Abstract
Axonal injury to retinal ganglion cells, a defined central neuron, induces a burst of intracellular superoxide anion that precedes externalization of membrane phosphatidylserine and subsequent apoptotic cell death. Dismutation of superoxide prevents the signal and delays loss of these cells, consistent with superoxide being necessary for transduction of the axotomy signal. However, phosphatidylserine externalization is a relatively late step in apoptosis, and it is possible that the superoxide burst is not an early axotomy signal but rather a result of cytochrome c release from the mitochondrial inner membrane with consequent accumulation of reduced intermediates. Other possibilities are that both superoxide generation and cytochrome c release are induced in parallel by axotomy, or that cytochrome c release potentiates the effect of the superoxide burst. To distinguish these various possibilities, serum-deprived neuronal retinal cells were assayed in vitro for superoxide elevation and release of cytochrome c from mitochondria, and the distribution of these two markers across a large number of cells used to model the temporal ordering of events. Based on this model of factor-dependent cell death, superoxide precedes, and possibly potentiates, cytochrome c release, and thus the former is likely an early signal for certain types of neuronal apoptosis in the central nervous system.
Keywords: Superoxide anion, Apoptosis signaling, Retinal ganglion cells, Growth factor deprivation
Introduction
Serum deprivation induces apoptosis in many cell types [1–3], due to a lack of necessary nutrients and trophic factors, with activation of the intrinsic (caspase 9-dependent) apoptotic pathway and release of cytochrome c. Serum deprivation is therefore a model for substrate-and/or neurotrophin-deprivation apoptosis. Serum deprivation-induced death can be prevented by drugs or other manipulations that scavenge or inactivate reactive oxygen species, implicating them in apoptosis signaling under these conditions [4].
Retinal ganglion cells (RGCs) are central neurons that transmit visual signals from the retina to the brain. RGCs undergo apoptosis after axonal injury [5]. Apoptosis also occurs if RGCs are deprived of critical growth factors, either in vitro by removal of factors from the medium [6], in vivo by blockage of retrograde transport [7], or removal of the tectal target [8]. Previous work in our laboratory showed that mitochondrially generated superoxide anion is an intracellular signal for cell death in primary RGCs after axotomy, a condition causing both axonal injury and deprivation of tectum-derived growth factors, but is not inhibited by extracellular delivery of brain-derived neurotrophic factor, ciliary neurotrophic factor, insulin, or forskolin [9]. Signaling of RGC death after axotomy by superoxide also occurs in vivo, using an optic nerve transection model [10].
Superoxide generation could therefore serve as the critical link between RGC axotomy and induction of RGC apoptosis independent of neurotrophin deprivation, as is seen in cerebral ischemia [11, 12]. However, superoxide generation could be a result of apoptosis, instead of causing it. This uncertainty arises from lack of data regarding the timing of the superoxide burst relative to other critical events in apoptosis, such as cytochrome c release from the mitochondrial matrix. To better understand the role of superoxide in apoptosis induction in growth factor deprivation, we assessed the signaling of serum deprivation-induced apoptosis in a transformed neuronal precursor cell line (RGC-5), and elucidated whether it was acting upstream or downstream to cytochrome c release from mitochondria.
Materials and methods
Materials
Dulbecco’s modified eagle’s medium (DMEM) and penicillin/streptomycin were from Cellgro (Manassas, VA). Fetal bovine serum was from Gemini Bio-Products (West Sacramento, CA). Poly-L-lysine, polyethylene glycol-conjugated superoxide dismutase (PEG–SOD), p-coumaric acid, and luminol were from Sigma (St. Louis, MO). Staurosporine was from Alexis Biochemicals (San Diego, CA). Etoposide was from MP Biomedicals (Solon, OH). Hydroethidine (HEt), calcein-AM, mouse monoclonal antibody against the 39 kDa subunit of complex I, mouse monoclonal antibody against native cytochrome c (clone 6H2.B4), Alexa Fluor 488 goat anti-mouse antibody, and NuPAGE 4–12% Bis–Tris gels were from Invitrogen (Carlsbad, CA). Mouse monoclonal antibody against denatured cytochrome c (clone 7H8.2C12) was from BD Biosciences (San Diego, CA). Affinity-purified peroxidase-conjugated goat anti-mouse IgG was from Jackson ImmunoResearch (West Grove, PA). All other reagents were from Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
Cell culture and serum deprivation
RGC-5 cells were obtained from Neeraj Agarwal, Ph.D. They were originally described as being derived from rat retinal neuronal precursor cells [13], but our and another laboratory’s mtDNA genotyping has revealed them to be of murine origin [14]. They were cultured at 37°C, 5% CO2 in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in 8-well chambered coverglass slides (Nunc, Rochester, NY) treated with poly-L-lysine. Prior to serum deprivation, all cells were cultured for 24 h followed by differentiation to a neuronal phenotype with 316 nM staurosporine [15]. Four hours after differentiation, cells were either left undisturbed, switched to a serum-free medium, or treated with etoposide, a known inducer of apoptosis through disruption of the mitochondrial membrane. Polyethylene glycol conjugated superoxide dismutase (PEG–SOD; 300 U/ml), a cell-permeable enzymatic scavenger of superoxide, was added to some serum-deprived conditions.
