Abstract
Resistance to obesity is becoming an exception rather than the norm, and understanding mechanisms that lead some to remain lean in spite of an obesigenic environment is critical if we are to find new ways to reverse this trend. Levels of energy intake and physical activity both contribute to body weight management, but it is challenging for most to adopt major long-term changes in either factor. Physical activity outside of formal exercise, also referred to as activity of daily living, and in stricter form, spontaneous physical activity (SPA), may be an attractive modifiable variable for obesity prevention. In this review, we discuss individual variability in SPA and NEAT (nonexercise thermogenesis, or the energy expended by SPA) and its relationship to obesity resistance. The hypothalamic neuropeptide orexin (hypocretin) may play a key role in regulating SPA and NEAT. We discuss how elevated orexin signaling capacity, in the context of a brain network modulating SPA, may play a major role in defining individual variability in SPA and NEAT. Greater activation of this SPA network leads to a lower propensity for fat mass gain and therefore may be an attractive target for obesity prevention and therapy.
Keywords: orexin, obesity, spontaneous physical activity, nonexercise activity thermogenesis, energy expenditure
Obesity and individual variability
Obesity is a condition defined by a chronic excess of body fat1 and is positively correlated with shorter life expectancy, metabolic syndrome, type 2 diabetes, and coronary heart disease.2 Obesity has become a public health issue, as its incidence in adults and children has increased in the last two decades across both developed and underdeveloped societies.3–5
Humans show large variation in their susceptibility to obesity, which is determined by both environmental and genetic factors.6–9 A major factor in determining this variability is physical activity, and specifically a component of overall energy expenditure known as nonexercise induced thermogenesis (NEAT).10–12 NEAT includes all forms of energy expenditure not associated with formal exercise, such as standing and fidgeting.10,13–15 The complementary concept of spontaneous physical activity (SPA) is used to describe “any type of physical activity that does not qualify as voluntary exercise.”16,17 Both SPA and NEAT have a heredability component.17,18
SPA and NEAT are not interchangeable but are complementary concepts: NEAT refers to energy expenditure while SPA describes the types of physical activity that result in NEAT. Given the association between SPA and NEAT, it is not surprising that variability in SPA also contributes to variability in sensitivity to obesity. For example, lean people spend larger amounts of time standing (approximately two hours daily) than do obese people.12 While the contribution of NEAT to weight gain resistance may seem small, it can be significant. For example, one study showed that after overfeeding humans with 1,000 kcal daily for eight weeks, fat mass gain was significantly and negatively correlated with the increase in SPA and NEAT. Importantly, there was no change in volitional exercise, and no relationship between the observed change in fat mass and basal metabolism or postprandial thermogenesis.11
The neural mechanisms that underlie human variability in SPA are distributed processes involving multiple brain regions, neurotransmitters and neuropeptides, including cholecystokinin, corticotrophin releasing hormone, neuromedin, neuropeptide Y (NPY), leptin, and orexin (also known as hypocretin).19 While all are important, in this review we focus on the biological role of central orexin peptides and their receptors with respect to their role in obesity and obesity resistance.
Orexin peptides and their receptors
The orexins are two closely related peptides, orexin A (OXA, hypocretin 1) and orexin B (OXB, hypocretin 2) that are produced by cleavage from a single propeptide.20,21 In mammals, the majority of CNS orexin peptides are synthesized in neurons located in the lateral hypothalamus and perifornical area. The hypothalamic orexin neurons are glutamatergic neurons with tonic firing, low-threshold spike on recovery from hyperpolarization and little spike adaptation.22,23 Recently, the existence of orexin neuronal subpopulations has been proposed based on morphological and electrophysiological evidence.24
The orexin peptides act through two G protein-coupled receptors, orexin receptor type 1 (OX1R, hypocretin receptor 1) and orexin receptor type 2 (OX2R, hypocretin receptor 2).20,21 Both orexin receptor subtypes can bind to OXA and OXB, but with differential affinity: OX1R has a higher affinity for OXA, while OX2R has equal affinity for either orexin peptide.20,25 Activation of both receptor subtypes leads to an increase in neuronal firing and an increase in intracellular calcium.26–30 Preadministration of the OX1R antagonist SB334867 can block OXA-induced SPA and NEAT,16,31–34 suggesting an important role for OX1R in mediating SPA and NEAT; however, OX2R involvement has not been ruled out.
An important characteristic of the orexin neurons are their projections to multiple brain regions.35–39 Neuroanatomical studies have shown that the orexin neurons have collateral projections within the CNS,40–42 transsynaptically collateral CNS efferents,43 or collateral efferents to both CNS regions and brown adipose tissue.44 The distribution pattern of the orexin receptors reflects the widespread projections of orexin neurons, as both orexin receptor subtypes are expressed throughout the brain. The orexin receptors show distinctive, yet overlapping patterns of expression, with a good agreement between mRNA and protein data.45–50 However, studies addressing colocalization of the orexin receptor subtypes at a cellular level are lacking. The wide distribution of the orexin receptors and orexinergic fibers initially suggested the orexin system was involved in multiple physiological processes, and current research supports a role for orexin in the control of arousal and sleep, reward, stress, and energy homeostasis.51–56
The main contribution of the orexin peptides to energy metabolism is elegantly exemplified in a mouse model that exhibits postnatal loss of orexin neurons.57 In these mice, the orexin promoter drives expression of the neurodegenerative gene ataxin-3, leading to progressive loss of the orexin neurons during development. These mice exhibit hypophagia, lower levels of SPA, and develop spontaneous onset obesity when fed a regular diet.57,58 These results suggest that one primary function of the orexin peptides is to drive energy expenditure, although they can also modulate food intake. Additional support for this idea comes from another mouse model in which the β-actin cytomegalovirus promoter drives overexpression of the orexin peptides.59 Consistent with the role of orexin in promoting energy expenditure, these mice show resistance to high-fat diet–induced obesity.60
Orexin-mediated signaling
As discussed above, the orexin peptides exert their effects by binding to two closely related G protein-coupled receptors. In vitro and in vivo models show that orexin signaling is of an excitatory nature at the cellular level. Increased intracellular Ca2+ influx has been accepted as the most immediate cellular response to orexin receptor activation in both overexpression and in vivo models.25 Signaling responses for orexin receptors and their specific G-α subunit activation are currently under intense investigation. The activation of either orexin receptor can be coupled to Gq, Gi/o, or Gs G-α subunit proteins, which can modulate ion channels and exchangers to induce neuronal depolarization.28,61–70 Thus far, increased cellular activity by either OX1R and OX2R can be mediated by modulation of nonselective cationic currents (NSCC), voltage-gated calcium channels, the Na+/Ca2+ exchanger, and inwardly rectifying potassium channels.27,28,30,71–79
The type of intracellular mechanism triggered by activation of the receptors appears to be cell dependent. For example, in nucleus accumbens and nucleus of the solitary tract, OX1R/OX2R mediated depolarization requires a simultaneous decrease in K+ conductance and increase in NSCC.26,73 In GABAergic neurons from the arcuate nucleus, it occurs through a decrease in K+ conductance and activation of the Na+/Ca2+ exchanger.80 Finally, there are differences in the temporal profile of intracellular Ca2+ increases after OX1R/OX2R activation between neurons from the dorsal raphe and laterodorsal tegmental areas.29,81
The specific mechanisms involved in orexin mediated second messenger cascades, and their physiological relevance to obesity, are relatively undefined. Homogeneous overexpression models with human OX1R have revealed alterations in adenylyl cyclase activity via Gi/o, Gs, and Gq subunits but differ in their potency.25,61 OXA can also activate extracellular signal-regulated kinases (ERK1/2) and p38 mitogen-activated phosphate kinase (MAPK) in recombinant and adrenal cell culture models.82 OXA activation of either OX1R/OX2R in cells overexpressing either receptor can elicit the activation of ERK1/2 and p38 MAPK via multiple G-α subunits.70 Food deprivation in Wistar rats has also revealed differences in G-α subunit activation in hypothalamic tissue homogenates in response to OXA.83 However, discrete hypothalamic OXA-induced G-α subunit signaling responses for either OX1R or OX2R have yet to be determined when coexpressed.
