Abstract
Pichia pastoris is a methylotrophic yeast that has been genetically engineered to express over one thousand heterologous proteins valued for industrial, pharmaceutical and basic research purposes. In most cases, the 5′ untranslated region (UTR) of the alcohol oxidase 1 (AOX1) gene is fused to the coding sequence of the recombinant gene for protein expression in this yeast. Because the effect of the AOX1 5′UTR on protein expression is not known, site-directed mutagenesis was performed in order to decrease or increase the length of this region. Both of these types of changes were shown to affect translational efficiency, not transcript stability. While increasing the length of the 5′UTR clearly decreased expression of a β-galactosidase reporter in a proportional manner, a deletion analysis demonstrated that the AOX1 5′UTR contains a complex mixture of both positive and negative cis-acting elements, suggesting that the construction of a synthetic 5′UTR optimized for a higher level of expression may be challenging.
Keywords: Pichia pastoris, Recombinant protein expression, Translational regulation, Untranslated regions, mRNA
1. Introduction
Recombinant proteins serve many uses in our society ranging from therapeutic agents to industrial enzymes. Since the early 1990s, the methylotrophic yeast, Pichia pastoris, has been an excellent system for heterologous protein production, with over one thousand recombinant proteins having been produced in this yeast (Cereghino and Cregg, 2000; Lin-Cereghino et al., 2007). One key to its success is the alcohol oxidase 1 promoter (AOX1), which is used to drive the expression of the recombinant protein (Ellis et al., 1985; Koutz et al., 1989). The AOX1 promoter is tightly repressed in cells grown in glucose medium but induced over 1000-fold in methanol medium (Lin-Cereghino et al., 2006). For expression of a specific recombinant protein, usually the coding sequence of the foreign gene is first inserted into a polylinker located between the AOX1 promoter with its 5′ untranslated region (5′UTR) and the AOX1 3′UTR of the expression cassette of a vector (Lin Cereghino et al., 2001). The construct is then transformed into competent P. pastoris cells, where it integrates usually by homologous recombination, and transformants are induced on methanol medium to trigger transcription of the hybrid gene. The resulting mRNA, containing the AOX1 5′UTR, the foreign coding region and the AOX1 3′UTR, is then bound at the cap by an initiation complex, which consists of the 40S ribosomal subunit and eukaryotic initiation factors (eIFs) (Merrick, 2010; Pesole et al., 2001; Sonenberg and Hinnebusch, 2009). The 5′UTR is consequently scanned by the translation initiation complex in a 5′ to 3′ direction until the first AUG is encountered (Merrick, 2010). At this point, the 60S ribosomal subunit normally joins the complex, an 80S complex is formed, and translation commences.
In order to increase the yield of the recombinant protein, most investigators have focused on altering the AOX1 promoter region or alternatively using a different promoter, such as glyceraldehyde-3-phosphate dehydrogenase (GAP) (Hartner et al., 2008; Qin et al., 2011). Little is known about the contribution of the AOX1 5′UTR to the expression of recombinant proteins. The 5′UTR is the region of mRNA encoded by DNA between the transcription start site and the first ATG in the gene (Lawless et al., 2009). In many eukaryotic genes, the 5′UTR has a regulatory function by affecting either the stability or the translational efficiency of the mRNA (Mokdad-Gargouri et al., 2001; Park et al., 2007; Sonenberg and Hinnebusch, 2009; Warringer et al., 2010; Wicksteed et al., 2007). The 5′UTR can exert an effect on translation efficiency either by adopting a secondary structure which can influence the rate of ribosome movement or by binding trans-acting factors that affect the function of the translation machinery (Chatterjee and Pal, 2009; Mignone et al., 2002; Mittelmeier and Dieckmann, 1995). Some eukaryotic 5′UTRs even contain sequences called internal ribosome entry sites (IRES), which allow the translation initiation complex with the 40S to skip over the cap and bind nearer to the first AUG in a cap-independent manner (Baird et al., 2006; Mokrejs et al., 2009). However, at the present time, IRES are defined by functional criteria only. Although some contain a Y-shaped stem-loop just upstream of the AUG initiation codon, there is no identified consensus sequence (Lopez-Lastra et al., 2005; Mignone et al., 2002). 5′UTR structure, thus, has the potential to influence protein expression, but much of this potential has yet to be understood.
Even though the AOX1 5′UTR is found in most P. pastoris expression vectors, to date no systematic studies have been done to determine if the AOX1 5′UTR plays a significant role in controlling protein expression. Because the 114 nucleotide-long 5′UTR is unusually long for yeast 5′UTRs (most are shorter than 50 nucleotides) (Lawless et al., 2009), this sequence drew our interest. Our first goal was to determine if the AOX1 5′UTR contains sequence elements that influence protein production by acting on transcript stability or translatability. We envisioned using this data to engineer an artificial 5′UTR to provide enhanced protein production. For instance, mutagenesis of a 5′UTR in Aspergillus oryzae increased expression of a GUS reporter up to eight-fold (Koda et al., 2004). Our second goal was to comprehend how increases in the length of 5′UTR affect expression of heterologous proteins. Often when investigators are inserting their coding sequences into expression vectors, they may add a large amount of foreign sequence upstream of the ATG or use a restriction site located in the 3′ region of a polylinker (Bagga, 2008; Grillo et al., 2010). Both of these actions lengthen the 5′UTR. Although it was reported that an increase of 36 nucleotides in the AOX1 5′UTR reduced the expression of human serum albumin by about 50-fold (Sreekrishna, 1993), no systematic analysis has been performed to correlate the effect of incremental 5′UTR extension with protein expression levels.
To meet these two goals, site-directed mutagenesis was carried out on the AOX1 5′UTR region fused to a β-galactosidase reporter. β-galactosidase assays were performed to measure the effect of these mutations on protein expression while northern analysis was utilized to explore the possibility that these mutations affected transcript stability. Although increasing the length of the 5′UTR had a clear effect on protein expression, our results also suggest that the AOX1 5′UTR contains an intricate network of both positive and negative cis-acting elements that affect the translational efficiency of the messenger mRNA.
2. Materials and methods
2.1. Strains, media and reagents
P. pastoris strain yJC100 (wild type) is a derivative of the original wild-type P. pastoris strain NRRL Y11430 (North Regional Research Laboratories, US Department of Agriculture, Peoria, IL) and has been described previously (Lin-Cereghino et al., 2006). yJC100 cells were cultured in either YPD medium (1% yeast extract, 2% peptone, and 2% glucose), YND (minimal medium with 1% dextrose) or YNM (minimal medium with 0.5% methanol) (Cregg and Madden, 1988; Cregg et al., 1985).
Recombinant DNA manipulations were carried out in the Escherichia coli strain TOP10 (Invitrogen Corp., Carlsbad, CA). TOP10 cells were cultured in LB medium (0.5% yeast extract, 1% glucose, and 0.5% NaCl) at 37 °C. Zeocin was added to LB medium at the final concentration of 25 µg/mL Zeocin for plasmid selection. Recombinant DNA methods, including bacterial transformation, were performed essentially as described (Sambrook et al., 1989). Plasmid DNA was purified from E. coli cultures using a QIAprep Spin Miniprep Kit (Qiagen, Chatsworth, CA). PCR products were purified with the QIAprep PCR Cleanup Kit (Qiagen, Chatsworth, CA) prior to restriction digestion. Restriction enzymes were purchased from MBI Fermentas (Hanover, MD). DNA digested with restriction enzymes was resolved in TBE agarose gels. DNA fragments were purified from agarose gels by using the Geneclean II kit (Qbiogene, Carlsbad, CA). Chromosomal DNA from P. pastoris transformants was prepared using the OmniPrep™ kit from GenoTechnology, Inc. (St. Louis, MO). Oligonucleotides were synthesized by Sigma Genosys (Plano, TX). All mutated sites and ligation junctions in newly synthesized vectors were confirmed by DNA sequencing (Geneway Research, Hayward, CA).