Detection of superoxide levels and cytochrome c localization
At 72 h after serum deprivation, cells were stained with 1 μg/ml HEt for 15 min in phosphate buffered saline (PBS) to detect the presence of the superoxide anion. After HEt staining, cells were rinsed with PBS and fixed with 4% paraformaldehyde for 10 min. Cells were again rinsed in PBS and washed with ice cold methanol for 5 min, followed by a PBS rinse and incubation for 30 min in Starting Block-T20 blocking buffer (Thermo Scientific, Rockford, IL). Fixed and blocked cells were incubated overnight with mouse monoclonal antibody against cytochrome c at 4 °C at 10 μg/ml in blocking buffer. Cells were rinsed three times with PBS and incubated at room temperature in Alexa Fluor 488 goat anti-mouse antibody at 8 μg/ml for 2 h. The cells were again rinsed in PBS three times, and sealed under cover slips with VectaShield (Vector Laboratories, Burlingame, CA). Cytochrome c was localized by immunofluorescence, and cells were photographed for quantitative and qualitative analysis.
Measurement of superoxide levels and cytochrome c localization
NIH ImageJ software was used to measure intensity of fluorescence in HEt-incubated cells to determine superoxide levels with respect to background. Cytochrome c release was assessed based on the absence or presence of punctuate fluorescent staining, indicating the presence of cytochrome c inside the mitochondria. Cells were enumerated based on their relative superoxide levels (low vs. high) and cytochrome c localization (mitochondrial vs. extramitochondrial), and classified into one of four categories: low superoxide/mitochondrial cytochrome c; low superoxide/extramitochondrial cytochrome c; high super-oxide/mitochondrial cytochrome c; or high superoxide/extramitochondrial cytochrome c (Fig. 1).
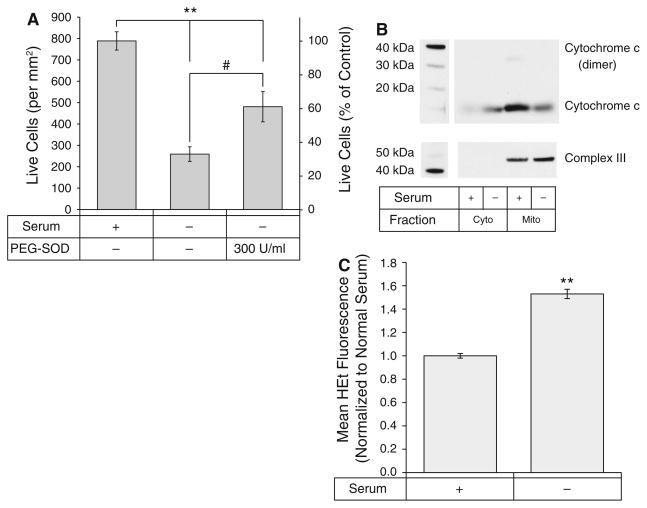
Fig. 1.
Serum deprivation induces apoptosis in a superoxide-dependent manner. A Viability of serum-deprived differentiated RGC-5 cells in the absence and presence of PEG–SOD (300 U/ml) compared to cells cultured in complete media. **p < 0.01 compared to complete media. #p < 0.05 compared to serum deprivation. B Cytochrome c release from mitochondria is induced by serum-deprivation. C Superoxide levels increased after serum deprivation
Immunoblotting for cytochrome c localization
To confirm cytochrome c release from mitochondria, RGC-5 cells were cultured in 150 cm2 tissue culture flasks and serum deprived as described above. After 72 h, cells were trypsinized, washed, and mitochondrial protein fractions prepared using Mitochondrial Isolation Kit for Cultured Cells (Thermo Scientific, Rockwell, IL), and cytosolic fractions reserved. Equal amounts of protein were resolved on Novex 4–20% Tris–Gly gels (Invitrogen), and transferred overnight at 150 mA to nitrocellulose membranes. Membranes were blocked in 0.5% milk protein in Tris–buffered saline containing 0.05% Tween-20 (TBS–T) at 4 °C overnight, then probed with antibodies against either denatured cytochrome c (1 μg/ml) or the core II subunit of ubiquinol cytochrome c oxidoreductase (Complex III; 0.5 μg/ml) at room temperature for 1 h. Blots were rinsed three times for 10 min with TBS–T, followed by probing with peroxidase-conjugated donkey anti-mouse IgG (0.04 μg/ml) at room temperature for 1 h. After washing, blots were incubated for ECL with 100 mM Tris–HCl, 225 μM p-coumaric acid, 1.25 mM luminol, and 3 mM H2O2 for 1 min, and used to expose X-ray film.
