Abstract
Background
The overexpression of Aurora A kinase (AurA) has been reported in various malignancies, including acute myeloid leukemia (AML). However, the expression of AurA and the effects of AurA inhibition in cancer stem cells are not yet fully understood. We investigated the expression and inhibition of AurA in AML stem cells (CD34+/CD38-).
Methods
Expression of AurA was investigated in cell lines (NB4 and KG1) that express high levels of CD34 and low levels of CD38. Primary AML cells were harvested from 8 patients. The expression of AurA and cell death induced by inhibition of AurA were analyzed in CD34+/CD38- cells.
Results
AurA was shown to be overexpressed in both primary AML cells and leukemia stem cells (LSCs) compared to normal hematopoietic stem cells. Inhibition of AurA plus cytarabine treatment in LSCs resulted in increased cytotoxicity compared to cytarabine treatment alone. Additional stimulation with granulocyte-colony stimulating factor (G-CSF) increased the cell death caused by AurA inhibition plus cytarabine treatment.
Conclusion
To our knowledge, this is the first report describing increased expression of AurA in LSCs. Our results suggest that selective AurA inhibition may be used to reduce LSCs, and this reduction may be enhanced by stimulation with G-CSF. Further exploration of relationship between nuclear factor kappa-B and AurA inhibition and the potential of AurA inhibition for use in leukemia treatment is needed.
Keywords: Acute myeloid leukemia, Leukemia stem cell, Aurora kinase
INTRODUCTION
Aurora kinases are a family of serine/threonine kinases that play essential roles in chromosome alignment, separation, and cytokinesis during mitosis and cell division [1, 2]. The overexpression of Aurora A kinase (AurA) leads to the amplification of centrosomes, inhibition of cytokinesis, and aneuploidy, which can be observed in various human cancers, including hepatocellular carcinoma, ovarian carcinoma, and hematological malignancies [3-7]. Although data on the roles of AurA in malignancy are accumulating and selective Aurora kinase inhibitors are under development in clinical trials [8, 9], the role of AurA in cancer stem cells has not been fully investigated. Thus far, reports on AurA in cancer stem cells examined malignancies of epithelial origin; AurA inhibition resulted in reduced activity of nuclear factor kappa-B (NF-κB) and anti-apoptotic Bcl-2 family members [10, 11].
Acute myeloid leukemia (AML) is a heterogeneous group of clonal hematopoietic neoplasms. Most patients achieve complete remission after a single round of chemotherapy, but nearly half of the patients relapse, and only 20-30% of patients achieve long-term disease-free survival. Relapse is related to residual disease and development of chemotherapy resistance. Leukemia stem cells (LSCs) are thought to have the capacity of self-renewal and the ability to initiate and maintain leukemia; thus, these cells may underlie relapses in AML. LSCs are widely hypothesized to exist as a small population among the CD34+/CD38- fraction of leukemia cells. CD44, CLL-1, and CD96 are also increased in LSCs. LSCs exhibit intrinsic resistance to treatment, which is attributed to their quiescent state and to the increased expression of abnormal drug transporters [12, 13].
We previously reported the enhancement of cytarabine (Ara-C)-induced cell death by AurA inhibition in leukemia cell lines. Cell death was increased by a non-caspase-dependent mitotic catastrophe [14]. To define AurA expression in LSCs and to identify the effects of AurA inhibition on the reduction of LSCs, we investigated the effect of selective AurA inhibition in LSCs from AML patients.
MATERIALS AND METHODS
1. Cell isolation, culture, and treatment
The human leukemia cell lines, KG1 (American Type Culture Collection, Manassas, VA, USA) and NB4 (DSMZ - German Collection of Microorganisms and Cell Cultures), and primary AML cells isolated from patients were used. Bone marrow mononuclear cells were isolated from anticoagulated bone marrow specimens aspirated from patients by Ficoll-Hypaque density gradient centrifugation. Cells were cultured in RPMI-1640 (Life Technologies, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, UT, USA) and 1% penicillin/streptomycin (Invitrogen Corporation, Carlsbad, CA, USA) in a humidified atmosphere containing 5% CO2 at 37℃. Growing cells were treated with different concentrations of clinical-grade Ara-C (Pharmacia/Upjohn, St. Quentin, France) alone or in combination with the Aurora kinase inhibitor, C1368 (Sigma-Aldrich, St. Louis, MO, USA).