The relevance of OX1R/OX2R in obesity has been exemplified in the obesity resistant (OR) and obesity prone (OP) rats. OR rats have higher basal levels of both intrinsic SPA and OXA-induced SPA following injections into the rostral lateral hypothalamic area (rLH) than OP or Sprague Dawley rats.34 Increased OXA sensitivity in OR rats appears to be due to an increase in receptor abundance compared to OP rats. While a difference in receptor density may address OXA sensitivity in OR rats, receptor functionality may also help explain the influence of orexin on SPA. Some aspects of orexin receptor sensitivity, distribution, and intracellular signaling mechanisms important in mediating OXA effects on SPA are currently under investigation using the OP or OR rat and other polygenic models of obesity. One such possibility under investigation is that that rLH orexin receptors in OR rats couple to Gs rather than Gi/o proteins, while the opposite occurs in OP rats.
Orexin in an animal model of obesity resistance
Levin et al. showed that when fed a high-fat diet a tertile of outbred Sprague Dawley rats gained no more weight than chow-fed controls.84 These diet-induced obese rats and their weight-gain resistant counterparts, referred to as diet resistant, were selectively bred by a commercial vendor for over ten years,85,86 resulting in the current OP/OR polygenic model of obesity. The OP and OR rats have divergent weight gain profiles despite inconsistently observed differences in energy intake.34,85,86 While early studies demonstrated that obese rats had a dampened feeding response to satiety-promoting agents such as leptin87 and insulin,88 it was clear that other neural modulators as well as differences in SPA likely contributed to the polygenic obesity observed in OR and OP rats. As the orexins modulate SPA,31,32,89 these findings underscored the potential significance of orexin as a neural modulator regulating body weight in this rodent model.
Like the outbred diet-resistant rats, the selectively bred OR rats exhibit lower body weight and fat mass gain on a low-fat diet and gain less weight when fed high-fat diet relative to their obesity-prone counterparts.33,34,85,90 These OR rats consume significantly fewer absolute calories, but they consume statistically more calories when calculated on a per gram body mass basis.34 Differences in SPA between OP and OR rats suggested by an early study91 were confirmed by tracking SPA levels in several groups of OP and OR rats at various ages using a chamber that tracks activity in the x, y, and z axes using infrared beams.34,90,92,93 OR rats display more ambulatory and vertical movement independent of age or the presence of food,34,90,92 and this finding has been consistent across different groups of OR and OP rats.92 Subsequent studies revealed that this greater SPA was associated with greater energy expenditure,92 a lower propensity to gain fat mass throughout development, maturation, and aging,90 and maintenance of higher SPA levels after high-fat diet feeding.33
OXA-induced SPA is associated with a dose-dependent increase in energy expenditure.31 Together with our previous studies showing OXA-induced hyperphagia following rLH infusion,94,95 we hypothesized that heightened responses to SPA-promoting agents and a dampened response to feeding-stimulatory agents such as OXA would perpetuate the lean phenotype in OR rats. To test this, OR and OP rats with chronically implanted guide cannulae targeting the rLH were given graded doses of OXA. In separate experiments, SPA and food intake were measured postinjection in young and adult rats. As expected, OR rats had greater OXA-induced SPA independent of age,34 but OR rats also had greater OXA-induced food intake per gram body mass than OP rats (also independent of age).34 This increase in caloric intake fits with the above-described enhanced 24 hour basal caloric intake in OR versus OP rats, and the idea that any behavioral effect of OXA in OR rats may be heightened due to higher OXA signaling capacity in OR rats (described in more detail below). OR rats maintain a lean phenotype over time, suggesting that the negative caloric benefit of OXA-induced SPA appears to outweigh the positive calories due to OXA-induced hyperphagia. A potential contributing mechanism for this is the observed longer duration of OXA action on SPA relative to that on food intake.89 Further supporting the idea that OR rats have higher endogenous SPA, we later showed that OR rats are also more sensitive to other SPA-promoting stimuli including caloric restriction96 and appear to be intrinsically protected from treatments that lower SPA, such as high-fat diet feeding. We and others have shown that in contrast to OP rats, which display lower SPA levels after high-fat diet consumption, OR rats maintain high basal SPA levels and have greater OXA-induced SPA after high fat diet feeding.97 Most importantly, we also showed that rLH-OXA increases energy expenditure,93 and others found that daily OXA treatment reduces body weight by increasing SPA.98 These findings support the hypothesis that elevated energy expenditure due to SPA-promoting agents such as OXA and defense from SPA-dampening treatments protects against excessive adiposity gain in OR rats.
