Abstract
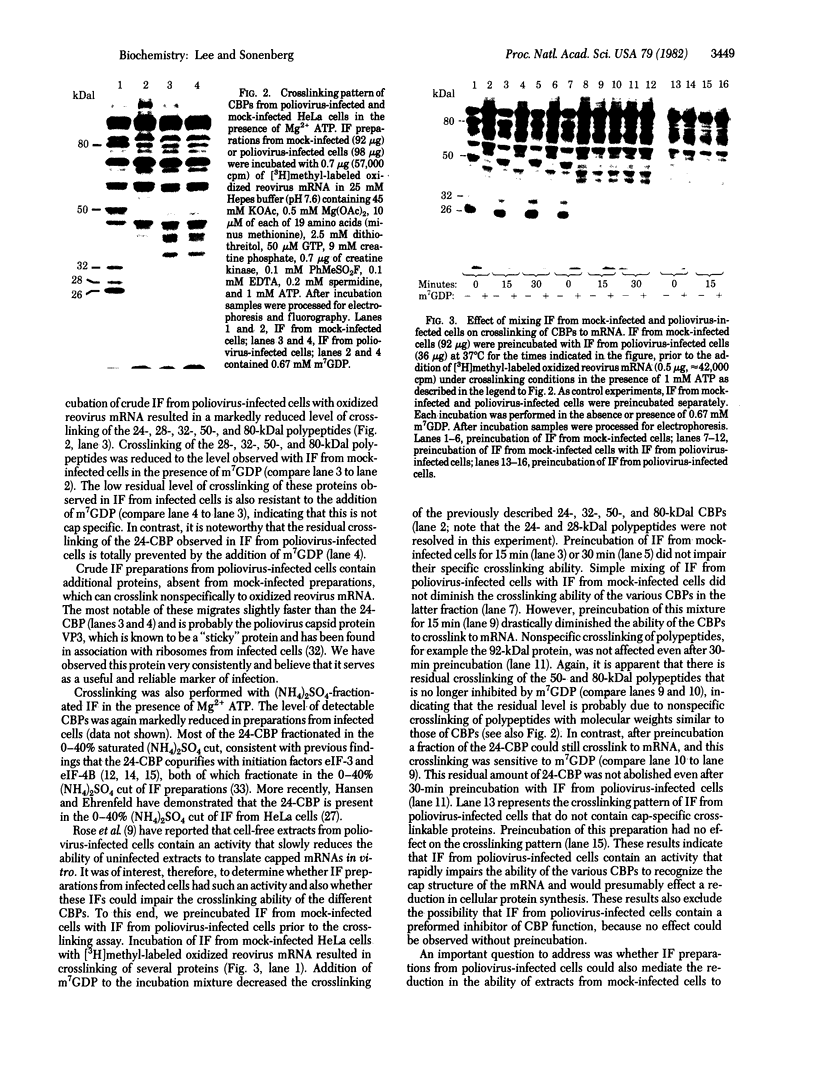
Infection of HeLa cells with poliovirus results in a rapid shut-off of host protein synthesis. It has been suggested that inactivation of a protein that binds to the cap structure of cellular mRNAs would explain the selective inhibition of host protein synthesis because the naturally uncapped poliovirus RNA can be translated by a cap-independent mechanism. To test directly for the presence of cap-binding proteins in poliovirus-infected and mock-infected cells, we analyzed initiation factor preparations for their ability to specifically crosslink to the 5' cap structure of oxidized reovirus mRNA. The data presented here show that the crosslinking ability of the different cap-binding proteins (24-, 28-, 32-, 50-, and 80-kilodalton polypeptides) is reduced in preparations from poliovirus-infected as compared to mock-infected cells. This reduction correlates with the inability of initiation factor preparations from infected cells to restore translation of capped mRNAs in extracts of poliovirus-infected cells. In addition, initiation factor preparations from poliovirus-infected cells have the ability to rapidly inactivate cap-binding proteins and can also impair the restoring activity of initiation factors from mock-infected cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Trachsel H., Sonenberg N., Shatkin A. J., Lodish H. F. Characterization of rabbit reticulocyte factor(s) that stimulates the translation of mRNAs lacking 5'-terminal 7-methylguanosine. J Biol Chem. 1979 Mar 10;254(5):1440–1443. [PubMed] [Google Scholar]
- Bonatti S., Sonenberg N., Shatkin A. J., Cancedda R. Restricted initiation of protein synthesis on the potentially polycistronic Sindbis virus 42 S RNA. J Biol Chem. 1980 Dec 10;255(23):11473–11477. [PubMed] [Google Scholar]
- Brown B. A., Ehrenfeld E. Initiation factor preparations from poliovirus-infected cells restrict translation in reticulocyte lysates. Virology. 1980 Jun;103(2):327–339. doi: 10.1016/0042-6822(80)90191-9. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Shatkin A. J. Ribosome-protected fragments from sindbis 42-S and 26-S RNAs. Eur J Biochem. 1979 Feb 15;94(1):41–50. doi: 10.1111/j.1432-1033.1979.tb12869.x. [DOI] [PubMed] [Google Scholar]
- Detjen B. M., Jen G., Thach R. E. Encephalomyocarditis viral RNA can be translated under conditions of poliovirus-induced translation shutoff in vivo. J Virol. 1981 May;38(2):777–781. doi: 10.1128/jvi.38.2.777-781.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Virus-induced interference in heterologously infected HeLa cells. J Virol. 1972 Jan;9(1):22–28. doi: 10.1128/jvi.9.1.22-28.