2.2. Construction of plasmids
The coding sequence the lacZ gene was amplified from pGC181 (Lin-Cereghino et al., 2006) with the primers chriss5bgal2 CAAGAATTCATGCCAGGGGATCCCGTCGTT and 0103R CAAGCGGCCGCTTTTTGACACCAGACCAA. The resulting PCR fragment was restricted with the enzymes EcoRI and NotI and then ligated into the corresponding sites of pPICZB (Invitrogen Corp, Carlsbad, CA) to yield pCS1, the parent deletion plasmid. All deletion plasmids were created by site-directed mutagenesis using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The parent addition plasmid, pLR1, was created from pCS1. The plasmid pLR1 and pCS1 are identical except pLR1 has a SpeI restriction site inserted in the 5′UTR for use in vector construction. Plasmid pLR1 was created using the QuikChange II XL Site-Directed Mutagenesis Kit with primers Laura 1a GAGAAGATCAAAAAACAACTAGTTATTCGAAACGAGG and Laura 1b CCTCGTTTTCGAATAACTAGTTGTTTTTTGATCTTCTC. All addition plasmids were produced from pLR1 using the double oligonucleotide reannealing technique (DORT) (see below). pAH201, in which the AOX1 5′UTR was replaced by the GAP 5′UTR, was constructed by removing the 140 bp HindIII-EcoRI fragment of pCS1 and inserting the following two complementary oligonucleotides Amyswapgaptop CCTTTTTTTTTATCATCACTAGTAGCTTACTTTCATAATGCG and Amyswapgapbottom CGCAATTATGAAAGTAAGCTACTAGTGATGATAAAAAAAAAGG with the DORT.
2.3. Site-directed mutagenesis
Deletions of the AOX1 5′UTR were generated using QuikChange II XL Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA). Primers were designed to anneal to the parent plasmid, pCS1, and “loop” out an area of the 5′UTR creating a deletion. The primers used in this work, which were HPLC purified, are listed in Table 1. Mutagenesis was performed according to manufacturer's directions. Briefly, the primers were diluted to a concentration of 25 ng/µL and the following reaction mixture was set up: 5 µL of 10× reaction buffer, 1 µL of pCS1, 5 µL of each primer, 1 µL of 10 mM dNTP, 3 µL of Quik-Solution, 1 µL of Pfu Turbo DNA polymerase, and sterile water to a final volume of 50 µL. The cycling parameters were a) one cycle of 95 °C for 5 min, b) eighteen cycles of 95 °C for 30 s, 55 °C for 60 s, 68 °C for 7 min and c) one cycle of 68 °C for 10 min. After temperature cycling, 1 µL DpnI was added directly to the reaction mix and incubated at 37 °C for 1 h to destroy the methylated, parental DNA. The final step of the mutagenesis was the transformation of the mutated plasmid reaction into One Shot TOP 10 Ultra competent E. coli cells (Invitrogen, Carlsbad, CA) and selection on LB plates supplemented with Zeocin® (25 µg/mL).
Table 1.
Primers used to make deletion constructs.
| Primer name | Sequence (5′-3′) | Plasmid |
|---|---|---|
| CS11fow | GCTTACTTTCATAATTGCTTTTGATTTTAACGACTTTTAACG | pCSΔ94-73 |
| CS11rev | CGTTAAAAGTCGTTAAAATCAAAAGCAATTATGAAAGTAAGC | |
| CS12fow | GCTTACTTTCATAATTGCCGACAACTTGAGAAGATCAAAAAAC | pCSΔ94-51 |
| CS12rev | GTTTTTTGATCTTCTCAAGTTGTCGGCAATTATGAAAGTAAGC | |
| CS13fow | GCTTACTTTCATAATTGCAAAAAACAACTAATTATTCGAAACGAGG | pCSΔ94-33 |
| CS13rev | CCTCGTTTCGAATAATTAGTTGTTTTTTGCAATTATGAAAGTAAGC | |
| CS14fow | GCTTACTTTCATAATTGCCGAAACGAGGAATTCATGCCAGGGG | pCSΔ94-16 |
| CS14rev | CCCCTGGCATGAATTCCTCGTTTCGGCAATTATGAAAGTAAGC | |
| CS15fow | CTTTTTTTTTATCATCATTATTAGCCGAAACGAGGAATTCATGCCAGGGG | pCSΔ110-16 |
| CS15rev | CCCCTGGCATGAATTCCTCGTTTCGGCTAATAATGATGATAAAAAAAAAG | |
| CS25fow | GCTTACTTTCATAATTGCGATTTTAACGACTTTTAACGAC | pCSΔ94-69 |
| CS25rev | GTCGTTAAAAGTCGTTAAAATCGCAATTATGAAAGTAAGC | |
| CS30fow | GCTTACTTTCATAATTGCTAACGACTTTTAACGAC | pCSΔ94-64 |
| CS30rev | GTCGTTAAAAGTCGTTAGCAATTATGAAAGTAAGC | |
| AMYAOX5UTRdel5TOP | GGTTCCAATTGACAAGCATTTTAACGAC | pCSΔ69-73 |
| AMYAOX5UTRdel5BOTTOM | GTCGTTAAAATGCTTGTCAATTGGAACC | |
| AMYAOX5UTRdel9TOP | GCGACTGGTTCCAATTGACAATTTAACGACTTTTAACG | pCSΔ67-75 |
| AMYAOX5UTRdel9BOTTOM | CGTTAAAAGTCGTTAAATTGTCAATTGGAACCAGTCGC | |
| PAH15top | CTAGTAGCTTACTTTCATAATTGCACA | pCSΔ94-80 |
| PAH15bottom | AGCTTGTGCAATTATGAAAGTAAGCTA | |
| TM31-43TOP | GACAAGCTTTTGATTTCGACAACTTGAGAAG | pCSΔ63-51 |
| TM31-43BOT | CTTCTCAAGTTGTCGAAATCAAAAGCTTGTC | |
| MN62-79TOP | CAACTTGAGAAGATCCGAAACGAGGAATTC | pCSΔ32-16 |
| MN62-79BOT | GAATTCCTCGTTTCGGATCTTCTCAAGTTG | |
| KO74-86TOP | GATCAAAAAACAACTAATAGGAATTCATGCCAG | pCSΔ9-19 |
| KO74-86BOT | CTGGCATGAATTCCTATTAGTTGTTTTTTGATC | |
| MNKOTM25-28DELBOT | CGTTAAAAGTCGTTAAAAAAGCTTGTCAATTGG | pCSΔ67-70 |
| MNKOTM25-28DELTOP | CCAATTGACAAGCTTTTTTAACGACTTTTAACG | |
| MNKOTM66-73DELTOP | CTTGAGAAGATCAAAAATTATTCGAAACGAGG | pCSΔ22-29 |
| MNKOTM66-73DELBOT | CCTCGTTTCGAATAATTTTTGATCTTCTCAAG |
2.4. Double oligo reannealing technique (DORT)
All addition plasmids were produced using the DORT to insert polymers of adenine (A) nucleotides into the AOX1 5′UTR. The pairs of complementary oligonucleotides (Table 2) were designed in a manner that would create SpeI and EcoRI “sticky ends” when annealed, which would allow them to insert into the respective sites located in pLR1. Briefly, single-stranded oligonucleotides were diluted to a concentration of 1 µg/µL, and 1 L of each oligonucleotide was added to 99 µL of sterile water in a 1.5 mL microfuge tube. Samples were heated at 100 °C for 5 min and then allowed to cool to room temperature, allowing annealing of the oligonucleotides. 3 µL (100 ng) of digested, purified pLR1 vector was combined with 5 µL (50–100 ng) of annealed oligonucleotides, 9 µL of sterile water, and 2 µL of Fermentas 10× ligase buffer and 1 µL of DNA ligase. The ligation reactions were incubated at room temperature overnight and then used to transform competent E. coli cells.