To understand whether differences in responsivity to OXA are driven by greater orexin signaling at the level of the peptide or the receptor, we analyzed mRNA data for prepro-orexin, OX1R, and OX2R from brain micropunches in OR and OP rats. Our data show that relative to OP rats, OR rats have greater orexin receptor mRNA in the rLH despite similar levels of preproorexin in the rLH or within whole hypothalamus.34 This elevated receptor mRNA is mirrored by elevated receptor peptide levels in OR rats (Fig. 1). We later showed greater orexin receptor mRNA in the dorsal raphe, locus coeruleus, and ventrolateral preoptic area, in addition to better sleep quality in OR rats, which would be expected to contribute to the favorable weight status observed.99 Together with our earlier work, these data suggest that elevated orexin receptor mRNA within distinct brain sites function to create a brain-wide orexin signaling network at the level of the receptor that perpetuates heightened basal and OXA-stimulated SPA levels, which attenuates adiposity gain in OR rats.
Figure 1.
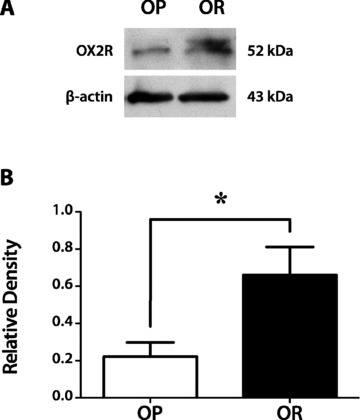
Difference in orexin 2 receptor (OX2R) protein levels between obesity-prone (OP) and obesity-resistant (OR) rats on chow diet. (A) Western blot analysis of caudal lateral hypothalamus (cLH) of OP and OR rats. The 52–53 kDA band is the predicted size of OXR2. Samples were normalized to β-actin. (B) Densitometric analysis of the Western blot data for all OP and OR rats. N = 4/group; *P < 0.05.
There are many animal models developed to amplify divergent locomotor activity, wheel running, or aerobic capacity.17,100 These models help clarify the role of overall physical activity level in obesity, but they do not mirror models varying in SPA, as exercise has been shown to have a strong motivational component, whereas SPA may not. A recent series of studies undertaken by Novak et al.101,102 in rodents selectively bred for high and low wheel running capacity (HCR and LCR, respectively)103 have shown that HCR rats also have greater SPA, and basal and activity-induced energy expenditure.102 Furthermore, like OR rats, HCR rats have heightened SPA following OXA infusion in the hypothalamic PVN.101 However, in contrast to OR rats, greater OXA-induced SPA in HCR rats is accompanied by greater OXA content but not increased orexin receptor mRNA in the perifornical lateral hypothalamus.101
It is possible that the differences in orexin function between animal models bred for high exercise and the OP and OR rats arise from long-term effects of high fat consumption in this particular model. In itself, this makes the OP and OR rats a more appropiate model for studies of diet-induced obesity. The evidence from the OP and OR model suggests that the interaction between high fat consumption and the orexins is determined by individual susceptibility to high fat consumption, which in turn might be determined by baseline orexin signaling through particular brain regions, including the rLH. In summary, while animal models of high exercise may share some of the same orexin signaling characteristics as that of high SPA models, there are likely differences in the regulatory control of orexin between these models, which might be a consequence of the high-fat intake used to derive the OP and OR rats.
Orexin and sleep
As mentioned above, OR rats, with higher orexin signaling capacity, also exhibit more consolidated sleep relative to OP rats. The overall distribution of orexin fibers in the brain has suggested that the orexins play a role in a number of systems, including the maintenance of arousal.104,105 Orexin fibers have been shown to project to several brain nuclei implicated in the control of sleep state.35–39 Application of OXA in the locus coeruleus104,106 and lateral preoptic area107 of the rat increase wakefulness, primarily through a decrease in rapid eye movement (REM) sleep.106 Activity in locus coeruleus (LC) neurons increases following application of OXA.104–106 More recently, direct stimulation of electrical activity in orexin neurons using optogenetic techniques was shown to induce wakefulness in sleeping mice.108
In addition to projecting to sleep-wake nuclei, orexin cells receive input from brain systems involved in regulation of sleep-wakefulness. In mammals, circadian organization of activity including sleep-wake behavior is regulated by the endogenous clock located in the suprachiasmatic nucleus (SCN).109 Orexin cell bodies receive both limited direct contact from the SCN,110 as well as substantial indirect contact from the SCN via the medial preoptic area and the subparaventricular zone.111,112 Orexin neurons show circadian patterns of activation,113 and ablation of the SCN eliminates rhythmicity of orexin release.114 Introduction of chemicals known to increase arousal in rats, such as methamphetamines or the anti narcoleptic drug modafanil, increase nuclear Fos expression in orexin cell bodies.115,116 Furthermore, increasing the behavioral arousal of rats by sleep deprivation induced due to handling also increases the expression of nuclear Fos in OXA cells.116 Finally, in a diurnal rodent model, Fos expression patterns in orexin neurons are correlated with individual variation in the timing of daily wheel running activity.117 The orexins thus appear to be capable of both receiving information related to the arousal state of the animal, and relaying arousal information to other nuclei known to promote wakefulness.
The association between the orexins and arousal was strengthened by the discovery that sleep disorder narcolepsy is associated with a defect in the orexin system.57,115 While it is clear from this evidence that orexin is not necessary for wakefulness, data suggest that orexin is important in maintaining high levels of arousal, and that one major function of the orexin system is to stabilize sleep–wake transitions. Furthermore, there is a recognition that orexin activity may be incompatible with sleep, as direct activation of orexin neurons causes wakefulness in rodents,108 and silencing of orexin neuronal activity during the inactive period results in slow wave sleep.118
Two comorbidities associated with narcolepsy—cataplexy and obesity—help shed light on the importance of orexin in normal physiology. Early studies of orexin effects suggested that orexin results in the activation of motor activity,119,120 and it is well established that physical activity is correlated with both activation of orexin neurons 116,117 and increases in OXA release.121 In narcoleptic individuals, cataplexy (defined as a loss of muscle tone) is often triggered by emotional stress or physical exertion,122 and is preceded by a reduction of neuronal firing in the LC.123 Injection of OXA into the locus coeruleus activates LC neurons104–106 and increases muscle tone.124 Promotion of motor activity may thus be one important function of orexin, and this orexin-induced activity may be coupled strongly to behavioral state to maintain normal motor tone during periods of emotional or physical stress.
