Abstract
The contribution of auditory cortex to spatial information processing was explored behaviourally in adult ferrets by reversibly deactivating different cortical areas by subdural placement of a polymer that released the GABAA agonist muscimol over a period of weeks. The spatial extent and time course of cortical inactivation were determined electrophysiologically. Muscimol-Elvax was placed bilaterally over the anterior (AEG), middle (MEG) or posterior ectosylvian gyrus (PEG), so that different regions of the auditory cortex could be deactivated in different cases. Sound localization accuracy in the horizontal plane was assessed by measuring both the initial head orienting and approach-to-target responses made by the animals. Head orienting behaviour was unaffected by silencing any region of the auditory cortex, whereas the accuracy of approach-to-target responses to brief sounds (40 ms noise bursts) was reduced by muscimol-Elvax but not by drug-free implants. Modest but significant localization impairments were observed after deactivating the MEG, AEG or PEG, although the largest deficits were produced in animals in which the MEG, where the primary auditory fields are located, was silenced. We also examined experience-induced spatial plasticity by reversibly plugging one ear. In control animals, localization accuracy for both approach-to-target and head orienting responses was initially impaired by monaural occlusion, but recovered with training over the next few days. Deactivating any part of the auditory cortex resulted in less complete recovery than in controls, with the largest deficits observed after silencing the higher-level cortical areas in the AEG and PEG. Although suggesting that each region of auditory cortex contributes to spatial learning, differences in the localization deficits and degree of adaptation between groups imply a regional specialization in the processing of spatial information across the auditory cortex.
Key points
The cerebral cortex plays a critical role in perception and in learning-induced plasticity.
We show that reversibly silencing any of the main regions of auditory cortex impairs the ability of adult ferrets to localize sound, with the largest deficit seen after deactivating the primary fields.
Although these animals had no trouble localizing longer sound bursts, their performance dropped considerably when auditory spatial cues were altered by occluding one ear with an earplug.
In contrast to control ferrets, which recovered their localization abilities with intensive training, adaptation to an earplug was impaired following cortical inactivation, with the greatest disruption in plasticity observed after silencing higher-level cortical areas.
These findings imply regional differences in the processing of spatial information across the auditory cortex.
Introduction
Numerous lesion studies, using different species and behavioural paradigms, have explored the role of auditory cortex in the localization (e.g. Neff et al. 1956; Jenkins & Masterton, 1982; Jenkins & Merzenich, 1984; Kavanagh & Kelly, 1987; Heffner & Heffner, 1990; Nodal et al. 2010) and discrimination of sounds (e.g. Kelly & Whitfield, 1971; Heffner & Heffner, 1986; Ohl et al. 1999; Harrington et al. 2001). These studies either used large aspiration lesions that included primary and much of non-primary auditory cortex or focused only on the primary auditory cortex (A1), and therefore did not address the question of whether different regions of auditory cortex have distinct functions. The existence of separate regions specialized for sound identification or localization is suggested by functional imaging (Alain et al. 2001; Hart et al. 2004), electrophysiological (Tian et al. 2001) and anatomical studies (Kaas & Hackett, 1999), as well as by the behavioural deficits observed when particular non-primary cortical areas are damaged (Adriani et al. 2003) or temporarily inactivated (Lomber & Malhotra, 2008). However, recording studies have shown that sensitivity to both spatial and non-spatial features is distributed over multiple cortical areas (Harrington et al. 2008; Recanzone, 2008; Bizley et al. 2009; Walker et al. 2011), indicating that these properties are not fully segregated. The extent to which a division of labour exists within the auditory cortex therefore remains a matter of controversy.
One approach to this issue is to examine the relative contributions of different cortical areas to stimulus-specific learning, on the basis that neurons in cortical areas that are specialized for a particular task might be expected to show a greater involvement than those in other areas when behavioural performance improves with training. Perceptual learning is often associated with changes in the response properties of neurons in early sensory cortex. For example, training-induced plasticity has been demonstrated in the primary somatosensory (Recanzone et al. 1992; Xerri et al. 2005), auditory (Recanzone et al. 1993; Polley et al. 2006; Schnupp et al. 2006) and visual (Crist et al. 2001; Schoups et al. 2001; Hua et al. 2010) cortices. However, it has been argued that the physiological changes observed in primary visual cortex are insufficient to account for the observed improvements in behaviour (Ghose, 2004; but see also Gilbert et al. 2009), whereas response selectivity does change with training in higher cortical areas that are more specialized for processing the trained stimulus feature (Zohary et al. 1994; Kobatake et al. 1998; Yang & Maunsell, 2004; Raiguel et al. 2006).
In addition to enabling an improvement in performance with training, the plasticity of sensory processing in adulthood allows adaptation to abnormal inputs. This is most clearly illustrated in the context of sound localization, which relies on the detection and interpretation of spatial cues present in the sound waves that arrive at the two ears. Adult listeners can learn to accommodate substantially altered cues, produced, for example, by occluding one ear (Kacelnik et al. 2006; Kumpik et al. 2010; Irving et al. 2011) or by reshaping one or both external ears (Hofman et al. 1998; Van Wanrooij & Van Opstal, 2005). We have previously shown that the ability of ferrets to relearn to localize sound in the presence of a unilateral earplug requires an intact auditory cortex (Nodal et al. 2010), and, more specifically, layer V corticocollicular projection neurons (Bajo et al. 2010a), but it is unknown whether particular cortical areas are responsible for this process.
In this study, we examined the effects of deactivating different regions of the auditory cortex, using muscimol-releasing Elvax, on the ability of ferrets to localize sound in the horizontal plane and to adapt to the altered spatial cues produced by reversibly occluding one ear. Electrophysiological studies have shown that the majority of neurons in the auditory cortex of this species are sensitive to both spatial and non-spatial sound attributes (Bizley et al. 2009). However, spatial sensitivity is greatest in A1 (Bizley & King, 2008; Walker et al. 2011) and in the anterior dorsal field (Bizley & King, 2008), suggesting that silencing these areas should have the largest behavioural effects. Although differences in the localization deficits and degree of adaptation were observed depending on which region of the cortex was silenced, our data suggest that much of the auditory cortex is engaged during these tasks.
Methods
Animals
All procedures involving animals were performed following University of Oxford Committee on Animal Care and Ethical Review approval and under licence from the UK Home Office in accordance with the Animals (Scientific Procedures) Act 1986. Twenty-six adult pigmented ferrets (Mustela putorius furo) from our breeding colony were used in this study.
Sound localization abilities of the ferrets were tested by positive conditioning using water as a reward as previously reported (Kacelnik et al. 2006; Nodal et al. 2008, 2010). Briefly, animals were tested in blocks that lasted up to 14 consecutive days with breaks between blocks of ≥4 days during which they were given free access to water and food.
Approach-to-target behaviour
The localization task was carried out in a circular arena of 70 cm radius enclosed by a hemispheric mesh dome and located inside a double-walled testing chamber lined with acoustic foam (MelaTech; Hodgson & Hodgson Ltd, Melton Mowbray, UK). Twelve loudspeakers and their associated water spouts were positioned at 30 deg intervals around the perimeter and hidden from the animal by a muslin curtain (Supplementary Fig. 1). An animal self initiated the trials by licking a centrally positioned waterspout. This ensured that its head was positioned at the centre of the arena, facing the speaker at 0 deg, when sound stimuli were delivered. Speakers to the animal's left were denoted by negative numbers and those to the right by positive numbers.
All stimuli were broadband noise bursts (with a low-pass cut-off frequency of 30 kHz) generated afresh on each trial using Tucker-Davis Technologies (Alachua, FL, USA) System 2 hardware. The stimuli were filtered using the inverse transfer function for each speaker in order to obtain a flat spectrum, and matched for overall level across the different speakers. Data were collected using sound durations of 2000, 1000, 500, 200, 100 and 40 ms. In each testing session, the sound duration was kept constant while the level was roved pseudorandomly from trial to trial in 7 dB steps from 56 to 84 dB sound pressure level (SPL). This was done in order to disrupt potential level cues arising from the acoustic shadowing caused by the animal's body and which might otherwise allow target localization based on the relative loudness of the stimulus.
Only correctly judged responses were rewarded. To reduce the possibility of bias towards particular speaker locations, an incorrect response was followed by a correction trial (same stimulus and location) up to two times. If the animal continued to mislocalize the sound, an easy trial (comprising a continuous series of noise bursts from the same location) was presented. Neither the correction nor the easy trials were included in the analysis. Although we did not condition the animals to make a prompt response, we observed that for >98% of the trials the time to respond was <5 s. As in previous studies, we therefore assumed that the very few trials taking >5 s to complete reflected a lack of attention and excluded these from the analysis (Nodal et al. 2008).