Table 2.
Primers used to make addition constructs.
| Primer name | Sequence (5′-3′) | Plasmid |
|---|---|---|
| 5AOXfun10allAtop | CTAGTAAAAAAAAAAG | pLR10 |
| 5AOXfun10allTbot | AATTCTTTTTTTTTTA | |
| 5AOXfun20allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAG | pLR20 |
| 5AOXfun20allTbot | AATTCTTTTTTTTTTTTTTTTTTTTA | |
| Fun30allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA | pLR30 |
| Fun30allTbot | AATTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTA | |
| 5AOXfun33allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAG | pLR33 |
| 5AOXfun33allTbot | AATTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTA | |
| 5AOXfun36allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAG | pLR36 |
| 5AOXfun36allTbot | AATTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTA | |
| Fun40allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAG | pLR40 |
| Fun40allTbot | AATTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTA | |
| Fun45allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAG | pLR45 |
| Fun45allTbot | AATTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTA | |
| Fun50allAtop | CTAGTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAG | pLR50 |
| Fun50allTbot | AATTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTA | |
| 5AOXfun50allGtop | CTAGTGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGG | pLR50G |
| 5AOXfun50allGbottom | AATTCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCA |
2.5. P. pastoris transformation
Transformations of P. pastoris were done by the electrotransformation method as described (Cregg and Russell, 1998; Lin-Cereghino et al., 2007). Plasmids were linearized within the AOX1 promoter with the restriction enzyme Mph1103I, purified with the QIAprep Spin PCR Purification Kit (Qiagen, Valencia, CA) and electroporated into competent yJC100 cells. Transformed cells were allowed to recover for 1 h in 1 mL of a 50% 1 M sorbitol/50% YPD solution at 30 °C and then plated on selective medium containing Zeocin® at 100 µg/mL for selection of transformants. Transformed colonies were then purified by streaking for isolated colonies on selective medium.
2.6. Colony PCR
The rapid colony PCR method, used to confirm the presence of the recombinant lacZ gene in transformed P. pastoris cells, has been previously described (Thor et al., 2005). The primers used for amplification were AOX5seqchris TCAAAATTTAACTGTTCTAACC and JN0107qPCRev CCTGCCATAAAAGAAACTGTTACCC.
2.7. Real-time PCR
Real-time PCR reactions were composed of 10 µL of 2× reaction buffer from the DyNAmo SYBR Green qPCR kit distributed by New England BioLabs (Beverly, MA), 10 ng of genomic DNA, and 0.3 µM primers in a 20 µL total reaction volume. PCR reactions were carried out in an MJ MiniOpticon (BioRad Corp, Hercules, CA) with the following parameters (1 cycle of 95 °C for 5 min, 40 cycles of 94 °C for 1 min, 59 °C for 1 min, 72 °C for 1 min). Primers used to amplify the lacZ target gene were JN0106qPCRF CGTTGCTGCATAAACCGACTAC and JN0107qPCRev CCTGCCATAAAAGAAACTGTTACCC. Primers used to amplify the MET2 (Thor et al., 2005) reference gene were mets100 CGTTCTCGCAACTCTTTCGAA and metas100 CAATGGCATCAGTTATGACGGAAG. All samples were performed in duplicate, and all samples were tested several times in different experiments as done previously (Lin-Cereghino et al., 2008). Q-gene software was utilized to analyze real-time PCR data (Muller et al., 2002).
2.8. Small scale induction of β-galactosidase in P. pastoris
On the first day each strain was picked with a sterile wooden stick and inoculated into 5 mL of YPD in 50 mL conical tubes. The conicals were then placed in shaker incubator at 30 °C and 225 rpm for 24 h. On the second day, a one to ten dilution (100 µL of cell solution +900 µL water) was made, and the optical density at 600 nm was determined using GENESYS™2 (Spectronic®, Rochester, NY) spectrophotometer. A volume containing approximately 0.01 OD600 units was then used to inoculate 10 mL of YND medium, which was grown overnight at the same temperature and rpm as before. On the third day, after the optical density was determined, 10 OD600 units of yeast culture were centrifuged at 6000 rpm for 5 min. The supernatant was then removed, and the cell pellet was resuspended in 10 mL of YNM. These cultures were placed in shaker incubator at 30 °C and 225 rpm overnight to induce β-galactosidase expression. On the fourth day, after the optical density was determined, the cells were centrifuged at 6000 rpm for 5 min, washed in 1 mL of water, re-centrifuged and stored at −80 °C. Cells were then used for preparation of cell free extracts or for isolation of total RNA. At least two strains containing one copy of each plasmid were assayed at least three times to determine average β-galactosidase values as well as standard deviations.
2.9. Generating a cell free extract of P. pastoris
The frozen cells were thawed at 37 °C for 2 min. 500 µL of chilled PBS and Yeast/Fungal Protease Arrest Cocktail (G Biosciences, Saint Louis, MO) was added to each pellet. After 150 µL of chilled glass beads (500–600 µm size) were added to each sample, the lips of tubes were cleaned with cotton swabs so that the tubes could seal well. The tubes were closed and vortexed for 2 min. The tubes were then spun at 13,200 rpm for 5 min at 4 °C. The supernatant was transferred to a fresh tube and kept on ice. Protein concentrations were determined using the Pierce (Rockford, IL) Bicinchoninic Acid (BCA) Protein Assay kit with bovine serum albumin as a standard The extracts were then stored at −80 °C.
2.10. β-galactosidase assays
β-galactosidase activity in cell free extracts was measured using standard protocols (Lin-Cereghino et al., 2006; Sambrook et al., 1989).
2.11. Northern analysis
P. pastoris RNA was prepared by a standard procedure adapted for total yeast RNA isolation (Cereghino et al., 1995). Samples containing 10 µg of total RNA were loaded into the wells of 1.5% agarose/denaturing formaldehyde gels and electrophoresed for separation. 18S and 26S rRNA bands were visualized and quantitated with Alpha Innotech ChemiImager 5500 (Alpha Innotech, San Leandro, CA). Transfer of RNA to MagnaGraph nylon membranes (Osmonics, Westborough, MA), cross-linking, prehybridization, hybridization, high stringency washing and imaging were all performed following a standard procedure (Sambrook et al., 1989). A biotinylated DNA probe to the lacZ gene was synthesized utilizing the BioPrime DNA Labeling System (Invitrogen, Carlsbad, CA). Labeled DNA was separated from unincorporated label by using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA). Approximately 400 ng of purified biotinylated DNA fragment was denatured by heating at 95 °C for 5 min, chilled on ice for 2 min and then added as a hybridization probe. The nylon membranes were washed and visualized according to the instructions of the Southern-Light Detection Kit (Tropix, Bedford, MA) with an Alpha Innotech ChemiImager 5500 (Alpha Innotech, San Leandro, CA).
2.12. Computer-aided analysis of yeast RNA structure
Sequences for all constructs were entered into UTR Scan database (http://www.ba.itb.cnr.it/BIG/UTRScan/) and RegRNA (http://regrna.mbc.nctu.edu.tw/) to identify putative regulatory sequences in the 5′UTRs. IRESite (http://iresite.org) was used to search for possible internal ribosome entry sites. RNA secondary structures and relative ΔG values were predicted at 30 °C using the Vienna RNA Secondary Structure Prediction version 1.5 (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) (Gruber et al., 2008).
