Abstract
Aims/hypothesis
Type 1 diabetes is the most frequent endocrine disease in children, with 65,000 children diagnosed worldwide every year. Up to 80% of these children present with diabetic ketoacidosis (DKA), which is associated with both short-term risks and long-term consequences. This study aimed to characterise the worldwide variation in presentation of type 1 diabetes to inform future interventions to reduce this excess morbidity and mortality.
Methods
This was a systematic review of studies indexed on PubMed, EMBASE, Web of Science, Scopus or CINAHL before March 2011 that included unselected groups of children presenting with new-onset type 1 diabetes, reported the proportion presenting with DKA and used a definition of DKA based on measurement of pH or bicarbonate.
Results
Sixty-five studies of cohorts comprising over 29,000 children in 31 countries were included. The frequency of DKA at diagnosis ranged from 12.8% to 80%, with highest frequencies in the United Arab Emirates, Saudi Arabia and Romania, and the lowest in Sweden, the Slovak Republic and Canada. Multivariable modelling showed the frequency of DKA was inversely associated with gross domestic product, latitude and background incidence of type 1 diabetes.
Conclusions/interpretation
This is the first description of the variation in frequency of DKA at presentation of type 1 diabetes in children across countries. It demonstrates large variations that may, at least in part, be explained by different levels of disease awareness and healthcare provision and suggests ways to decrease the excess morbidity and mortality associated with DKA at diagnosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-012-2690-2) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Children, Diabetic ketoacidosis, Diagnosis, Epidemiology, Systematic review, Type 1 diabetes, Worldwide
Introduction
Diabetic ketoacidosis (DKA) is a metabolic derangement characterised by the triad of hyperglycaemia, acidosis and ketosis that occurs in the presence of very low levels of effective insulin action. It is the leading cause of mortality in children with type 1 diabetes [1, 2] and is associated with increased morbidity and healthcare expenditure: DKA at diagnosis is associated with a lower frequency of partial remission [3, 4] and less residual beta cell function [5, 6], and the mean excess medical expenditure for one episode of DKA is over US$3,500 [7]. The psychological effect of DKA at onset of type 1 diabetes has not been studied, but children later hospitalised with DKA have a higher number of psychiatric disorders, lower self-esteem and social competence and worse relationships with their parents [8].
Worldwide, approximately 65,000 children aged under 15 years develop type 1 diabetes each year [9] and up to 80% present with DKA. The worldwide variation in the incidence of type 1 diabetes in children has been well characterised by the WHO’s Diabetes Mondiale (DiaMond) project [9, 10], using standardised incidence data from 57 countries from 1990 onwards. Annual age-adjusted incidence varies over 350-fold, ranging from 0.1/100,000 in China to 40.9/100,000 in Finland [9]. However, there is less evidence concerning the frequency of DKA at diagnosis. Data from 24 centres in Europe collected as part of the EURODIAB [11] project reported DKA frequency at diagnosis that varied from 11% to 67%, and an inverse correlation between background incidence of type 1 diabetes and frequency of DKA in 11 of the 24 centres. The variation in frequency of DKA at diagnosis of type 1 diabetes in children worldwide, however, remains largely uncharacterised.
Characterising this variation is an important step towards identifying possible explanations for the differences and potentially informing the design of interventions to decrease rates of DKA. We have previously shown that, at an individual level, younger age, diagnostic error, ethnic minority status, lack of health insurance, lower body mass index, preceding infection, and delayed treatment are all associated with an increased risk of DKA, while having a first-degree relative with type 1 diabetes at the time of diagnosis and higher parental education appear to be protective [12]. We therefore aimed to systematically review the effects of country characteristics on the frequency of DKA to provide the first comprehensive synthesis of worldwide data on the frequency of DKA and explore possible reasons for the variation.
Methods
Search strategy
The search strategy has been previously described [12]. We searched five electronic bibliographic databases (PubMed, EMBASE, Web of Science, Scopus and CINAHL) from inception to March 2011 using a combination of subject headings and free text incorporating ‘diabetic ketoacidosis’, ‘diabetes and ketoacidosis’ and ‘diagnosis’ and limited to infants, children or adolescents (see electronic supplementary material [ESM] Methods for complete search strategy). We also manually screened the reference lists of all included papers.
Study selection
Included studies fulfilled all of the following criteria: (1) published as a primary research paper in a peer-reviewed journal; (2) included cohorts of children and young people presenting with new-onset type 1 diabetes who had not been selected based on other characteristics; (3) reported the proportion of children presenting with DKA; and (4) included a measurement of either pH or bicarbonate in the definition of DKA. We chose to include studies that defined DKA based on measurement of either pH or bicarbonate as we wanted the search strategy to be broad enough to include studies from different international settings but rigorous enough to ensure that we were consistently identifying those children with objective evidence of metabolic derangement. We excluded studies of only highly selected groups, for example neonates, children being treated with high-dose steroids or receiving chemotherapy, as well as drug trials and conference proceedings. No restrictions were made for language, sample size or period of study, and we included all studies that included children and young people up to age 21.
One reviewer (JUS) performed the search and screened the titles and abstracts to exclude papers that were clearly not relevant. Full-text articles were reviewed by at least two reviewers (JUS and FMW/MT) to assess eligibility, and consensus was used to resolve any disagreement between researchers. When either the definition of DKA was not given or we were unable to adequately interpret the data presented, we contacted authors for clarification. We also contacted the authors if papers reported collective data from multiple centres in order to obtain disaggregated data.
Quality assessment
We applied the Critical Appraisal Skills Programme guidelines for case–control and cohort studies as a framework for quality assessment and excluded studies with major limitations in methods or reporting [13]. Data concerning study size, period of study, design (prospective or retrospective) and method of case identification were extracted and used as markers of quality for analysis.
Data extraction and synthesis
Characteristics of included studies were extracted independently by at least two researchers (JUS and FMW/MT) using a standardised form. These included markers of quality as well as the country or region of the study, eligible age range and definition of DKA. For studies that recruited children over more than one time period, we used data only from the most recent period, where possible. If this was not possible, data from all time periods were combined.
Analysis
Multivariable regression analysis was performed using R (version 2.15.1) with frequency of DKA as the dependent variable and four features of the country where the study was performed (background incidence of type 1 diabetes, gross domestic product [GDP], expenditure on healthcare as a percentage of GDP, and latitude) as independent variables. In addition, we included four study-level characteristics (study size, period of study, design and method of case identification) in the regression analysis. The inclusion of study size provided a weighting for each study and adjustment for potential confounding by the number of children included in each study. The period of study, design and method of case identification in turn allowed adjustment for changes in the background incidence and diagnosis of type 1 diabetes over time, the possibility of recording and recall bias in retrospective studies and the rigour of case identification. At the country level, the background incidence of type 1 diabetes was included as it has been shown to be related to the frequency of DKA. GDP and expenditure on healthcare as a percentage of GDP were included to explore the effects of economic and healthcare factors on the frequency of DKA, and latitude because the incidence of type 1 diabetes has been reported to vary with latitude [14, 15]. Other potential confounding factors identified previously, such as age, ethnicity and family history of type 1 diabetes could not be included as few studies reported on these individual characteristics of recruited children.
We defined study size as the total number of children with type 1 diabetes included in the study. The study period was converted to a numerical scale using the mid-year of each study and numbering each year upwards from zero for the earliest study. Study design and method of case identification were dichotomised as follows: design as prospective or retrospective; and method of case identification as internal hospital or clinic records (children recruited by examining hospital or clinic records or by requesting local healthcare workers to examine records to provide a list) or external register, database or surveillance cohort (children identified via externally held registers or databases or through established surveillance cohorts). In the last group, the external registers and databases included registers for drug reimbursement, registration for exemption from payment for medication, national drug registries and systems for insulin delivery. This distinction was selected as we felt studies that relied solely on internal hospital or clinic records were at greater risk of inclusion bias.
The background incidence of type 1 diabetes was obtained from the included studies or those performed by the same research group that reported rates for the same population. When this was not possible, a literature search was performed by first reviewing the reference list of the study and then searching PubMed for studies reporting DKA incidence in the city, region or country. Where possible, incidence data from the same city or region were used, but where such data were not available, data for the whole country or the closest region in the country for which data were available were used. Data were not available for two countries (Turkey and the United Arab Emirates), so values from neighbouring countries (Jordan and Oman) were used, as in the International Diabetes Federation Diabetes Atlas [16]. To allow for changes in the background incidence of type 1 diabetes over time, values were only used when the period of collection of the incidence data included the mid-year of the study period. If values were available for individual years, the incidence in the mid-year of the study period was used.
The latitude of the location of each study was obtained from the online World Atlas (www.worldatlas.com, accessed 23 June 2012) and the modulus of the latitude used for analysis. When studies were nationwide or included multiple centres within a country, the latitude of the mid-point of the country was used.
We obtained the GDP for each country for the mid-year of each study from the International Monetary Fund World Economic Outlook Database (www.imf.org/external/pubs/ft/weo/2011/01/weodata/index.aspx, accessed 30 Oct 2011). For two countries (the Slovak Republic and Slovenia) values were not available for the mid-year of the study (1991) so values from 1993 and 1992, respectively, were used which were still within the period of study.
The expenditure on healthcare as a percentage of GDP was obtained for the mid-year of each study using the WHO Global Health Observatory Data Repository (www.who.int/gho, accessed 23 June 2012) for studies after 1995, and the Organisation for Economic Co-operation and Development Health Data (http://stats.oecd.org, accessed 23 June 2012) for those before 1995 (when WHO data were not available). Data were not available for the mid-year of the study for ten studies, so values from 1995 were used, which were still within 2 years of the study period. Data were not available for any years within 2 years of the period of study for one study (Table 2).