Obesity is a comorbidity of narcolepsy in both human and animal models.57,122,125 Both human and animal subjects with narcolepsy eat less as would be expected given the association between orexins and feeding behavior.57,125 Yet the effect of reduced caloric consumption is offset by decreases in physical activity, as narcoleptic individuals exhibit a significantly elevated body mass index relative to nonnarcoleptic patients.122 In human subjects, while the total time spent awake is not reduced, there is a decreased amplitude of circadian activity patterns, consistent with reduced overall physical activity.126 In a mouse model of narcolepsy, in which orexin neurons are ablated postnatally, physical activity during the active (but not the resting) phase is reduced in affected animals,57 and these animals subsequently become obese. These human and animal studies demonstrate that the effects of orexin on sleep–wake patterns and physical activity are consistent with the idea that orexin is a neuropeptide conveying resistance to obesity.
Instability of sleep patterns is known to contribute to weight gain.127,128 In this light, it could be argued that weight gain in narcolepsy is more due to disturbance of sleep than to reductions in activity due to lack of orexin signaling. However, evidence from a diurnal rodent model and from laboratory rats suggests that orexin-associated activity can be altered without disturbing total sleep. Wheel running activity in some Nile grass rats can occur exclusively at night, during the inactive phase, while in others wheel running follows the normal diurnal pattern.129 While Fos activation in orexin neurons occurs only during the light period in day-active animals, in night-active grass rats Fos is elevated both during nightly activity bouts and during the day.117 Behavioral measures of sleep in these animals showed that while the timing of sleep was changed in night-active animals due to wheel running at night, neither total sleep nor duration of sleep bouts differed between groups.130 Finally, previously unpublished data from our laboratory show that in OP and OR rats the effects of orexin on activity and arousal are not inextricably linked. Application of exogenous orexin significantly increases physical activity in OR relative to OP rats; however, recordings of total sleep using implanted EEG/EMG transmitters showed that this increase in activity was not caused by increased arousal or reduced sleep time in OR rats (Fig. 2). Importantly, while both sets of rats had increased time spent in wakefulness, there was not a greater increase in OR rats relative to OP rats. This lack of difference between OP and OR rats in time spent awake after orexin treatment suggests that SPA following orexin is not merely due to increased wakefulness; the SPA effect in OR rats is in addition to the wake-promoting effect of orexin.
Figure 2.
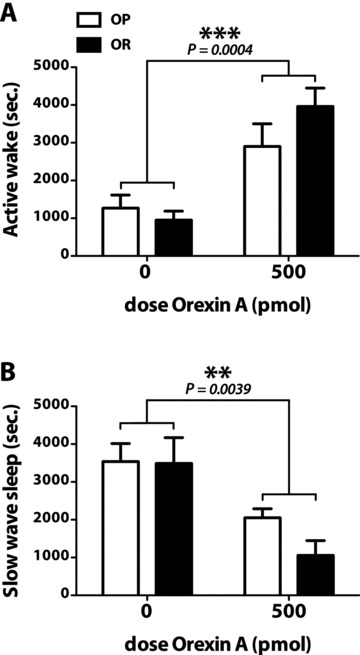
Total active wake (A) and slow wave sleep (B), in seconds, for obesity-prone (OP) and obesty-resistant (OR) rats for a 2-h period after treatment with vehicle or 500 pmol orexin A (OXA). In both panels, there is a significant overall effect of OXA on increasing active wake (P = 0.0004) and decreasing quiet sleep (P = 0.0039), respectively. However, there is no difference for either wake or sleep between OP and OR rats within treatment groups, despite the fact that OR rats show significantly higher levels of spontaneous physical activity following OXA treatment. N = 6/group; data are means ± SEM. **P < 0.005; ***P < 0.0005.
Translating SPA to energy expenditure
An important consideration is the relevance of SPA to overall energy expenditure. Energy expenditure (EE) comprises at least four main components, including basal metabolic rate (BMR; 60–70% of EE), which is the minimal energy required to maintain life, including heart beat, respiration, endocrine secretion, and kidney filtration. Diet-induced thermogenesis constitutes about 10% of total EE and includes energy related to the digestion, absorption, and metabolism of foods. Adaptive thermogenesis ranges from 10% to 15% of total EE and is related to adjustments in energy expenditure due to environment changes (e.g., shivering). Physical activity is the most variable of these components, ranging from 6% to 10% of total EE.131
SPA is neither a part of BMR nor a part of exercise physical activity. Therefore, it may be an attractive obesity target. SPA in humans was identified as early as 1954, and defined as a component of energy expenditure.132 Ravussin et al.15 showed that SPA in a human respiratory chamber averaged 348 kcal/day and, importantly, identified a large range in values: 100–700 kcal/day. Clearly, this wide range in values among humans suggests that there could be a large range in the weight gain response to overfeeding in humans, as demonstrated by Levine et al.11 Zurlo et al.133 showed that levels of SPA clustered in families, and could prospectively help explain propensity for weight gain in males, which suggests heritability of SPA. As discussed above, the idea that SPA level is an intrinsic heritable trait has recently been strengthened by Levine et al., in his study showing that lean humans stand and ambulate for approximately two hours daily more than obese, which is not affected by weight loss or weight gain, in the obese and lean respectively.11 That spontaneous physical activity levels differ between mouse strains134 also suggests that SPA is an intrinsic, inherited trait that varies within animal species.