Initial head orienting response
In addition to the approach-to-target responses, we measured the initial head orienting response (50 Hz sampling rate for one second) following stimulus onset by using an overhead infrared-sensitive camera and video contrast detection device (HVS Image, London, UK) to track the angular location of a self-adhesive reflective strip attached along the midline of the animal's head (Supplementary Fig. 1). In contrast to the approach-to-target responses, where the animals had to select which of the 12 loudspeaker/reward–spout combinations most closely matched the perceived sound direction, the initial head turns were unconstrained by the location of the reward spouts and therefore provided a more absolute measure of sound localization accuracy. The animal was considered to have initiated an orienting response if a head movement in the same direction was recorded over three consecutive frames, with the time of the first frame taken as the latency of the movement. The initial head turn was considered over when a change in the direction of the movement was recorded. The final head bearing was calculated as the mean angle from the last three frames of this initial movement. Trials were excluded if the initial head angle (at the time of sound onset) deviated by >7 deg from straight ahead or if the head movement latency exceeded 500 ms.
Elvax preparation
Elvax sheets embedded with muscimol (Tocris Cookson, Bristol, UK) were prepared following previous descriptions (Smith et al. 1995, 2004). Briefly, beads of the ethylene–vinyl acetate copolymer Elvax 40-W (Elvax 40P; Du Pont) were washed for 24 h in several alcohol gradients (5 times in 95% and 5 times in 100%). The beads then were filtered, dried and stored in the dark at room temperature. Washed Elvax beads (0.5 g) were dissolved in dichloromethane (Sigma-Aldrich) at room temperature to give a 10% w/v solution. A 100 μl sample of either double-distilled water (for control implants) or an aqueous solution of muscimol was added to the Elvax/dichloromethane solution. Aqueous muscimol solutions were produced by dissolving muscimol in one equivalent of 10 m NaOH(aq) and making-up the volume to 100 μl with double-distilled water. Muscimol ‘concentrations’ in Elvax were taken to be the concentration in the Elvax/dichloromethane/aqueous suspension; thus, addition of 100 μl of 3.75 m muscimol(aq) gave rise to ‘75 mm muscimol’ Elvax. In order to visualize the polymer slices for implantation, 50 μl of 5% Fast Green(aq) (Allied Chemical) was also added to the Elvax mixture. The resulting suspension was immediately poured in a slow, steady stream onto a horizontal glass plate resting on dry ice. After 20 min, the Elvax sheet was peeled off the plate with cold forceps and freeze-dried overnight (−60°C, 10–4 atm). The dry Elvax pancake (approximately 2 mm deep) was sectioned on a vibrating-blade microtome at 200 μm. Sections were stored on filter paper at 4°C prior to implantation.
Surgical procedures
Animals were initially anaesthetized by an intramuscular administration of a mixture of 0.022 mg kg−1 medetomidine hydrochloride (Domitor; Pfizer Ltd) and 5 mg kg−1 ketamine (Ketaset; Fort Dodge Animal Health) and maintained with 0.5–1.5% isoflurane (IsoFlo; Abbott Laboratories Ltd). Fluid (saline 5 ml h−1) was provided i.v., and the animal's core temperature, ECG and end-tidal CO2 were maintained throughout the procedure.
Other drugs administered were atropine (0.006 mg kg−1; Atrocare; Animalcare Ltd) to reduce bronchial secretions and buprenorphine (0.03 mg kg−1; Vetergesic; Alstoe Animal Health) and meloxicam (0.2 mg kg−1; Metacam; Boehringer Ingelheim) to provide perisurgical analgesia. Finally, local anaesthetic (Elma; Astra Zeneca) was applied to the stereotaxic pressure points and carbomer (Viscotears; Lewis Pharmaceuticals Ltd) to the eyes. Once the head was fixed in the stereotaxic frame, the skull over the ectosylvian gyrus (EG), where the auditory cortex lies, was exposed and craniotomies were drilled bilaterally. A slit made in the dura mater allowed the sheet of Elvax polmer to be inserted over the appropriate region of cortex or removed from it.
Once the temporal muscles and skin were repositioned and sutured the animal was allowed to recover from anaesthesia. Postsurgical analgesia was provided by administering meloxicam and buprenorphine for 2 days, followed by meloxicam alone for 3 more days.
Experimental design
Twenty-one animals were trained and tested in our approach-to-target task (Supplementary Fig. 1) and, once they showed stable sound localization behaviour, were assigned to one of four experimental groups. The control group (n = 6) received bilateral subdural implants of drug-free Elvax polymer over the middle EG (MEG). The other three groups received bilateral subdural implants of Elvax loaded with muscimol (75 μm) over different regions of the ectosylvian gyrus: the MEG (n = 4), the posterior EG (PEG) (n = 5) and the anterior EG (AEG) (n = 6). These morphological subdivisions were easily identified based on the sulcal pattern exposed by the craniotomy and allowed consistency in the placement of the implants without requiring electrophysiological recordings that might have caused local damage to the underlying cortex.
The polymer placements were not intended to target specific cortical fields within each region of the EG since these are broadly similar in their physiological response properties (Nelken et al. 2004, 2008; Bizley et al. 2005, 2007a) and connectivity patterns (Bajo et al. 2007, 2010b; Bizley et al. 2007a). Thus, the MEG comprises the primary fields, the primary auditory cortex (A1) and anterior auditory field (AAF), whose neurons are tonotopically organized and have short response latencies. The PEG contains the posterior pseudosylvian field (PPF) and posterior suprasylvian field (PSF), which are also tonotopically organized, but are characterized by more sustained and stimulus-dependent firing patterns and a greater tendency to exhibit tuning for both sound frequency and level than in the primary fields. Finally, the AEG includes the anterior dorsal field (ADF) and anterior ventral field (AVF), which are not tonotopically organized and (especially AVF) contain particularly high numbers of visually responsive neurons.
All animals were allowed to recover for 5 days after the surgery for Elvax implantation before behavioural testing was resumed. Their ability to localize bursts of broadband sound of different durations and levels was then tested again during the following 7–10 days, after which we assessed during the following 2 weeks the contribution of the inactivated cortex to training-induced plasticity of spatial hearing. This was done by occluding the external auditory meatus of the left ear under medetomidine hydrochloride sedation (0.1 mg kg−1 body weight i.m.) with a foam plug (E.A.R., 3M), which was held in place by filling the concha of the external ear with Otoform-K2 silicone impression material (Dreve Otoplastik, Unna, Germany). Acoustical measurements indicated that these earplugs produced 40–50 dB of attenuation at frequencies of >3.5 kHz, which rolled off gradually at lower frequencies. We have previously shown that, with appropriate behavioural training, ferrets can relearn to localize noise bursts of both 1000 ms and 40 ms duration after occluding one ear in this fashion (Kacelnik et al. 2006). As in our earlier studies in which parts of the cortex (Nodal et al. 2010) or its descending projections (Bajo et al. 2010a) were ablated, we focused here on adaptation at 1000 ms. This is because (a) cortical lesions impair the localization of 40 ms, but not 1000 ms, noise bursts, therefore confounding the interpretation of any effects on learning, and (b) monaural occlusion results in a particularly large change in performance for the longer stimuli, thereby facilitating a comparison of the effects of deactivating different regions of auditory cortex on the recovery of both approach-to-target and head orienting behaviour.
Data analysis
Our software registered the reward spout licked by the animal and converted this to a percentage correct-score and error magnitude and direction for each trial. These values, along with the head movement data and the associated stimulus parameters (location, duration and level), were exported to Microsoft Excel for further analysis and presentation. Statistical analysis was done with SPSS software (SPSS Inc., Chicago, IL, USA).
The localization behaviour of the animals was further quantified by calculating the mutual information (MI) between target and response locations using the following formula:
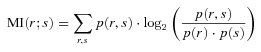 |
where r is the response location or final head bearing, s is the target location, MI(r; s) is the MI between r and s, p(r, s) is the joint probability of r and s, and is equivalent to p(r | s) p(s), where p(s) and p(r) are obtained from the overall distribution of target locations and response locations, respectively. This takes into account the distribution of the responses for each target location, not just whether the response was correct or not.
Electrophysiology
To ensure the effectiveness of the muscimol-Elvax polymer in silencing cortical activity, all batches were tested before implantation in acute electrophysiological experiments (n = 4). In one additional animal, we also tested the long-term extent of the cortical inactivation by carrying out a recording experiment 3 weeks after bilateral muscimol-Elvax implantation over auditory cortex, corresponding to the stage where most of the behavioural data had been collected in the other implanted ferrets. More extensive recordings from the auditory and visual cortices of ferrets implanted chronically with muscimol-Elvax have been published previously (Smith et al. 2004; Bizley et al. 2007b).
Electrophysiological recordings were carried out in an anechoic chamber from A1 of these five anaesthetized ferrets. Cortical activity was measured using single shank Michigan electrodes (Neuronexus, Ann Arbor, MI, USA) with 16 recording sites spaced at 150 μm intervals. The probes were oriented approximately orthogonal to the cortical surface, so that recordings could be made over the full depth of the cortex, both prior to the application of the polymer and again following implantation, via a hole in the Elvax sheet, in the acute experiments (Fig. 1A) or beneath the polymer once it had been removed in the chronic experiment. Recordings were also made beyond the area covered by the implant to determine the spatial extent of the inactivated cortex. Unfrozen broadband noise bursts (100 ms duration) were generated using Tucker-Davis Technologies System 3 hardware and Matlab (The Mathworks Inc., Natick, MA, USA) and presented at a range of levels (30–80 dB SPL) through pairs of Panasonic earphone drivers (RPHV297), which were mounted on plastic otoscope speculae inserted into each ear canal.