Assessment of cell viability
Cells were plated in 96-well tissue culture plates in sextuplicate or greater at a density of 125 cells/mm2. Approximately 24 h after plating, cells were differentiated by addition of staurosporine to a final concentration of 316 nM. Four hours after differentiation, media was aspirated from wells using a 25 gauge needle and replaced with fresh media or serum-free medium, both containing staurosporine at 316 nM. Some serum-deprived wells additionally received PEG–SOD in serum-free medium to a final concentration of 300 U/ml. At 72 h after deprivation, the medium was aspirated and replaced with 50 μl of phosphate-buffered saline containing 1 μg/ml calcein-AM. Plates were returned to a 37°C humidified incubator for 30 min. Three randomly chosen fields per well were photographed on an Axiovert 135 microscope under epifluorescence at a resolution of 3,008 × 2,000 pixels and an exposure time of 1.6 s. Live (calcein-positive) cells per field were counted using ImageJ software and averaged. Pictures were batch analyzed using a macro containing the following actions: subtract background, threshold, erode, erode, watershed, and analyze particles between the sizes of 1,000 square pixels and infinity. Automated counts were periodically checked against manual counts with good agreement. Relative counts are presented with respect to control.
Statistics
Significance testing of comparisons between groups of continuous variables were with Student’s t. Fisher’s exact test was used for 2 × 2 contingency tables. Chi-squared with Yates’s correction was used for 2 × n contingency tables.
Results
Serum deprivation leads to cell death, cytochrome c release from mitochondria, and elevated superoxide levels
In order to explore the sequence of apoptosis signaling in RGC-5 neuronal precursor cells, it was first necessary to characterize the serum deprivation model for inducing cell death in these cells. Our first aim was to verify that serum deprivation led to apoptosis in RGC-5 cells. Cells were differentiated with staurosporine (316 nM) to halt proliferation, a necessary step to distinguish cell death from decreased proliferation in serum-deprived conditions. Staurosporine at these doses causes RGC-5 cells to extend neurites and stop dividing for the first 24 h [15]. Four hours later the serum-containing media was aspirated and replaced with serum-free media containing staurosporine. Three days after serum deprivation, viability was assessed by calcein-AM fluorescence. Serum deprivation led to a significant decrease in cell viability compared to control (100 ± 2.7% vs. 32.9 ± 4.3%; p < 0.001), which was mitigated by treatment with PEG–SOD (61.0 ± 9.0%; p = 0.02). (Fig. 1A). The apoptotic nature of this cell death was confirmed through immunoblotting of mitochondrial and cytosolic fractions of differentiated RGC-5 cells cultured in serum and serum-deprived conditions. Immunoblotting (Fig. 1B) confirmed a loss of cytochrome c in the mitochondrial fraction of serum-deprived cells, and increased levels of cytochrome c in the cytosolic fraction. Immunoblotting for complex III was used to verify equal loading and purity of mitochondrial fractions.
Having established that serum deprivation of RGC-5 cells induces apoptosis characterized by cytochrome c release and that this apoptosis could be rescued by PEG–SOD, we examined intracellular superoxide levels in serum-deprived cells. Cells were incubated with dihydroethidium (HEt), fixed, and fluorescent intensity quantified. Fluorescence levels in each experiment were normalized to the mean fluorescence of cells cultured in complete media. Serum deprivation resulted in a significant increase in superoxide levels in differentiated RGC-5 cells compared to control cells at 72 h (1.53 ± 0.04 vs. 1.00 ± 0.02; p < 0.0001) (Fig. 1C). Together with the previous results, this suggests that superoxide plays a role in apoptotic signaling after serum deprivation in RGC-5 cells.