Table 2.
Frequency of DKA at diagnosis of type 1 diabetes along with characteristics of the country of each study
| Author | Country | DKA (%) | Latitudea | GDP (PPP) per capita (US$)b | Expenditure on healthcare (% of GDP)c | Annual incidence of T1DM (cases/100,000) |
|---|---|---|---|---|---|---|
| Abduljabbar et al, 2010 [35] | Saudi Arabia | 40 | 26.17 | 16,784.47 | 3.34 | 27.52 [35] |
| Abdul-Rasoul et al, 2010 [18] | Kuwait | 37.7 | 29.3375 | 35,631.59 | 3.23 | 22.3 [86] |
| Al Khawari et al, 1997 [36] | Kuwait | 49 | 29.3375 | 36,723.92 | 3.76 | 15.4 [87] |
| Al Magamsi et al, 2004 [37] | Saudi Arabia | 55.2 | 24.27 | 16,227.01 | 2.96 | 18.05 [35] |
| Alvi et al, 2001 [38] | UK | 27 | 52.29 | 17,082.07 | 6.3 | 17.7 [9] |
| Barák et al, 2006 [39] | Slovak Republic | 15 | 48.8 | 13,566.39 | 5.82 | 13.6 [88] |
| Blanc et al, 2003 [40] | France | 54 | 48.51 | Data not available | Data not available | 8.5 [10] |
| Böber et al, 2001 [41] | Turkey | 29 | 38.25 | 6,226.56 | 2.7 | 3.2 [89] |
| Bowden et al, 2008 [3] | USA | 32.9 | 40.25 | 40,450.62 | 15.71 | 23.9 [90] |
| Bui et al, 2010 [42] | Canada | 18.6 | 51.15 | 24,534.2 | 8.79 | 29.7 [42] |
| Campbell-Stokes et al, 2005 [43] | New Zealand | 29 | 41 | 18,636.32 | 7.53 | 17.9 [43] |
| Charemska et al, 2003 [44] | Poland | 38 | 53.46 | 10,305.36 | 5.52 | 13 [86] |
| Charron-Prochownik et al, 1995 [45] | USA | 30 | 40.26 | 13,599.99 | 9.37 | 14.6 [91] |
| Fernández Castañer et al, 1996 [5] | Spain | 44 | 41.23 | 12,121.95 | 6 | 10.6 [92] |
| Habib, 2005 [46] | Saudi Arabia | 55.3 | 24.27 | 16,784.47 | 3.34 | 18.05 [35] |
| Hanas et al, 2007 [47] | Sweden | 16 | 62 | 28,443.74 | 9.23 | 44.2 [93] |
| Hekkala et al, 2007 [48] | Finland | 22.4 | 65 | 16,283.62 | 8.8 | 36.5 [48] |
| Hekkala et al, 2010 [49] | Finland | 19.4 | 64 | 27,358.8 | 8.15 | 54 [94] |
| Hodgson et al, 2006 [50] | Chile | 37 | 33.28 | 7,594.38 | 6.71 | 4.02 [95] |
| Jackson et al, 2001 [51] | New Zealand | 41.7 | 36.5 | 16,494.09 | 7.07 | 13.7 [9] |
| Kapellen et al, 2001 [52] | Germany | 29.8 | 51.2 | 23,454.27 | 10.27 | 15.4 [9] |
| Karjalainen et al, 1989 [53] | Finland | 24.4 | 65 | 11,673.04 | 6.7 | 34.1 [96] |
| Kulaylat et al, 2001 [54] | Saudi Arabia | 77 | 22.17 | 15,587.46 | 2.96 | 12.3 [97] |
| Lévy-Marchal et al, 2001 (E1)d [11] | Iceland | 30 | 65 | 19,234.79 | 8 | 13.9 [11] |
| Lévy-Marchal et al, 2001 (H1)d [11] | The Netherlands | 28.6 | 52.3 | 20,073.13 | 8.2 | 12.5 [11] |
| Lévy-Marchal et al, 2001 (K1)d [11] | Lithuania | 41.4 | 56 | 7,125.21 | 5.37 | 7.6 [11] |
| Lévy-Marchal et al, 2001 (M1)d [11] | Germany | 25.6 | 51.13 | 19,501.64 | 9.9 | 13.2 [11] |
| Lévy-Marchal et al, 2001 (R1)d [11] | Romania | 67 | 44.26 | 5,034.02 | 3.49 | 4.8 [11] |
| Lévy-Marchal et al, 2001 (W1)d [11] | Poland | 54.2 | 52 | 5,609.77 | 6 | 7 [11] |
| Lévy-Marchal et al, 2001 (Y1)d [11] | Slovenia | 28.6 | 46.07 | 10,757.33 | 7.45 | 8.5 [11] |
| Lévy-Marchal et al, 2001 (Z1)d [11] | Slovak Republic | 35.6 | 48.6667 | 7,448.81 | 6.06 | 9.2 [11] |
| Mallare et al, 2003 [55] | USA | 38 | 40.42 | 29,076.55 | 13.5 | 16.1 [86] |
| Maniatis et al, 2005 [56] | USA | 28.4 | 39.44 | 36,949.99 | 14.82 | 23.9 [90] |
| Mayer-Davies et al, 2009 [57] | USA | 25.2 | 38 | 38,324.38 | 15.67 | 18.3 [98] |
| Mylnarski et al, 2003 [58] | Poland | 54.7 | 51.45 | 9,623.8 | 5.73 | 13 [86] |
| Neu et al, 2003 [59] | Germany | 26.3 | 48.39 | 20,282.77 | 9.6 | 12.5 [59] |
| Newfield et al, 2009 [60] | USA | 27.2 | 32.42 | 33,501.68 | 13.35 | 16.1 [86] |
| Olak-Białoń et al, 2007 [61] | Poland | 33 | 50.15 | 12,700.47 | 6.2 | 17.7 [99] |
| Pawlowicz et al, 2009 [62] | Poland | 32.9 | 54.17 | 11,058.57 | 6.34 | 13.09 [100] |
| Pinero-Martinez et al, 1995 [63] | Spain | 61.81 | 40.25 | 11,154.8 | 5.4 | 11.3 [101] |
| Pinkney et al, 1994 [64] | UK | 26 | 51.45 | 16,789.25 | 5.9 | 17.7 [9] |
| Pocecco and Nassimbeni 1993 [65] | Italy | 41.1 | 46.13 | 15,100.95 | 7.3 | 9.8 [65] |
| Prisco et al, 2006 [66] | Italy | 32.2 | 42.8333 | 26,419.73 | 8.31 | 14.78 [102] |
| Pronina et al, 2008 [67] | Russia | 30 | 55.44 | 7,737.08 | 5.42 | 12.9 [67] |
| Punnose et al, 2002 [68] | United Arab Emirates | 80 | 24.12 | 24,076.5 | 2.64 | 2.62 [103] |
| Quinn et al, 2006 [69] | USA | 43.7 | 42.21 | 26,906.53 | 13.6 | 16.1 [86] |
| Rewers et al, 2008 [70] | USA and Hawaii | 30 | 38 | 38,324.38 | 15.67 | 18.3 [98] |
| Roche et al, 2005 [71] | Ireland | 25 | 53 | 21,675.15 | 6.26 | 16.3 [104] |
| Rosenbauer et al, 2002 [72] | Germany | 53.8 | 51.25 | 21,320.48 | 9.8 | 14.3 [72] |
| Sadaskaite-Kuehne et al, 2002 [17] | Sweden | 14.5 | 55.59 | 22,282.05 | 8.03 | 31.7 [105] |
| Sadaskaite-Kuehne et al, 2002 [17] | Lithuania | 34.6 | 56 | 7,887.59 | 6.08 | 8 [105] |
| Salman, 1991 [73] | Saudi Arabia | 67.3 | 24.42 | 12,826.07 | Data not available | 3.8 [97] |
| Samuelsson et al, 2005 [28] | Sweden | 12.8 | 55.59 | 17,498.73 | 8.2 | 28.9 [105] |
| Savova et al, 1996 [74] | Bulgaria | 35.3 | 42.41 | 5,675.1 | 5.23 | 6.99 [106] |
| Schober et al, 2010 [75] | Austria | 37.2 | 47.333 | 25,958.99 | 9.93 | 10.3 [107] |
| Sebastiani Annicchiarico and Guglielmi, 1992 [76] | Italy | 35.65 | 41.39 | 16,190.78 | 7.3 | 6.97 [76] |
| Smith et al, 1998 [77] | UK | 27 | 53.28 | 18,210.23 | 6.8 | 17.7 [9] |
| Soliman et al, 1997 [78] | Oman | 41.7 | 21.3 | 12,177.94 | 3.64 | 2.45 [78] |
| Soltész et al, 1997 [79] | Hungary | 23 | 47 | 8,578.35 | 8.1 | 9.1 [10] |
| Sundaram et al, 2009 [80] | UK | 27.2 | 52.29 | 32,083.71 | 8.24 | 26.3 [108] |
| Tahirovic et al, 2007 [81] | Bosnia and Herzegovina | 48 | 44.31 | 2,817.00 | 7.9 | 7.1 [109] |
| Ting et al, 2007 [82] | Taiwan | 65 | 25.5 | 11,886.09 | 3.54 | 3.75 [110] |
| Vehik et al, 2009 [83] | USA | 27 | 39.33 | 38,324.38 | 15.67 | 23.9 [90] |
| Veijola et al, 1996 [84] | Finland | 21.7 | 64 | 13,753.51 | 7.3 | 35.3 [111] |
| Xin et al, 2010 [85] | China | 41.9 | 41.48 | 4,748.66 | 4.55 | Data not available |
aLatitude of the location of each study from the online World Atlas
bGDP for the mid-year of each study from the International Monetary Fund World Economic Outlook Database
cExpenditure on healthcare as a percentage of GDP for the mid-year of each study from the WHO Global Health Observatory Data Repository for studies when the mid-year was after 1995 and the Organisation for Economic Co-operation and Development Health Data for those before 1995
dCodes refer to abbreviations used for specific datasets in [11]
T1DM, type 1 diabetes mellitus
Generalised linear modelling for proportion data and standard linear modelling showed significant overdispersion and heteroscedasticity, respectively. Box–Cox analysis suggested a log transformation and this was found to model the data well. We therefore used multivariable linear regression of log transformed DKA frequencies against the explanatory variables. Stepwise removal of non-statistically significant variables with ANOVA testing at each step showed that removal of any of the variables did not affect the others.