Despite large differences in body fat, energy intake, and body size, OR and OP rats expend a similar number of absolute kilocalories.92 This suggests that OR rats are less efficient in their calorie use, as they are expending relatively large amounts of calories to support their relatively smaller energy needs related to their reduced body circumference and body fat, which affect levels of heat loss. This supports the idea that elevated SPA and the resultant NEAT in OR rats contributes to their obesity resistant phenotype.34
When considering the therapeutic potential of SPA, a practical consideration is the comparability of the SPA difference between OP and OR rats to that in obesity-prone versus obesity-resistant humans. Calories expended via spontaneous physical activity correlate with whole body energy expenditure in humans and in animals.14,92 However, are the extra calories mediated by SPA in an OR rat, when compared to that in humans, enough to make meaningful changes in body weight? Based on indirect calorimetry studies, we estimate the energy flux in a rat to be about 100 kcal/day; SPA differences between OP and OR rats, when corrected for lean mass, amount to about 4 kcal/day or 4%.92 In humans, a 100 kcal energy gap (that level of energy intake above the daily requirement to maintain a stable body weight) per day, or 5% for a person in balance at 2,000 kcal/day, can lead to a 10-lb weight differential over one year.135 Based on this synthesis, we conclude that it is clear that SPA differences explaining obesity resistance in rodents can be relevant for human body weight control. Further, orexin-mediated mechanisms identified here may explain the differential body weight gain response to overfeeding in humans.11 For example, a prior human study showed that gain or loss of 10–20 pounds resulted in linear changes in energy expenditure, and that the majority of the change was specifically in nonresting energy expenditure (i.e., NEAT).136 We know that physical activity is correlated with both activation of orexin neurons116,117 and increases in OXA release,121 although the pathways through which this is effected are largely undefined. Thus increased SPA (either endogenous or artificial) could also lead to feedback mechanisms that maintain higher SPA levels in the future.
As discussed elsewhere in this review, orexin enhances feeding behavior and physical activity in a site-specific manner, with some sites conveying information regarding eating behavior, others activity, yet others both or neither. As our data and others show, the energetic consequence of these two behavioral outputs, when added up on a caloric basis, may result in negative energy balance and reduce body weight. In other words, the calories taken in by the effects of the orexin signal are outweighed by those expended via physical activity.
Networks regulating spontaneous physical activity
Orexin plays a key role in an interdependent distributed brain network that regulates spontaneous physical activity. A significant number of brain sites that participate in this regulatory network in the forebrain and hindbrain have been identified, and there are a number of neurotransmitters that participate in this network, as depicted in Figure 3. The SPA network is distinct from the brain pathways that regulate purposeful activity, although the final common pathway leading to movement is clearly shared. Many of the brain sites that participate in the SPA network also participate in regulatory networks for food intake and other aspects of energy balance such as thermogenesis, but based both on distribution and on functional responses to stimulation, including orexin stimulation, it is clear that the SPA network is different from the networks that otherwise regulate energy balance.
Figure 3.
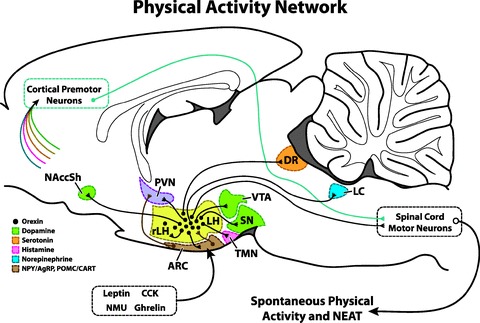
Overview of the involvement of orexin in a neural network regulating nonexercise activity thermogenesis (NEAT), indicating a sample of the brain areas, neuropeptides, and transmitters involved. Colors correspond to specific neuropeptide/hormone as follows: Black circles/projections: orexin; green, dopamine; orange, serotonin; pink, histamine; blue, norepinephrine; brown, neuropeptide Y/agouti-related protein (NPY/AgRP) and proopiomelanocortin/cocaine and amphetamine-related transcript (POMC/CART). Signals from all of these areas have the potential to influence cortical premotor neurons (indicated by arrows), and feedback from premotor neurons and orexinergic projections may interact to drive SPA. See the text for details. CCK, cholecystokinin; DR, dorsal raphe; LC, locus coeruleus; LH, lateral hypothalamus; NAccSH, shell of nucleus accumbens; NMU, neuromedin U; PVN, hypothalamic paraventricular nucleus; VTA, ventral tegmental area; rLH, rostral LH; SN, substantia nigra; TMN, tuberomammillary nucleus. Brain areas are not all to scale, and connections and neuropeptides/transmitters indicated are not all inclusive. Not all connections shown are discussed in this review. Figure modified from work by Kotz et al.92
Orexin is a unique contributor to this distributed SPA regulatory network by virtue of its position and projections. Orexin is made in one relatively small area of the hypothalamus, involving the caudal lateral hypothalamus and adjacent perifornical area.20,21 From these sites, orexin projects throughout the brain. Orexin neurons project throughout the hypothalamus, including the paraventricular, arcuate, rostrolateral, perifornical, and ventromedial areas, as well as to several extrahypothalamic sites, including the septal nuclei, bed nucleus of the stria terminalis, paraventricular, and reunions nuclei of the thalamus, zona incerta, subthalamic nucleus, central gray, substantia nigra, dorsal raphe nuclei, parabrachial nucleus, locus coeruleus, medullary reticular formation, area postrema, and nucleus of the solitary tract.92
The sites receiving orexin signal vary considerably with respect to the primary function associated with that site.53 Further, it is likely that in many sites the orexin effect on SPA is partially overlapping with other known orexin actions such as attention and wakefulness. While the functional outcomes of orexin action vary considerably from site to site, the production of SPA is common across many sites, and is widely distributed.92 This organization, involving a focused site of origin with wide distribution of effect, greatly enhances the potential potency of orexin as a regulator of spontaneous physical activity.