3. Results and discussion
3.1. Substitution of the AOX1 5′UTR with the GAP 5′UTR
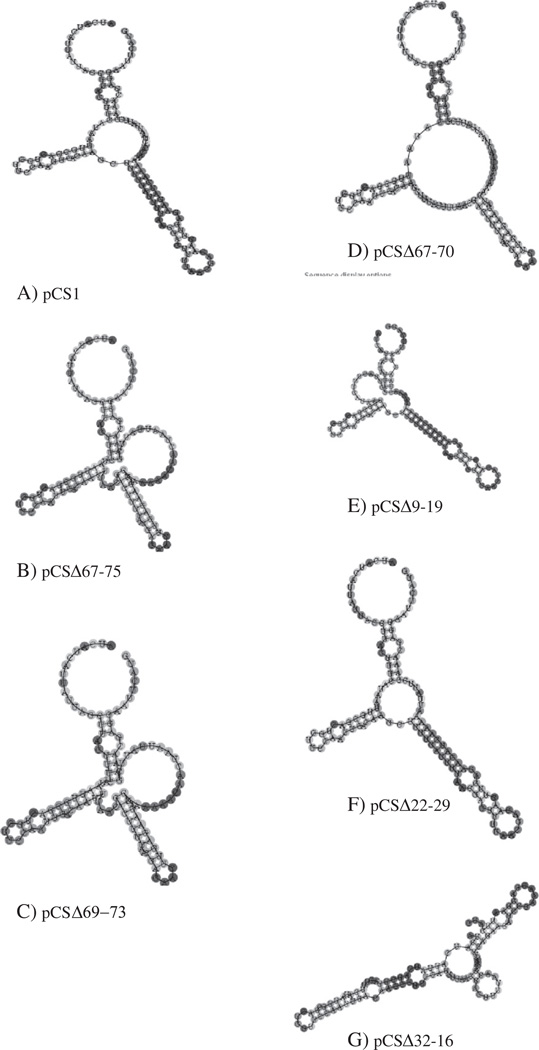
When P. pastoris cells are grown on methanol medium, the alcohol oxidase mRNA can comprise up to 5% of polyadenylated RNA and the resultant peptide may reach 30% of total cellular protein (Lin Cereghino et al., 2001). Although the induction of the AOX1 promoter is well-studied, it is not clear if the AOX1 5′UTR enhances or inhibits protein production under methanol growth conditions relative to other 5′UTRs. Our primary goal was to characterize how the structure of this region affects the level of protein expression. Computer-aided RNA modeling predicted that the AOX1 5′UTR, with the temperature parameters set at 30 °C, the growth temperature for P. pastoris, folded into a structure with three hairpins radiating from a central loop region (Fig. 1A). However, this figure should be viewed as an illustration of the extensive secondary structure of its 5′UTR as opposed to a proven structure. The ΔG of this structure, which is approximately −30 kcal/mol, suggests that it is a moderately stable structure, but it would not be expected to stall the migration of the 40S ribosomal subunit, which would require a ΔG of at least −50 kcal/mol (Mignone et al., 2002). Although such moderately stable motifs can be targets for RNA binding proteins, a bioinformatic analysis of the AOX1 5′UTR found no significant similarity to any known RNA regulatory sequences or potential IRES sites (Grillo et al., 2010).
Fig. 1.
Predicted secondary structure of 5′UTRs encoded by plasmid deletion constructs.
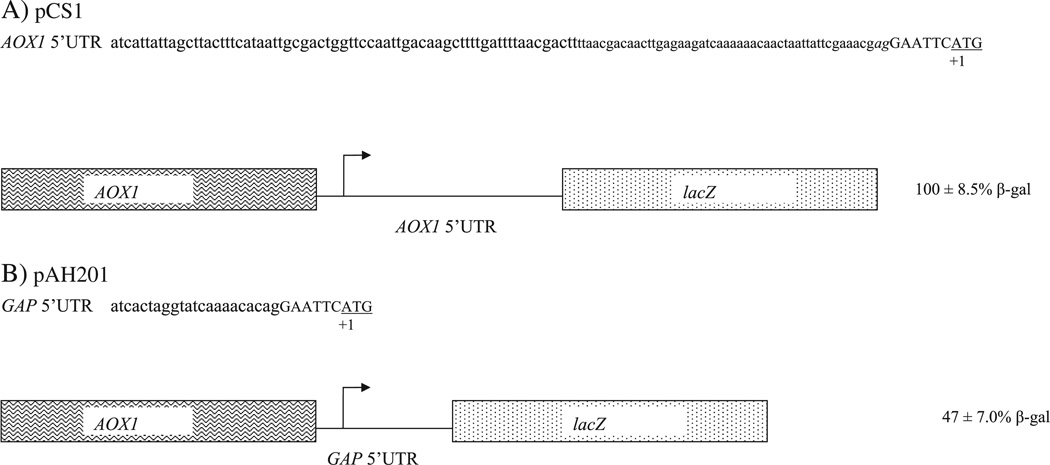
With little to go on, we desired to determine if the AOX1 5′UTR had a relative inhibitory or activating effect on protein expression. To pursue this objective, β-galactosidase, the product of the bacterial lacZ gene, was used as reporter since it had been proven reliable for promoter induction studies in P. pastoris (Lin-Cereghino et al., 2006). The lacZ coding sequence was inserted into pPIC ZB (Invitrogen, Carlsbad, CA), a commercially available vector widely used for expression of recombinant intracellular proteins. The β-galactosidase coding sequence was inserted at EcoRI site, the most 5′ site in the polylinker. Relative to the AUG of the coding sequence, this made the AOX1 5′UTR 122 nucleotides long (114 native 5′UTR nucleotides, 2 nucleotide linker and 6 nucleotide restriction site). This plasmid, named pCS1, would provide expression of intracellular β-galactosidase protein, which could be quantified by a convenient assay (Fig. 2A). Once all of the constructs were made and verified by sequencing, they were transformed into yJC100 P. pastoris yeast cells. In our studies, strains carrying pCS1 or mutant vectors were grown on glucose containing medium to generate biomass but to repress the AOX1 promoter. To induce its transcription, the cells were consequently shifted to methanol containing medium for 24 h, after which β-galactosidase specific activity was determined in the intracellular fraction, using the averages of at least three trials on single copy strains. Twenty four hours of induction is considered optimal for the production of intracellular recombinant proteins (Lin-Cereghino et al., 2007). The level of reporter provided by translation of the mRNA with the 122 nucleotide 5′UTR in pCS1 was considered wild type, 100% (Fig. 2A). When the AOX1 5′UTR was removed from pCS1 and replaced with the 5′UTR of GAP (glyceraldehyde phosphate dehydrogenase gene) (Waterham et al., 1997), another highly expressed gene, to create pAH201, a drop of approximately 50% in β-galactosidase activity was observed (Fig. 2B). This result suggested, relative to the GAP 5′UTR, that the AOX1 5′UTR enhanced protein expression and was overall a positive-acting element.
Fig. 2.
Comparison of the AOX1 5′UTR and GAP 5′UTR. A. The native 114 nucleotide sequence is in lower case, the two nucleotide linker is italicized, and the EcoR1 site is in capital letters. The initiation codon is underlined. The AOX1 5′UTR indicated is encoded by pCS1 plasmid, fused to the lacZ coding sequence and under the controls of the AOX1 promoter. B. The GAP 5′UTR is indicated. The EcoR1 site is in capital letters, and the initiation codon is underlined. The AOX1 5′UTR indicated is encoded by pAH201 plasmid, fused to the lacZ coding sequence and under the control of the AOX1 promoter. Strains harboring pCS1 and pAH201 were grown on glucose containing medium, shifted to methanol-containing medium, and harvested after 24 h. After extracts were made from the cell pellets, the β-galactosidase activity and total protein concentrations were determined for these intracellular fractions to determine the specific activity of the reporter. The values represent averages as well as standard deviations of at least three trials on single copy strains.