Results
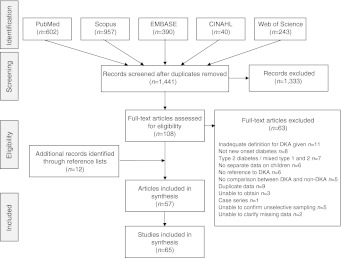
After duplicates were removed, the search identified 1,441 papers, of which 1,333 were excluded as clearly irrelevant and a further 63 after full-text assessment. There was complete agreement between researchers throughout this process. The most common reasons for exclusion were failure to use a definition of DKA that included either pH or bicarbonate, duplication of data or no data for children with new-onset diabetes (Fig. 1). Two studies were excluded based on quality alone. The first because we were unable to adequately interpret the numerical data after contacting the author, and the second because of a large amount of missing data. A further 12 papers were identified through citation searching. One paper compared incidence of type 1 diabetes at presentation between south-east Sweden and Lithuania and so is reported as two studies [17]. Another [11] reported the mean frequency of DKA across 24 centres in Europe. After contacting the authors we were able to obtain data for 11 of these centres individually. Results from three of these centres had been reported in other included papers in greater detail, leaving eight studies for inclusion from that paper. This review is therefore based on 65 studies.
Fig. 1.
PRISMA (www.prisma-statement.org) flow diagram
The 65 included studies provided data on over 29,000 children (range 10 to 3,947) from 31 countries across five continents (two from Asia, 21 from Europe, three from the Middle East, two from North America, two from Oceania, and one from South America) (Table 1). Notably, many large countries, such as South American countries, India, China and Japan, are not represented and there are no studies for the whole continent of Africa. All included studies were cohort studies. Most recruited children retrospectively from hospital or clinic records, with study lengths ranging from 1 to 17 years, and the periods of study covering a 30 year period from 1978 to 2008. Most included children up to age 15 (n = 29), 16 (n = 5) or 18 years (n = 13), with eight studies including young people between 18 and 21 years and one only those under 6 years. There was a wide range of definitions of DKA, all of which included either pH ≤7.2 to <7.36 or bicarbonate <15 to ≤21 mmol/l.
Table 1.
Characteristics of included studies
| Author | Country | Study size | Eligible age range (years) | Period of study | Design | Number of centres | Method(s) of case identification | Definition(s) of DKA | Ascertainment (%)a |
|---|---|---|---|---|---|---|---|---|---|
| Abduljabbar et al, 2010 [35] | Saudi Arabia | 438 | 0 to <15 | 1990–2007 | R | 1 medical services organisation for oil company | (1) Hospital paediatric diabetes registry; (2) registry of children admitted with diabetes | pH <7.3 and/or HCO3 <15 mmol/l | 100 |
| Abdul-Rasoul et al, 2010 [18] | Kuwait | 677 | 0 to <12 | 2000–2006 | R | Nationwide | Hospital records | pH <7.3 and/or HCO3 <15 mmol/l with ketonuria and glucose > 11 mmol/l | 93.9 |
| Al Khawari et al, 1997 [36] | Kuwait | 243 | 0 to <15 | 1992–1995 | P | Nationwide | (1) Kuwait IDDM register; (2) hospital records; (3) diabetic clinic mandatory registry | pH <7.3 and/or HCO3 <18 mmol/l and hyperglycaemia and ketonuria | 92 |
| Al Magamsi et al, 2004 [37] | Saudi Arabia | 230 | 0 to <15 | 1992–2001 | R | 1 maternity and children’s hospital | Hospital records | Glucose >14 mmol/l and pH <7.3 or bicarbonate <15 mmol/l in the presence of ketonuria | |
| Alvi et al, 2001 [38] | UK | 328 | 0 to 15 | 1987–1996 | R | Regional | (1) Local paediatricians; (2) general practitioners and diabetes nurse specialists | pH ≤7.25 or HCO3 ≤15 mmol/l in the presence of hyperglycaemia and ketonuria | |
| Barák et al, 2006 [39] | Slovak Republic | 323 | Not given | 2002–2005 | R | 1 diabetology centre and 1 children's hospital | Hospital and clinic records | pH <7.3, HCO3 <15 mmol/l, glucose >13.9 mmol/l and ketonuria | |
| Blanc et al, 2003 [40] | France | 72 | 0 to <18 | Not given | P | 1 endocrinology and diabetes department | Hospital records | pH <7.35 | |
| Böber et al, 2001 [41] | Turkey | 62 | 0 to <18 | 1991–1998 | R | 1 paediatric endocrinology department | Hospital records | pH <7.3 and HCO3 <15 mmol/l | |
| Bowden et al, 2008 [3] | USA | 152 | 0 to ? | 2004 | R | 1 children’s hospital | Hospital records | HCO3 <15 mmol/l and ketonuria and hyperglycaemia | |
| Bui et al, 2010 [42] | Canada | 3,947 | 0 to <18 | 1994–2000 | R | Regional | (1) Health insurance plan; (2) database of health and long-term care; (3) discharge abstract database | ICD-9-CM diagnostic codes 250.1–250.3 | |
| Campbell-Stokes et al, 2005 [43] | New Zealand | 298 | 0 to 15 | 1999–2000 | R | Regional | (1) New Zealand Paediatric Surveillance Unit; (2) paediatricians; (3) hospital discharges from New Zealand Health Information Service | pH <7.3 | 95.2 |
| Charemska et al, 2003 [44] | Poland | 158 | 0 to <19 | 1998–2002 | R | 1 children’s hospital | Clinic records | pH ≤7.3 and HCO3 ≤18 mmol/l | |
| Charron-Prochownik et al, 1995 [45] | USA | 89 | 0 to <14 | 1978–1985 | P | 1 endocrinology unit | Unit records | pH ≤7.3 | 68.7 |
| Fernández Castañer et al, 1996 [5] | Spain | 50 | 0 to 18 | 1986–1991 | R | 1 endocrinology unit | Unit records | HCO3 <15 mmol/l | |
| Habib, 2005 [46] | Saudi Arabia | 311 | 0 to 15 | 1992–2004 | R | 1 maternity and children’s hospital | Hospital records | Glucose >14 mmol/l and pH <7.3 or HCO3 <15 mmol/l in presence of ketonuria | |
| Hanas et al, 2007 [47] | Sweden | 149 | 0 to <18 | 2000–2004 | P | Nationwide | National Paediatric Diabetes Registry | pH <7.3 | |
| Hekkala et al, 2007 [48] | Finland | 585 | 0 to <15 | 1982–2001 | R | 1 paediatric department | Hospital and clinic register | pH <7.3 and/or HCO3 <15 mmol/l | |
| Hekkala et al, 2010 [49] | Finland | 1616 | 0 to <15 | 2002–2005 | R | 27 centres | (1) Paediatric diabetes register; (2) hospital records | pH <7.3 | 97.6 |
| Hodgson et al, 2006 [50] | Chile | 97 | 0 to <17 | 1988–2003 | R | 1 hospital | Hospital records | pH <7.3, HCO3 <15 mmol/l and ketonaemia | |
| Jackson et al, 2001 [51] | New Zealand | 70 | 0 to 15 | 1995–1996 | R | 1 children’s hospital | (1) Hospital records; (2) regional diabetes database; (3) laboratory staff | pH <7.35 | |
| Kapellen et al, 2001 [52] | Germany | 104 | 0 to <18 | 1995–1999 | R | 1 children’s hospital | Hospital records | pH <7.3, glucose >250 mg/dl (> 13.9 mmol/l) and HCO3 <15 mmol/l | |
| Karjalainen et al, 1989 [53] | Finland | 82 | 0 to <19 | 1983–1986 | R | 1 hospital | Hospital records | pH <7.35 | 90.1 |
| Kulaylat et al, 2001 [54] | Saudi Arabia | 46 | 0 to 15 | 1986–1997 | R | 1 hospital | Hospital records | pH <7.35 or tCO2 <21 mmol/l | |
| Lévy-Marchal et al, 2001 (E1)b [11] | Iceland | 10 | 0 to <15 | 1989–1994 | P | Nationwide | Incidence surveillance cohort | pH <7.3 | 100 |
| Lévy-Marchal et al, 2001 (H1)b [11] | The Netherlands | 49 | 0 to <15 | 1989–1994 | P | 5 regions | Incidence surveillance cohort | pH <7.3 | 80.3 |
| Lévy-Marchal et al, 2001 (K1)b [11] | Lithuania | 58 | 0 to <15 | 1989–1994 | P | Nationwide | Incidence surveillance cohort | pH <7.3 | 93.5 |
| Lévy-Marchal et al, 2001 (M1)b [11] | Germany | 43 | 0 to <15 | 1989–1994 | P | Regional | Incidence surveillance cohort | pH <7.3 | 91.5 |
| Lévy-Marchal et al, 2001 (R1)b [11] | Romania | 21 | 0 to <15 | 1989–1994 | P | Regional | Incidence surveillance cohort | pH <7.3 | 95.5 |
| Lévy-Marchal et al, 2001 (W1)b [11] | Poland | 59 | 0 to <15 | 1989–1994 | P | 8 regions | Incidence surveillance cohort | pH <7.3 | 62.8 |
| Lévy-Marchal et al, 2001 (Y1)b [11] | Slovenia | 21 | 0 to <15 | 1989–1994 | P | Nationwide | Incidence surveillance cohort | pH <7.3 | 100 |
