maternal mortality rates among sub-populations within an individual country or across time that arise from geographic, socioeconomic, educational, and health access diversity. Thus, a DSMB should use its expertise and the best available data to select the most locally appropriate background rates for anticipated adverse event calculations. Second, pregnant women under the active surveillance of a clinical trial tend to have better access to medical care and therefore superior pregnancy outcomes relative to the general population. As in all mathematical models and systematic reviews, we are limited by the quality of the data informing our calculations. Many countries in Sub-Saharan Africa contribute incomplete vital registration data to systematic analyses of maternal mortality, stillbirths, and neonatal mortality [3], [44], [45] on which our calculations are based. Additionally, while our search included English, French, and Portuguese language publications, it ignores publications from sub-Saharan African countries in journals not included in MEDLINE.
The global public health community awaits with great anticipation the results of several ongoing clinical trials in developing countries that explore whether maternal immunization improves pregnancy outcomes and enhances young infant survival. In some of these trials, investigators have chosen to avoid the use of pure placebo in the control group, instead providing a licensed vaccine with an independent potential benefit that does not influence the primary outcome. For example, in an ongoing clinical trial in Mali assessing the effectiveness of maternal influenza immunization against influenza in mothers and their infants, the pregnant women randomized to the control group receive quadrivalent meningococcal conjugate vaccine (NCT01430689). In such situations where for ethical reasons the study design does not include a true placebo group, reliable background rates of adverse pregnancy outcomes are invaluable to help distinguish between vaccine benefits and safety concerns. Further studies that clarify locally relevant, population-based background rates of adverse pregnancy outcomes will improve safety assessment of maternal interventions.
Supporting Information
Meta-analysis of the proportion of maternal deaths that occur before delivery, during labor and delivery or within 24 hours, and 24 hours to 42 days post-partum.
(DOCX)
The incidence of low birth weight and prematurity in 104 studies in Sub-Saharan Africa published between 1992 and 2011.
(DOCX)
Acknowledgments
We would like to thank the Centre pour le Développement des Vaccins, Mali (in particular, Dr. Fadima Haidara, Dr. Moussa Doumbia, Dr. Fatoumata Diallo, and Dr. Flanon Coulibaly) for their advice and support throughout this project. We would also like to thank the participants, communities, and study teams involved in an ongoing trial of maternal influenza immunization in Bamako, Mali, as well as the Mali Ministry of Health and staff at the Gabriel Touré Teaching Hospital.
Funding Statement
Lauren Orenstein's participation was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship, the Infectious Disease Society of America Medical Scholarship, and Emory University School of Medicine. Evan Orenstein's participation was supported by the Benjamin H. Kean Traveling Fellowship in Tropical Medicine, the Infectious Disease Society of America Medical Scholarship, and Emory University School of Medicine. Additional support was provided by grant OPP1002744 from the Bill & Melinda Gates Foundation (M. M. Levine, Principal Investigator). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, et al. (2011) Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 378: 1139–1165. [DOI] [PubMed] [Google Scholar]
- 2.Chou D, Inoue M, Mathers C, Oestergaard M, (2010) Trends in Maternal Mortality: 1990–2008. Geneva: World Health Organization.
- 3. Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, et al. (2010) Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 375: 1609–1623. [DOI] [PubMed] [Google Scholar]
- 4. Louie JK, Acosta M, Jamieson DJ, Honein MA (2010) Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 362: 27–35. [DOI] [PubMed] [Google Scholar]
- 5. Rahman M, Chen LC, Chakraborty J, Yunus M, Chowdhury AI, et al. (1982) Use of tetanus toxoid for the prevention of neonatal tetanus. 1. Reduction of neonatal mortality by immunization of non-pregnant and pregnant women in rural Bangladesh. Bull World Health Organ 60: 261–267. [PMC free article] [PubMed] [Google Scholar]
- 6. Vandelaer J, Birmingham M, Gasse F, Kurian M, Shaw C, et al. (2003) Tetanus in developing countries: an update on the Maternal and Neonatal Tetanus Elimination Initiative. Vaccine 21: 3442–3445. [DOI] [PubMed] [Google Scholar]
- 7. Roper MH, Vandelaer JH, Gasse FL (2007) Maternal and neonatal tetanus. Lancet 370: 1947–1959. [DOI] [PubMed] [Google Scholar]
- 8. Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M (2011) Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ 342: d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, et al. (2011) Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med 8: e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhoff MC (2011) In: Levine MM, editor.