3.2. Incremental deletion analysis
Several previous studies have demonstrated that both inhibitory and activating regions may be present in the same 5′UTR (Mittelmeier and Dieckmann, 1995; Sonenberg and Hinnebusch, 2009; Yamanaka et al., 1999). Because we hypothesized that AOX1 5′UTR also contained both positive and negative acting regions, a conventional deletion analysis was performed to identify these motifs. In these constructs, sequences close to the transcription start were not altered because such changes might alter the transcription initiation process and affect transcription rate. In addition, once these plasmids were made and transformed into P. pastoris, real time PCR was used to confirm that all strains had an equivalent copy number of deletion plasmids because elevated copy numbers may, in some circumstances, lead to higher β-galactosidase activities regardless of the 5′ UTR structure (data not shown).
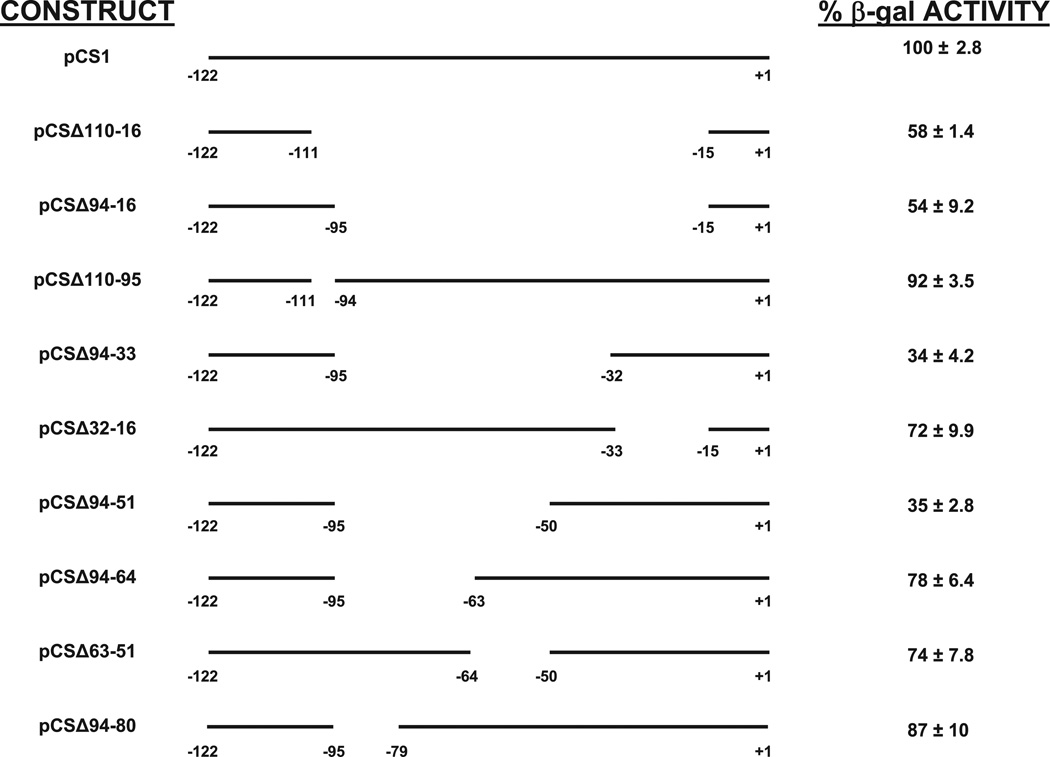
Our strategy for the deletion analysis was to first remove almost the entire 5′UTR region and then incrementally add back portions of it. The first deletion, which lacked 96 nucleotides, from −16 to −110, of the AOX1 5′UTR (pCSΔ110-16) displayed about a 50% reduction in β-galactosidase activity (Fig. 3). This result, which was numerically consistent with the result of the GAP 5′UTR substitution (Fig. 2B), suggested that this large internal region which had been removed from the 5′ UTR, housed sequences that stimulated protein expression. Insertion of 16 nucleotides (−110 to −95) to create (pCSΔ94-16) had only minor effect on reporter protein expression (Fig. 3). Furthermore, ensuring that its effect was not masked by other deleted nucleotides, the deletion of this sequence alone from the AOX1 5′UTR (pCSΔ110-95) had no significant effect, only a drop to 92% of pCS1 specific activity (Fig. 3). Because of these two results, all further constructs contained this region from the transcription start to nucleotide −95.
Fig. 3.
β-galactosidase activities of deleted AOX1 5′UTRs. P. pastoris strains expressing the mutant 5′UTRs fused to lacZ coding sequence were induced on methanol growth medium, and the relative intracellular specific activities of β-galactosidase (β-galactosidase activity/µg total intracellular protein) were determined. 100% corresponds to the specific activity of pCS1, which encodes the full length AOX1 5′UTR. The values represent averages as well as standard deviations of at least three trials on single copy strains.
Insertion of nucleotides −16 to −32 to the pCSΔ94-16 to create pCSΔ94-33 caused a large drop in reporter expression from 54% to 34% of wild type, intimating that nucleotides −16 to −32 contained negative-acting sequences (Fig. 3). Therefore, we expected that removal of this region would increase expression. However, when this region alone was deleted from the wild type 5′UTR to produce pCSΔ32-16, expression surprisingly decreased to about 72% of the pCS1 level (Fig. 3). This suggested that amore complex regulatory mechanism was present, perhaps requiring an interaction between nucleotides −16 to −32 and some sequence between nucleotides −33 and −94.
Continuing our systematic deletion analysis, we observed that insertion of nucleotides −33 to −50 to create pCSΔ94-51 had practically no effect on β-galactosidase expression, remaining at about 35% of wt expression (Fig. 3). However, the addition of nucleotides −51 to −63 to produce pCSΔ94-64 caused reporter expression to rise from 35 to 78% of wild type levels. Because this appeared to be a positive acting element, we expected that a deletion of this region alone would decrease expression by a large amount. We found that its removal in pCSΔ63-51 caused a reduction of expression to about 74% of pCS1 levels, confirming our hypothesis (Fig. 3). Furthermore, adding nucleotides −66 to −79 to produce pCSΔ94-80 had a relatively small effect on reporter activity (from 78% to 87%), suggesting that this region contained only a minor positive-acting region. Therefore, the most salient results of the initial deletion analysis were that regions of −16 to −32 and −51 to −63 played a significant role in regulation when they were present in the context of a large deletion as in pCSΔ94-33 and pCSΔ94-64. However, deletion of these regions by themselves, pCSΔ63-51 and pCSΔ32-16, had a smaller effect on protein expression than expected. Moreover, our studies indicate that many sequences in this relatively large 5′UTR could be deleted with little effect and seem to exert only a minor influence on protein expression.
3.3. Deletion analysis based on secondary structure predictions
Along with an incremental deletion analysis, we also used predicted secondary structures based on an RNA fold program (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) to explore which regions could influence structure and presumably the function of the 5′UTR in protein production (Bagga, 2008; Grillo et al., 2010). As described above, this analysis suggested the 5′UTR folded into a structure with three hairpins radiating from a central loop region (Fig. 1A). Our attention was drawn to the sequence between −65 to −75 because these nucleotides apparently played a key role in the formation of the largest hairpin. Three mutants were constructed to alter this structure: pCSΔ67-75, pCSΔ69-73, and pCSΔ67-70. These changes were predicted to significantly affect the overall secondary structure (Figs. 1B–D). However, for all the constructs, the β-galactosidase activity for strains carrying these constructs was approximately 90% of wild type levels, suggesting that they played only a minor role (Table 3).