| Lévy-Marchal et al, 2001 (Z1)b [11] | Slovak Republic | 104 | 0 to <15 | 1989–1994 | P | Nationwide | Incidence surveillance cohort | pH <7.3 | 86.7 |
| Mallare et al, 2003 [55] | USA | 139 | 0 to <19 | 1995–1998 | R | 1 children’s hospital | Hospital records | pH <7.3 | 81.3 |
| Maniatis et al, 2005 [56] | USA | 359 | 0 to <18 | 2002–2003 | R | 1 diabetes centre | Diabetes centre records | pH <7.3 and HCO3 <15 mmol/l | 93.7 |
| Mayer-Davies et al, 2009 [57] | USA | 436 | 0 to <20 | 2002–2005 | P | 6 clinical centres | (1) Reporting network of clinics and healthcare providers; (2) hospital discharge, billing and paediatric endocrinology case lists; (3) mailed survey to providers likely to see children not included in above | pH <7.25 (venous) or <7.3 (arterial/capillary) or HCO3 < 15 mmol/l or ICD-9 code 250.1 at discharge or diagnosis of DKA in medical notes | |
| Mylnarski et al, 2003 [58] | Poland | 106 | 0 to <19 | 1997–2001 | P | 1 diabetes centre | Hospital records | pH <7.35 | |
| Neu et al, 2003 [59] | Germany | 2,121 | 0 to <15 | 1987–1997 | R | 31 paediatric departments and 1 diabetes centre | (1) Hospital records ; (2) questionnaire to members of Diabetic Patients Association | Glucose > 250 mg/dl (>13.9 mmol/l), pH <7.3 or HCO3 <15 mmol/l and ketonuria | 97.2 |
| Newfield et al, 2009 [60] | USA | 136 | 0 to <18 | 1998–2001 | R | 1 children’s hospital | Hospital database | pH <7.3 or HCO3 <15 mmol/l | |
| Olak-Białoń et al, 2007 [61] | Poland | 186 | 0 to <18 | 2004–2005 | R | 1 children’s endocrinology and diabetes centre | Clinic records | pH <7.3, HCO3 <18 mmol/l, ketonuria and glucose >250 mg/dl (> 13.9 mmol/l) | |
| Pawlowicz et al, 2009 [62] | Poland | 474 | 0 to <17 | 1999–2005 | R | 1 paediatric endocrinology department | (1) Hospital records; (2) regional diabetic outpatient clinics | pH <7.3 and HCO3 <15 mmol/l | 99.73 |
| Pinero-Martinez et al, 1995 [63] | Spain | 74 | 0 to <15 | 1983–1992 | R | 1 hospital | Hospital records | pH <7.3 | |
| Pinkney et al, 1994 [64] | UK | 95 | 0 to <21 | 1990 | P | Regional | Hospital register | pH ≤7.35 or HCO3 ≤21.0 mmol/l | |
| Pocecco and Nassimbeni 1993 [65] | Italy | 73 | 0 to <17 | 1987–1990 | R | 14 paediatric departments and 14 diabetologic services | (1) Departmental records; (2) central register for all patients receiving drug reimbursement | pH <7.36 | 98 |
| Prisco et al, 2006 [66] | Italy | 118 | 0 to <19 | 2003 | P | 7 territorial reference hospitals | Hospital records | pH <7.3 and glucose >250 mg/dl (> 13.9 mmol/l) and capillary ketone bodies >3 mmol/l | 98 |
| Pronina et al, 2008 [67] | Russia | 2,031 | 0 to <15 | 1996–2005 | P | 4 largest children’s hospitals in Moscow | (1) Departmental and hospital records; (2) registration for exemption from payment for medication | Bicarbonate <10 mmol/l and pH <7.3 | 94 |
| Punnose et al, 2002 [68] | United Arab Emirates | 35 | 0 to 18 | 1990–1998 | R | 1 hospital | Hospital records | Glucose >205 mg/dl (> 11.4 mmol/l) and HCO3 <15 mmol/l with ketonuria ++ or more | |
| Quinn et al, 2006 [69] | USA | 247 | 0 to <6 | 1990–1999 | R | 1 children’s hospital | Hospital records | Glucose >300 mg/dl (> 16.7 mmol/l), pH <7.3 and/or HCO3 or tCO2 <15 mmol/l | |
| Rewers et al, 2008 [70] | USA and Hawaii | 1,656 | 0 to 20 | 2002–2004 | R | Regional | Rapid reporting network of clinics and healthcare providers | pH <7.25 (venous) or <7.3 (arterial/capillary) or HCO3 <15 mmol/l or ICD-9 code 250.1 at discharge or diagnosis of DKA in chart | 77 |
| Roche et al, 2005 [71] | Ireland | 197 | 0 to <15 | 1997–1998 | P | Nationwide | (1) Irish paediatric surveillance unit; (2) national survey of adult physicians and endocrinologists | Glucose >15 mmol/l, urinary ketones +2, pH <7.2, HCO3 <15 mmol/l and clinical symptoms | 90.7 |
| Rosenbauer et al, 2002 [72] | Germany | 262 | 0 to <15 | 1993–1995 | R | 41 paediatric and diabetes departments | (1) Active clinic-based surveillance system; (2) yearly surveillance among paediatric, general and internal medicine practices | pH ≤7.35 | 92.5 |
| Sadaskaite-Kuehne et al, 2002 [17] | Sweden | 401 | 0 to <16 | 1995–1999 | P | 12 hospitals | Hospital records | pH ≤7.2 plus hyperglycaemia and ketonuria | 83.4 South-east Sweden, 49.5 Skane region |
| Sadaskaite-Kuehne et al, 2002 [17] | Lithuania | 286 | 0 to <16 | 1996–2000 | P | Nationwide | Hospital records | pH ≤7.2 plus hyperglycaemia and ketonuria | 100 |
| Salman 1991 [73] | Saudi Arabia | 110 | 0 to <13 | 1985–1989 | R | 1 children’s hospital | Hospital records | HCO3 <15 mmol/l and glucose >15 mmol/l and ketonuria and clinical features | |
| Samuelsson et al, 2005 [28] | Sweden | 1,903 | 0 to <16 | 1977–2001 | R | 7 paediatric clinics | (1) Medical records; (2) Swedish Diabetes Register | pH ≤7.3 | 78.5 |
| Savova et al, 1996 [74] | Bulgaria | 1,248 | 0 to <18 | 1974–1996 | R | 1 children’s hospital | (1) Hospital records; (2) national centralised system of insulin delivery | pH <7.34 or acidotic breathing | |
| Schober et al, 2010 [75] | Austria | 3,331 | 0 to 15 | 1989–2008 | P | Nationwide | Network covering all paediatric hospitals, wards and diabetologists | pH <7.3 | >93 |
| Sebastiani Annicchiarico and Guglielmi, 1992 [76] | Italy | 117 | 0 to <15 | 1989–1990 | P | 51 local health units and 71 hospitals | Basic incidence surveillance cohort | pH <7.3 | |
| Smith et al, 1998 [77] | UK | 79 | 0 to <16 | 1990–1996 | R | 1 children’s hospital | Clinic records | pH <7.3 or HCO3 <18 mmol/l | 90 |
| Soliman et al, 1997 [78] | Oman | 60 | 0 to <15 | 1990–1993 | P | Regional (10 hospitals) | Diabetologists and paediatricians in the regions | pH <7.35 | |
| Soltész et al, 1997 [79] | Hungary | 168 | 0 to 15 | 1994 | P | Nationwide | Incidence surveillance cohort | pH <7.20 | 91 |
| Sundaram et al, 2009 [80] | UK | 99 | 0 to <16 | 2004–2007 | R | 1 children’s hospital | Hospital database | pH <7.3 or HCO3 <15 mmol/l and blood glucose >11 mmol/l and ketonaemia ± ketonuria | |
| Tahirovic et al, 2007 [81] | Bosnia and Herzegovina | 100 | 0 to ≤14 | 1990–2005 | R | 1 children’s hospital | (1) Hospital diabetes register; (2) hospital records | pH <7.3 and HCO3 <15 mmol/l | 91.7 |
| Ting et al, 2007 [82] | Taiwan | 304 | 0 to <18 | 1979–2006 | R | 1 paediatric department | Hospital records | Glucose >200 mg/dl (>11.1 mmol/l) and pH <7.3 and/or HCO3 <15 mmol/l and ketonuria | |
| Vehik et al, 2009 [83] | USA | 712 | 2 to <18 | 2002–2004 | R | Regional | Rapid reporting network of clinics and healthcare providers | pH <7.3 or HCO3 <18 mmol/l or physician-diagnosed DKA episode at diagnosis | 75–76 |
| Veijola et al, 1996 [84] | Finland | 801 | 0 to <15 | 1986–1989 | P | Nationwide | (1) Diabetes nurses; (2) national central drug registry | pH <7.3 | |
| Xin et al, 2010 [85] | China | 203 | 0 to <15 | 2004–2008 | R | 1 hospital | Hospital records | pH <7.3 or HCO3 <15 mmol/l and glucose >14 mmol/l in the presence of ketonuria |
aEstimates of the ascertainment given in the original study, where available
bCodes refer to abbreviations used for specific datasets in [11]
IDDM, insulin-dependent diabetes mellitus; P, prospective; R, retrospective; tCO2, total CO2
Frequency of DKA across countries
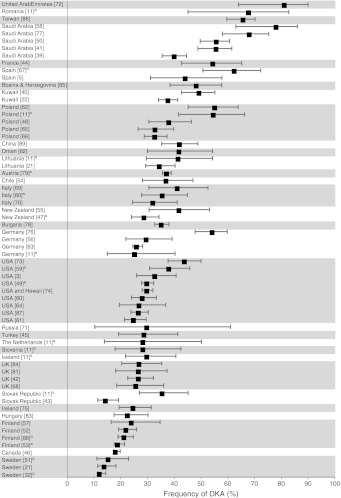
Table 2 shows the frequency of DKA at diagnosis in each study, together with the background incidence of type 1 diabetes, latitude, GDP and expenditure on healthcare as a percentage of the country’s GDP. The frequency of DKA varied sixfold, from 12.8% in Sweden to 80% in the United Arab Emirates and from 16% to 67% when only those studies defining DKA by pH < 7.3 were included. Ranking of countries according to frequency of DKA (Fig. 2) demonstrated that the highest frequencies were seen in the United Arab Emirates (80%), Romania (67%), Taiwan (65%) and Saudi Arabia (59%) and the lowest in Sweden (14%), Canada (18.6%), Finland (22%) and Hungary (23%).