Figure 1. Muscimol-Elvax silences cortical neurons.
A, photograph of a ferret brain used for electrophysiological recordings showing the location of the muscimol-Elvax sheet. Recordings were made in the underlying cortex with the Elvax in place (via each of the two holes indicated) and in the immediately adjacent cortex. B, stack of peristimulus time histograms showing the neural activity recorded beneath the implant in response to 100 ms noise bursts (stimulus duration indicated by the grey rectangle) delivered to the contralateral ear at different times following the application of the muscimol-Elvax implant. Both driven responses (defined as spike counts in the 50 ms window after stimulus onset that were significantly different (P < 0.05, paired t test) from those in a 50 ms window starting 800 ms after stimulus onset) and spontaneous activity gradually disappear over the following 90 min. C, coronal section through the MEG showing an example of one of the electrode penetrations. The borders of the cortical layers are indicated by dashed lines and the schematic diagram next to the photomicrograph shows the approximate location of the recording sites, 150 μm apart, along the probe, which spanned the whole depth of the cortex. Calibration bar, 250 μm. D, photograph of a ferret brain illustrating the different implantation locations over the MEG, AEG or PEG in the animals used for behavioural testing; the Elvax sheets were implanted bilaterally over one of these regions in individual animals. A1, primary auditory cortex; AAF, anterior auditory field; ADF, anterior dorsal field; AEG, Anterior Ectosylvian Gyrus; AVF, anterior ventral field; MEG, middle ectosylvian gyrus; PEG, posterior ectosylvian gyrus; PPF, posterior pseudosylvian field; PSF, posterior suprasylvian field; pss, pseudosylvian sulcus; sss, suprasylvian sulcus; VP, ventroposterior field.
Off-line spike sorting was performed using ‘spikemonger’, an in-house software package for Matlab. Candidate spikes were identified as voltage-threshold crossing events. The recorded waveforms on sets of five neighbouring channels were clustered using an automated expectation maximization algorithm; any given spike typically produced measurable signals on one or two of these channels. The resulting clusters were analysed manually to eliminate artifactual clusters (such as waveforms whose shapes were very similar over the whole cortical depth). No attempt was made to distinguish single-unit from multi-unit activity.
Histological procedures
To evaluate whether the muscimol-Elvax implants had any effect on the morphology of the auditory cortex, histological analysis was carried out in most cases (MEG implants, n = 1; PEG implants; n = 5; AEG implants, n = 4) once the behavioural testing was complete. Some of the first animals to receive MEG implants (n = 3) were subsequently used in another study for electrophysiologically guided aspiration of A1. In those cases, no sign of cortical atrophy was observed when the craniotomy was opened above the MEG, and the frequency tuning and thresholds of the recorded neurons did not differ in any obvious way from those in control animals.
When the behavioural tests were finished, the animals were sedated with medetomidine hydrochloride (0.1 mg (kg body weight)−1 i.m.) and overdosed with sodium pentobarbital (2 ml of 200 mg ml−1 Euthatal, i.p.; Merial Animal Health Ltd) prior to being perfused with 300 ml saline solution (0.9% NaCl, pH 7.2), followed by 1 litre of freshly prepared fixative (4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.2).
The brain was extracted from the skull and left under gentle agitation in 30% sucrose solution in 0.1 m PB at 5°C for 48 h. Coronal sections at 40 μm thickness covering the full extent of the EG were cut using a freezing microtome and three sets of serial sections were collected in 0.1 m PB. The first set of sections was Nissl stained with 0.5% cresyl violet. The second set was used for selectively staining neurons with mouse anti-neuronal nuclei protein (NeuN) monoclonal antibody (MAB377, Millipore Corp.). For the third set of serial sections, we used the neurofilament H non-phosphorylated (SMI32) mouse monoclonal antibody (Covance Research Products Inc.) to stain only non-phosphorylated epitopes on the medium (170 kDa) and heavy (200 kDa) molecular weight subunits of neurofilament H (Sternberger & Sternberger, 1983). Protocols used for NeuN and SMI32 immunohistochemistry in the ferret have been reported previously (Bajo et al. 2007, 2010a,b)
Histological analysis
Examination and analysis of the sections were carried out using Neurolucida (MBF Bioscience, MicroBrightField, Williston, VT, USA) image-analysis software and a Leica DMR microscope (Leica Microsystems). Photomicrographs were taken at the microscope using a digital camera (Microfire, Olympus America Inc.).
The thickness of the cortex and its layers was measured in Nissl-stained sections taken from the three main regions (MEG, PEG and AEG) where the implants were placed (see Fig. 1D). Ten random measurements, perpendicular to the cortical layers and avoiding the surrounding sulci, were taken for each region, side of the brain and animal. Comparisons between implanted and non-implanted cortex were carried out using Student's t test with SPSS software. All measurements are given as the mean ± standard deviation. In a subset of three animals, one for each of the regions of the auditory cortex implanted with muscimol-Elvax, we also measured the thickness of the different cortical layers in NeuN-stained sections.
Results
Cortical inactivation by muscimol-Elvax
Acute recording experiments were performed to test the effectiveness of each batch of muscimol-Elvax prior to implantation in trained animals (Fig. 1). Recordings were first made in the MEG before the Elvax was applied and responses to sound were typically found on at least 12 of the 16 recording sites on the linear electrode arrays. In keeping with previous reports (Bizley et al. 2005), these were characterized by short latency (≤25 ms) onset responses with thresholds around 40 dB SPL. The electrode was then removed and the muscimol-Elvax placed sub-durally over the MEG. An electrode was lowered into the cortex through a hole made previously in the Elvax (Fig. 1A) and the implant and exposed cortex were covered with 2% agar to provide stability and to keep the implant moist.
Neural activity was monitored every 10–15 min (Fig. 1B) from positions that spanned the full depth of the cortex (Fig. 1C). As with the pre-implantation recordings, auditory responses were initially recorded on at least 12 of the recording sites. However, ∼30 min following implantation, both driven and spontaneous activity were clearly reduced at the two to three most superficial sites, whereas activity at deeper recording sites remained unchanged. Over the next few hours, progressively deeper recording sites became unresponsive. Deactivation of the cortex did not progress with depth in a linear manner, as recording sites located around the middle of the probe were usually the last to be silenced. Subsequent histological analysis confirmed that those sites corresponded to intermediate cortical layers (Fig. 1C), where most of the thalamic afferents terminate.
Recordings made beyond the edges of the implant (≥500 μm) continued to register normal acoustically driven activity even after the region of cortex beneath the implant had been deactivated. This is consistent with previous electrophysiological and pharmacological data (Smith et al. 2004; Bizley et al. 2007b) showing that the release of muscimol from Elvax is largely restricted to the underlying cortex. This is therefore an appropriate method for examining the contribution of specific regions of cortex to behavioural tasks. After activity had been lost on all 16 electrode channels, the implant was removed and further recordings made in the same region of the MEG. Normal activity resumed 1–3 h later.
In one animal (Fig. 2) we examined the longer term effects of implanting muscimol-Elvax to ensure that cortical inactivation lasted long enough for the animals to complete their behavioural testing. Muscimol-Elvax implants were implanted bilaterally over the MEG and 3 weeks later recordings were made as before. We began by recording adjacent to the implant (electrode penetrations 1 and 2 in the right auditory cortex; Fig. 2A, and penetrations 15 and 16 on the left side; Fig. 2B). Acoustically driven or spontaneous activity was observed on all recording sites for each of these electrode penetrations, again showing that lateral diffusion of muscimol from the Elvax implant was very limited. This is illustrated by the black (auditory response) and grey (spontaneous activity) boxes at the different recording sites for penetrations 1 and 2 in Fig. 2C and for penetrations 15 and 16 in Fig. 2D.
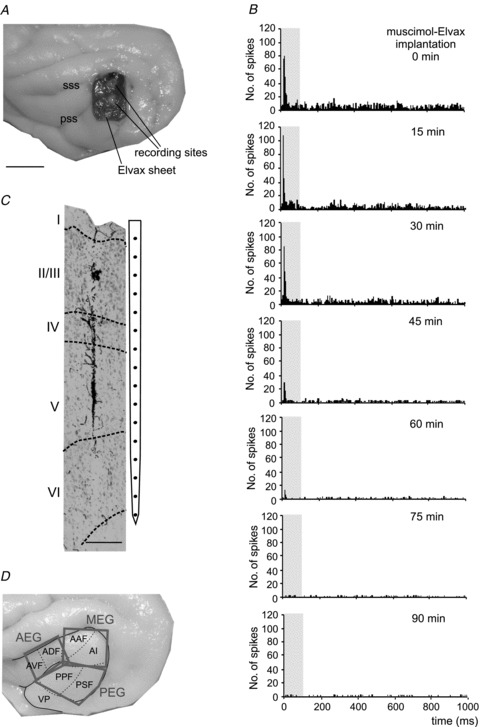
Figure 2. Long term inactivation of the auditory cortex by muscimol-Elvax implants.