Cytochrome c release from the mitochondria occurs during a transient increase in superoxide levels
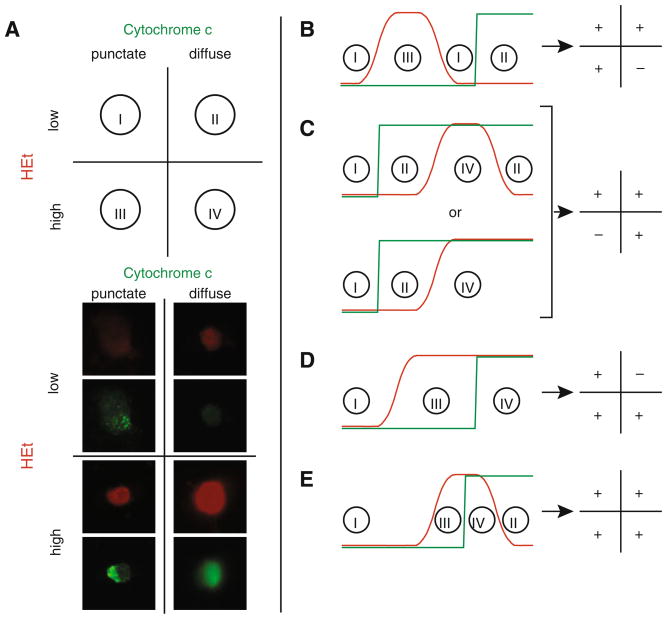
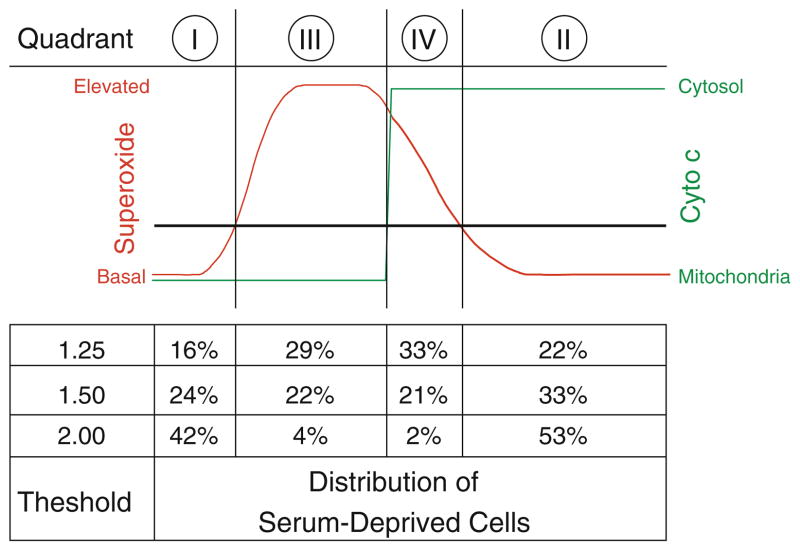
Having demonstrated elevation in intracellular superoxide levels after serum deprivation, we sought to determine the relative timing of cytochrome c release with respect to the increase in superoxide levels. We labeled cells with HEt to measure superoxide, and immunostained for cytochrome c in order to assess its localization in the mitochondria or cytosol of normal and serum-deprived cells. This allowed classification of individual cells into one of four quadrants: low superoxide with mitochondrial cytochrome c localization (quadrant I), low superoxide with cytosolic cytochrome c (quadrant II), high superoxide with mitochondrial cytochrome c localization (quadrant III), and high superoxide with cytosolic cytochrome c (quadrant IV) (Fig. 2A).
Fig. 2.
Relative timing of cytochrome c release and superoxide elevation. A Cells were classified into one of four quadrants based on superoxide levels, as assessed by HEt fluorescence, and cytochrome c localization. Cells representative of each combination, in appropriate quadrants, for a superoxide threshold value of 1.5. B–E There are four experimentally distinguishable possibilities for the relative release of cytochrome c with respect to elevated superoxide levels: B superoxide levels (red) can rise and fall prior to cytochrome c release (green); C cytochrome c can be released prior to the rise (and fall) of superoxide levels; D superoxide levels can become elevated and remain at high levels, followed by cytochrome c release; or E cytochrome c release can occur while superoxide levels are elevated, followed by a decrease in superoxide. Regions on the timing graph matching each quadrant are identified, and the expected distribution pattern for each scheme is provided (Color figure online)
Because release of cytochrome c from mitochondria is irreversible, there are a limited number of hypothetical schemes depicting how it could be linked to superoxide release (Fig. 2B–E). In the first scheme, a burst in superoxide completely precedes the release of cytochrome c (Fig. 2B). This sequence of events would eliminate the possibility of cells in quadrant IV, i.e. those with both elevated superoxide levels and cytosolic cytochrome c, and would imply that generation of superoxide is an upstream signal preceding cytochrome c release. In the second scheme, cytochrome c release induces an increase in superoxide (Fig. 2C). This sequence of events would eliminate the possibility of cells in quadrant III i.e. those with high superoxide levels and punctate (mitochondrial) cytochrome c, because elevated superoxide levels would not be seen prior to cytochrome c release in this scenario. In the third scheme, a sustained elevation in superoxide levels precedes cytochrome c release, with high levels of superoxide sustained until cell death occurs (Fig. 2D). With this scheme there should be no cells in quadrant II, i.e. low superoxide and released (cytoplasmic) cytochrome c. In the fourth scheme, cytochrome c is released during a burst of superoxide (Fig. 2E). With this scheme there should be cells in all four quadrants. For each of the four possible schemes, we expect a shift in the distribution of cells from primarily quadrant I (healthy cells) to the other quadrants following serum deprivation.