Fig. 2.
Plot of the frequency (±95% CI) of DKA at diagnosis of type 1 diabetes per study, grouped in countries in descending order of the average frequency of DKA per country. aStudies defining DKA as pH < 7.3
Multivariable linear regression
Three studies had incomplete data (Table 2) and were, therefore, excluded from the regression modelling. Initial linear modelling showed that GDP and latitude were strongly collinear and therefore it was only possible to include one. Latitude was chosen as it explained more of the variation.
The final model (Table 3) shows that latitude and background incidence of type 1 diabetes were significantly associated with frequency of DKA, with frequency decreasing progressively with distance from the equator and in areas with a higher background incidence of type 1 diabetes. Although not reaching statistical significance, there was also an inverse association between expenditure on healthcare as a percentage of GDP and frequency of DKA. No significant associations were found for study size, period of study, design or method of case identification. These associations were the same when latitude was replaced with GDP, showing that GDP is also inversely associated with frequency of DKA.
Table 3.
Results of multivariate linear regression model (loge[frequency] = 4.5 − 0.000080 × study size–0.0023 × period of study + 0.10 × design + 0.050 × method of case identification − 0.022 × expenditure on healthcare as a percentage of GDP − 0.013 × latitude − 0.014 × background incidence of type 1 diabetes)
| Variable | Coefficient | 95% CI | p value |
|---|---|---|---|
| (Intercept) | 4.5 | 4.4, 4.5 | |
| Study size | −0.000080 | −0.0011, 0.00094 | 0.13 |
| Period of study | −0.0023 | −0.016, 0.012 | 0.75 |
| Design | 0.10 | −0.073, 0.27 | 0.26 |
| Method of case identification | 0.050 | −0.12, 0.22 | 0.58 |
| Expenditure on healthcare as a percentage of GDP | −0.022 | −0.045, 0.00030 | 0.058 |
| Latitude | −0.013 | −0.020, (−0.0050) | 0.0020 |
| Background incidence of T1DM | −0.014 | −0.022, (−0.0052) | 0.0028 |
T1DM, type 1 diabetes
After adjusting for study size, period of study, design, method of case identification, expenditure on healthcare as a percentage of GDP, and latitude, the frequency of DKA decreased by approximately 10% as the annual background incidence increased from 10 to 30 cases per 100,000, with greater changes at lower background rates. R 2 for the model was 0.56, indicating that a large amount of the variation was explained by these factors.
Discussion
Principal findings
This systematic review provides the first comprehensive synthesis of the international variation in frequency of DKA at presentation of type 1 diabetes in children. The frequency of DKA at diagnosis ranges from 12.8% to 80%, and is lowest in Sweden, the Slovak Republic and Canada and highest in the United Arab Emirates, Saudi Arabia and Romania. The frequency of DKA is lower in countries where the background incidence of type 1 diabetes is higher and in countries further from the equator and with a lower GDP. Although not statistically significant, there is also an association (p = 0.058) between frequency of DKA and expenditure on healthcare as a percentage of GDP. The association with background incidence may be due to increased awareness of the condition and hence earlier detection, or it may reflect a different subtype of disease. The association with latitude may similarly reflect different subtypes of disease or geographical factors and is also likely to include socioeconomic factors, including GDP and health expenditure. The importance of these findings is that they suggest the variation may, at least in part, be explained by different levels of awareness of the disease and healthcare provision. Given that these factors are amenable to change, there is the potential to decrease the excess morbidity, mortality and healthcare expenditure associated with DKA at diagnosis.
Strengths and limitations
Our broad inclusion criteria and systematic search encompassing multiple databases, not limited by language or study size, provided data on over 29,000 children from 31 countries. While this allows us to make comparisons across multiple countries, it also increases the heterogeneity of the included studies. In most studies, the primary source of ascertainment was internal hospital records or notifications by paediatricians and family doctors, with only 19 studies including a secondary source. Furthermore, many of the studies were retrospective and relied on hospital records for the data and so are subject to recording and recall bias. However, these factors (method of case identification and study design) and study size and period were not associated with the variation in frequency of DKA, suggesting that they are unlikely to account for the differences seen. This cannot, however, adjust for variations in ascertainment of both children with type 1 diabetes and those with DKA. It also fails to take account of other potential confounding factors such as age, ethnicity and family history of type 1 diabetes, which have been described previously [12]. Unfortunately, few studies reported individual characteristics of recruited children so these could not be included in the modelling.
Included studies also used a wide range of definitions of DKA, reflecting different international settings and periods of study. Only one [18] used the current diagnostic criteria for DKA published by the International Society for Paediatric and Adolescent Diabetes [19]. However, our inclusion criteria limited studies to those with a measurement of pH (pH ≤ 7.2 to <7.36) and/or bicarbonate (<15 to ≤ 21 mmol/l) and so, while being broad enough to include studies from different countries, consistently identified those with worse metabolic derangements. Although the absolute frequency of DKA may vary with different definitions, it is unlikely that this alone would account for the variations seen. The wide range of definitions used precluded comprehensive sensitivity analyses, but inclusion of only those studies defining DKA by a pH < 7.3 (n = 18) still demonstrated a variation from 16% (in Sweden) to 67% (in Romania) (Fig. 2).
Although the included studies represented 31 countries, 21 of these—providing data for 18,164 (62%) of the children—were European. The USA and Canada accounted for a further 7,873 (27%), with only 3,122 (11%) children from outside these countries. Many large countries, including South American countries, India, China and Japan, were also not represented and no studies came from any country in Africa, where DKA is known to be a major problem [20]. This lack of data is itself an important finding of this review, but limits the range of included values of latitude, GDP and expenditure on healthcare as a percentage of GDP, and the extent to which the conclusions can be generalised worldwide.
Possible causal explanations for variation and comparison with existing literature
Although the nature of this study prevents statements of causality and the quality of the data limits the conclusions that can be drawn, there are a number of explanations for why these observations might be causal. The inverse relationship between frequency of DKA and background incidence of type 1 diabetes worldwide is consistent with previous reports of this relationship in Europe [11]. This may reflect the overall awareness of the condition in a given country along with the capability of its healthcare system to quickly initiate the appropriate treatment following diagnosis [21]. Better disease recognition through improved awareness of diabetes is also supported by the findings that children from families with higher parental education are less likely to present in DKA and having a first-degree relative with diabetes is associated with an up to six-fold decreased risk of DKA at diagnosis [12]. However, it remains unclear why some children present in DKA while others do not and whether the development of DKA is a consequence of patient or clinician delays, or whether it indicates a particularly aggressive form of diabetes [22]. It is possible that the observed associations reflect heterogeneity in the underlying disease, with more aggressive disease in those countries with lower incidence. This phenomenon may also explain the considerable geographic variation in the prevalence of long-term diabetic complications [21].
There are also a number of possible explanations for the association of frequency of DKA and latitude. The most likely is that latitude represents a group of characteristics for each country, including economy, healthcare provision, access to healthcare and disease burden. The co-linearity between latitude and GDP suggests that a large proportion of the variation in DKA is due to differences in a country’s economic position and healthcare provision, with a higher frequency of DKA in countries with lower GDP and hence lower expenditure on healthcare. This is not surprising as there is robust evidence for a similar relationship between life expectancy and GDP [23], and poorer countries account for the largest share of the global burden of disease [24]. Several ecological studies have also shown that the type 1 diabetes incidence rates correlate strongly with indicators of national prosperity such as GDP and low infant mortality [25, 26] and that microalbuminuria and neuropathy are significantly associated with health system performance, gross national investment per capita and purchasing power [27]. However, some of the effect could reflect true geographical differences. The increased frequencies of DKA in countries nearer the equator may be because hotter climates lead to more rapid dehydration and metabolic decompensation, particularly in young children, who have less metabolic reserve [28]. Finally, there is also growing evidence for a role for vitamin D in the pathogenesis of type 1 diabetes. The active metabolite of vitamin D, 1,25-dihydroxyvitamin D, is a potent immunomodulator that enhances the production and secretion of insulin from beta cells, and vitamin D deficiency in utero and early childhood is associated with an increased risk of type 1 diabetes [29–32]. Although children living near the equator are less likely to be vitamin D deficient, differences in levels of vitamin D may contribute to the variation seen.