A and B, photographs of the two cortical hemispheres of a ferret showing the locations of the muscimol-Elvax implants (rectangles) and of the recordings made 3 weeks after implantation. C and D, the boxes in each column correspond to different recording sites along an individual probe arranged from superficial to deep. The different columns of boxes refer to different recording sites whose locations are indicated by the corresponding numbers in A and B. The relative time at which the recordings were made is shown at the top of each column and refers to the time from explantation of the muscimol-Elvax sheet. The recordings for the first two columns in each hemisphere (#1 and #2 in C and #15 and #16 in D) were made to one side of the Elvax sheets while they were still in place. All the other recordings were made after explantation in the region of cortex previously occupied by the Elvax. Whereas driven (black boxes) or spontaneous (grey boxes) activity was found at all cortical depths adjacent to the Elvax, no neural activity (white boxes) was present in the great majority of the initial recordings made in the cortex previously covered by the implants. However, auditory responses gradually returned over time and were found at nearly all recording sites after ∼80 min following Elvax removal in the left auditory cortex (D) and 140 min on the right side (C). E, photomicrograph of a coronal section of the left hemisphere in which the tracts of penetrations #20 and #27 are visible. Calibration bars, 1 mm in A and B; 0.5 mm in E. A, anterior; D, dorsal; M, medial; P, posterior; ps, pseudosylvian sulcus; sss, suprasylvian sulcus.
The muscimol-Elvax was then removed, one side at a time, and a total of 11 penetrations were made in the area previously occupied by the implant on the left side (Fig. 2A) and 12 on the right side (Fig. 2B). Very little or no neural activity was recorded in the auditory cortex in the majority of penetrations made during at least the first hour following the removal of the implant (indicated by the white boxes in Fig. 2C and D). Although the spacing between recording sites (150 μm) suggested that the full cortical depth should have been sampled in these penetrations, we extended two of the tracks (no. 20 and no. 27) into the white matter to confirm that this was the case (Fig. 2E).
The time course of the recovery of cortical activity varied between the two hemispheres and was in line with the data from the acute experiments. In the case of the left auditory cortex, driven or spontaneous activity was initially recorded at the most superficial sites and was found over the full depth after ∼1 h (Fig. 2D). By contrast, the right auditory cortex took longer to recover, with activity returning at all depths ∼2 h following removal of the implant (Fig. 2C). As a control for loss of responsiveness due to a change in the condition of the animal, every 30–60 min we went back to one of the cortical locations adjacent to where the Elvax had been located and verified that normal driven activity was still present. Histological processing of the brain confirmed the locations of the recording sites in the auditory cortex (Fig. 2E). Together, these electrophysiological data indicate that the region beneath the implant had been effectively deactivated for the full 3 weeks that the muscimol-Elvax had been in place in this instance.
Normal sound localization behaviour
The pre-implantation data from all 21 animals used for behavioural testing are summarized in Fig. 3. In keeping with previous studies of auditory localization in the ferret (Nodal et al. 2008), no differences in the accuracy of either approach-to-target (ANOVA, F(4,465) = 0.835; P = 0.504) or initial head orienting responses (ANOVA, F(4,82) = 1.873; P = 0.123) were observed within the range of sound levels (56–84 dB SPL) tested. Data for both measures of localization performance were therefore pooled across sound level for all subsequent analyses.
Figure 3. Sound localization behaviour before Elvax implantation.
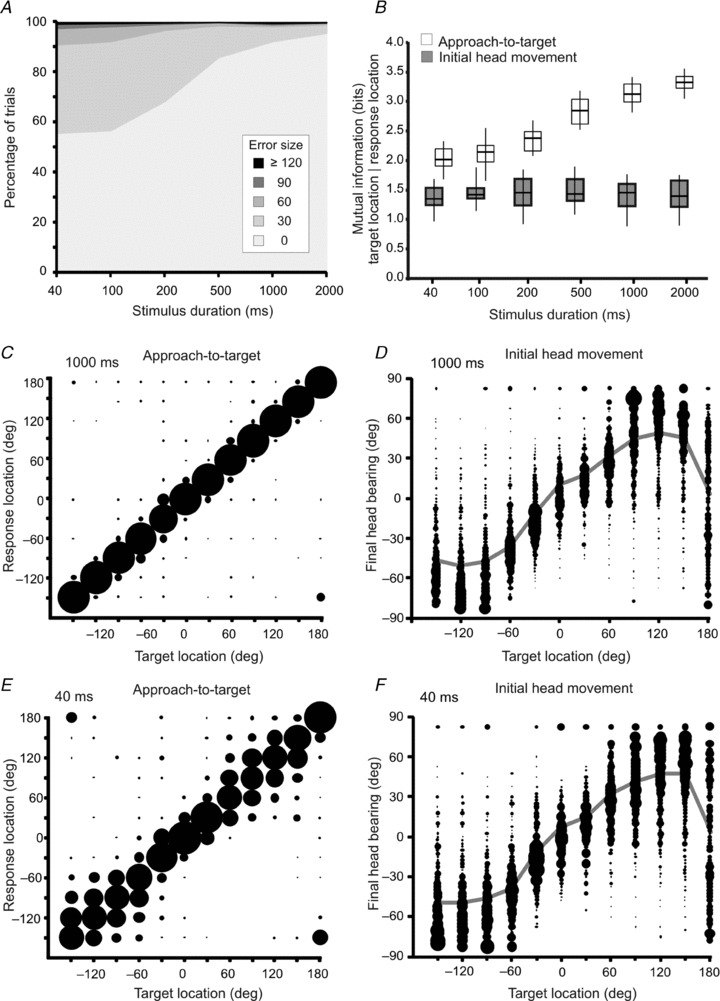
A, overall percentage across all target locations of trials classified by their error size for the different stimulus durations tested (2000 to 40 ms). The incidence of incorrect trials and the size of the errors increased as the stimulus duration was reduced. B, mutual information (MI) values between target location and response location for the approach-to-target and head orienting responses at different stimulus durations. The MI between target and response location increased with stimulus duration for the approach-to-target task, indicating that the animals became more accurate, but remained constant for the relationship between final head bearing and target location. Boxes represent the inter-quartile range, the horizontal line the median and the vertical line the full range of MI values. C and E, stimulus–response plots showing the distribution of approach-to-target responses for two stimulus durations, 1000 ms (C) and 40 ms (E). For each target location the size of the dots is proportional to the probability of responses to different target locations. The corresponding percentage of correct responses and MI values for these stimulus durations (1000 and 40 ms) are shown in panels A and B, respectively. D and F, stimulus–response plots showing the distribution of final head bearings (binned in steps of 7.5 deg) for each target location. The size of the dots is proportional to the response probability. The grey lines represent the mean final bearing for each target location.
Approach-to-target localization accuracy, as measured by the percentage of correct scores across the 12 target locations, declined as the duration of the stimulus was reduced from an average of ∼95% correct for 2000 ms to ∼56% correct for 40 ms, (ANOVA, F(5,15) = 260.678; P < 0.001) (Fig. 3A). The MI between target and response location mirrored the percentage of correct responses, becoming smaller as the stimuli were reduced in length from 2000 ms to 40 ms (ANOVA, F(5,102) = 91.689; P < 0.001) (Fig. 3B). The approach-to-target behaviour also became less precise as the stimulus duration was reduced, as indicated by the greater spread in the responses (Fig. 3C and E) and the larger errors made (Fig. 3A) at shorter stimulus durations. These effects of stimulus duration were location dependent as the performance of the animals was unchanged in the frontal region of space (±30 deg), but declined for briefer sounds at more lateral and posterior locations. Extremely few left-right errors were made, as sounds were hardly ever mislocalized across the midline.
In contrast to the approach-to-target behaviour, the distribution of the sound-evoked head orienting responses made before the animals left the central start platform was unaffected by stimulus duration (Fig. 3D and F), and the MI between target location and final head bearing remained unaltered as the duration was reduced from 2000 ms to 40 ms (Fig. 3B). The latency of the initial head orienting response was 179.64 ± 109.94 ms. This suggests that the spatial cues provided by the onset of the target sound are used to drive this behaviour, which could therefore explain the lack of any effect of stimulus duration on the accuracy of these movements. For speaker locations in the frontal hemifield, the amplitude of the head orienting responses increased linearly with the eccentricity of the target locations, though consistently undershooting them up to a maximum value of ∼±60 deg for target angles ≥90 deg (Fig. 3D and F).