Orexin neurons receive input from a number of sites throughout the brain that are thought to influence expression of the orexin signal. The lateral hypothalamus receives afferents from cortical structures, including the prefrontal/orbitofrontal, insular, and olfactory cortices; limbic sites, including the amygdala, the hippocampal formation, and the shell of the nucleus accumbens; and from brainstem sites, including the nucleus of the solitary tract.137 Projections from other parts of hypothalamus include those from arcuate nucleus proopiomelanocortin (POMC)/cocaine and amphetamine-related transcript (CART) and NPY/agouti-related protein (AgRP) neurons.138,139 In addition, there is connectivity within the lateral hypothalamic area, notably projections from anterior to posterior portions.140 Whether all of these lateral hypothalamic projections play a significant role in regulating the activity of orexin neurons specifically is not yet determined, but a strong network of local lateral hypothalamic interneurons indicates the possibility of influences even by projections that do not directly synapse on orexin neurons themselves.140 Tracing studies have specifically identified projections to lateral hypothalamic orexin neurons from several regions of the amygdala, nucleus accumbens shell, bed nucleus of the stria terminalis, laterodorsal tegmental area, basal forebrain cholinergic neurons, GABAergic neurons in the preoptic area, and serotonergic neurons in the median/paramedian raphe nuclei.138
Orexin neurons are in a baseline intrinsic state of depolarized activity141 and are highly influenced by local conditions in an intralateral hypothalamic local network.140 The functional effects of the many afferents, and the associated neural function and neurotransmitters to orexin neurons, are underexploration. Application of the cholinergic agent carbachol activates many orexin neurons, indicating that the cholinergic input to orexin neurons from basal forebrain is excitatory.138 Cholinergic input from the laterodorsal tegmental area may also be excitatory to orexin neurons. Input of an as yet unidentified chemical type from the amygdala and bed nucleus of stria terminalis may also stimulate orexin neurons.138 CRF release from projections originating in the hypothalamic paraventricular nucleus also activates orexin neurons.142 Inhibitory influences on orexin neurons come from the preoptic area and from the serotonergic neurons in the median raphe.138
The physiological conditions that are known to affect orexin neurons include suppression of activity by glucose,143 along with prominent activation by hypoglycemia144 and by food restriction.145 There is evidence that low glucose states may be directly sensed by orexin neurons,143 although the likelihood of low glucose signals originating in other glucose sensing neurons with projections to orexin neurons is substantial. Intracellular Foxa2 signaling from the insulin receptor may be a mechanism allowing orexin neurons to sense the state of glucose and possibly short-term nutrition.146 Orexin neurons also receive local projections from leptin receptor bearing neurons, providing a means for translating the state of leptin signaling to the orexin neurons, and leptin action in lateral hypothalamus increases orexin action and decreases food intake.147 Leptin and energy state sensing arcuate neurons that express POMC/CART and NPY/AgRP also project to lateral hypothalamic neurons.138,139 A recent study has shown that amino acids, particularly nonessential amino acids, can stimulate orexin neurons directly through action on potassium channels and amino acid transporters.148 The stimulation provided by amino acids may be potent enough to overcome inhibition by glucose.148 Additional energy and nutrient related information may come to orexin neurons, perhaps directly, from the gut hormones ghrelin and glucagon-like peptide 1, both of which appear to activate orexin neurons in direct administration studies.120,149 Orexin neurons also receive input about physiological stress–related information, likely through a corticotrophin releasing hormone pathway.142 The integration of nutrition-related and other signals in orexin neurons, or in a network of which orexin is part, has not been defined.
Orexin signaling pathways can be further modified by the level of orexin-receptor expression in brain sites receiving orexin efferents. The conditions that lead to modulation of orexin receptor expression are incompletely defined. One example is the difference in orexin-receptor expression in a variety of brain sites associated with the difference between orexin-induced SPA response in polygenic OP and OR rat strains,90,99 as described elsewhere in this review.
Many brain areas contribute to SPA, and all of these areas operate in a network; thus activity in one area, affected by environmental cues or physiology as discussed above, influences firing patterns in other areas. Behavioral studies of SPA can determine the output of specific brain activity, improving understanding of the brain sites and neurotransmitter systems that are most important. The existing literature, however, is not always interpretable in a straightforward way. Locomotor activity measured in a beam-break chamber (as has been done for SPA measures) has also been used to assess nonspecific drug effects, as in studies of drugs of abuse. Similarly, low locomotor activity has been used as a diagnostic criterion for depression or illness in rats and mice.150 Thus, the data must be interpreted with care and in many cases repeated in a new context for full understanding.
Orexin A injected in almost all brain areas increases SPA, contrasting with feeding behavior that is stimulated after injection into only some of the same sites.92 The time course of action is different for the feeding and activity effects of orexin A, so the presence of one behavior (feeding or SPA) does not depend upon the other.95 Whether OXA-induced SPA is derived from orexin-enhanced wakefulness is not clear, but as discussed elsewhere in this review it appears likely that energy expenditure produced from SPA occurs after the initial waking event.
Many neurotransmitters have been shown to influence SPA in the network into which orexin action is projecting, although most of the available studies reporting on locomotor activity do not directly consider SPA itself, so at present some inferences must be made. Neurotransmitters that are likely to affect SPA include cholecystokinin, corticotrophin releasing hormone, neuromedin, NPY, leptin, and orexin.19 The current state of the evidence does not permit straightforward interpretation of the direction of effect for these neurotransmitters since there is disparate evidence based in part on site and type of administration.19 In general, it appears that in most situations each of these neurotransmitters can stimulate SPA, but orexin is the most consistent across all brain sites and types of stimulation. Little is presently known about the interactions of orexin with these other neurotransmitters with respect to the regulation of SPA.
Ultimately the output pathways for the SPA regulatory network must share engagement of the motor control pathways with voluntary movement brain mechanisms. The brain locations where these movement regulatory pathways begin to overlap have not yet been defined. It is likely that in part there are projections from forebrain structures, including the hypothalamus, to spinal motor neurons. An interesting possibility is that the SPA regulatory network engages cortical areas involved in voluntary motor control. The wide pattern of projections of the orexin neurons throughout the brain could mediate this function. In addition, a projection pathway from the accumbens through cortical premotor neurons and out to the spinal motor neurons has also been implicated.92
Conclusion
Brain mechanisms mediate SPA and NEAT (Fig. 3), and the understanding of this concept is beginning to shed light on new ways to target obesity prevention and treatment. Studies of orexin and its role in obesity resistance show that stimulation of orexin receptors may be an attractive therapy for altering the course of excess body weight gain with aging, and also demonstrate that modulating SPA and NEAT has important consequences for obesity resistance. The knowledge of this can guide human obesity therapy immediately, as the option to include more low-level activity throughout one's day is likely more feasible advice to prevent and treat obesity than standard approaches that repeatedly fail over time. The greater challenge is in how to use information on brain SPA and NEAT networks to provide better pharmaceutical and/or other therapeutic approaches for treatment of obesity. The rapid development of new neurochemical methods of altering brain neurophysiology and recent advances in computer/brain interface technologies provide confidence that knowledge of brain SPA and NEAT networks could be therapeutically applied in the near future.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 2.Must A, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, et al. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 7.Forbes GB, et al. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br. J. Nutr. 1986;56:1–9. doi: 10.1079/bjn19860080. [DOI] [PubMed] [Google Scholar]