Unanswered questions and implications for future research
We found little or no data on the frequency of DKA, or even type 1 diabetes, for large areas of the world, particularly Africa and South and East Asia. Infectious diseases currently dominate childhood morbidity and mortality in these regions, so reliable indicators of diabetes and DKA may only be possible with strengthened epidemiological surveillance of the growing burden of non-communicable diseases [9].
The factors addressed in this review also only account for a proportion of the international variation, suggesting the need for further research using standardised data including factors known to affect the frequency of DKA and other potential contributors, e.g. access to healthcare, population density, genetics, patient education and healthcare resources for diabetes. We also need to understand better the causes and long-term consequences of DKA: in particular, whether DKA simply reflects delayed recognition and treatment and whether long-term clinical findings merely indicate greater beta cell destruction at that moment, or reflect a more aggressive form of diabetes.
Nevertheless, the finding that in some countries the frequency of DKA is less than 15% and that the variation may, at least in part, be explained by different levels of awareness of the disease and healthcare provision, suggests there is considerable scope to decrease the excess morbidity, mortality and healthcare expenditure associated with DKA at diagnosis of type 1 diabetes. The effect that campaigns to improve awareness can have locally has been demonstrated from a community intervention in Italy, in which posters were displayed in schools, and paediatricians were provided with blood glucometers and cards listing guidelines for early diabetes diagnosis to give to parents; the frequency of DKA at diagnosis fell from 78% to 12.5% [33, 34]. This review provides support for the development and evaluation of further country-specific diabetes education programmes, particularly in those countries where the frequency at diagnosis is highest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 144 kb)
Acknowledgements
We thank I. Kuhn, Reader Services Librarian, University of Cambridge Medical Library, for her help developing the search strategy and D. Dunger and S. Griffin for helpful advice throughout the study and, together with J. Mant, for commenting on the manuscript. We also thank the EURODIAB co-ordinators, C. Lévy-Marchal and A. Green, for providing data for individual centres within the EURODIAB study.
Funding
JUS is supported by a National Institute for Health Research Academic Clinical Fellowship, FMW and AE are employed by the University of Cambridge, and MT is funded by a Career Development Fellowship supported by the National Institute for Health Research. This report is independent research and the views expressed in this publication are those of the authors and not necessarily of the NHS, the National Institute for Health Research or the Department of Health.
Duality of interest
All authors declare no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Contribution statement
JUS performed the literature search, selected articles for inclusion, extracted the data, planned the analysis and wrote the first draft of the manuscript. FMW and MT selected articles for inclusion, extracted the data and reviewed and edited the manuscript. AE performed the statistical analysis and reviewed and edited the manuscript. All authors approved the final version.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Abbreviations
- DKA
Diabetic ketoacidosis
- GDP
Gross domestic product
References
- 1.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999;81:318–396. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scibilia J, Finegold D, Dorman J, Becker D, Drash A. Why do children with diabetes die? Acta Endocrinol. 1986;279(Suppl):326–333. doi: 10.1530/acta.0.112s326. [DOI] [PubMed] [Google Scholar]
- 3.Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008;9:197–201. doi: 10.1111/j.1399-5448.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Rasoul M, Habib H, Al-Khouly M. “The honeymoon phase” in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101–107. doi: 10.1111/j.1399-543X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez Castaner M, Montana E, Camps I, et al. Ketoacidosis at diagnosis is predictive of lower residual beta-cell function and poor metabolic control in type 1 diabetes. Diabetes Metabol. 1996;22:349–355. [PubMed] [Google Scholar]
- 6.Fernández Castañer M, Gonzálbez J, Carrera MJ, et al. The influence of clinical presentation and metabolic control of insulin dependent diabetes in the evolution of residual insulin secretion. A prospective study at five years. Medicina Clínica. 1997;109:328–332. [PubMed] [Google Scholar]
- 7.Shrestha SS, Zhang P, Barker L, Imperatore G. Medical expenditures associated with diabetes acute complications in privately insured U.S. youth. Diabetes Care. 2010;33:2617–2622. doi: 10.2337/dc10-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liss DS, Waller DA, Kennard BD, McIntire D, Capra P, Stephens J. Psychiatric illness and family support in children and adolescents with diabetic ketoacidosis: a controlled study. J Am Acad Child Adolesc Psychiatr. 1998;37:536–544. doi: 10.1097/00004583-199805000-00016. [DOI] [PubMed] [Google Scholar]
- 9.DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 10.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–1526. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 11.Lévy-Marchal C, Patterson CC, Green A. Geographical variation of presentation at diagnosis of type I diabetes in children: The EURODIAB study. Diabetologia. 2001;44:B75–B80. doi: 10.1007/PL00002958. [DOI] [PubMed] [Google Scholar]
- 12.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092. doi: 10.1136/bmj.d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health Resource Unit (1998) Critical appraisal skills. Available from www.york.ac.uk/inst/crd/, accessed 30 Oct 2011
- 14.Sloka S, Grant M, Newhook LA. The geospatial relation between UV solar radiation and type 1 diabetes in Newfoundland. Acta Diabetologica. 2010;47:73–78. doi: 10.1007/s00592-009-0100-0. [DOI] [PubMed] [Google Scholar]
- 15.Staples JA, Ponsonby A-L, Lim LL-Y, McMichael AJ. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: latitude, regional ultraviolet radiation, and disease prevalence. Environ Heal Perspect. 2003;111:518–523. doi: 10.1289/ehp.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Diabetes Federation (2009) IDF Atlas, 4th edn. Available from www.idf.org/node/23640, accessed 30 Oct 2011
- 17.Sadauskaite-Kuehne V, Samuelsson U, Jasinskiene E, et al. Severity at onset of childhood type 1 diabetes in countries with high and low incidence of the condition. Diabetes Res Clin Pract. 2002;55:247–254. doi: 10.1016/S0168-8227(01)00328-X. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Rasoul M, Al-Mahdi M, Al-Qattan H, et al. Ketoacidosis at presentation of type 1 diabetes in children in Kuwait: frequency and clinical characteristics. Pediatr Diabetes. 2010;11:351–356. doi: 10.1111/j.1399-5448.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolfsdorf J, Craig M, Daneman D, et al. ISPAD Clinical Practice Consensus Guidelines 2009 Compendium: diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10:118–133. doi: 10.1111/j.1399-5448.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Majaliwa ES, Munubhi E, Ramaiya K, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar es Salaam, Tanzania. Diabetes Care. 2007;30:2187–2192. doi: 10.2337/dc07-0594. [DOI] [PubMed] [Google Scholar]
- 21.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev. 2010;9:A355–A365. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Neu A, Ehehalt S, Willasch A, Kehrer M, Hub R, Ranke MB. Varying clinical presentations at onset of type 1 diabetes mellitus in children–epidemiological evidence for different subtypes of the disease? Pediatr Diabetes. 2001;2:157–53. doi: 10.1034/j.1399-5448.2001.20402.x. [DOI] [PubMed] [Google Scholar]
- 23.Swift R. The relationship between health and GDP in OECD countries in the very long run. Heal Econ. 2011;20:306–322. doi: 10.1002/hec.1590. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization World Health Statistics 2007: Ten statistical highlights in global public health. Available from www.who.int/gho/publications/world_health_statistics/whostat2007_10highlights.pdf, accessed 14 Jan 2012
- 25.Patterson CC, Dahlquist G, Soltész G, Green A. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44(Suppl 3):B9–B16. doi: 10.1007/PL00002961. [DOI] [PubMed] [Google Scholar]
- 26.Haynes A, Bulsara MK, Bower C, Codde JP, Jones TW, Davis EA. Independent effects of socioeconomic status and place of residence on the incidence of childhood type 1 diabetes in Western Australia. Pediatr Diabetes. 2006;7:94–100. doi: 10.1111/j.1399-543X.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 27.Walsh MG, Zgibor J, Songer T, Borch-Johnsen K, Orchard TJ. The socioeconomic correlates of global complication prevalence in type 1 diabetes (T1D): a multinational comparison. Diabetes Res Clin Pract. 2005;70:143–150. doi: 10.1016/j.diabres.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson U, Stenhammar L. Clinical characteristics at onset of type 1 diabetes in children diagnosed between 1977 and 2001 in the south-east region of Sweden. Diabetes Res Clin Pract. 2005;68:49–55. doi: 10.1016/j.diabres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin d during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61:175–178. doi: 10.2337/db11-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holick MF. Diabetes and the vitamin d connection. Curr Diabetes Rep. 2008;8:393–398. doi: 10.1007/s11892-008-0068-0. [DOI] [PubMed] [Google Scholar]