2. Antibodies
Mouse monoclonal antibodies against poly ADP-ribose polymerase (PharMingen, San Diego, CA, USA) and α-tubulin (Upstate Biotechnology, Lake Placid, NY, USA) were used. Rabbit polyclonal antibodies against phospho(p)-AurA, Wnt-1, beta-catenin, Gli-1, Gli-2, p-Akt, p-MEK, p-ERK, p-p38 (Cell Signaling Technology, Beverly, MA, USA), caspase-3 (PharMingen), and NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used.
3. Assessment of cell viability and apoptosis
To assess the viability of LSCs, cells were labeled with anti-CD34-ECD (BD Biosciences) and anti-CD38-PerCP (PharMingen) for 30 min, and then resuspended in phosphate-buffered saline (PBS) containing 50 µg/mL propidium iodide (PI; Sigma-Aldrich, St. Louis, MO, USA), prior to flow cytometric analysis. Cell death was defined based on changes in the permeability of PI. Trypan blue staining and light microscopic examination were also used for assessing cell viability. Apoptosis was detected with an annexin V-binding assay and flow cytometric analysis. Labeled cells were resuspended in 100 µL of annexin V-binding buffer and incubated with annexin V-fluorescein isothiocyanate and anti-7-aminoactinomycin (Beckman Coulter) for 20 min at room temperature prior to flow cytometric analysis. The percentage of apoptotic cells was determined by quantifying the annexin V-positive cells using gates set for total ungated cells, CD34+/CD38- cells, and CD34+/CD38+ cells by flow cytometric analysis on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, BDIS, San Jose, CA, USA).
4. Cell cycle analysis
After the indicated treatments, cells were harvested, washed with PBS, and fixed in 70% ethanol at -20℃ for 16 h. Fixed cells were washed twice with PBS, and resuspended in cell cycle buffer (0.38 mM sodium citrate, 0.5 mg/mL RNase A, 0.01 mg/mL PI) at a concentration of 1×106 cells/mL. Cell cycle analysis was performed by flow cytometric analysis.
5. Western blot analysis
Cells were lysed in 100 µL of lysis buffer and sonicated briefly. Homogenized lysates were quantified with a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein (20 µg) were boiled for 10 min, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. After blocking in 0.05% PBS-T and 5% bovine serum albumin for 1 h, the membranes were incubated with primary antibodies for 2 h at room temperature. The membranes were then washed 4 times in PBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 1 h at room temperature. They were then washed with Tris-buffered saline with Tween, and proteins were visualized using enhanced chemiluminescence (Amersham Bioscience). Quantitative densitometric analysis was performed using a luminescent image analyzer (LAS-1000 Plus, Fuji Film, Ltd.) and TINA software version 2.10e (Raytest Isotopenmessgeraete GmbH, Straubenhardt, Germany).
6. Statistical analysis
Statistical analysis was performed using the Student's t-test. Differences were defined as statistically significant when P<0.05. Values are expressed as mean±standard deviation.