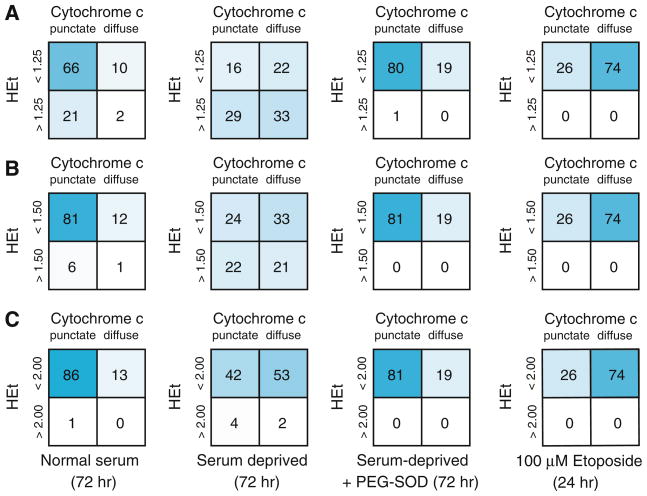
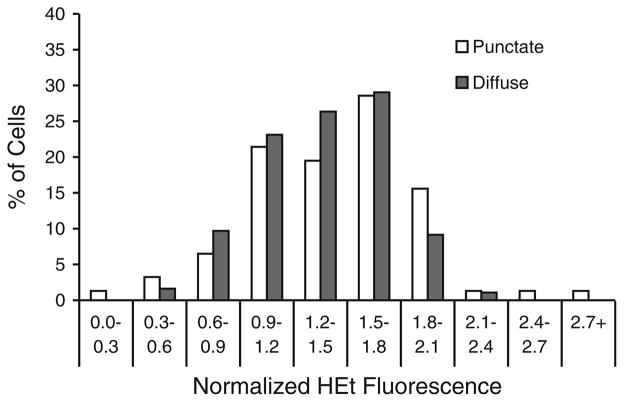
Using a threshold value of 1.5—representing superoxide increasing greater than 50% over basal levels in complete medium [9]—we saw a decrease in cells classified into quadrant I, to 23.5% from 80.7%, and a corresponding increase in the three other quadrants (II: 33.2% vs. 12.0%; III: 21.8 vs. 6.4%; IV: 21.5 vs. 0.9%; p < 0.0001 by Fisher exact test). Similar shifts were seen for threshold values of 1.25 (p < 0.0001) and 2.00 (p < 0.0001) (Fig. 3A–C), indicating that the results did not depend on the choice of a threshold for the superoxide elevation. Additionally, comparison of mean HEt fluorescence levels between cells prior to and following cytochrome c release after serum deprivation showed no significant difference in the mean cell superoxide levels between the two groups (1.43 ± 0.04 vs. 1.37 ± 0.03 in cells with punctate and diffuse staining, respectively; p = 0.2), nor in distributions of per cell HEt fluorescence (Fig. 4). These results are consistent with the superoxide burst overlapping with the early stages of cytochrome c release from mitochondria, and rule out the superoxide burst following cytochrome c release.
Fig. 3.
Classification of cells into quadrants based on superoxide and cytochrome c states. Cells are classified into the four quadrants to determine a relative timing for the events involved in serum deprivation-induced apoptosis. Serum-deprived RGC-5 cells show a significant shift in distribution away from the low superoxide/particulate cytochrome c state with threshold HEt fluorescence values of A 1.25, B 1.5, and C 2.0
Fig. 4.
Elevation of superoxide in serum-deprived cells is not dependent on cytochrome c localization. Histogram showing the distribution of normalized HEt fluorescence values at 72 h in serum-deprived RGC-5 cells before (open bars) and after (gray bars) cytochrome c release. There was no significant difference in mean fluorescence levels between the two groups (p = 0.20 by chi-square testing)
To prove that the signaling of cytochrome c release was superoxide-dependent, pegylated superoxide dismutase (PEG–SOD) was used to scavenge intracellular superoxide. The addition of PEG–SOD eliminated the rise in superoxide (mean normalized value of 0.62 ± 0.03 vs. 1.31 ± 0.06; p <0.001), and significantly reduced the fraction of cells undergoing cytochrome c release—the sum of quadrants II and IV—to 19.3% compared to 54.7% for serum deprivation alone (p <0.0001) (Fig. 3). PEG–SOD also returned the distribution of serum-deprived cells to a level statistically indistinguishable from normal serum (p = 0.6).