- 8.Mustelin L, et al. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int. J. Obes. 2009;33:29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 10.Levine JA. Non-exercise activity thermogenesis (NEAT) Best Pract. Res. Clin. Endocrinol. Metab. 2002;16:679–702. doi: 10.1053/beem.2002.0227. [DOI] [PubMed] [Google Scholar]
- 11.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 12.Levine JA, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 13.Fruhbeck G. Does a NEAT difference in energy expenditure lead to obesity? Lancet. 2005;366:615–616. doi: 10.1016/S0140-6736(05)66834-1. [DOI] [PubMed] [Google Scholar]
- 14.Ravussin E. Physiology. A NEAT way to control weight? Science. 2005;307:530–531. doi: 10.1126/science.1108597. [DOI] [PubMed] [Google Scholar]
- 15.Snitker S, Tataranni PA, Ravussin E. Spontaneous physical activity in a respiratory chamber is correlated to habitual physical activity. Int. J. Obes. Relat. Metab. Disord. 2001;25:1481–1486. doi: 10.1038/sj.ijo.0801746. [DOI] [PubMed] [Google Scholar]
- 16.Kotz CM, et al. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006;142:29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Garland T, Jr, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J. Exp. Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dishman RK. Gene-physical activity interactions in the etiology of obesity: behavioral considerations. Obesity. 2008;16(Suppl 3):S60–S65. doi: 10.1038/oby.2008.520. [DOI] [PubMed] [Google Scholar]
- 19.Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87:71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 21.de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J. Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, et al. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 24.Schone C, et al. Dichotomous cellular properties of mouse orexin/hypocretin neurons. J. Physiol. 2011;589:2767–2779. doi: 10.1113/jphysiol.2011.208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammoun S, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- 26.Yang B, Ferguson AV. Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J. Neurophysiol. 2003;89:2167–2175. doi: 10.1152/jn.01088.2002. [DOI] [PubMed] [Google Scholar]
- 27.van den Pol AN, et al. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson KP, et al. Orexin-A-induced Ca2+ entry: evidence for involvement of trpc channels and protein kinase C regulation. J. Biol. Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- 29.Uramura K, et al. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2 +signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–1889. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- 30.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J. Physiol. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiwaki K, et al. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am. J. Physiol. Endocrinol. Metab. 2004;286:E551–559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 32.Kotz CM, et al. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul. Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 33.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2006;290:E396–403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- 34.Teske JA, et al. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R889–899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 35.Date Y, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mintz EM, et al. Distribution of hypocretin-(orexin) immunoreactivity in the central nervous system of Syrian hamsters (Mesocricetus auratus) J. Chem. Neuroanat. 2001;21:225–238. doi: 10.1016/s0891-0618(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 37.Moore RY, Abrahamson EA, Van Den Pol A. The hypocretin neuron system: an arousal system in the human brain. Arch. Ital. Biol. 2001;139:195–205. [PubMed] [Google Scholar]
- 38.Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav. Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espana RA, et al. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J. Comp. Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 41.Ciriello J, et al. Identification of neurons containing orexin-B (hypocretin-2) immunoreactivity in limbic structures. Brain Res. 2003;967:123–131. doi: 10.1016/s0006-8993(02)04233-6. [DOI] [PubMed] [Google Scholar]
- 42.Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- 43.Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience. 2003;122:541–550. doi: 10.1016/j.neuroscience.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Oldfield BJ, et al. Lateral hypothalamic ‘command neurons’ with axonal projections to regions involved in both feeding and thermogenesis. Eur. J. Neurosci. 2007;25:2404–2412. doi: 10.1111/j.1460-9568.2007.05429.x. [DOI] [PubMed] [Google Scholar]
- 45.Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul. Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 46.Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res. Mol. Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 47.Hervieu GJ, et al. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 48.Marcus JN, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 49.Sunter D, et al. Orexins: effects on behavior and localisation of orexin receptor 2 messenger ribonucleic acid in the rat brainstem. Brain Res. 2001;907:27–34. doi: 10.1016/s0006-8993(01)02344-7. [DOI] [PubMed] [Google Scholar]
- 50.Trivedi P, et al. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 51.Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol. Behav. 2006;88:294–301. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med. Rev. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol. Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 57.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 58.Akiyama M, et al. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur. J. Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- 59.Mieda M, et al. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc. Natl. Acad. Sci. USA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Funato H, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ammoun S, et al. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol. Endocrinol. 2006;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- 62.Ekholm ME, Johansson L, Kukkonen JP. IP3-independent signalling of OX1 orexin/hypocretin receptors to Ca2+ influx and ERK. Biochem. Biophys. Res. Commun. 2007;353:475–480. doi: 10.1016/j.bbrc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 63.Johansson L, Ekholm ME, Kukkonen JP. Regulation of OX1 orexin/hypocretin receptor-coupling to phospholipase C by Ca2 +influx. Br. J. Pharmacol. 2007;150:97–104. doi: 10.1038/sj.bjp.0706959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson L, Ekholm ME, Kukkonen JP. Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol. Life Sci. 2008;65:1948–1956. doi: 10.1007/s00018-008-8206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kukkonen JP, Akerman KE. Orexin receptors couple to Ca2+ channels different from store-operated Ca2+ channels. Neuroreport. 2001;12:2017–2020. doi: 10.1097/00001756-200107030-00046. [DOI] [PubMed] [Google Scholar]
- 66.Lund PE, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J. Biol. Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 67.Magga J, et al. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem. Pharmacol. 2006;71:827–836. doi: 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 68.Nasman J, et al. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J. Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peltonen HM, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptors. Biochem. Biophys. Res. Commun. 2009;385:408–412. doi: 10.1016/j.bbrc.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 70.Tang J, et al. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Hoang QV, et al. Effects of orexin (hypocretin) on GIRK channels. J. Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- 72.Hoang QV, et al. Orexin (hypocretin) effects on constitutively active inward rectifier K +channels in cultured nucleus basalis neurons. J. Neurophysiol. 2004;92:3183–3191. doi: 10.1152/jn.01222.2003. [DOI] [PubMed] [Google Scholar]
- 73.Mukai K, et al. Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: an in vitro study. Peptides. 2009;30:1487–1496. doi: 10.1016/j.peptides.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 74.van den Pol AN, et al. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J. Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samson WK, et al. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul. Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 76.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J. Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bayer L, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J. Neurosci. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bisetti A, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J. Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium–calcium exchanger. J. Neurosci. 2003;23:4951–4957. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohlmeier KA, Inoue T, Leonard CS. Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J. Neurophysiol. 2004;92:221–235. doi: 10.1152/jn.00076.2004. [DOI] [PubMed] [Google Scholar]