Table 3.
β-galactosidase activities of deletion constructs based on predicted secondary structure.
| Construct | Percent activity |
|---|---|
| pCS1 | 100 ± 2.2 |
| pCSΔ67-75 | 88 ± 7.1 |
| pCSΔ69-73 | 89 ± 1.4 |
| pCSΔ67-70 | 92 ± 2.0 |
| pCSΔ9-19 | 72 ± 4.6 |
| pCSΔ22-29 | 120 ± 2.6 |
In addition, the secondary structure prediction program suggested that the region between nucleotides −8 and −30 was involved in the formation of the central loop and a hairpin adjacent to the initiation codon. Because the loop seemed crowded in this region, we hypothesized that small deletions in this area may reduce strain of crowding and alter the ribosome–mRNA interaction. pCSΔ9-19, which had a very different predicted structure (Fig. 1E), showed an almost 30% decrease in reporter expression. In contrast, pCSΔ22-29 (Fig. 1F), which maintained practically the same predicted structure as pCS1 but with less crowding in the central loop, demonstrated an increase of about 20% in protein expression, intimating that this deletion may have improved either the translational efficiency or stability of the mRNA (Table 3). This data is surprising given that deletion of nucleotides −16 to −32 reduced protein expression by about 30% (pCSΔ32-16, Fig. 3). However, the modest increase in the deletion size of nine nucleotides (from pCSΔ22-29 to pCSΔ32-16) greatly disrupted the predicted secondary structures, which may explain this rather large drop in reporter expression (Figs. 1F and G). Thus, the AOX1 5′UTR cannot be simply dissected into motifs that have purely a positive or negative effect on translation but must be viewed on a larger level where motifs act in concert.
3.4. Addition analysis
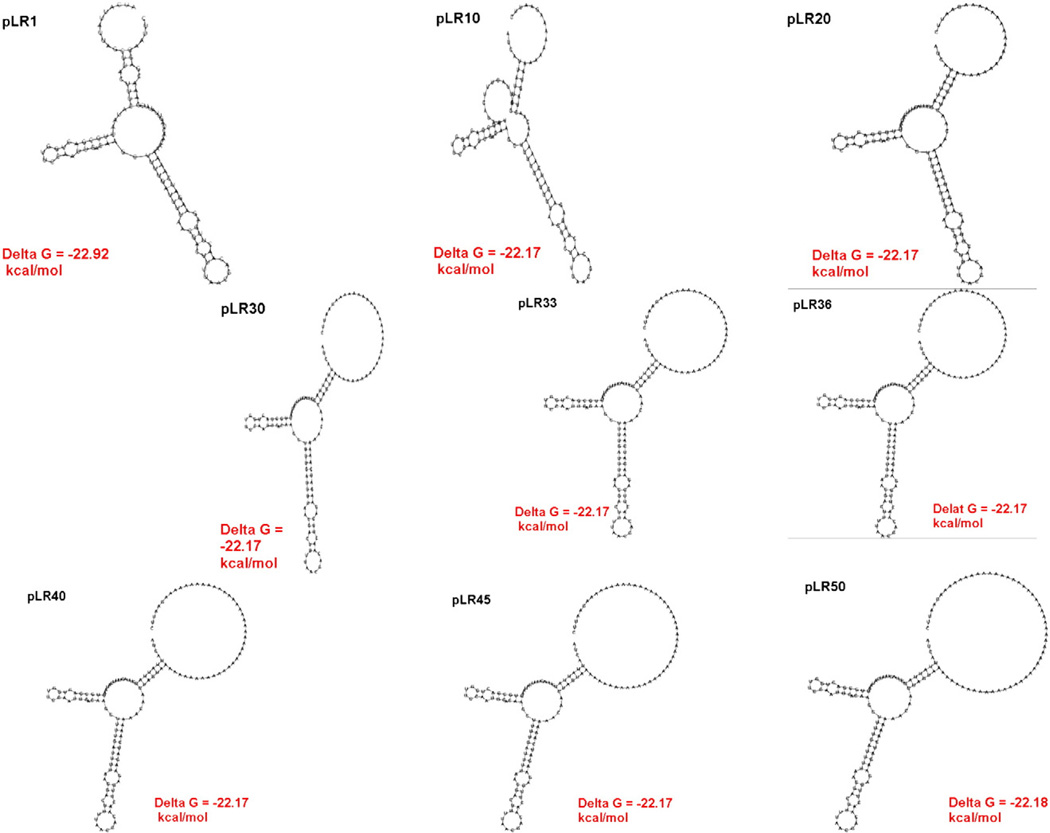
Our second goal was to comprehend how increases in the length of the AOX1 5′UTR affect expression of heterologous proteins. Often when investigators are inserting their coding sequences into the expression cassettes of plasmids such as pPICZB, they may include large amount of foreign sequence upstream of the ATG or use a 3′ restriction site in a polylinker. Either case makes the AOX1 5′UTR substantially longer than 122 nucleotides. Because the sequences directly adjacent to the first AUG in the mRNA have been shown to influence translation rate (Asano and Sachs, 2007; Kozak, 2005), insertions were placed distant from the initiation codon because of our focus on the effect of lengthening the 5′UTR, not changing initiation codon context. Therefore, to mimic increases in length produced by subcloning, we first created a SpeI restriction site in the 5′UTR of pCS1 by mutating an A to a G at nucleotide −21 and then systematically added increasing numbers of A nucleotides, using this restriction site (Table 4). As done in our deletion analysis, real time PCR was first used to confirm that all strains contained one copy of each expression vector, and these cells were then subjected to methanol induction and β-galactosidase activity assay to asses the effects of elongated 5′UTR on the expression rate. Table 4 shows the results from all addition constructs. First, the mutagenesis of 1 nucleotide to create a SpeI site in pLR1 had very little effect on β-galactosidase expression. β-galactosidase activity was reduced by approximately 34% by inserting a segment of 10 A nucleotides compared to the pCS1 strain. Addition of 20 nucleotides decreased expression to about one third of wild type levels. Further increases in 5′UTR length resulted in a steady decrease in reporter activity, notably with the addition of 40 nucleotides causing an 86% reduction and the addition of 50 nucleotides triggering a 93% decline compared to wild type.
Table 4.
β-galactosidase activities of 5′UTR constructs with additions.