- 31.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin N Am. 2010;39:419–446. doi: 10.1016/j.ecl.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 33.Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care. 1999;22:7–9. doi: 10.2337/diacare.22.1.7. [DOI] [PubMed] [Google Scholar]
- 34.Vanelli M, Chiari G, Lacava S, Iovane B. Campaign for diabetic ketoacidosis prevention still effective 8 years later. Diabetes Care. 2007;30:e12. doi: 10.2337/dc07-0059. [DOI] [PubMed] [Google Scholar]
- 35.Abduljabbar MA, Aljubeh JM, Amalraj A, Cherian MP. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi Med J. 2010;31:413–418. [PubMed] [Google Scholar]
- 36.Al Khawari M, Shaltout A, Qabazard M, et al. Incidence and severity of ketoacidosis in childhood-onset diabetes in Kuwait. Kuwait Diabetes Study Group. Diabetes Res Clin Pract. 1997;35:123–128. doi: 10.1016/S0168-8227(96)01365-4. [DOI] [PubMed] [Google Scholar]
- 37.Al Magamsi M, Habib H. Clinical presentation of childhood type 1 diabetes mellitus in the Al-Madina region of Saudi Arabia. Pediatr Diabetes. 2004;5:95–98. doi: 10.1111/j.1399-543X.2004.00046.x. [DOI] [PubMed] [Google Scholar]
- 38.Alvi NS, Davies P, Kirk JMW, Shaw NJ, Lane S. Diabetic ketoacidosis in Asian children. Arch Dis Child. 2001;85:60–62. doi: 10.1136/adc.85.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barák L, Jančová E, Staník J, Karovič D, Csomor D, Šagát T, Benedeková M. Diabetic ketoacidosis. Diabetická ketoacidóza. 2006;61:599–602. [Google Scholar]
- 40.Blanc N, Lucidarme N, Tubiana-Rufi N. Factors associated to ketoacidosis at diagnosis of type 1 diabetes in children. Arch Pediatr. 2003;10:320–325. doi: 10.1016/S0929-693X(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 41.Böber E, Dündar B, Büyükgebiz A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. Pediatr Endocrinol Metabol. 2001;14:435–441. doi: 10.1515/jpem.2001.14.4.435. [DOI] [PubMed] [Google Scholar]
- 42.Bui H, To T, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156:472–477. doi: 10.1016/j.jpeds.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Campbell-Stokes PL, Taylor BJ. Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia. 2005;48:643–648. doi: 10.1007/s00125-005-1697-3. [DOI] [PubMed] [Google Scholar]
- 44.Charemska D, Przybyszewski B, Klonowska B. Estimation of the severity of metabolic disorders in children with newly diagnosed insulin dependent diabetes mellitus (IDDM) Med Wieku Rozwoj. 2003;7:261–270. [PubMed] [Google Scholar]
- 45.Charron-Prochownik D, Kovacs M, Obrosky DS, Ho V. Illness characteristics and psychosocial and demographic correlates of illness severity at onset of insulin-dependent diabetes mellitus among school-age children. J Pediatr Nurs. 1995;10:354–359. doi: 10.1016/S0882-5963(05)80032-6. [DOI] [PubMed] [Google Scholar]
- 46.Habib HS. Frequency and clinical characteristics of ketoacidosis at onset of childhood type 1 diabetes mellitus in Northwest Saudi Arabia. Saudi Med J. 2005;26:1936–1939. [PubMed] [Google Scholar]
- 47.Hanas R, Lindgren F, Lindblad B. Diabetic ketoacidosis and cerebral oedema in Sweden—a 2-year paediatric population study. Diabet Med. 2007;24:1080–1085. doi: 10.1111/j.1464-5491.2007.02200.x. [DOI] [PubMed] [Google Scholar]
- 48.Hekkala A, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care. 2007;30:861–866. doi: 10.2337/dc06-2281. [DOI] [PubMed] [Google Scholar]
- 49.Hekkala A, Reunanen A, Koski M, Knip M, Veijola R. Age-related differences in the frequency of ketoacidosis at diagnosis of type 1 diabetes in children and adolescents. Diabetes Care. 2010;33:1500–1502. doi: 10.2337/dc09-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodgson BMI, Ossa AJC, Velasco FN, Urrejola NP, Arteaga Ll A. Clinical picture at the onset of type 1 diabetes mellitus in children. Rev Med Chile. 2006;134:1535–1540. doi: 10.4067/s0034-98872006001200007. [DOI] [PubMed] [Google Scholar]
- 51.Jackson W, Hofman PL, Robinson EM, Elliot RB, Pilcher CC, Cutfield WS. The changing presentation of children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes. 2001;2:154–159. doi: 10.1034/j.1399-5448.2001.20403.x. [DOI] [PubMed] [Google Scholar]
- 52.Kapellen TM, Galler A, Nietzschmann U, Schille R, Kiess W. Prevalence of diabetic ketoacidosis in newly diagnosed children and adolescents with type 1 diabetes mellitus. Experience of a center for pediatric diabetology in Germany. Monatsschrift fur Kinderheilkunde. 2001;149:679–682. doi: 10.1007/s001120170122. [DOI] [Google Scholar]
- 53.Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med. 1989;320:881–886. doi: 10.1056/NEJM198904063201401. [DOI] [PubMed] [Google Scholar]
- 54.Kulaylat NA, Narchi H. Clinical picture of childhood type 1 diabetes mellitus in the Eastern Province of Saudi Arabia. Pediatr Diabetes. 2001;2:43–47. doi: 10.1046/j.1399-543x.2001.020108.x. [DOI] [PubMed] [Google Scholar]
- 55.Mallare JT, Cordice CC, Ryan BA, Carey DE, Kreitzer PM, Frank GR. Identifying risk factors for the development of diabetic ketoacidosis in new onset type 1 diabetes mellitus. Clin Pediatr (Phila) 2003;42:591–597. doi: 10.1177/000992280304200704. [DOI] [PubMed] [Google Scholar]
- 56.Maniatis AK, Goehrig SH, Gao D, Rewers A, Walravens P, Klingensmith GJ. Increased incidence and severity of diabetic ketoacidosis among uninsured children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes. 2005;6:79–83. doi: 10.1111/j.1399-543X.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 57.Mayer-Davis EJ, Beyer J, Bell RA, et al. Diabetes in African American youth. Diabetes Care. 2009;32:S112–S122. doi: 10.2337/dc09-S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mylnarski W, Zmyslowska A, Kubryn I, Perenc M, Bodalski J. Factors involved in ketoacidosis at the onset of type 1 diabetes in childhood. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2003;9:23–28. [PubMed] [Google Scholar]
- 59.Neu A, Willasch A, Ehehalt S, et al. Ketoacidosis at onset of type 1 diabetes mellitus in children - frequency and clinical presentation. Pediatr Diabetes. 2003;4:77–81. doi: 10.1034/j.1399-5448.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 60.Newfield RS, Cohen D, Capparelli EV, Shragg P. Rapid weight gain in children soon after diagnosis of type 1 diabetes: is there room for concern? Pediatr Diabetes. 2009;10:310–315. doi: 10.1111/j.1399-5448.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 61.Olak-Białoń B, Deja G, Jarosz-Chobot P, Buczkowska EO. The occurrence and analysis of chosen risk factors of DKA among children with new onset of DMT1. Wieku Rozwoj. 2007;13:85–90. [PubMed] [Google Scholar]
- 62.Pawlowicz M, Birkholz D, Niedzwiecki M, Balcerska A. Difficulties or mistakes in diagnosing type 1 diabetes in children?—demographic factors influencing delayed diagnosis. Pediatr Diabetes. 2009;10:542–549. doi: 10.1111/j.1399-5448.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 63.Pinero Martinez E, Ruibal Francisco JL, Bueno Lozano G, Reverte Blanc F. Clinical and analytical aspects of insulin-dependent diabetes mellitus during childhood. Our experience in the decade 1983–1992. Anales Espanoles de Pediatria. 1995;43:265–269. [Google Scholar]
- 64.Pinkney JH, Bingley PJ, Sawtell PA, Dunger DB, Gale EAM. Presentation and progress of childhood diabetes mellitus: a prospective population-based study. Diabetologia. 1994;37:70–74. doi: 10.1007/BF00428780. [DOI] [PubMed] [Google Scholar]
- 65.Pocecco M, Nassimbeni G. Distribution of new cases of insulin-dependent diabetes mellitus (IDDM) by age, sex, seasonality, and clinical characteristics at onset in youngsters from the Friuli Venezia Giulia region from 1987 to 1990. Pediatr Med Chir. 1993;15:489–492. [PubMed] [Google Scholar]
- 66.Prisco F, Picardi A, Iafusco D, et al. Blood ketone bodies in patients with recent-onset type 1 diabetes (a multicenter study) Pediatr Diabetes. 2006;7:223–228. doi: 10.1111/j.1399-5448.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 67.Pronina EA, Petraikina EE, Antsiferov MB, et al. A 10-year (1996–2005) prospective study of the incidence of type 1 diabetes in Moscow in the age group 0–14 years. Diabet Med. 2008;25:956–959. doi: 10.1111/j.1464-5491.2008.02508.x. [DOI] [PubMed] [Google Scholar]
- 68.Punnose J, Agarwal MM, El Khadir A, Devadas K, Mugamer IT. Childhood and adolescent diabetes mellitus in Arabs residing in the United Arab Emirates. Diabetes Res Clin Pract. 2002;55:29–33. doi: 10.1016/S0168-8227(01)00267-4. [DOI] [PubMed] [Google Scholar]