As another means of testing whether the increase in superoxide levels was a cause or a result of apoptosis, apoptosis was directly induced with the topoisomerase II inhibitor etoposide, and superoxide levels and cytochrome c localization measured [16]. Analysis of etoposide-treated cells (100 μM, 24 h) showed an increase in cytochrome c release independent of superoxide production. Etoposide also induced a significant decrease in viability compared to control (27.7 ± 0.8% vs. 100 ± 1.0%; p < 0.0001), and this was not rescued by co-incubation with PEG–SOD (22.1 ± 1.0%). Together these results imply that superoxide production in this neuronal precursor cell line is a cause and not a result of apoptosis.
Discussion
These data indicate that growth factor-deprived neuronal cells increase superoxide levels before release of mitochondrial release of cytochrome c. These results are in agreement with previous studies demonstrating an asynchronous rise in superoxide levels in axotomized primary RGCs [9]. In that study, cells could be classified into two categories: cells undergoing a superoxide burst (>50% above mean control levels), and cells remaining at control superoxide levels. The fraction of cells with an intracellular superoxide burst increased substantially after both axotomy and optic nerve crush, and dismutation of superoxide achieved by intracellularly delivered PEG–SOD significantly increased RGC survival [17].
These in vitro and similar in vivo [10] results are consistent with superoxide serving as a signaling molecule for the induction of apoptosis after axotomy. However, it is also possible that superoxide elevation is the result of apoptosis itself, e.g. cytochrome c release and inhibition of mitochondrial electron flow leading to one-electron reduction of O2 [18]. In order to determine the relative timing of superoxide production and cytochrome c release, we systematically assessed these markers in neuronal precursor cells after serum deprivation, and found that cytochrome c release occurred only in the later stages of the superoxide burst.
The timing of superoxide-dependent apoptotic pathways is controversial. We previously showed that serum deprivation induces superoxide in neuronal RGC-5 cells, and that scavenging of reactive oxygen species is protective in this model [19]. Axonal injury of retinal ganglion cells also specifically induces superoxide in vivo [10, 19], and scavenging of superoxide is neuroprotective—all indicative of superoxide as an early, pre-commitment phase in apoptosis, occurring prior to the irreversible release of cytochrome c. In vivo imaging of intracellular RGC superoxide and annexin V binding demonstrated that the superoxide burst preceded phosphatidylserine exposure [10]. Based on the results of the present study and previous research, we suggest that the order of events in axotomy-induced apoptosis is superoxide production, then cytochrome c release, and finally phosphatidylserine exposure. This agrees with the ordering of events reported in a variety of models for studying apoptosis, including Hep2G cells treated with sodium selenite [20], Jurkat T cells after addition of exogenous C(2)-ceramide [21], HeLa cells after ultraviolet light exposure [22], and cerebral ischemia in mice deficient in SOD-2 [11].
Conversely, Cai and Jones reported an altered order of these steps in staurosporine-induced apoptosis [23], with cytochrome c release preceding oxidation, as measured by intracellular redox potential. Phosphatidylserine exposure was seen preceding cytochrome c release after treatment with staurosporine, etoposide, and tumor necrosis factor in a different cell line [24]. Others found that peroxidation of phosphatidylserine is an early oxidative marker occurring prior to release of cytochrome c in the same model as Cai and Jones, suggesting a small but highly specific oxidative event prior very early in the cell death process [25]. However, the inability of the antioxidant N-acetylcysteine (NAC) to rescue cells from staurosporine-induced apoptosis by Cai and Jones more likely suggests that that process may proceed by a superoxide-independent pathway. Chauhan et al. demonstrated in multiple myeloma cells that induction of a superoxide-dependent apoptotic pathway can be rescued with NAC, preventing both cell death and the release of cytochrome c and Smac, while NAC is not effective in preventing cell death or Smac release in a superoxide-independent (dexamethasone) pathway [26]. While all the components of the apoptotic pathway are activated in both the superoxide-dependent and superoxide-independent pathways, it may be the sequence of these first steps that differentiates the two modalities, and the subsequent activation of “skipped” steps in one versus the other may be signs of an inherent redundancy to ensure that once initiated, apoptosis proceeds to caspase activation and eventual cell death.
Given that intracellular cytochrome c distribution is most accurately studied with immunocytochemistry, it was necessary to use a cross-sectional method for correlating superoxide levels with cytochrome c release. To do this, we delineated four distinct schemes that could account for the relative timing of increased superoxide production and cytochrome c release after serum deprivation (Fig. 2). Each of these four schemes would predict a different pattern of cells belonging to each of four quadrants—low superoxide/mitochondrial cytochrome c localization (quadrant I), low superoxide/cytosolic cytochrome c (quadrant II), high superoxide/mitochondrial cytochrome c (quadrant III), and high superoxide/cytosolic cytochrome c (quadrant IV).