- 11. Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR (1998) Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 148: 1094–1102. [DOI] [PubMed] [Google Scholar]
- 12. Moretti ME, Bar-Oz B, Fried S, Koren G (2005) Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology 16: 216–219. [DOI] [PubMed] [Google Scholar]
- 13. Levine MM (1974) Live-virus vaccines in pregnancy. Risks and recommendations. Lancet 2: 34–38. [DOI] [PubMed] [Google Scholar]
- 14. Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, et al. (2011) Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 165: 104–111. [DOI] [PubMed] [Google Scholar]
- 15. Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, et al. (2008) Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 359: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 16. Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD (1994) Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol 1: 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simister NE (2003) Placental transport of immunoglobulin G. Vaccine 21: 3365–3369. [DOI] [PubMed] [Google Scholar]
- 18. van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, et al. (2010) Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J 29: 801–805. [DOI] [PubMed] [Google Scholar]
- 19. Healy CM, Baker CJ (2006) Prospects for prevention of childhood infections by maternal immunization. Curr Opin Infect Dis 19: 271–276. [DOI] [PubMed] [Google Scholar]
- 20.Ahman E, Zupan J (2007) Neonatal and perinatal mortality : country, regional and global estimates 2004. Geneva: World Health Organization.
- 21. Bouvier-Colle MH, Ouedraogo C, Dumont A, Vangeenderhuysen C, Salanave B, et al. (2001) Maternal mortality in West Africa. Rates, causes and substandard care from a prospective survey. Acta Obstet Gynecol Scand 80: 113–119. [PubMed] [Google Scholar]
- 22. Cham M, Vangen S, Sundby J (2007) Maternal deaths in rural Gambia. Glob Public Health 2: 359–372. [DOI] [PubMed] [Google Scholar]
- 23. Greenwood AM, Greenwood BM, Bradley AK, Williams K, Shenton FC, et al. (1987) A prospective survey of the outcome of pregnancy in a rural area of the Gambia. Bull World Health Organ 65: 635–643. [PMC free article] [PubMed] [Google Scholar]
- 24. Hoj L, Stensballe J, Aaby P (1999) Maternal mortality in Guinea-Bissau: the use of verbal autopsy in a multi-ethnic population. Int J Epidemiol 28: 70–76. [DOI] [PubMed] [Google Scholar]
- 25. Martey JO, Djan JO, Twum S, Browne EN, Opoku SA (1994) Maternal mortality and related factors in Ejisu District, Ghana. East Afr Med J 71: 656–660. [PubMed] [Google Scholar]
- 26. McDermott JM, Slutsker L, Steketee RW, Wirima JJ, Breman JG, et al. (1996) Prospective assessment of mortality among a cohort of pregnant women in rural Malawi. Am J Trop Med Hyg 55: 66–70. [DOI] [PubMed] [Google Scholar]
- 27. Walraven G, Telfer M, Rowley J, Ronsmans C (2000) Maternal mortality in rural Gambia: levels, causes and contributing factors. Bull World Health Organ 78: 603–613. [PMC free article] [PubMed] [Google Scholar]
- 28. Naghavi M, Makela S, Foreman K, O'Brien J, Pourmalek F, et al. (2010) Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronsmans C, Filippi V (2004) Learning from severe maternal morbidity. In: Organization WH, editor. Beyond the Numbers: Reviewing Maternal Deaths and Complications to Make Pregnancy Safer. Geneva: WHO.