| Construct | Percent activity | 5′UTR |
|---|---|---|
| pCS1 | 100 ± 2.8 | __________________________ |
| pLR1 | 98 ± 8.5 | __________________SpeI ____ |
| pLR10 | 66 ± 3.5 | _____________AAAAAAAAAA_____ |
| pLR20 | 35 ± 10 | _____________AAAAAAAAAAAAAAAAAAAA____ |
| pLR30 | 24 ± 4.2 | _____________AAAAAAAAAAAAAAAAAAAAAAAAAAAAAA_____ |
| pLR33 | 11 ± 0.1 | _____________AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA_____ |
| pLR36 | 14 ± 1.4 | _____________AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA_____ |
| pLR40 | 14 ± 1.7 | _____________AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA_____ |
| pLR45 | 11 ± 5.3 | _____________AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA____ |
| pLR50 | 7 ± 3.5 | _____________AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA____ |
| pLR50G | 3 ± 1.4 | ____________GGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGG___ |
Based on our RNA modeling data, secondary structure did not change that significantly as the 5′UTR was lengthened. The additions merely increase the size of the unstructured loop bordering region adjacent to the initiation codon (Fig. 4). Several previous reports have proposed that the longer the 5′UTR, the greater the chance that translation initiation complex will disassociate from the mRNA as it scans from the cap to the first AUG (Sreekrishna, 1993; Tuller et al., 2009). This would explain an incremental drop off in β-galactosidase with additions of 20–50 nucleotides. While the insertion of 50 nucleotides had a dramatic effect, the insertion of multiple adenines may have partly stimulated translation because it could have mimicked the poly A tail found in the 3′ UTR, which binds proteins to increase translation efficiency (Sonenberg and Hinnebusch, 2009; Tarun and Sachs, 1995). In an effort to see if the type of nucleotide influenced this effect, we also inserted 50Gs into the SpeI site to analyze its effect on protein expression compared to 50As. The 50G insertion reduced β-galactosidase levels to about 3% of wild type levels (Table 4), which suggests that the type of nucleotide does not have a large effect. The rather dramatic decrease initially in reporter activity with the addition of 20 nucleotides (100% to about 35%) is surprising, but it emphasizes that even small increases in AOX1 5′UTR length can severely disturb protein expression. Taken together, these results emphasize that using the most 5′ restriction sites in the polylinker and avoiding bringing in nucleotides 5′ of the ATG of the foreign coding sequence are important considerations to obtain efficient production of intracellular recombinant proteins. However, it is not clear whether this extension in the 5′UTR reduced protein expression by influencing translation efficiency or RNA stability.
Fig. 4.
Predicted secondary structure of 5′UTRs encoded by poly A addition constructs.
3.5. Northern analysis
To determine whether modifying the 5′UTR length affected the stability or translational efficiency of the AOX1 5′UTR-β-galactosidase mRNA, a northern blot was performed on representative samples of deletion and addition constructs that were induced in methanol. The steady state level of an mRNA species depends on its rate of synthesis (transcription) versus its rate of destruction (degradation). Previous studies have demonstrated that modifying the 5′UTR may affect a transcript's degradation rate, which influences the amount of translated protein (Mignone et al., 2002; Wang et al., 2005). We assumed that since the region around the transcription initiation site and promoter were not changed in our deletion and addition experiments, then the rate of transcription must be equivalent among all the constructs. Thus, if degradation was reduced or increased for a particular construct, the steady state level of the message should be affected, resulting in a change that can be seen on northern blot. Total RNA was extracted from strains induced on methanol and resolved on agarose gels (Fig. 5). The 26S ribosomal RNA signal was used to normalize for equal loading among all the sample RNAs. The samples were then hybridized to a lacZ probe to measure the amount of message encoding β-galactosidase. The intensities of these bands were quantitated with respect to 26S rRNA signal (Fig. 5). Our results demonstrate that the β-galactosidase mRNA levels are approximately equal in all the strains with the modified 5′UTRs, indicating that these deletions and insertions in the 5′UTR did not affect the normal messages' stability. Therefore, these results suggest that these mutations in the AOX1 5′UTR affected translational efficiency, not RNA stability.
Fig. 5.
Northern analysis of representative AOX1 5′UTR mutant constructs. A. Strains containing the indicated plasmids were induced on methanol medium and harvested after 24 h. Total RNA was extracted and resolved on a formaldehyde-agarose gel and transferred to a nitrocellulose membrane. The nitrocellulose blot was then hybridized with a biotin-labeled lacZ probe and processed with a Tropix Southern-Star detection system. B. The formaldehyde-agarose gel was photographed prior to transfer to record the intensity of the 26S ribosomal RNA (rRNA) bands, which indicate comparative loading. The intensity of the lacZ band in A and the 26S rRNA band in B were measured. The numerical value of the lacZ signal was divided by the numerical value of the rRNA band to provide lacZ mRNA values standardized for equal loading.
4. Conclusions
Using the cap-dependent model for the initiation of translation, one can speculate on how changes in the AOX1 5′UTR affected translational efficiency. During the cap-dependent process in other eukaryotes, the 7-methyl-guanylate cap (m7G) serves as a “flag” were the 40S ribosomal subunit with eukaryotic initiation factors (eIFs) are recruited to initiate contact with the mRNA. Another key player in this recruitment process is a 3-subunit complex of eIF4E (cap-binding protein), eIF4A (RNA helicase activity), and eIF4G (interacts with other various proteins) collectively referred to as eIF4F (Lopez-Lastra et al., 2005). The eIF4A peptide functions to unwind secondary structures (Mignone et al., 2002; Pestova and Kolupaeva, 2002). Although an actual structure was not determined for the AOX1 5′UTR in our work, computer aided modeling programs provided a framework to base our deletion analysis. The deletions, which changed the predicted secondary structure of the AOX1 5′UTR, may have affected the efficiency by which P. pastoris eIF4A unwinds this region. Because there is competition for the limited translation factors in the cell (Merrick, 2010), another possibility is that the structure of the 5′UTR changed in a way that decreased the affinity for certain proteins that bound the mRNA or helped scan the message. With so many proteins involved in the translation initiation complex (Sonenberg and Hinnebusch, 2009), there are many possibilities that can explain why deleting or adding nucleotides from this region changes translation efficiency. Regardless of the reason, our results demonstrate that the effect of these deletions on translational regulation is highly dependent on position of the deletion, not the total length of the 5′ UTR. However, this regulation is not straightforward. For instance, the presence of nucleotides −16 to −32 decreased expression in the absence of nucleotides −33 to −94, suggesting that it had a negative effect on translational efficiency. Although one would expect that the deletion of−16 to −32 from the full-length 5′UTR would increase expression of the β-galactosidase reporter, we found that its removal by itself actually reduced expression. On the other hand, a deletion of an internal sequence, between −22 and −29, increased reporter activity. Taken together, these results suggest that a much more complex regulatory system is present, perhaps involving interaction between RNA binding proteins and the three dimensional structure of the AOX1 5′UTR. While one 5′UTR mutant (pCSΔ22-29) gave a higher level of protein expression than the wild type 5′UTR, the fact that all our other constructs gave lower reporter activities suggest that the normal AOX1 5′UTR may be already nearly optimal for translational efficiency. This raises the prospect that although the creation of a synthetic AOX1 5′UTR would be extremely desirable for optimizing recombinant expression, it will be more difficult than simply fusing multimers of positive-acting sequences and deleting negative-acting motifs from the wild type 5′UTR. It will require further studies to pinpoint and elucidate the exact molecular mechanisms behind these translational controls.
Acknowledgments
This work was supported by NIH-AREA grant GM65882-03 to J. L.-C. and G. P. L.-C. Z. L. was supported with intramural research funds from the Department of Chemistry, University of the Pacific. A. H. was recipient of a Pacific Summer Undergraduate Research Fellowship.
Abbreviations
- AOX1
alcohol oxidase 1
- UTR
untranslated region
- nt
nucleotides
- eIF
eukaryotic initiation factor.