At baseline, cells are in quadrant I, with background levels of superoxide and cytochrome c maintained within mitochondria. Our data demonstrate that at 72 h after serum deprivation, cells shift from quadrant I to quadrants II, III, and IV. This pattern indicates a timeline for apoptotic signaling consistent with cytochrome c release occurring in the latter half of the superoxide burst, after superoxide levels have peaked. If cytochrome c release were instead to precede the superoxide burst, then there should be no cells in quadrant III (high superoxide but mitochondrial cytochrome c), contrary to what was observed. Given that 22% of the cells were in quadrant III and 21% in quadrant IV (Fig. 5), then high superoxide levels were present in cells before and after cytochrome c release. This suggests that the superoxide burst precedes and partly overlaps cytochrome c release.
Fig. 5.
Proposed scheme for the time-course of a superoxide burst and cytochrome c release. The distribution of cells among the four quadrants is consistent with the scheme where the release of cytochrome c occurs during the later portion of the superoxide burst. Increasing the threshold for the burst of superoxide decreased the proportion of cells classified into quadrants 3 and 4. The schematic is descriptive and is not to scale
Cytochrome c release is considered an early apoptotic commitment point. Our observation that the superoxide burst precedes cytochrome c release is consistent with the ability of PEG–SOD to rescue primary RGCs after axotomy in culture [17], as well as in vivo [10]. Furthermore, treatment of cells with etoposide for 24 h resulted in cytochrome c release without a corresponding increase in superoxide levels, consistent with etoposide inducing apoptosis in a superoxide-independent manner [16], and confirming that cytochrome c release alone does not induce superoxide production.
This study has inherent limitations. It is controversial whether RGC-5 cells are truly RGCs, and all that can be established with certainty is that they are neuronal precursor cells which can be differentiated to express cell-surface and morphological markers consistent with more mature neurons [15, 27]. We used serum withdrawal to trigger apoptosis, which deprives cells of multiple growth factors, a pathway similar but not identical to what occurs when neurons undergo axotomy-induced deprivation of target-derived factors. RGC-5 cells respond to serum deprivation by undergoing apoptosis via a cytochrome c-dependent pathway. These results are therefore applicable to the underlying mechanism and signaling of apoptosis after serum deprivation in a neuronal cell line, but not necessarily axotomy.
A possible source of bias in this study is the selection of the threshold value for defining a superoxide burst. The number of cells in quadrants III and IV is entirely dependent on this threshold value. In our prior work with primary RGCs, a threshold value of 1.5 (indicating superoxide present at 50% greater than basal levels) was sufficient for detecting an increase in the proportion of cells undergoing a superoxide burst [9]. In that study, however, up to 10% of RGCs from retinas not undergoing optic nerve crush had basal levels above threshold. This is likely due to inherent variability in basal superoxide production, and therefore it is not surprising that we observed a small fraction of RGC-5 cells above this level when incubated in complete medium. In the present study 7.4% of cells cultured in complete medium had superoxide levels greater than the threshold value of 1.5, consistent with results in primary RGCs. To further address this possible bias, we analyzed the data using two other threshold values (1.25 and 2.0). Although the raw percentages of cells in each quadrant shifted with different choices of threshold values, the change in distribution induced by serum deprivation was statistically independent of which threshold value was chosen.
A possible confounding factor is that the differentiating agent, staurosporine, induces apoptosis at high concentrations. Low levels of apoptosis were seen even at the concentration (316 nM) used in this study, with 12.9% of cells cultured in complete media undergoing apoptosis by 72 h, based on cytochrome c release into the cytoplasm. On the other hand, although this is higher than what is normally seen with undifferentiated cells in complete media (approximately 5%) [28], it still is significantly lower than the 54.7% of serum-deprived cells that released mitochondrial cytochrome c.
In summary, superoxide production in serum-deprived neuronal cells occurs before cytochrome c release, and thus is an early signal in triggering apoptosis. How superoxide is generated and how it transduces the apoptosis signal is unclear, but presumably preventing or reversing superoxide signaling before neurons become committed to an apoptotic fate would be potentially useful as a therapy for trophic factor-deprived neurons in the central nervous system [19].
Acknowledgments
Grant Support: NIH R21 EY017970 and P30EY016665, Retina Research Foundation, and an unrestricted departmental grant from Research to Prevent Blindness, Inc. LAL is a Canada Research Chair of Ophthalmology and Visual Sciences.