RESULTS
1. Effect of Ara-C and an the AurA inhibitor on the NB4 leukemia cell line
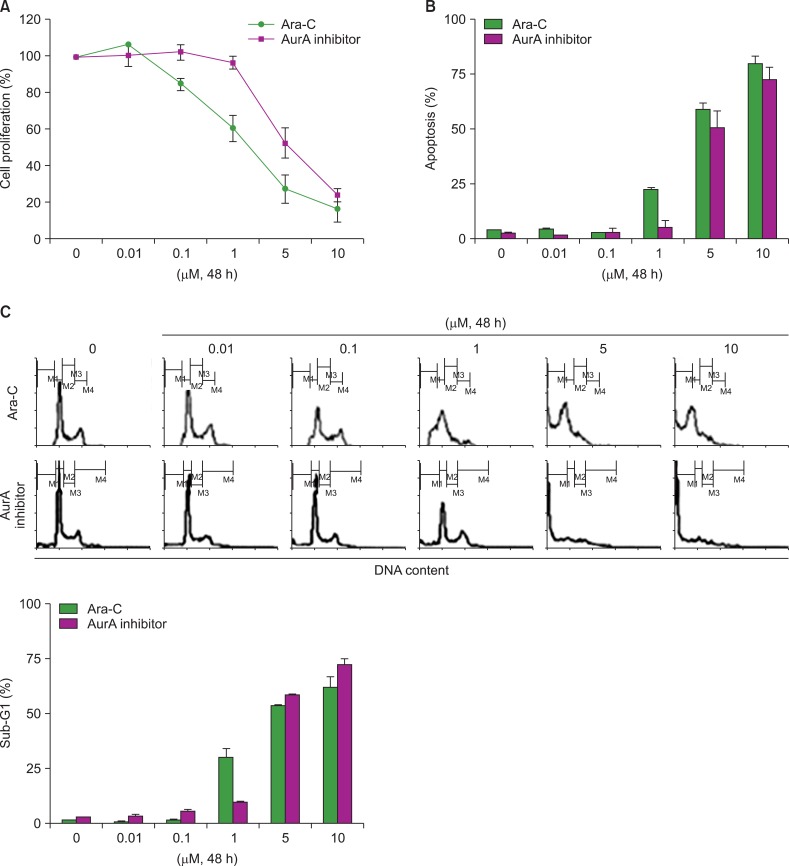
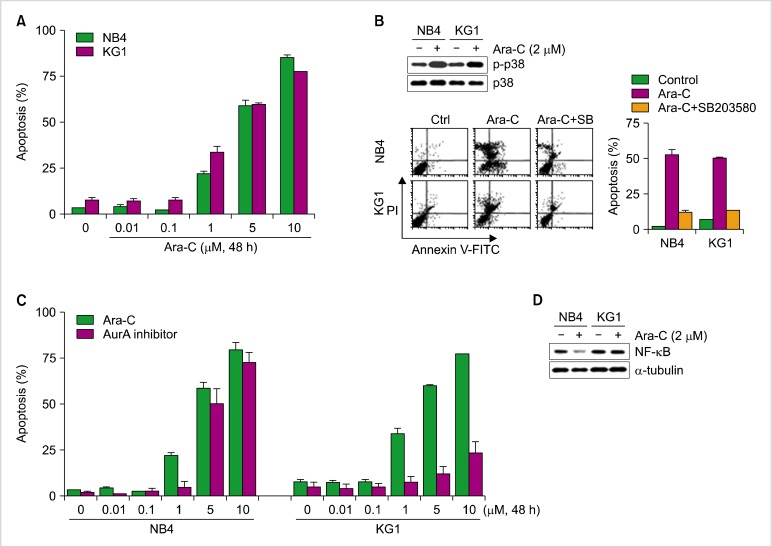
We examined the sensitivity of the NB4 leukemia cell line to single treatment with Ara-C or an the AurA inhibitor, and to co-treatment with both Ara-C and the AurA inhibitor. Cells were treated with different concentrations of Ara-C or the AurA inhibitor for 48 h. Cell proliferation, apoptosis, and cell cycle analyses were performed. Inhibition of cell proliferation was first detected in Ara-C-treated cultures at 1 µM (60.5±7.5%) and in AurA-treated cells at 5 µM (52.15±8.3%, Fig. 1A). Both Ara-C and the AurA inhibitor increased the proportion of apoptotic cells in a dose-dependent manner (Fig. 1B). Cell cycle analysis revealed that the fraction of sub-G1 phase cells began to increase at the same concentration at which apoptosis started to increase (Fig. 1C).
Fig. 1.
Ara-C- or AurA inhibition-induced cell death in the NB4 cell line. (A) NB4 cells were incubated with different concentrations of Ara-C and AurA inhibitor for 48 h. After treatment, cell proliferation was analyzed as described. (B) The proportions of apoptotic cells after treatment with Ara-C or AurA inhibitor at various concentrations for 48 h (P>0.05 for all concentrations). (C) Representative histogram of cell cycle analysis after treatment with various concentrations of Ara-C or AurA inhibitor (P>0.05 for all concentrations).