- 31. Say L, Pattinson RC, Gulmezoglu AM (2004) WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, et al. (1991) New Ballard Score, expanded to include extremely premature infants. J Pediatr 119: 417–423. [DOI] [PubMed] [Google Scholar]
- 33. Dubowitz LM, Dubowitz V, Goldberg C (1970) Clinical assessment of gestational age in the newborn infant. J Pediatr 77: 1–10. [DOI] [PubMed] [Google Scholar]
- 34. Eregie CO (2000) A new method for maturity determination in newborn infants. J Trop Pediatr 46: 140–144. [DOI] [PubMed] [Google Scholar]
- 35. Farr V, Mitchell RG (1969) Estimation of gestational age in the newborn infant. Comparison between birth weight and maturity scoring in infants premature by weight. Am J Obstet Gynecol 103: 380–383. [DOI] [PubMed] [Google Scholar]
- 36. Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J (2002) Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990–1998). Arch Gynecol Obstet 266: 163–167. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization (2004) International Statistical Classification of Diseases and Health Related Problems. Geneva.
- 38. Mantel GD, Buchmann E, Rees H, Pattinson RC (1998) Severe acute maternal morbidity: a pilot study of a definition for a near-miss. Br J Obstet Gynaecol 105: 985–990. [DOI] [PubMed] [Google Scholar]
- 39. Filippi V, Ronsmans C, Gohou V, Goufodji S, Lardi M, et al. (2005) Maternity wards or emergency obstetric rooms? Incidence of near-miss events in African hospitals. Acta Obstet Gynecol Scand 84: 11–16. [DOI] [PubMed] [Google Scholar]
- 40. Kaye DK, Kakaire O, Osinde MO (2011) Systematic review of the magnitude and case fatality ratio for severe maternal morbidity in sub-Saharan Africa between 1995 and 2010. BMC Pregnancy Childbirth 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demographic and Health Surveys (2012). Calverton, MD.
- 42.United Nations Children's Fund (2012). New York, NY.
- 43.United Nations Children's Fund (2011) The State of the World's Children 2011. New York.
- 44. Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, et al. (2011) National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 45. Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, et al. (2010) Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet 375: 1988–2008. [DOI] [PubMed] [Google Scholar]
- 46. Okong P, Byamugisha J, Mirembe F, Byaruhanga R, Bergstrom S (2006) Audit of severe maternal morbidity in Uganda–implications for quality of obstetric care. Acta Obstet Gynecol Scand 85: 797–804. [DOI] [PubMed] [Google Scholar]
- 47. Gandhi MN, Welz T, Ronsmans C (2004) Severe acute maternal morbidity in rural South Africa. Int J Gynaecol Obstet 87: 180–187. [DOI] [PubMed] [Google Scholar]
- 48. Cochet L, Pattinson RC, Macdonald AP (2003) Severe acute maternal morbidity and maternal death audit–a rapid diagnostic tool for evaluating maternal care. S Afr Med J 93: 700–702. [PubMed] [Google Scholar]
- 49. van den Akker T, van Rhenen J, Mwagomba B, Lommerse K, Vinkhumbo S, et al. (2011) Reduction of severe acute maternal morbidity and maternal mortality in Thyolo District, Malawi: the impact of obstetric audit. PLoS One 6: e20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vandecruys HI, Pattinson RC, Macdonald AP, Mantel GD (2002) Severe acute maternal morbidity and mortality in the Pretoria Academic Complex: changing patterns over 4 years. Eur J Obstet Gynecol Reprod Biol 102: 6–10. [DOI] [PubMed] [Google Scholar]
- 51. Ali AA, Khojali A, Okud A, Adam GK, Adam I (2011) Maternal near-miss in a rural hospital in Sudan. BMC Pregnancy Childbirth 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mayi-Tsonga S, Meye JF, Tagne A, Ndombi I, Diallo T, et al. (2007) [Audit of the severe obstetrical morbidity (near miss) in Gabon]. Sante 17: 111–115. [PubMed] [Google Scholar]
