Abstract
We encountered a patient with cystoid macular edema (CME) secondary to paclitaxel use. A 57-year-old man presented with gradual decreased bilateral vision. His chemotherapeutic regimen consisted of bevacizumab, paclitaxel (175 mg/m2 for 5 months), and carboplatin. Optical coherence tomography imaging revealed bilateral CME greater than 500 µm. However, one year later, visual acuity was improved, best-corrected Snellen visual acuity was 40 / 80 in each eye, and CME was spontaneously improved. Our study confirmed that macular edema associated with paclitaxel use shows spontaneous resolution and improvement of visual acuity after a change of chemotherapeutic regimen.
Keywords: Macular edema, Paclitaxel
Macular edema is a condition that is usually secondary to an underlying disease and that causes severe visual impairment. It is suspected that cystoid macular edema (CME) is a result of breakdown of the blood-retinal barrier or accumulation of fluid in the intracellular space [1]. We report a patient with CME secondary to paclitaxel (Taxol; Bristol-Meyers Squibb Co., New York, NY, USA) use.
Case Report
A 57-year-old man presented with gradual decreased bilateral vision. The past medical history was significant for stage 4 lung adenocarcinoma. His chemotherapeutic regimen consisted of bevacizumab, paclitaxel (175 mg/m2 for 5 months), and carboplatin. Six cycles of chemotherapy over five months has been performed and followed by monthly intravenous bevacizumab injection for maintenance therapy. On initial examination, best-corrected Snellen visual acuity was 20 / 80 in each eye. Anterior segment examination showed normal findings. Findings of fundus examination were within normal limits except for bilateral cystic change of the macula. Fluorescein angiograms of the right eye showed weak hyperfluorescence in the parafoveal area in the late frame, demonstrating macular edema. Optical coherence tomography (OCT) imaging revealed bilateral CME with a foveal thickness greater than 500 µm (Fig. 1). No central scotoma was found in visual field examination, and b-wave amplitude was diminished in scotopic and mesopic electroretinogram. The CME was thought to be secondary to paclitaxel use, which had been discontinued for one month at that time. Intravitreal bevacizumab injection in the right eye and intravitreal bevacizumab plus triamcinolone injection in the left eye were performed. Six weeks later, intravitreal triamcinolone injection in the right eye was performed. Visual acuity had not improved, and Snellen visual acuity was 20 / 80 in each eye. CME was unchanged in OCT image in both eyes (Fig. 1). However, one year later, visual acuity was improved, best-corrected Snellen visual acuity was 40 / 80 in each eye, and CME was spontaneously improved (Fig. 2).
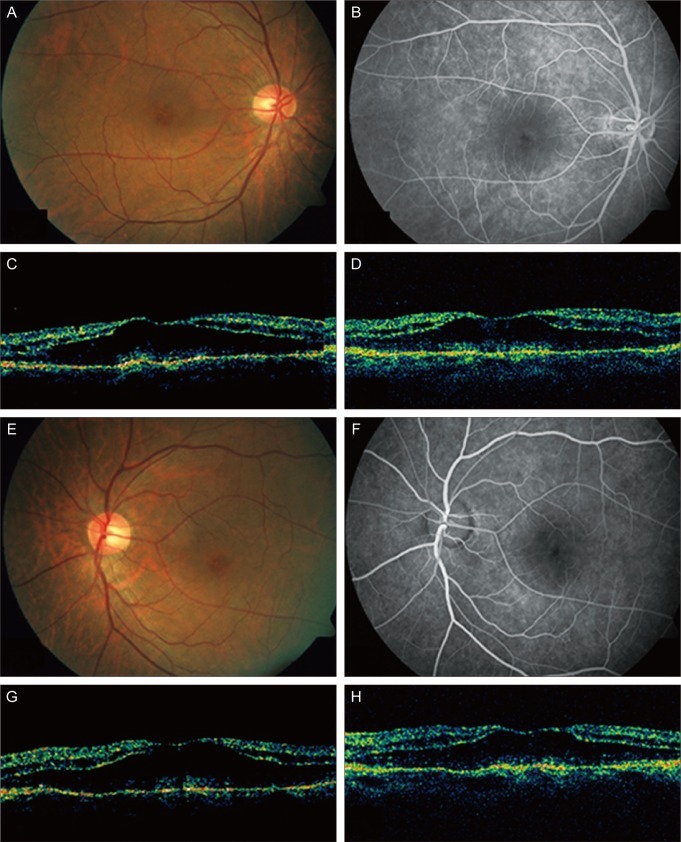
Fig. 1.