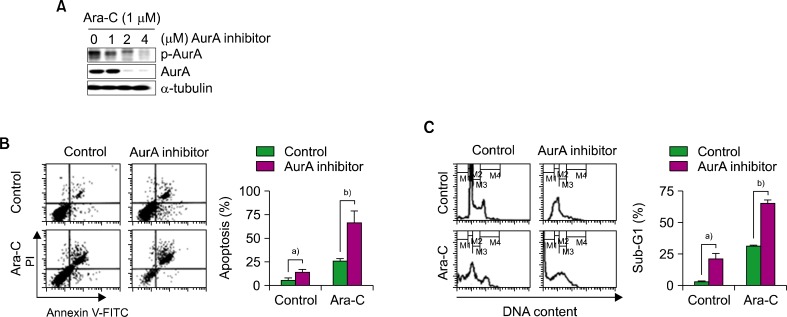
2. Ara-C/the AurA inhibitor co-treatment enhanced cell death in the NB4 leukemia cell line
To determine the optimal co-treatment concentrations of Ara-C and the AurA inhibitor, we identified concentrations of the AurA inhibitor that reduced p-AurA expression during co-treatment with Ara-C by western blotting. At an the AurA inhibitor concentration of 2 µM, both p-AurA and total AurA protein expression were decreased (Fig. 2A). Then, NB4 cells were co-treated with 1 µM of Ara-C and 2 µM of the AurA for 48 h and analyzed for apoptosis and cell cycle characteristics. Co-treatment increased the rate of apoptosis compared to Ara-C alone or the AurA inhibitor alone (co-treatment, 66.23±11.93%; Ara-C alone, 25.38±2.45%; the AurA inhibitor, 13.82±1.82%; P<0.05; Fig. 2B). The proportion of cells in sub-G1 phase increased more in co-treated cells (co-treatment, 65.5±1.74%; Ara-C alone, 31.17±0.72%; the AurA inhibitor alone, 20.96±4.17%; P<0.05; Fig. 2C).
Fig. 2.
Effect of co-treatment with Ara-C and AurA inhibitor in NB4 cells. (A) Expression of p-AurA and total AurA protein after co-treatment with Ara-C (1 µM) and various concentrations of AurA inhibitor (0, 1, 2, and 4 µM) for 48 h. (B) Analysis of apoptosis after co-treatment with Ara-C (1 µM) and AurA inhibitor (2 µM). a)P<0.05, b)P<0.05. (C) Cell cycle analysis after co-treatment with Ara-C (1 µM) and AurA inhibitor (2 µM). a)P<0.05, b)P<0.05.
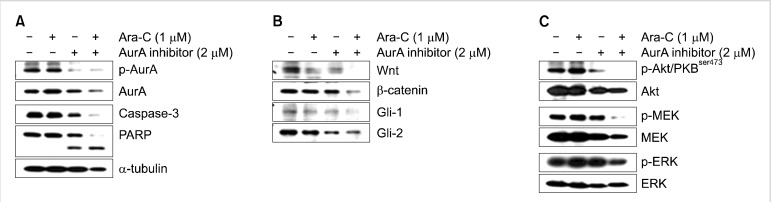
We previously reported that Ara-C/the AurA inhibitor induced mitochondria-mediated, caspase-dependent apoptosis in Ara-C-sensitive cell lines, and non-caspase-dependent mitotic catastrophe in Ara-C-resistant cell lines. To identify changes in signaling pathways in cancer stem cells treated with Ara-C and the the AurA inhibitor, we examined the expression of p-Akt, p-MEK, p-ERK, Wnt, β-catenin, Gli-1, and Gli-2 by western blot analysis after co-treatment of NB4 cells. Expression of Wnt1 and β-catenin decreased synergistically (Fig. 3B), while p-Akt, p-MEK, and p-ERK were further decreased by co-treatment (Fig. 3C). However, no synergistic effect of co-treatment was observed for Gli expression (Fig. 3B).
Fig. 3.