Effect of cortical inactivation on localization behaviour
The overall pattern observed for the approach-to-target behaviour remained unchanged after deactivating any of the three major subdivisions of the EG (Fig. 4). In fact, for long (≥100 ms) durations, no differences were observed following implantation of muscimol-Elvax over any of the subdivisions of the EG in the percentage of correct trials or in the size of the errors made on incorrect trials (Wilcoxon signed rank test for paired comparisons of pre- and post-implantation data within each experimental group, P > 0.05).
Figure 4. Effect of cortical inactivation on the localization of 40 ms noise bursts.
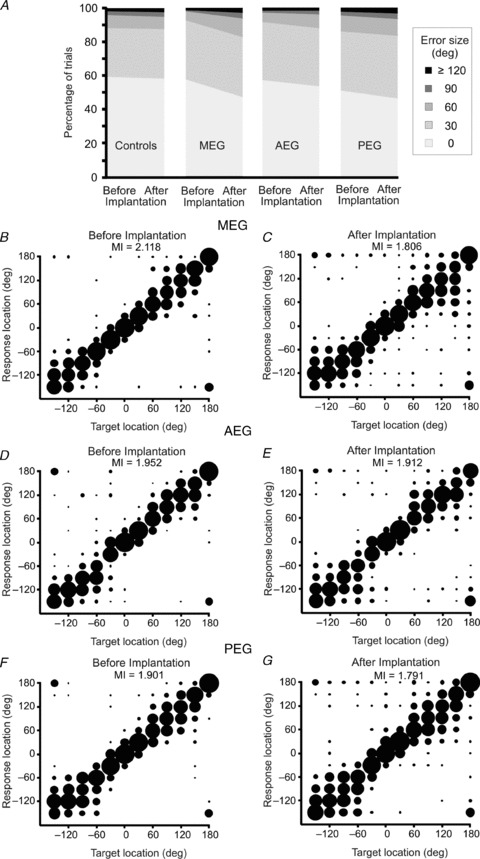
A, distribution of trials classified by error size before and after bilateral implantation of either drug-free Elvax (Controls) or muscimol-Elvax over the cortical regions indicated (MEG, AEG or PEG). B–G, stimulus–response plots showing the approach-to-target performance before (left panels) and after (right panels) implanting muscimol-Elvax bilaterally over the MEG (B and C), AEG (D and E) or PEG (F and G). The MI (in bits) between target location and response location is given in each panel. Stimulus–response plots for the control group are not shown as there were no differences following Elvax implantation (MI = 1.921 bits before implantation and 1.973 bits after implantation).
However, for brief target sounds (40 ms), placement of muscimol-Elvax implants over the MEG, AEG and PEG all resulted in small localization deficits, as indicated by a reduction in the percentage of correct trials (Fig. 4A) and in the MI between stimulus and response location (Fig. 4B–G). By contrast, no changes in performance were observed in the animals with drug-free Elvax implants (Fig. 4A, first column). After silencing the MEG, the percentage of correct trials fell from 58.7 ± 6.5% to 47.8 ± 6.6% (t(3) = 2.398, P = 0.048), while the incidence of front–back errors (sounds presented in the frontal hemifield that are mislocalized into the posterior ipsilateral hemifield or vice versa) rose from 2.3 ± 0.6% to 7.4 ± 1.6% (t(3) = −4.88, P = 0.008). These changes in performance are in line with our previous data in which A1 was reversibly inactivated (Smith et al. 2004). Although inactivation of non-primary auditory regions in the AEG and PEG had a similar effect, the only significant difference between the pre- and post-implantation scores was an increase in front–back errors after inactivation of the AEG (t(3) = −8.54; P = 0.002).
However, individual measures, like the percentage of correct scores, fail to take into account possible differences in the types of localization errors made by the animals. We therefore constructed two-way contingency tables for each group to test if the distribution frequencies of all trials, classified by their error size (0 deg, 30 deg, 60 deg, 90 deg or ≥120 deg), changed after deactivating different regions of the auditory cortex. This analysis revealed a significant difference in the distribution of localization errors for 40 ms sounds before and after muscimol-Elvax implantation over all three cortical regions (MEG: χ2(6) = 202.6, P < 0.0001; AEG: χ2(6) = 42.1, P < 0.0001: PEG χ2(6) = 33.1, P < 0.0001), whereas no difference was found for the control group (χ2(4) = 1.24, P = 0.87). These findings therefore indicate that deactivating any of the main auditory cortical regions impairs the localization of brief sounds, although the largest effects were seen when the primary areas in the MEG were silenced.
The initial head orienting responses appeared to be unaffected by inactivation of any of the cortical areas. No differences were found for the final head bearings among the different experimental groups before and after insertion of the implants (ANOVA, F(2,81028) = 2.376; P = 0.296). Although suggesting that acoustic orientation behaviour might not require the integrity of the auditory cortex, it is possible that any modest localization deficits like those apparent in the approach responses might have been obscured given that the average amplitude of the head turns was very similar for target locations between 90 deg and 150 deg from the frontal midline.
Effect of cortical inactivation on sound localization plasticity
Occluding one ear alters the spatial cue values corresponding to different directions in space and therefore disrupts localization accuracy in ferrets (Kacelnik et al. 2006; Irving et al. 2011) and humans (Kumpik et al. 2010). Near normal localization performance can recover over a few days, however, so long as appropriate behavioural training is provided.
A repeated measures ANOVA revealed a significant effect of day of wearing the earplug (F(8,5) = 5.263, P = 0.042) and of experimental group (F(3,12) = 3.945, P = 0.036) on the percentage correct scores. The control ferrets that received drug-free Elvax implants showed the same adaptive behaviour previously reported for normal animals (Fig. 5A). Thus, insertion of a unilateral earplug resulted in a reduction in the percentage of correct responses for 1000 ms stimuli from >90% to ∼30%, which reflected an increase both in the number of errors made and in the magnitude of those errors. Approach-to-target behaviour was degraded at all target locations, but particularly in the hemifield ipsilateral to the occluded left ear (Supplementary Fig. 2A). The performance of the control ferrets steadily improved in the days following insertion of the earplug, resulting in a progressive reduction in the disparity between the animals’ apparent perceived location and the actual target location. In accordance with our previous work, the earplug was typically left in place for 9 days, with the animals undergoing two testing sessions per day. By the end of this period, the average score of the control ferrets had risen to ∼70% correct (Figs 5A, Supplementary Fig. 2A). The earplug was then removed, which immediately resulted in approach-to-target scores very close to those achieved in the pre-plug sessions (Fig. 5A). The performance of these control animals changed significantly over this sequence of testing (ANOVA (F(3,27) = 39.44, P < 0.001), with post hoc analysis revealing that the scores on the last day of earplugging were higher than those achieved when the ear was first plugged (P < 0.01), but no different from those made in the post-plug session (P = 0.125).
Figure 5. Time course of adaptation to a unilateral earplug.
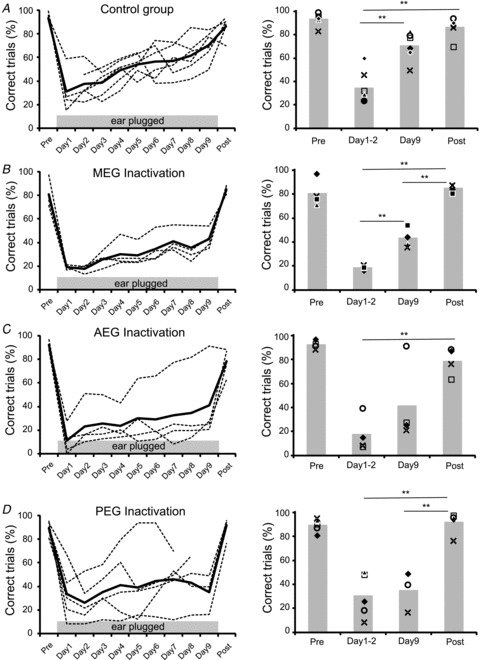
A–D, percentage of correct trials measured every day from individual animals and averaged across all speaker locations are shown by the dashed lines, with the mean performance for each group indicated by the continuous lines. Data are shown prior to insertion of an earplug in the left ear (Pre), on each of the 9 days that the earplug was in place (Days 1–9), and following its removal (Post). A, drug-free controls. B, muscimol-Elvax over the MEG. C, muscimol-Elvax over the AEG. D, muscimol-Elvax over the PEG. The histogram plots on the right show the mean percentage of correct responses in the Pre and Post earplug sessions and at the beginning (Day 1–2) and end (Day 9) of the period of monaural occlusion. Data from individual animals are indicated by the different symbols. Significant differences revealed by ANOVA and post hoc Scheffé's test are shown by the asterisks (P < 0.01). For all groups, the Day 1–2 earplugging scores were also significantly lower than both Pre and Post earplugging scores.