References
- Asano K, Sachs MS. Translation factor control of ribosome conformation during start codon selection. Genes Dev. 2007;21:1280–1287. doi: 10.1101/gad.1562707. [DOI] [PubMed] [Google Scholar]
- Bagga PS. Bioinformatics approaches for studying untranslated regions of mRNAs. Methods Mol. Biol. 2008;419:1–21. doi: 10.1007/978-1-59745-033-1_1. [DOI] [PubMed] [Google Scholar]
- Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA. 2006;12:1755–1785. doi: 10.1261/rna.157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000;24:45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Cereghino GP, Atencio DP, Saghbini M, Beiner J, Scheffler IE. Glucose-dependent turnover of the mRNAs encoding succinate dehydrogenase peptides in Saccharomyces cerevisiae: sequence elements in the 5′ untranslated region of the Ip mRNA play a dominant role. Mol. Biol. Cell. 1995;6:1125–1143. doi: 10.1091/mbc.6.9.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Pal JK. Role of 5′- and 3′-untranslated regions of mRNAs in human diseases. Biol. Cell. 2009;101:251–262. doi: 10.1042/BC20080104. [DOI] [PubMed] [Google Scholar]
- Cregg JM, Madden KR. Development of the methylotrophic yeast, Pichia pastoris, as a host system for the production of foreign proteins. Dev. Ind. Microbiol. 1988;29:33–41. [Google Scholar]
- Cregg JM, Russell KA. Transformation. In: Higgins DR, Cregg JM, editors. Pichia Protocols. Totowa, New Jersey: Humana Press; 1998. pp. 27–39. [Google Scholar]
- Cregg JM, Barringer KJ, Hessler AY, Madden KR. Pichia pastoris as a host system for transformations. Mol. Cell. Biol. 1985;5:3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SB, Brust PF, Koutz PJ, Waters AF, Harpold MM, Gingeras TR. Isolation of alcohol oxidase and two other methanol regulatable genes from the yeast Pichia pastoris. Mol. Cell. Biol. 1985;5:1111–1121. doi: 10.1128/mcb.5.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo G, et al. UTRdb and UTRsite (RELEASE 2010): a collection of sequences and regulatory motifs of the untranslated regions of eukaryotic mRNAs. Nucleic Acids Res. 2010;38:D75–D80. doi: 10.1093/nar/gkp902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AR, Lorenz R, Bernhart SH, Neubock R, Hofacker IL. The Vienna RNA websuite. Nucleic Acids Res. 2008;36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner FS, et al. Promoter library designed for fine-tuned gene expression in Pichia pastoris. Nucleic Acids Res. 2008;36:e76. doi: 10.1093/nar/gkn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda A, Minetoki T, Ozeki K, Hirotsune M. Translation efficiency mediated by the 5′ untranslated region greatly affects protein production in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2004;66:291–296. doi: 10.1007/s00253-004-1681-8. [DOI] [PubMed] [Google Scholar]
- Koutz P, Davis GR, Stillman C, Barringer K, Cregg J, Thill G. Structural comparison of the Pichia pastoris alcohol oxidase genes. Yeast. 1989;5:167–177. doi: 10.1002/yea.320050306. [DOI] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene. 2005;361:13–37. doi: 10.1016/j.gene.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Lawless C, et al. Upstream sequence elements direct post-transcriptional regulation of gene expression under stress conditions in yeast. BMC Genomics. 2009;10:7. doi: 10.1186/1471-2164-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Cereghino GP, Sunga AJ, Lin Cereghino J, Cregg JM. Expression of foreign genes in the yeast Pichia pastoris. Genet. Eng. (N. Y.) 2001;23:157–169. doi: 10.1007/0-306-47572-3_9. [DOI] [PubMed] [Google Scholar]
- Lin-Cereghino GP, et al. Mxr1p, a key regulator of the methanol utilization pathway and peroxisomal genes in Pichia pastoris. Mol. Cell. Biol. 2006;26:883–897. doi: 10.1128/MCB.26.3.883-897.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Cereghino GP, Leung W, Lin-Cereghino J. Expression of protein in Pichia pastoris. In: Dyson M, Durocher Y, editors. Expression Systems: Methods Express. Oxfordshire: Scion Publishing Limited; 2007. pp. 123–145. [Google Scholar]
- Lin-Cereghino J, et al. Direct selection of Pichia pastoris expression strains using new G418 resistance vectors. Yeast. 2008;25:293–299. doi: 10.1002/yea.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lastra M, Rivas A, Barria MI. Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation. Biol. Res. 2005;38:121–146. doi: 10.4067/s0716-97602005000200003. [DOI] [PubMed] [Google Scholar]
- Merrick WC. Eukaryotic protein synthesis: still a mystery. J. Biol. Chem. 2010;285:21197–21201. doi: 10.1074/jbc.R110.111476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone F, Gissi C, Liuni S, Pesole G. Untranslated regions of mRNAs. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-3-reviews0004. REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelmeier TM, Dieckmann CL. In vivo analysis of sequences required for translation of cytochrome b transcripts in yeast mitochondria. Mol. Cell. Biol. 1995;15:780–789. doi: 10.1128/mcb.15.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad-Gargouri R, Belhadj K, Gargouri A. Translational control of human p53 expression in yeast mediated by 5′-UTR–ORF structural interaction. Nucleic Acids Res. 2001;29:1222–1227. doi: 10.1093/nar/29.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrejs M, Masek T, Vopalensky V, Hlubucek P, Delbos P, Pospisek M. IRE-Site—a tool for the examination of viral and cellular internal ribosome entry sites. Nucleic Acids Res. 2009;38:D131–D136. doi: 10.1093/nar/gkp981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1374. 1376, 1378–1379. [PubMed] [Google Scholar]
- Park YS, et al. Design of 5′-untranslated region variants for tunable expression in Escherichia coli. Biochem. Biophys. Res. Commun. 2007;356:136–141. doi: 10.1016/j.bbrc.2007.02.127. [DOI] [PubMed] [Google Scholar]
- Pesole G, Mignone F, Gissi C, Grillo G, Licciulli F, Liuni S. Structural and functional features of eukaryotic mRNA untranslated regions. Gene. 2001;276:73–81. doi: 10.1016/s0378-1119(01)00674-6. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 2002;16:2906–2922. doi: 10.1101/gad.1020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Qian J, Yao G, Zhuang Y, Zhang S, Chu J. GAP promoter library for fine-tuning of gene expression in Pichia pastoris. Appl. Environ. Microbiol. 2011;77:3600–3608. doi: 10.1128/AEM.02843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Handbook. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekrishna K. Strategies for optimizing protein expression and secretion in the methylotrophic yeast Pichia pastoris. In: Baltz R, GD H, Skatrud P, editors. Industrial Microorganisms: Basic and Applied Molecular Genetics. Washington, DC: American Society for Microbiology; 1993. pp. 119–126. [Google Scholar]
- Tarun SZ, Jr, Sachs AB. A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev. 1995;9:2997–3007. doi: 10.1101/gad.9.23.2997. [DOI] [PubMed] [Google Scholar]
- Thor D, et al. Cloning and characterization of the Pichia pastoris MET2 gene as a selectable marker. FEMS Yeast Res. 2005;5:935–942. doi: 10.1016/j.femsyr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Tuller T, Ruppin E, Kupiec M. Properties of untranslated regions of the S. cerevisiae genome. BMC Genomics. 2009;10:391. doi: 10.1186/1471-2164-10-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Guo X, Floros J. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L497–L508. doi: 10.1152/ajplung.00100.2005. [DOI] [PubMed] [Google Scholar]
- Warringer J, Hult M, Regot S, Posas F, Sunnerhagen P. The HOG pathway dictates the short-term translational response after hyperosmotic shock. Mol. Biol. Cell. 2010;21:3080–3092. doi: 10.1091/mbc.E10-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Digan ME, Koutz PJ, Lair SV, Cregg JM. Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene. 1997;186:37–44. doi: 10.1016/s0378-1119(96)00675-0. [DOI] [PubMed] [Google Scholar]
- Wicksteed B, Uchizono Y, Alarcon C, McCuaig JF, Shalev A, Rhodes CJ. A cis-element in the 5′ untranslated region of the preproinsulin mRNA (ppIGE) is required for glucose regulation of proinsulin translation. Cell Metab. 2007;5:221–227. doi: 10.1016/j.cmet.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Mitta M, Inouye M. Mutation analysis of the 5′ untranslated region of the cold shock cspA mRNA of Escherichia coli. J. Bacteriol. 1999;181:6284–6291. doi: 10.1128/jb.181.20.6284-6291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]