- 53. Nyamtema AS, de Jong AB, Urassa DP, van Roosmalen J (2011) Using audit to enhance quality of maternity care in resource limited countries: lessons learnt from rural Tanzania. BMC Pregnancy Childbirth 11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prual A, Bouvier-Colle MH, de Bernis L, Breart G (2000) Severe maternal morbidity from direct obstetric causes in West Africa: incidence and case fatality rates. Bull World Health Organ 78: 593–602. [PMC free article] [PubMed] [Google Scholar]
- 55. Prual A, Huguet D, Garbin O, Rabe G (1998) Severe obstetric morbidity of the third trimester, delivery and early puerperium in Niamey (Niger). Afr J Reprod Health 2: 10–19. [PubMed] [Google Scholar]
- 56. Gessessew A (2007) Maternal complications–in a zonal hospital. Ethiop Med J 45: 47–54. [PubMed] [Google Scholar]
- 57. Lori JR, Starke AE (2012) A critical analysis of maternal morbidity and mortality in Liberia, West Africa. Midwifery 28: 67–72. [DOI] [PubMed] [Google Scholar]
- 58. Oladapo OT, Ariba AJ, Odusoga OL (2007) Changing patterns of emergency obstetric care at a Nigerian University hospital. Int J Gynaecol Obstet 98: 278–284. [DOI] [PubMed] [Google Scholar]
- 59. Cham M, Sundby J, Vangen S (2009) Fetal outcome in severe maternal morbidity: too many stillbirths. Acta Obstet Gynecol Scand 88: 343–349. [DOI] [PubMed] [Google Scholar]
- 60. Ahuka OL, Toko RM, Omanga FU, Tshimpanga BJ (2006) Congenital malformations in the North-Eastern Democratic Republic of Congo during Civil War. East Afr Med J 83: 95–99. [DOI] [PubMed] [Google Scholar]
- 61. Sukhani S, Patel YK, Chintu C (1977) Major congenital malformations in neonates at U.T.H. Lusaka Zambia. Med J Zambia 11: 127–138. [PubMed] [Google Scholar]
- 62. Embree JE, Braddick M, Datta P, Muriithi J, Hoff C, et al. (1989) Lack of correlation of maternal human immunodeficiency virus infection with neonatal malformations. Pediatr Infect Dis J 8: 700–704. [DOI] [PubMed] [Google Scholar]
- 63. Shija JK, Kingo AR (1985) A prospective clinical study of congenital anomalies seen at Harare Central Hospital, Zimbabwe. Cent Afr J Med 31: 145–148. [PubMed] [Google Scholar]
- 64. Delport SD, Christianson AL, van den Berg HJ, Wolmarans L, Gericke GS (1995) Congenital anomalies in black South African liveborn neonates at an urban academic hospital. S Afr Med J 85: 11–15. [PubMed] [Google Scholar]
- 65. Abudu OO, Uguru V, Olude O (1988) Contribution of congenital malformation to perinatal mortality in Lagos, Nigeria. Int J Gynaecol Obstet 27: 63–67. [DOI] [PubMed] [Google Scholar]
- 66. Venter PA, Christianson AL, Hutamo CM, Makhura MP, Gericke GS (1995) Congenital anomalies in rural black South African neonates–a silent epidemic? S Afr Med J 85: 15–20. [PubMed] [Google Scholar]
- 67. Stevenson AC, Johnston HA, Stewart MI, Golding DR (1966) Congenital malformations. A report of a study of series of consecutive births in 24 centres. Bull World Health Organ 34 Suppl: 9–127. [PMC free article] [PubMed] [Google Scholar]
- 68. Khan AA, Ivanov I (1977) Congenital malformations in Zambian neonates. East Afr Med J 54: 632–636. [PubMed] [Google Scholar]
- 69. Gupta B (1969) Indicence of congenital malformations in Nigerian children. West Afr Med J Niger Pract 18: 22–27. [PubMed] [Google Scholar]
- 70. Bakare TI, Sowande OA, Adejuyigbe OO, Chinda JY, Usang UE (2009) Epidemiology of external birth defects in neonates in Southwestern Nigeria. Afr J Paediatr Surg 6: 28–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Meta-analysis of the proportion of maternal deaths that occur before delivery, during labor and delivery or within 24 hours, and 24 hours to 42 days post-partum.
(DOCX)
The incidence of low birth weight and prematurity in 104 studies in Sub-Saharan Africa published between 1992 and 2011.
(DOCX)