- 82.Wenzel J, et al. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1601–R1609. doi: 10.1152/ajpregu.91034.2008. [DOI] [PubMed] [Google Scholar]
- 83.Karteris E, et al. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1089–E1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- 84.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am. J. Physiol. 1989;256:R766–R771. doi: 10.1152/ajpregu.1989.256.3.R766. [DOI] [PubMed] [Google Scholar]
- 85.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R610–R618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- 86.Levin BE, et al. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am.J. Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 87.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R941–R948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 88.Clegg DJ, et al. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am. J. Physiol. Regul. Integr Comp. Physiol. 2005;288:R981–R986. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 89.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 90.Teske JA, Billington CJ, Kuskowksi MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. Int. J. Obes. (Lond) 2012;36(4):603–613. doi: 10.1038/ijo.2011.108. doi: 10.1038/ijo.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levin BE. Spontaneous motor activity during the development and maintenance of diet-induced obesity in the rat. Physiol. Behav. 1991;50:573–581. doi: 10.1016/0031-9384(91)90548-3. [DOI] [PubMed] [Google Scholar]
- 92.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 93.Teske JA, Billington CJ, Kotz CM. Hypocretin/orexin and energy expenditure. Acta Physiol. (Oxf) 2010;198:303–312. doi: 10.1111/j.1748-1716.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- 94.Sweet DC, et al. Feeding response to central orexins. Brain Res. 1999;821:535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- 95.Thorpe AJ, et al. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1409–1417. doi: 10.1152/ajpregu.00344.2002. [DOI] [PubMed] [Google Scholar]
- 96.Teske JA, Kotz CM. Effect of acute and chronic caloric restriction and metabolic glucoprivation on spontaneous physical activity in obesity-prone and obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R176–184. doi: 10.1152/ajpregu.90866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novak CM, Zhang M, Levine JA. Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity. Brain Res. 2007;1169:57–68. doi: 10.1016/j.brainres.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Novak CM, Levine JA. Daily intraparaventricular orexin—A treatment induces weight loss in rats. Obesity. 2009;17:1493–1498. doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mavanji V, et al. Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int. J. Obes. 2010;34:1576–1588. doi: 10.1038/ijo.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feder ME, et al. Locomotion in response to shifting climate zones: not so fast. Annu. Rev. Physiol. 2010;72:167–190. doi: 10.1146/annurev-physiol-021909-135804. [DOI] [PubMed] [Google Scholar]
- 101.Novak CM, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Hormo. Behav. 2010;58:355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Novak CM, et al. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PloS ONE. 2009;4:e5869. doi: 10.1371/journal.pone.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Britton SL, Koch LG. Animal genetic models for complex traits of physical capacity. Exe. Sport Sci. Rev. 2001;29:7–14. doi: 10.1097/00003677-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 104.Hagan JJ, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horvath TL, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 106.Bourgin P, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J. Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Methippara MM, et al. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11:3423–3426. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- 108.Adamantidis AR, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J. Biol. Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 110.Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- 111.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 112.Yoshida K, et al. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955:1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- 114.Zhang S, et al. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27:619–627. doi: 10.1093/sleep/27.4.619. [DOI] [PubMed] [Google Scholar]
- 115.Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 116.Estabrooke IV, et al. Fos expression in orexin neurons varies with behavioral state. J. Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 118.Tsunematsu T, et al. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J. Neurosci. 2011;31:10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ida T, et al. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- 120.Yamanaka A, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 121.Wu MF, et al. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1079–1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Overeem S, et al. Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J. Clin. Neurophysiol. 2001;18:78–105. doi: 10.1097/00004691-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu. Rev. Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kiyashchenko LI, et al. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schuld A, et al. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–1275. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 126.Middelkoop HA, et al. Circadian distribution of motor activity and immobility in narcolepsy: assessment with continuous motor activity monitoring. Psychophysiology. 1995;32:286–291. doi: 10.1111/j.1469-8986.1995.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 127.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spiegel K, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 129.Blanchong JA, et al. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J. Biol. Rhythms. 1999;14:364–377. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- 130.Schwartz MD, Smale L. Individual differences in rhythms of behavioral sleep and its neural substrates in Nile grass rats. J. Biol. Rhythms. 2005;20:526–537. doi: 10.1177/0748730405280924. [DOI] [PubMed] [Google Scholar]
- 131.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:599–605. doi: 10.1097/00075197-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 132.Widdowson EM, Edholm OG, Mc CR. The food intake and energy expenditure of cadets in training. Br. J. Nutr. 1954;8:147–155. doi: 10.1079/bjn19540023. [DOI] [PubMed] [Google Scholar]
- 133.Zurlo F, et al. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am. J. Physiol. 1992;263:E296–E300. doi: 10.1152/ajpendo.1992.263.2.E296. [DOI] [PubMed] [Google Scholar]
- 134.Tou JC, Wade CE. Determinants affecting physical activity levels in animal models. Exp. Biol. Med. 2002;227:587–600. doi: 10.1177/153537020222700806. [DOI] [PubMed] [Google Scholar]
- 135.Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am. J. Clin. Nutr. 2009;89:477–484. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- 136.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 137.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sakurai T, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 139.Kampe J, et al. An anatomic basis for the communication of hypothalamic, cortical and mesolimbic circuitry in the regulation of energy balance. Eur. J. Neurosci. 2009;30:415–430. doi: 10.1111/j.1460-9568.2009.06818.x. [DOI] [PubMed] [Google Scholar]
- 140.Burt J, et al. Local network regulation of orexin neurons in the lateral hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R572–R580. doi: 10.1152/ajpregu.00674.2010. [DOI] [PubMed] [Google Scholar]
- 141.Eggermann E, et al. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J. Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Winsky-Sommerer R, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williams RH, et al. Adaptive sugar sensors in hypothalamic feeding circuits. Proc. Natl. Acad. Sci. USA. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moriguchi T, et al. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci. Lett. 1999;264:101–104. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- 145.Cai XJ, et al. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- 146.Silva JP, et al. Regulation of adaptive behaviour during fasting by hypothalamic Foxa2. Nature. 2009;462:646–650. doi: 10.1038/nature08589. [DOI] [PubMed] [Google Scholar]
- 147.Louis GW, et al. Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J. Neurosci. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karnani MM, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 149.Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J. Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Malisch JL, et al. Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav. Genet. 2009;39:192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]