For all the experimental groups in which one of the subdivisions of the EG was deactivated, adaptation to the earplug was reduced compared to that seen in the control group (Figs. 5B–D and Supplementary Fig. 2). In order to quantify the rate of adaptation, we calculated the regression lines for the percentage of correct trials over the period of monaural occlusion (Fig. 5 and Supplementary Fig. 3). Although these regression lines had positive slopes in all groups, implying some recovery in performance in each case, only the slopes for the drug-free control group (ANOVA, F(1,51) = 48.22, P < 0.0001) (Fig. 5A and Supplementary Fig. 3) and for the ferrets in which MEG was deactivated (F(1,34) = 32.28, P < 0.0001) (Fig. 5B, Supplementary Fig. 3) were significantly different from zero. Like the control group, a significant improvement in the percentage correct scores was found between the beginning and the end of the earplugging period in the animals in which MEG had been deactivated (post hoc Scheffé's test, P < 0.01), but, unlike the controls, their scores on the last day of earplugging were substantially lower than those made in the post-plug session (P < 0.01) (Fig. 5B). Thus, silencing the primary auditory cortical areas in the MEG reduced the capacity of the ferrets to adapt to an earplug.
The AEG (Fig. 5C) and PEG (Fig. 5D) groups both included individual animals whose auditory localization accuracy in the presence of an earplug improved with training. Nevertheless, the slopes of the fitted regression lines were not significantly different from zero in either case (AEG: F(1,34) = 3.75, P = 0.057; PEG: F(1,40) = 1.30, P = 0.260), and no difference was found between the percentage of correct scores at the beginning and end of the period of monaural occlusion (post hoc Scheffé's test, P > 0.05). These animals therefore had a significantly impaired ability to relearn to localize sound in the presence of abnormal spatial cues.
As in the control animals, the initial insertion of the earplug caused a marked reduction in the percentage of correct responses, especially for stimulus locations on the ipsilateral side. No differences in the scores were found between any of the groups on day 2 of monaural occlusion (ANOVA, F(3,74) = 2.45, P = 0.071), whereas all three groups with muscimol-Elvax implants made significantly lower scores than the controls by day 9 (ANOVA F(3,74) = 8.997, P < 0.0001; post hoc Scheffé's test, P < 0.01) (Fig. 5 and Supplementary Fig. 2). Throughout the period of monaural occlusion, each of the groups with muscimol-Elvax made significantly more left-right errors for ipsilateral target locations than the control group (Supplementary Fig. 2, right column) (ANOVA, day 2: F(3,19) = 3.372, P = 0.045; day 9: F(3,74) = 8.54, P = 0.001). It seems that in the animals in which one of the regions of the EG had been deactivated, the binaural imbalance caused by the earplug uncovered a deficit in spatial processing even at a stimulus duration (1000 ms) where no impairment was apparent in the absence of an earplug.
Plugging one ear initially had a very similar effect on the head orienting behaviour of the ferrets with both drug-free (Fig. 6A) and muscimol-Elvax implants (Fig. 6B–D), in that the responses became more variable and, for ipsilateral targets, less accurate compared to those measured prior to earplugging. This is illustrated for all four groups on the second day of monaural occlusion by the increase in the magnitude of the head orienting errors on the left side (ipsilateral to the earplug) and the larger region bound by the confidence intervals. Similarly, the MI between target location and final head bearing fell when the left ear was first plugged. However, by day 9 of monaural occlusion, only the control group showed a recovery in head orienting accuracy close to the pre-plug value (Fig. 6A). By contrast, in all three groups in which part of the EG had been deactivated, the head orienting errors remained large at the end of this 9 day period and the MI between target location and final head bearing was still well below the corresponding pre-plug value (Fig. 6B–D). As with the approach-to-target responses, earplug removal in each of the experimental groups led to an immediate increase in MI to near pre-plug values.
Figure 6. Plots of the distribution of head orienting errors (target location minus final head bearing) for the different experimental groups before the insertion of the earplug (white) and on day 2 (pink and continuous line) and day 9 (green and dashed line) of wearing the earplug after bilateral implantation of drug-free Elvax over MEG (A) or muscimol-Elvax over the MEG (B), AEG (C) or PEG (D).
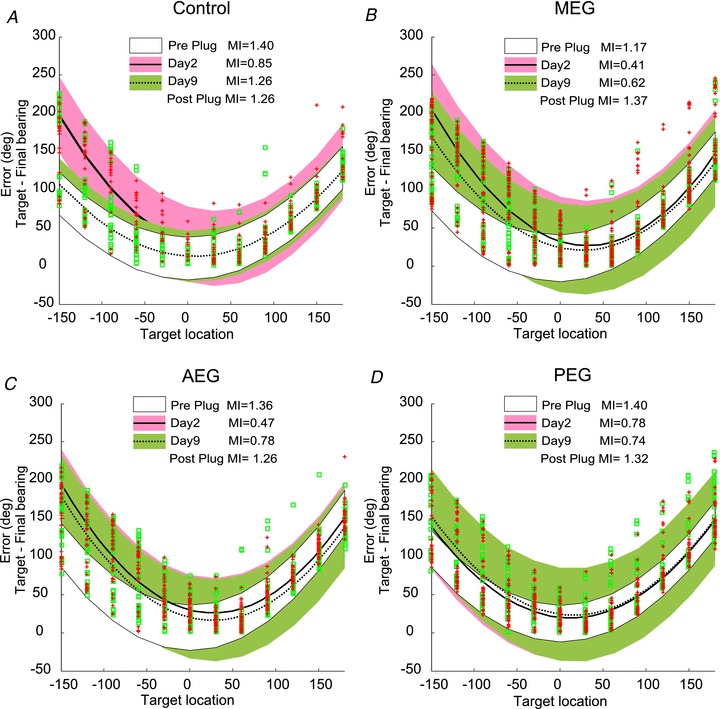
Quadratic polynomial curves were fitted using a robust least squares method (bisquare weights) to the data. Colour areas indicate the 95% confidence intervals in each case. Red crosses indicate trials on day 2 and green squares trials on day 9 of wearing an earplug. Trials before the insertion for the earplug have been omitted for clarity. Note that the confidence intervals for the preplug and day 9 earplug data almost entirely overlap in the control group (A), indicating near complete recovery in head orienting accuracy, whereas this is not the case for any of the groups with muscimol-Elvax (B–D).
Together, the approach-to-target and head orienting data indicate that inactivation of any of the three major subdivisions of the EG impaired training-induced plasticity of auditory localization, although the greatest learning deficits were found after silencing higher-level cortical areas in the AEG and PEG.
Evaluation of the auditory cortex beneath the Elvax implants
After the earplug had been removed and sound localization accuracy retested, we removed the Elvax under general anaesthesia and, 5 days later, carried out further behavioural testing. The small duration-dependent deficits in approach-to-target response accuracy were no longer present, and no differences in performance were found compared to the first pre-Elvax runs. This is illustrated by the lack of any difference between the pre-implantation and post-removal scores (percentage of correct responses at 40 ms: F(2,8) = 0.830, P = 0.389; percentage of front–back errors: F(2,8) = 2.15, P = 0.152) or between any of the groups (percentage of correct responses at 40 ms: F(2,8) = 2.842, P = 0.117; percentage of front-back errors: F(2,8) = 4.147, P = 0.058). Together with the lack of any change in performance in the drug-free group, these results indicate that the surgeries for Elvax implantation and explantation did not alter the localization abilities of the animals, and that the deficits observed in the experimental animals with muscimol-Elvax positioned over the auditory cortex were reversible.
Most of the animals were then perfused so that the auditory cortex could be examined histologically. In three cases that had received muscimol-Elvax implants over the MEG, this was not possible because these animals were subsequently used in another study for electrophysiologically guided aspiration of A1. In those cases, no sign of any cortical damage was apparent and the recorded neurons produced typically strong, short-latency responses that were tuned to specific sound frequencies.
In the other cases (n = 10), Nissl staining and NeuN immunohistochemistry were carried out in order to examine the cytoarchitectonic features of the auditory cortex and the morphology of the neurons found there, while the pattern of SMI32 immunopositive neurons allowed us to delimit the MEG from the AEG and PEG according to previous work in the ferret (Bajo et al. 2007). No abnormalities were found in the appearance of the neurons or in the laminar organization of the cortex in the regions beneath the muscimol-Elvax implant (Figs 7 and 8). Each of the cortical layers could be readily identified in Nissl (Fig. 7B) and NeuN (Fig. 7C, Fig. 8C) stained sections, and the appearance of SMI32-immunopositive pyramidal cells in layers III and V was the same in regions beneath and adjacent to the previously implanted Elvax sheet (Fig. 7D).
Figure 7. Anatomy of ferret auditory cortex.