(A-D) The right eye of the patient. (A) Before intravitreal bevacizumab plus triamcinolone injection, fundus showed cystic change of the macula. (B) Fluorescein angiograms (FA) showed hyperfluorescence in the macula in the late frame. (C) Optical coherence tomography (OCT) showed cystoid macular edema (CME). (D) Six weeks after the injection, CME was unchanged in OCT. (E-H) The left eye of the patient. (E) Before intravitreal bevacizumab plus triamcinolone injection, fundus showed cystic change of the macula. (F) FA showed hyperfluorescence in the macula in the late frame. (G) OCT showed CME. (H) Six weeks after the injection, CME was unchanged in OCT.
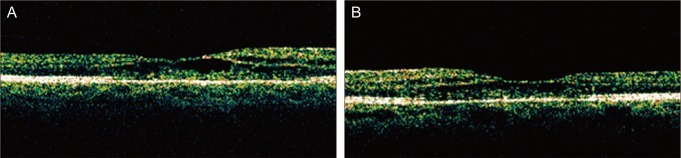
Fig. 2.
The right (A) and left (B) eyes of the patient. One year later, optical coherence tomography showed cystoid macular edema improved. The central retinal thickness was decreased from 502 µm to 246 µm in the right eye and from 513 µm to 228 µm in the left eye.
Discussion
Paclitaxel is an anticancer drug used to treat various malignancies including breast and lung cancers. Paclitaxel restricts microtubule mobility and inhibits mitosis. Toxic effects to bone marrow are the main adverse effect of this agent. Ophthalmic adverse effects include impaired visual acuity, photopsia, and optic neuropathy [2]. Teitelbaum and Tresley [3] have reported a case of macular edema associated with docetaxel use. Telander and Sarraf [4] also documented OCT findings of CME secondary to docetaxel therapy and treatment with oral acetazolamide because visual acuity was not improved after cessation of docetaxel therapy. Joshi and Garretson [5] reported a case of macular edema associated with paclitaxel use. Paclitaxel is an analogue of docetaxel with similar mechanisms of action. They described spontaneous resolution of CME and improvement of visual acuity after a change of chemotherapeutic regimen.
Our case is the first report to document a case of intravitreal bevacizumab plus triamcinolone injection in CME secondary to paclitaxel use. Intravitreal bevacizumab plus triamcinolone injection showed no improving effect on CME secondary to paclitaxel use, and it is suspected that systemic bevacizumab injection was not effective in the prevention and treatment of CME in this case based on the previous chemotherapeutic regimen. CME is usually associated with intraocular inflammation, venous occlusive disease, diabetic retinopathy, and cataract surgery. The pathophysiology of CME in this case is unclear, but we propose that CME secondary to paclitaxel use may be not associated with vascular endothelial grow factor or intraocular inflammation considering the lack of effectiveness of bevacizumab plus triamcinolone. As in the case of Joshi and Garretson [5], our study confirmed that macular edema associated with paclitaxel use shows spontaneous resolution and improvement of visual acuity after a change of chemotherapeutic regimen. Ophthalmic evaluation is needed in all patients using paclitaxel or docetaxel.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tso MO. Pathology of cystoid macular edema. Ophthalmology. 1982;89:902–915. doi: 10.1016/s0161-6420(82)34698-9. [DOI] [PubMed] [Google Scholar]
- 2.Hofstra LS, de Vries EG, Willemse PH. Ophthalmic toxicity following paclitaxel infusion. Ann Oncol. 1997;8:1053. doi: 10.1023/a:1008249230056. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum BA, Tresley DJ. Cystic maculopathy with normal capillary permeability secondary to docetaxel. Optom Vis Sci. 2003;80:277–279. doi: 10.1097/00006324-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Telander DG, Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22:151–153. doi: 10.1080/08820530701457373. [DOI] [PubMed] [Google Scholar]
- 5.Joshi MM, Garretson BR. Paclitaxel maculopathy. Arch Ophthalmol. 2007;125:709–710. doi: 10.1001/archopht.125.5.709. [DOI] [PubMed] [Google Scholar]