- 69.Quinn M, Fleischman A, Rosner B, Nigrin DJ, Wolfsdorf JI. Characteristics at diagnosis of type 1 diabetes in children younger than 6 years. J Pediatr. 2006;148:366–371. doi: 10.1016/j.jpeds.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 70.Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics. 2008;121:1258–1266. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 71.Roche EF, Menon A, Gill D, Hoey H. Clinical presentation of type 1 diabetes. Pediatr Diabetes. 2005;6:75–78. doi: 10.1111/j.1399-543X.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 72.Rosenbauer J, Icks A, Giani G. Clinical characteristics and predictors of severe ketoacidosis at onset of type 1 diabetes mellitus in children in a North Rhine-Westphalian region, Germany. J Pediatr Endocrinol Metabol. 2002;15:1137–1145. doi: 10.1515/jpem.2002.15.8.1137. [DOI] [PubMed] [Google Scholar]
- 73.Salman H, Abanamy A, Ghassan B, Khalil M. Insulin-dependent diabetes mellitus in children: familial and clinical patterns in Riyadh. Ann Saudi Med. 1991;11:302–306. doi: 10.5144/0256-4947.1991.302. [DOI] [PubMed] [Google Scholar]
- 74.Savova R, Popova G, Koprivarova K, et al. Clinical and laboratory characteristics of type I (insulin dependent) diabetes mellitus at presentation among Bulgarian children. Diabetes Res Clin Pract. 1996;34:S159–S163. doi: 10.1016/S0168-8227(96)90024-8. [DOI] [PubMed] [Google Scholar]
- 75.Schober E, Rami B, Waldhoer T. Diabetic ketoacidosis at diagnosis in Austrian children in 1989–2008: a population-based analysis. Diabetologia. 2010;53:1057–1061. doi: 10.1007/s00125-010-1704-1. [DOI] [PubMed] [Google Scholar]
- 76.Sebastiani Annicchiarico L, Guglielmi A. The EURODIAB experience in Lazio. Ann Ig. 1992;4:173–178. [PubMed] [Google Scholar]
- 77.Smith CP, Firth D, Bennett S, Howard C, Chisholm P. Ketoacidosis occurring in newly diagnosed and established diabetic children. Acta Paediatr. 1998;87:537–541. doi: 10.1111/j.1651-2227.1998.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 78.Soliman AT, al Salmi I, Asfour M. Mode of presentation and progress of childhood diabetes mellitus in the Sultanate of Oman. J Trop Pediatr. 1997;43:128–132. doi: 10.1093/tropej/43.3.128. [DOI] [PubMed] [Google Scholar]
- 79.Soltész G, Györkö BJ, Levy-Marchal C. Clinical diagnosis of childhood insulin dependent diabetes mellitus. Hungarian Epidemiological Group for Childhood Diabetes. Orv Hetil. 1997;138:7–9. [PubMed] [Google Scholar]
- 80.Sundaram PC, Day E, Kirk JM. Delayed diagnosis in type 1 diabetes mellitus. Arch Dis Child. 2009;94:151–152. doi: 10.1136/adc.2007.133405. [DOI] [PubMed] [Google Scholar]
- 81.Tahirovic H, Toromanovic A, Bacaj D, Hasanovic E. Ketoacidosis at onset of type 1 diabetes mellitus in children in Bosnia and Herzegovina: frequency and clinical presentation. J Pediatr Endocrinol Metabol. 2007;20:1137–1140. doi: 10.1515/JPEM.2007.20.10.1137. [DOI] [PubMed] [Google Scholar]
- 82.Ting WH, Huang CY, Lo FS, et al. Clinical and laboratory characteristics of type 1 diabetes in children and adolescents: experience from a medical center. Acta Paediatr Taiwan. 2007;48:119–124. [PubMed] [Google Scholar]
- 83.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith GJ, Dabelea D. Childhood growth and age at diagnosis with type 1 diabetes in Colorado young people. Diabet Med. 2009;26:961–967. doi: 10.1111/j.1464-5491.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 84.Veijola R, Reijonen H, Vähäsalo P, et al. HLA-DQB1-defined genetic susceptibility, beta cell autoimmunity, and metabolic characteristics in familial and nonfamilial insulin-dependent diabetes mellitus. J Clin Investig. 1996;98:2489–2495. doi: 10.1172/JCI119067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xin Y, Yang M, Chen XJ, Tong YJ, Zhang LH. Clinical features at the onset of childhood type 1 diabetes mellitus in Shenyang, China. J Paediatr Child Health. 2010;46:171–175. doi: 10.1111/j.1440-1754.2009.01657.x. [DOI] [PubMed] [Google Scholar]
- 86.Soltész G, Patterson C, Dahlquist G. Global trends in childhood type 1 diabetes. In: Gan D, editor. Diabetes Atlas. 3. Brussels: International Diabetes Federation; 2006. [Google Scholar]
- 87.Shaltout AA, Qabazard MA, Abdella NA, et al. High incidence of childhood-onset IDDM in Kuwait. Kuwait Study Group of Diabetes in Childhood. Diabetes Care. 1995;18:923–927. doi: 10.2337/diacare.18.7.923. [DOI] [PubMed] [Google Scholar]
- 88.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 89.Ajlouni K, Qusous Y, Khawaldeh AK, et al. Incidence of insulin-dependent diabetes mellitus in Jordanian children aged 0–14 y during 1992–1996. Acta Paediatr. 1999;88:11–13. doi: 10.1111/j.1651-2227.1999.tb14334.x. [DOI] [PubMed] [Google Scholar]
- 90.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 91.Rewers M, Stone RA, LaPorte RE, et al. Poisson regression modeling of temporal variation in incidence of childhood insulin-dependent diabetes mellitus in Allegheny County, Pennsylvania, and Wielkopolska, Poland, 1970–1985. Am J Epidemiol. 1989;129:569–581. doi: 10.1093/oxfordjournals.aje.a115169. [DOI] [PubMed] [Google Scholar]
- 92.Green A, Gale EA, Patterson CC. Incidence of childhood-onset insulin-dependent diabetes mellitus: the EURODIAB ACE Study. Lancet. 1992;339:905–909. doi: 10.1016/0140-6736(92)90938-Y. [DOI] [PubMed] [Google Scholar]
- 93.Dahlquist G (2004) The Swedish Childhood Diabetes Study Group. Data from The Swedish Childhood Diabetes Study Group (cited as reference 29 in [52])
- 94.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–S136. doi: 10.2337/diabetes.54.suppl_2.S125. [DOI] [PubMed] [Google Scholar]
- 95.Carrasco E, Pérez-Bravo F, Dorman J, Mondragón A, Santos JL. Increasing incidence of type 1 diabetes in population from Santiago of Chile: trends in a period of 18 years (1986–2003) Diabetes Metabol Res Rev. 2006;22:34–37. doi: 10.1002/dmrr.558. [DOI] [PubMed] [Google Scholar]
- 96.Padaiga Z, Tuomilehto J, Karvonen M, et al. Incidence trends in childhood onset IDDM in four countries around the Baltic sea during 1983–1992. Diabetologia. 1997;40:187–192. doi: 10.1007/s001250050661. [DOI] [PubMed] [Google Scholar]
- 97.Kulaylat NA, Narchi H. A twelve year study of the incidence of childhood type 1 diabetes mellitus in the Eastern Province of Saudi Arabia. J Pediatr Endocrinol Metabol. 2000;13:135–140. doi: 10.1515/JPEM.2000.13.2.135. [DOI] [PubMed] [Google Scholar]
- 98.Dabelea D, Bell RA, D’Agostino RB, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 99.Jarosz-Chobot P, Polanska J, Szadkowska A, et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia. 2011;54:508–515. doi: 10.1007/s00125-010-1993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pawlowicz M, Birkholz D, Niedźwiecki M. Czestosc wystepowania cukrzycy typu 1 wsrod dzieci w wojewodztwie pomorskimczy nadciaga wielka fala? Endokrynol Pediatr. 2007;6:70. [Google Scholar]
- 101.Serrano Ríos M, Moy CS, Martín Serrano R, et al. Incidence of type 1 (insulin-dependent) diabetes mellitus in subjects 0–14 years of age in the Comunidad of Madrid, Spain. Diabetologia. 1990;33:422–424. doi: 10.1007/BF00404093. [DOI] [PubMed] [Google Scholar]
- 102.Bruno G, Maule M, Merletti F, et al. Age-period-cohort analysis of 1990–2003 incidence time trends of childhood diabetes in Italy: the RIDI study. Diabetes. 2010;59:2281–2287. doi: 10.2337/db10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soliman AT, al-Salmi IS, Asfour MG. Epidemiology of childhood insulin-dependent diabetes mellitus in the Sultanate of Oman. Diabet Med. 1996;13:582–586. doi: 10.1002/(SICI)1096-9136(199606)13:6<582::AID-DIA114>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 104.Roche E, Menon A, Gill D, Hoey HMCV. National incidence of type 1 diabetes in childhood and adolescence. Ir Med J. 2002;95(115–116):118. [PubMed] [Google Scholar]
- 105.Pundziūte-Lyckå A, Dahlquist G, Urbonaite B, Zalinkevicius R. Time trend of childhood type 1 diabetes incidence in Lithuania and Sweden, 1983–2000. Acta Paediatr. 2004;93:1519–1524. doi: 10.1111/j.1651-2227.2004.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 106.Tzaneva V, Iotova V, Yotov Y. Significant urban/rural differences in the incidence of type 1 (insulin-dependent) diabetes mellitus among Bulgarian children (1982–1998) Pediatr Diabetes. 2001;2:103–108. doi: 10.1034/j.1399-5448.2001.002003103.x. [DOI] [PubMed] [Google Scholar]
- 107.Schober E, Rami B, Waldhoer T. Steep increase of incidence of childhood diabetes since 1999 in Austria. Time trend analysis 1979–2005. A nationwide study. Eur J Pediatr. 2008;167:293–297. doi: 10.1007/s00431-007-0480-5. [DOI] [PubMed] [Google Scholar]
- 108.Imkampe A-K, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991–2008. Diabet Med. 2011;28:811–814. doi: 10.1111/j.1464-5491.2011.03288.x. [DOI] [PubMed] [Google Scholar]
- 109.Tahirović H, Toromanović A. Incidence of type 1 diabetes mellitus in children in Tuzla Canton between 1995 and 2004. Eur J Pediatr. 2007;166:491–492. doi: 10.1007/s00431-006-0257-2. [DOI] [PubMed] [Google Scholar]
- 110.Tseng C-H. Incidence of type 1 diabetes mellitus in children aged 0–14 years during 1992–1996 in Taiwan. Acta Paediatr. 2008;97:392–393. doi: 10.1111/j.1651-2227.2008.00684.x. [DOI] [PubMed] [Google Scholar]
- 111.Tuomilehto J, Lounamaa R, Tuomilehto-Wolf E, et al. Epidemiology of childhood diabetes mellitus in Finland—background of a nationwide study of type 1 (insulin-dependent) diabetes mellitus. The Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1992;35:70–76. doi: 10.1007/BF00400854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 144 kb)