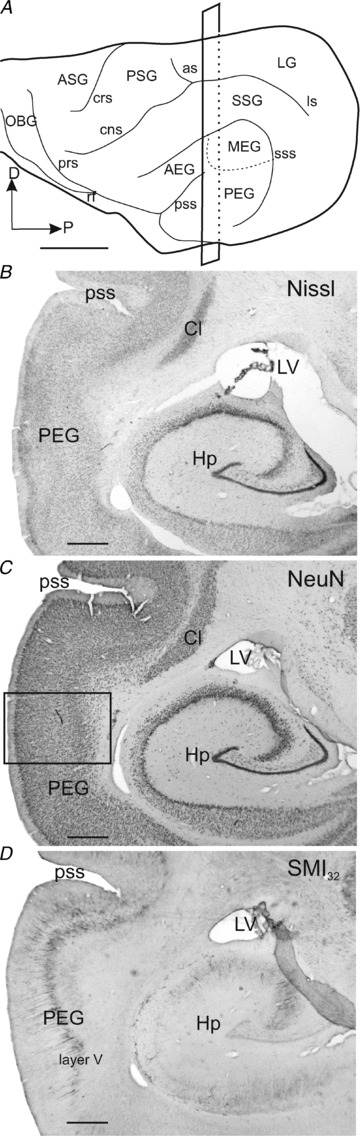
A, lateral view of the ferret cortex showing the ectosylvian gyrus where the auditory cortex is located and its three main regions. B–D, consecutive coronal sections were stained for three different neuronal markers, Nissl substance (B), NeuN (C) and SMI32 (D). The rectangle in A shows the cutting plane at the level of the photomicrographs in B–D. The rectangle in C indicates where the higher magnification image shown in Fig. 8C was taken. Scale bars, 5 mm in A and 1 mm in B–D. AEG, anterior ectosylvian gyrus; ASG, anterior sigmoid gyrus; as, ansinate sulcus; Cl, claustrum; cns, coronal sulcus; crs, cruciate sulcus; D, dorsal; Hp, hippocampus; LG, lateral gyrus; ls, lateral sulcus; LV, lateral ventricle; MEG, middle ectosylvian gyrus; NeuN, neuronal nuclei protein antibody; OBG, orbital gyrus; P, posterior; PEG, posterior ectosylvian gyrus; prs, presylvian sulcus; PSG, posterior sigmoid gyrus; pss, pseudosylvian sulcus; rf, rhinal fissure; SMI32, Neurofilament H non-phosphorylated; SSG, suprasylvian gyrus; sss, suprasylvian sulcus.
Figure 8. Muscimol-Elvax implants do not modify the thickness of cortical layers.
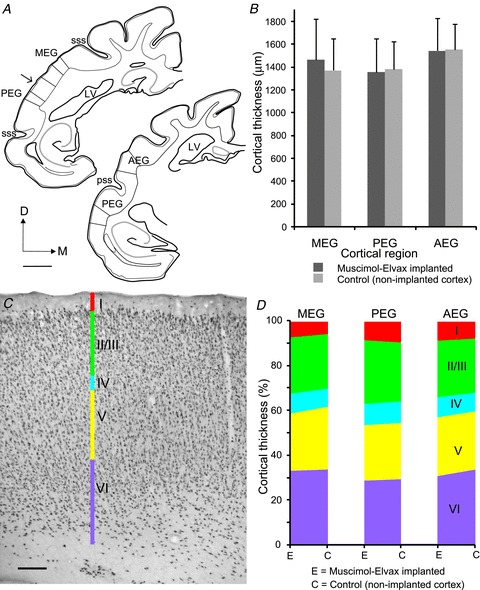
A, drawings of coronal sections of the left hemisphere at two antero-posterior levels of the EG showing where the cortical thickness was measured in the three different auditory regions. Scale bar, 2 mm. B, mean ± SD cortical thickness in the region where muscimol-Elvax was implanted and in a corresponding region of non-implanted auditory cortex from the same brain. C, photomicrograph of a coronal section showing NeuN immunoreactivity to reveal the different cortical layers in a region of the PEG where muscimol-Elvax had been implanted. Scale bar, 200 μm. D, relative thickness of each layer in the region where muscimol-Elvax was implanted and in a corresponding area of non-implanted auditory cortex from the same brain. AEG, anterior ectosylvian gyrus; D, dorsal; LV, lateral ventricle; M, medial; MEG, middle ectosylvian gyrus; PEG, posterior ectosylvian gyrus; pss, pseudosylvian sulcus; sss, suprasylvian sulcus.
To confirm that the presence of the Elvax had not compressed or distorted the cortex, we quantified its thickness in Nissl-stained sections of the MEG, PEG and AEG by taking 10 random measures in each region on the left and right sides of the brain. An additional 10 measures of the cortical thickness were taken in each hemisphere at the level of the cingular gyrus and used to normalize the data to allow for inter-animal differences in the angle of the cutting plane. In each case, sampling was restricted to the gyrii and avoided the sulci (Fig. 8A). The thickness estimates for each region of the auditory cortex are shown in Fig. 8B, subdivided according to whether the measurements were taken from locations over which the Elvax was implanted or not. No differences in thickness were found when these values were compared within each of the three regions (MEG: t(198) = 1.37, P = 0.174; PEG: t(192) = 0.68, P = 0.500; AEG: t(143) = 0.19, P = 0.846).
The thickness of each cortical layer was measured in NeuN-stained sections for one animal from each of the three groups in which muscimol-Elvax had been implanted over a different auditory region (Fig. 8C), and expressed as a percentage of the total cortical thickness for both implanted and adjacent regions of the EG (Fig. 8D). The values obtained for each region were very similar irrespective of whether muscimol-Elvax had been placed there or not.
The normal morphology of the auditory cortex is consistent with the appearance of the cortical vasculature and the resumption of cortical responses and normal sound localization behaviour following muscimol-Elvax removal. This confirms that the behavioural deficits observed while the Elvax was in place were due to the diffusion of muscimol from the implants and not to a non-specific lesion resulting from the presence of the implants.
Discussion
By reversibly deactivating different regions of the ferret auditory cortex using subdural muscimol-Elvax implants, we were able to reveal the relative contribution of these areas to the ability of the animals both to localize sound in the horizontal plane and to learn to accommodate a substantial change in the spatial cues that underlie this behaviour. Although our findings indicate that these functions are distributed across the auditory cortex, some regional specialization does exist with inactivation of the primary areas in the MEG producing the largest localization deficits under normal hearing conditions and silencing of all three regions, but particularly the higher-level areas in the AEG and PEG, compromising the learning normally observed after plugging one ear.
Deactivating specific cortical areas
Investigating the role of different auditory cortical areas in spatial processing and its recalibration by experience requires a method for deactivating localized regions of the brain continuously over the period of monaural occlusion. Temporary cooling has been used in cats to reveal that activity within A1, the posterior auditory field, the anterior ectosylvian sulcus and the dorsal zone of the auditory cortex is required for normal sound localization behaviour, whereas other auditory cortical fields do not contribute to this task (Malhotra & Lomber, 2007; Lomber & Malhotra, 2008; Malhotra et al. 2008). This method is less appropriate, however, when more prolonged inactivation is required. Moreover, recent work in guinea pigs has shown that placing a cooling probe on the auditory cortex can reduce neural activity across much of the ipsilateral cerebral hemisphere, indicating that this approach may not be suitable for producing regional inactivation in smaller animals (Coomber et al. 2011).
We therefore used muscimol-Elvax to deactivate the auditory cortex, which also allowed us to measure both the initial head orienting response and approach-to-target behaviour unencumbered by the complex set-up required in cooling studies. Our morphological measurements showed that this slow-release polymer did not damage the cortex, and our electrophysiological and behavioural data confirmed the results of previous studies (Smith et al. 2004; Bizley et al. 2007b) in showing that the functional changes produced by muscimol-Elvax implantation are reversible and are not seen with drug-free Elvax. This technique has been shown to silence the underlying neurons in all cortical layers for up to 6 weeks following implantation, with the region of inactivation spreading by ≤500 μm from the edges of the implant in the deeper layers and somewhat further in the superficial layers (Smith et al. 2004). The electrophysiological data reported in the present study are consistent with this and confirm that this method produces a localized, persistent and reversible inactivation of the brain. Consequently, placement of muscimol-Elvax over the MEG, AEG or PEG should have silenced neurons in specific areas of the ferret auditory cortex that have been characterized physiologically in other studies (Bizley et al. 2005, 2007a, 2009; Nelken et al. 2004, 2008).
The auditory cortex and sound localization
Deactivating the primary fields, A1 and AAF, located in the MEG, produced a modest but significant deficit in the localization of brief sounds. This impairment was observed only for approach-to-target behaviour and not the initial head orienting responses, and closely resembled that reported previously following either bilateral pharmacological inactivation (Smith et al. 2004) or aspiration lesions of A1 alone (Nodal et al. 2010). The dependence of this effect on the duration of the stimulus raises the possibility that cortical inactivation may impair the ability of the animals to remember the perceived location of the sound source.