Effect of Ara-C/AurA inhibitor co-treatment on the expression of caspase-3 and components of the Wnt/β-catenin, hedgehog, Akt/PKB, and MAPK pathways. (A) After co-treatment, NB4 cell lysates were subjected to western blotting to assess the expression of p-AurA and the cleavage of PARP. (B) After co-treatment, NB4 cell lysates were subjected to western blotting to assess the expression of Wnt-1, β-catenin, Gli-1, and Gli-2. (C) Expression of Akt, MEK, and ERK after co-treatment with Ara-C/AurA inhibitor.
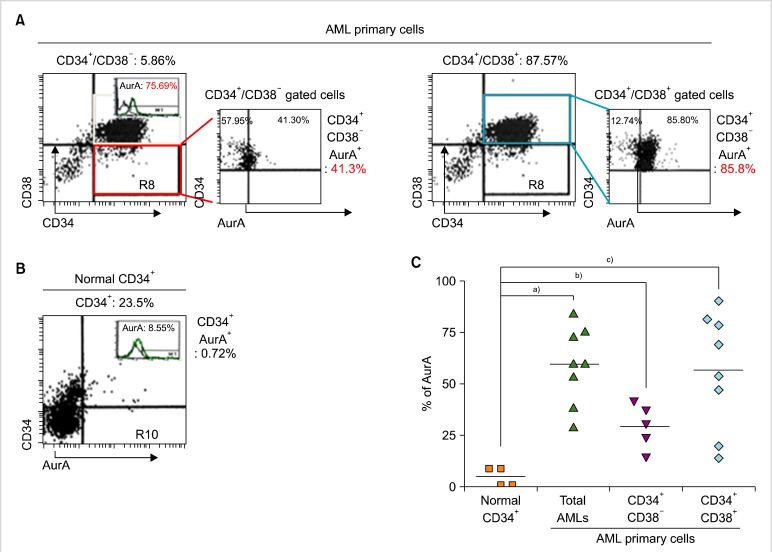
3. AurA expression in normal hematopoietic stem cells and LSCs
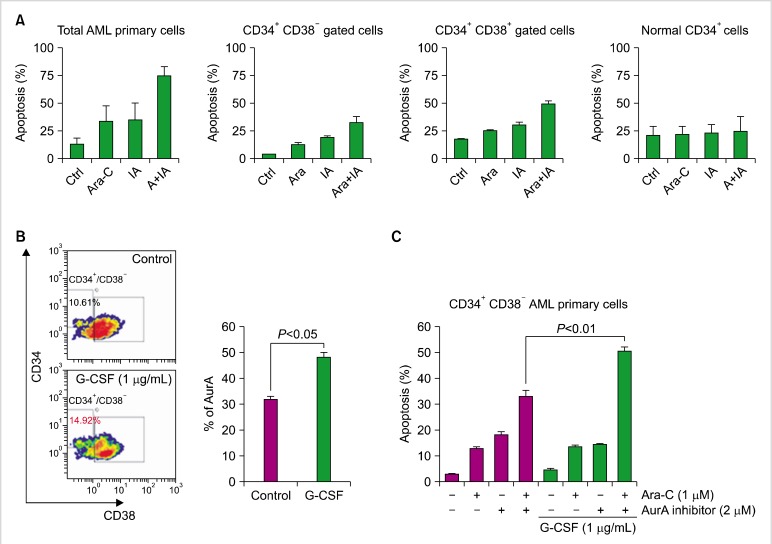
To examine the expression of AurA kinase in LSCs, we used primary AML cells from patients. Primary AML cells were divided into 2 groups, CD34+/CD38- cells (LSCs+) containing LSCs and CD34+/CD38+ cells (LSCs-), by immunophenotyping based on the expression of CD34 and CD38 (Fig. 4A). Compared to normal hematopoietic stem cells (HSCs), primary AML cells showed higher expression of AurA (normal, 4.7±2.27%; primary AML cells, 59.95±6.58%; Fig. 4A, B). The proportion of LSCs+ comprising LSCs was 10.55±5.33%. Both LSCs+ and LSCs- cells showed significantly higher expression of AurA compared to normal HSCs. In addition, LSCs- cells showed higher expression of AurA than did normal HSCs (LSCs+, 29.86±4.85%; LSCs-, 61.59±10.07%; Fig. 4C).
Fig. 4.