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Lund H. Untranslated vesicular stomatitis virus messenger RNA after poliovirus infection. Virology. 1977 Jul 15;80(2):297–308. doi: 10.1016/s0042-6822(77)80006-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Darnell J. E. Structural difference between the 5' termini of viral and cellular mRNA in poliovirus-infected cells: possible basis for the inhibition of host protein synthesis. J Virol. 1976 May;18(2):719–726. doi: 10.1128/jvi.18.2.719-726.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby D., Eaton M., Fellner P. Absence of 5' terminal capping in encephalomyocarditis virus RNA. Nucleic Acids Res. 1976 Oct;3(10):2771–2787. doi: 10.1093/nar/3.10.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Ehrenfeld E. Presence of the cap-binding protein in initiation factor preparations from poliovirus-infected HeLa cells. J Virol. 1981 May;38(2):438–445. doi: 10.1128/jvi.38.2.438-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Control of protein synthesis in extracts from poliovirus-infected cells. I. mRNA discrimination by crude initiation factors. J Virol. 1978 May;26(2):510–521. doi: 10.1128/jvi.26.2.510-521.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen G., Detjen B. M., Thach R. E. Shutoff of HeLa cell protein synthesis by encephalomyocarditis virus and poliovirus: a comparative study. J Virol. 1980 Jul;35(1):150–156. doi: 10.1128/jvi.35.1.150-156.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Goldstein E., Penman S. Poliovirus-induced inhibition of polypeptide initiation in vitro on native polyribosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1834–1838. doi: 10.1073/pnas.73.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M. S., Fraenkel-Conrat H. Translation of tobacco necrosis virus and its satellite in a cell-free wheat germ system. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2288–2292. doi: 10.1073/pnas.74.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. ATP/Mg++-dependent cross-linking of cap binding proteins to the 5' end of eukaryotic mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1643–1656. doi: 10.1093/nar/9.7.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Guertin D., Cleveland D., Trachsel H. Probing the function of the eucaryotic 5' cap structure by using a monoclonal antibody directed against cap-binding proteins. Cell. 1981 Dec;27(3 Pt 2):563–572. doi: 10.1016/0092-8674(81)90398-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. J., Cooper P. D. Poliovirus proteins associated with ribosomal structures in infected cells. Virology. 1974 May;59(1):1–20. doi: 10.1016/0042-6822(74)90201-3. [DOI] [PubMed] [Google Scholar]