Contributor Information
Christopher J. Lieven, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA
Katherine A. Thurber, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA
Emily J. Levin, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA
Leonard A. Levin, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA. Maisonneuve-Rosemont Hospital Research Center and Department of Ophthalmology, University of Montreal, Montreal, Canada
References
- 1.Charles I, Khalyfa A, Kumar DM, et al. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005;46:1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- 2.Rello S, Stockert JC, Moreno V, et al. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis. 2005;10:201–208. doi: 10.1007/s10495-005-6075-6. [DOI] [PubMed] [Google Scholar]
- 3.Hogg N, Browning J, Howard T, Winterford C, Fitzpatrick D, Gobe G. Apoptosis in vascular endothelial cells caused by serum deprivation, oxidative stress and transforming growth factor-beta. Endothelium. 1999;7:35–49. doi: 10.3109/10623329909165310. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Hyun DH, Halliwell B, Jenner P. Effect of over-expression of wild-type and mutant Cu/Zn-superoxide dismutases on oxidative stress and cell death induced by hydrogen peroxide, 4-hydroxynonenal or serum deprivation: potentiation of injury by ALS-related mutant superoxide dismutases and protection by Bcl-2. J Neurochem. 2001;78:209–220. doi: 10.1046/j.1471-4159.2001.00417.x. [DOI] [PubMed] [Google Scholar]
- 5.Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 8.Franklin Hughes W, La Velle A. The effects of early tectal lesions on development in the retinal gonglion cell layer of chick embryos. J Comp Neurol. 1975;163:265–283. doi: 10.1002/cne.901630303. [DOI] [PubMed] [Google Scholar]
- 9.Lieven CJ, Schlieve CR, Hoegger MJ, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest Ophthalmol Vis Sci. 2006;47:1477–1485. doi: 10.1167/iovs.05-0921. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010;133:2612–2625. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimura M, Morita-Fujimura Y, Kawase M, et al. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome c and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niizuma K, Yoshioka H, Chen H, et al. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010;1802:92–99. doi: 10.1016/j.bbadis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 14.Van Bergen NJ, Wood JP, Chidlow G, et al. Recharacterization of the RGC-5 retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2009;50:4267–4272. doi: 10.1167/iovs.09-3484. [DOI] [PubMed] [Google Scholar]
- 15.Frassetto LJ, Schlieve CR, Lieven CJ, et al. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 16.Gorman A, McGowan A, Cotter TG. Role of peroxide and superoxide anion during tumour cell apoptosis. FEBS Lett. 1997;404:27–33. doi: 10.1016/s0014-5793(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 17.Schlieve CR, Lieven CJ, Levin LA. Biochemical activity of reactive oxygen species scavengers do not predict retinal ganglion cell survival. Invest Ophthalmol Vis Sci. 2006;47:3878–3886. doi: 10.1167/iovs.05-1010. [DOI] [PubMed] [Google Scholar]
- 18.Luetjens CM, Bui NT, Sengpiel B, et al. Delayed mitochondrial dysfunction in excitotoxic neuron death: cytochrome c release and a secondary increase in superoxide production. J Neurosci. 2000;20:5715–5723. doi: 10.1523/JNEUROSCI.20-15-05715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanamori A, Catrinescu MM, Mahammed A, Gross Z, Levin LA. Neuroprotection against superoxide anion radical by metallocorroles in cellular and murine models of optic neuropathy. J Neurochem. 2010;114:488–498. doi: 10.1111/j.1471-4159.2010.06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen HM, Yang CF, Ding WX, Liu J, Ong CN. Superoxide radical-initiated apoptotic signalling pathway in selenite-treated HepG(2) cells: mitochondria serve as the main target. Free Radic Biol Med. 2001;30:9–21. doi: 10.1016/s0891-5849(00)00421-4. [DOI] [PubMed] [Google Scholar]
- 21.Hearps AC, Burrows J, Connor CE, Woods GM, Lowenthal RM, Ragg SJ. Mitochondrial cytochrome c release precedes transmembrane depolarisation and caspase-3 activation during ceramide-induced apoptosis of Jurkat T cells. Apoptosis. 2002;7:387–394. doi: 10.1023/a:1020034906200. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 24.Denecker G, Dooms H, Van Loo G, et al. Phosphatidyl serine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett. 2000;465:47–52. doi: 10.1016/s0014-5793(99)01702-0. [DOI] [PubMed] [Google Scholar]
- 25.Matsura T, Serinkan BF, Jiang J, Kagan VE. Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 2002;524:25–30. doi: 10.1016/s0014-5793(02)02990-3. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan D, Li G, Sattler M, et al. Superoxide-dependent and-independent mitochondrial signaling during apoptosis in multiple myeloma cells. Oncogene. 2003;22:6296–6300. doi: 10.1038/sj.onc.1206734. [DOI] [PubMed] [Google Scholar]
- 27.Lieven CJ, Millet LE, Hoegger MJ, Levin LA. Induction of axon and dendrite formation during early RGC-5 cell differentiation. Exp Eye Res. 2007;85:678–683. doi: 10.1016/j.exer.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Zhang Q, Wang J, et al. Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res. 2009;1279:131–138. doi: 10.1016/j.brainres.2009.04.054. [DOI] [PubMed] [Google Scholar]