Expression of AurA kinase in primary AML cells and normal hematopoietic cells. (A) Expression of AurA in subpopulations of CD34+/CD38- cells (LSCs+) containing LSCs and CD34+/CD38+ cells (LSCs-) containing non-LSC blasts, analyzed by flow cytometry. (B) Expression of AurA in normal hematopoietic stem cells analyzed by flow cytometry. (C) Comparison of AurA expression in normal hematopoietic stem cells, CD34+/CD38- cells, and CD34+/CD38+ cells. Symbols indicate the expression level in each specimen; bar indicates mean. a)P<0.05, b)P<0.05, c)P<0.05.
4. Increased cell death in LSCs+ induced by Ara-C/the AurA inhibitor co-treatment and granulocyte-colony stimulating factor (G-CSF)
Primary AML cells and normal bone marrow CD34+ cells were treated with Ara-C and the AurA inhibitor for 48 h and analyzed for cell death. Cell death was not increased by co-treatment in normal CD34+ cells, but co-treated primary AML cells showed markedly increased cell death compared to single treatment with Ara-C or the AurA inhibitor. The cell death rate in co-treated primary AML cells was 71.12±8.01%, compared to 32.64±4.09% for LSCs+ cells and 48.7±3.02% for LSCs- cells (Fig. 5A). To increase cytotoxicity in LSCs+ cell populations, G-CSF was administered before Ara-C/the AurA inhibitor co-treatment. Expression of AurA in LSCs+ cells increased from 32.14±0.7% to 48.19±1.92% (Fig. 5B). G-CSF-stimulated primary AML cells were then treated with Ara-C and the AurA inhibitor alone or in combination. Cell death in the co-treated LSCs+ population increased after G-CSF stimulation compared to single treatment with Ara-C or the AurA inhibitor (Fig. 5C, G-CSF stimulation, 50.30±1.87%; non-G-CSF, 32.64±2.71%).
Fig. 5.
Effect of Ara-C/the AurA inhibitor co-treatment on primary AML cells and normal hematopoietic cells, and effect of G-CSF priming on this co-treatment. Primary leukemia cells from patients were treated with Ara-C (1 µM)±the AurA inhibitor (2 µM) for 48 h. (A) Cells were evaluated for apoptosis by flow cytometry in total cells, CD34+/CD38- cells, and CD34+/CD38+ cells, and compared to normal CD34+ hematopoietic stem cells. Bars indicate SD. (B) Primary CD34+/CD38- cells containing LSCs were stimulated with G-CSF (1 µg/mL) for 7 days and analyzed for expression of AurA. Bars indicate SD. (C) CD34+/CD38- cells were stimulated with G-CSF for 7 days and then treated with or without Ara-C and the AurA inhibitor. Evaluation of apoptosis was performed by flow cytometry. Bars indicate SD.
5. Ara-C-induced cell death is related to p38 mitogen-activated protein kinase (p38MAPK) and NF-κB activation and might be related to decreased apoptosis induced by the AurA inhibitor in KG1 cells
In our previous report, we found that Ara-C-induced cell death is related to p38MAPK; our current data are consistent with that observation (Fig. 6A, B). Induction of apoptosis by Ara-C was similar in NB4 cells and KG1 cells, but responses to the AurA inhibitor were different. Specifically, the expression of NF-κB as detected by western blot analysis differed between NB4 and KG1 cells (Fig. 6D). NF-κB expression was decreased by Ara-C treatment in NB4 cells but was unaffected by the same treatment in KG1 cells.
Fig. 6.
Effect of Ara-C and the AurA inhibitor on NB4 and KG1 cells in relation to p38 MAP kinase activation and NF-κB protein. (A) Cells were treated with various concentrations (0.01, 0.1, 1, 5, and 10 µM) of Ara-C for 48 h, after which apoptosis was evaluated by flow cytometry. (B) Expression of p38 and phospho-p38 was evaluated after Ara-C (2 µM) treatment. Cells were treated with a selective inhibitor of p38, SB203580 (20 µM), for 3 h; next, the rates of apoptosis were compared. (C) NB4 and KG1 cells were treated with various concentrations of Ara-C or the AurA inhibitor (0.01, 0.1, 1, 5, and 10 µM) and then evaluated for apoptosis by flow cytometry. (D) Expression of NF-κB evaluated by western blot analysis.
DISCUSSION
Resistance to cytotoxic agents is one of the obstacles in the treatment of AML. We found that combined AurA inhibition and Ara-C treatment enhanced leukemic cell death in Ara-C-resistant leukemia cells by inducing apoptosis and mitotic catastrophe [14]. Because LSCs are also a major cause of relapse and resistance to treatment, we explored the effects of AurA inhibition in LSCs.
AurA is overexpressed in various cancers, including hematological malignancies [3-5, 8], and its overexpression is related to progression or poor prognosis subtypes in some malignancies [4, 6, 7, 15]. Therefore, inhibition of AurA by selective inhibitors is the subject of several clinical trials. However, AurA expression and the effects of AurA inhibition in cancer stem cells are not yet fully understood. In a few reports on cancer stem cells of epithelial origins, AurA expression was found to be upregulated, and inhibition of AurA led to cell cycle arrest or restricted cell growth [10, 11].
Most LSCs are known to exist as a fraction of the CD34+/CD38- population and are able to transmit AML to nonobese diabetic/severe combined immunodeficient mice [16]. Our data show that a CD34+/CD38- subpopulation of primary AML cells that contains LSCs exhibited significantly increased expression of AurA when compared to normal HSCs. Similarly, Ye et al. reported increased AurA expression in CD34+ blast cells from patients with AML or myelodysplastic syndrome [17]. Because the CD34+ population includes non-LSC blasts, to our knowledge, this is the first report on AurA expression in LSCs. As expected, AurA expression was high in non-LSC blast cells, which may be because non-LSC blasts are actively cycling, whereas the majority of LSCs are quiescent. Similar results have been seen in colorectal cancer stem cells (CR-CSC) and ovarian cancer stem cells (EOC stem cells) [10, 11]. Cammareri et al. [10] found that the expression of AurA in CR-CSC was lower than that in primary colorectal cancer cells. However, in our findings, LSCs expressed a significantly higher level of AurA than did normal HSCs, and upon AurA inhibition, LSCs showed increased apoptosis while AurA inhibition caused no changes in apoptosis in normal HSCs. We think this difference between normal HSCs and LSCs may be useful for developing treatments that target LSCs.
The role of AurA in LSCs is not yet fully defined. AurA exerted no transforming activity in normal colon cells by itself, but induced centrosomal amplification, which can result in oncogenesis with additional genetic changes [10]. Although the function of AurA in LSC survival has not been fully analyzed, experiments in both CR-CSC and EOC stem cells showed that silencing or inhibition of AurA was related to a decreased G1 phase population and an increased number of cells in G2/M arrest. Our results in LSCs were similar. Selective AurA inhibition increased the sub-G1 phase fraction in NB4 cells, and in K562, KG1, and U937 cells in another study [14].
Our previous report focused on the synergistic effects of Ara-C and the AurA inhibitor in leukemia cells and its effects on related molecular pathways. Here, we examined the effects of selective AurA inhibition on LSC survival. CD34+/CD38- populations containing LSCs, obtained from AML patients, showed increased apoptosis after treatment with either Ara-C or the AurA inhibitor, and the effect was significantly enhanced upon co-treatment with Ara-C and the AurA inhibitor. This co-treatment effect was greater in non-LSCs. Following stimulation with G-CSF, AurA expression increased, leading to enhanced apoptosis with Ara-C/AurA co-treatment. Because the main roles of AurA are in spindle formation, centrosome maturation, and duplication [2], this result may indicate that G-CSF stimulated some LSCs to enter the cell cycle or increased asymmetric cell division.
KG1 cells exhibited lower rates of apoptosis compared to NB4 cells following AurA inhibitor treatment. In both cell lines, Ara-C-induced cell death involved the activation of p38MAPK. In KG1 cells, NF-κB was constitutively activated after Ara-C treatment, perhaps explaining the lower apoptosis rates in these cells. In EOC stem cells [11], selective AurA inhibition resulted in reduced NF-κ activation. Guzman et al. reported increased NF-κB levels in CD34+ human AML cells [18], and Colado et al. tested bortezomib as an NF-κB inhibitor to overcome drug resistance in CD34+ immature myeloid leukemia cells [19]. Reduced apoptosis in KG1 cells induced by AurA inhibition may result from insufficient NF-κB inhibition. Because NF-κB activation levels differ in various cancers, the required dose of AurA inhibitor may vary among cell lines. Nevertheless, taken together, these results suggest that AurA inhibition and potent NF-κB activation can reduce the number of LSCs.
In summary, our study demonstrated increased expression of AurA in LSCs. Furthermore, our findings suggest the possibility that selective AurA inhibitors can be used to reduce the numbers of LSCs, an effect that can be enhanced by stimulation with G-CSF. Further exploration of the relationships between NF-κB and AurA inhibition, and the potential utility of AurA inhibition in leukemia treatment is needed.
Footnotes
This work was supported by National Research Foundation of Korea Grant 313-2007-2-E00246 funded by the Korean Government.
References
- 1.Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the auroras come into view. Curr Opin Cell Biol. 2003;15:672–683. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 3.Gritsko TM, Coppola D, Paciga JE, et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–1426. [PubMed] [Google Scholar]
- 4.Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004;10:2065–2071. doi: 10.1158/1078-0432.ccr-1057-03. [DOI] [PubMed] [Google Scholar]
- 5.Kurai M, Shiozawa T, Shih HC, et al. Expression of Aurora kinases A and B in normal, hyperplastic, and malignant human endometrium: Aurora B as a predictor for poor prognosis in endometrial carcinoma. Hum Pathol. 2005;36:1281–1288. doi: 10.1016/j.humpath.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Reiter R, Gais P, Jütting U, et al. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–5141. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]
- 7.Lassus H, Staff S, Leminen A, Isola J, Butzow R. Aurora-A overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecol Oncol. 2011;120:11–17. doi: 10.1016/j.ygyno.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Farag SS. The potential role of Aurora kinase inhibitors in haematological malignancies. Br J Haematol. 2011;155:561–579. doi: 10.1111/j.1365-2141.2011.08898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cammareri P, Scopelliti A, Todaro M, et al. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res. 2010;70:4655–4665. doi: 10.1158/0008-5472.CAN-09-3953. [DOI] [PubMed] [Google Scholar]
- 11.Chefetz I, Holmberg JC, Alvero AB, Visintin I, Mor G. Inhibition of Aurora-A kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFκB pathway. Cell Cycle. 2011;10:2206–2214. doi: 10.4161/cc.10.13.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito Y, Uchida N, Tanaka S, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testa U. Leukemia stem cells. Ann Hematol. 2011;90:245–271. doi: 10.1007/s00277-010-1118-7. [DOI] [PubMed] [Google Scholar]
- 14.Cheong JW, Jung HI, Eom JI, Kim SJ, Jeung HK, Min YH. Aurora-A kinase inhibition enhances the cytosine arabinoside-induced cell death in leukemia cells through apoptosis and mitotic catastrophe. Cancer Lett. 2010;297:171–181. doi: 10.1016/j.canlet.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Yang S, Zhang H, et al. Aurora-A as an independent molecular prognostic marker in gastric cancer. Oncol Rep. 2011;26:23–32. doi: 10.3892/or.2011.1250. [DOI] [PubMed] [Google Scholar]
- 16.Fajtova M, Babusikova O. Immunophenotype characterization of hematopoietic stem cells, progenitor cells restricted to myeloid lineage and their leukemia counterparts. Neoplasma. 2010;57:392–400. doi: 10.4149/neo_2010_05_392. [DOI] [PubMed] [Google Scholar]
- 17.Ye D, Garcia-Manero G, Kantarjian HM, et al. Analysis of Aurora kinase A expression in CD34(+) blast cells isolated from patients with myelodysplastic syndromes and acute myeloid leukemia. J Hematop. 2009;2:2–8. doi: 10.1007/s12308-008-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 19.Colado E, Alvarez-Fernández S, Maiso P, et al. The effect of the proteasome inhibitor bortezomib on acute myeloid leukemia cells and drug resistance associated with the CD34+ immature phenotype. Haematologica. 2008;93:57–66. doi: 10.3324/haematol.11666. [DOI] [PubMed] [Google Scholar]