Using a two-choice task, Kavanagh & Kelly (1987) found that complete bilateral lesions of the MEG produced a severe deficit in the ability of ferrets to localize single clicks in the left and right hemifields, without markedly affecting minimum audible angles around the midline. Although we also found that the performance of the ferrets was most affected in the lateral hemifield, our ferrets were still able to localize 40 ms noise bursts after deactivating the MEG. Unlike Kavanagh & Kelly (1987), we used a 12-speaker task so we could measure the distribution of responses throughout the horizontal plane, rather than simply how well the animals could discriminate between two sound source locations. Since we found that localization deficits were no longer apparent when we used noise bursts of ≥100 ms in duration, the particularly short stimuli employed by Kavanagh & Kelly (1987) may also have contributed to the difference in the severity of the localization deficits observed in these studies.
Previous studies in the ferret have shown that the magnitude of the localization deficits increases when larger areas of the auditory cortex, extending beyond the MEG, are removed (Kavanagh & Kelly, 1987; Nodal et al. 2010). Indeed, we found that larger lesions that included part of the AEG and PEG produced more substantial localization impairments, which were apparent not only in the approach-to-target behaviour but also in the accuracy of the head orienting responses, than those restricted to A1 (Nodal et al. 2010). This is consistent with behavioural (Malhotra & Lomber, 2007) and electrophysiological evidence (Harrington et al. 2008; Recanzone et al. 2000; Miller & Recanzone, 2009) in other species that non-primary cortical areas also contribute to auditory spatial processing.
Although our data show that deactivating each of the main regions of the ferret auditory cortex degrades sound localization accuracy, there was no indication that any of the non-primary areas plays a relatively larger role in this aspect of hearing. This contrasts with behavioural data from the cat showing that cooling of the posterior auditory field (Malhotra & Lomber, 2007; Lomber & Malhotra, 2008), anterior ectosylvian sulcus (Malhotra & Lomber, 2007) or dorsal zone of the auditory cortex (Malhotra et al. 2008) each resulted in impaired localization, whereas no deficits were observed when other cortical areas, such as the secondary auditory cortex or anterior auditory field, were deactivated. However, there is a fundamental difference between these methods for silencing cortical activity. Whereas the use of cooling probes allows the acute effects of cortical inactivation to be examined during a single testing session, slow-release polymers are more suited for examining the consequences of longer-term inactivation, which was necessary for the spatial learning part of our study.
While our electrophysiological measurements suggest that it is unlikely that the muscimol-Elvax placements would have had much effect on neighbouring areas of the auditory cortex, we cannot rule out the possibility that individual cortical fields might not have been completely deactivated, particularly if they extend into the adjacent sulci. This may have allowed a partial recovery in function while the Elvax was in place. Similarly, since neurons throughout the auditory cortex convey information about sound source location (Harrington et al. 2008; Bizley et al. 2009), it is possible that any transient deficits in performance produced by deactivating a particular region of cortex could have been missed due to unmasking of function involving other cortical areas. Although we found that muscimol-Elvax can silence the full depth of the underlying cortex, the extent of the inactivation has been shown to decline ∼6 weeks following implantation (Smith et al. 2004). However, this is unlikely to account for the modest effects that we observed in normal localization behaviour since (a) this was measured within the first 2 weeks of implantation, and (b) most of the animals implanted with muscimol-Elvax subsequently exhibited a substantial deficit in their ability to adapt to an earplug.
The finding that the effects of cortical inactivation on sound localization are more area specific in cats than in ferrets might also be a consequence of a difference between these species or in the behavioural protocols used. Furthermore, Chowdhury & DeAngelis (2008) reported that deactivating area MT impaired coarse depth discrimination in monkeys, but no longer did so after the animals were trained to discriminate fine differences in relative disparity, indicating that the order in which different tasks are learned may determine whether a given brain area continues to be necessary for task performance. In view of this, we were careful to train all our ferrets in exactly the same way, beginning first with the normal sound localization task followed by measurement of their ability to adapt to an earplug.
Resolution of differences in the regional specificity of the behavioural deficits produced by cortical inactivation will require that the same cooling technique used in the cat by Lomber and colleagues is applied to other species, so that the immediate consequences of deactivating particular cortical areas can be assessed. Nevertheless, the localization deficits observed in the present study are consistent with what is known physiologically about the functional organization of ferret auditory cortex. Measurements of the sensitivity of neurons in both core and belt cortical areas to stimulus periodicity, timbre and location suggest that spatial and non-spatial sound features form intermingled and distributed representations (Bizley et al. 2009). Although inconsistent with a functional division of labour among different cortical fields, significant regional differences in sensitivity to these features do exist. Thus, the most informative responses about sound azimuth are found in A1 and in ADF, which is part of the AEG (Bizley & King, 2008; Bizley et al. 2009; Walker et al. 2011). In keeping with this, the clearest impairments in sound localization behaviour were found in ferrets in which muscimol-Elvax had been implanted either over the MEG or, to a lesser extent, over the AEG.
The auditory cortex and learning
We further examined the role of different cortical areas in spatial processing by determining whether animals implanted with muscimol-Elvax could adapt to the altered localization cues produced by occluding one ear. This manipulation alters the interaural level differences and interaural time differences associated with each stimulus direction, as well as the spectral localization cues available through the occluded ear. So long as sufficient behavioural training is provided, localization accuracy recovers after a week or so of monaural occlusion. In both ferrets (Kacelnik et al. 2006) and humans (Kumpik et al. 2010), this process of adaptation appears to involve the reweighting of different cues, with subjects learning to rely more on the unaltered spectral cues provided by the open ear. This accounts for the lack of any substantial after-effect following earplug removal.
A number of studies have shown that auditory perceptual learning is accompanied by changes in the response properties of cortical neurons, with nearly all of these studies focusing exclusively on A1 (Dahmen & King, 2007). We have shown that bilateral cortical lesions, including those restricted to A1, prevent the training-induced plasticity normally seen when one ear is chronically occluded (Nodal et al. 2010). When layer V neurons that project from A1 to the inferior colliculus are removed in one hemisphere, spatial learning deficits are observed in the opposite hemifield (Bajo et al. 2010a). This therefore suggests that descending inputs from A1 to the auditory midbrain play a critical role in the adaptive reweighting of auditory localization cues that is required to maintain a stable percept in the presence of a unilateral hearing loss.
In keeping with these findings, inactivation of the primary fields in the MEG impaired the adaptation to an earplug. Although the performance of these animals at the end of the period of monaural occlusion was significantly below that of ferrets implanted with drug-free Elvax, a small but steady improvement was observed while the earplug was in place. This contrasts with the lack of any recovery in localization accuracy in ferrets with cortical lesions (Nodal et al. 2010). Assuming that the activity of neurons in the deeper cortical layers had not recovered beneath the muscimol-Elvax implants by the time these behavioural measurements were made, it seems likely that this difference is due to degeneration of neurons in other brain regions to which the lesioned cortex is connected.
Although the integrity of A1 and its descending connections is clearly important for auditory spatial learning, this does not exclude the possibility that other cortical areas are also involved. Indeed, visual perceptual learning is most commonly associated with cortical fields that lie beyond the primary visual cortex (Zohary et al. 1994; Kobatake et al. 1998; Yang & Maunsell, 2004). Our findings confirm that this is the case, since inactivation of either the AEG or PEG prevented any adaptation during the 9 day period of monaural occlusion. We cannot rule out the possibility that localization accuracy in these animals would have recovered with further training, potentially reflecting compensatory changes involving other brain regions. It would not be possible to use a slow-release polymer like muscimol-Elvax to investigate this, however, since neural activity starts to return ∼6 weeks following implantation (Smith et al. 2004).
Our results suggest that non-primary belt areas of the auditory cortex may be more important than core areas in this spatial learning task. Nevertheless, there was no indication of any further functional segregation within the auditory cortex. Although regional differences might be revealed in recording studies or following more localized inactivation, it is also possible that adaptive reweighting of different spatial cues relies upon a more extensive network of cortical areas than simpler localization tasks. Similar principles may also operate in other complex tasks, such as auditory scene analysis, which engages much of the auditory cortex irrespective of the cues used for streaming (Schadwinkel & Gutschalk, 2010).
Acknowledgments
This study was supported by the Wellcome Trust through a Principal Research Fellowship to A.J.K. We are very grateful to Patricia Cordery for preparing the Elvax, and to Susan Spires, Dan Kumpik, Nick Leach, Peter Keating and Rob Campbell for their contributions to the behavioural testing.
Glossary
- A1
primary auditory cortex
- AEG
anterior ectosylvian gyrus
- EG
ectosylvian gyrus
- MEG
middle ectosylvian gyrus
- MI
mutual information
- NeuN
neuronal nuclei protein
- PEG
posterior ectosylvian gyrus
- SMI32
neurofilament H non-phosphorylated
Author contributions
F.R.N., V.M.B. and A.J.K. designed the study and conducted the behavioural experiments. F.R.N. and V.M.B. carried out the electrophysiological recordings and analysed the data. V.M.B. performed the histological analysis. F.R.N., V.M.B. and A.J.K. wrote the article. All authors have approved the final version of this article.
Supplementary material
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
References
- Adriani M, Maeder P, Meuli R, Thiran AB, Frischknecht R, Villemure JG, Mayer J, Annoni JM, Bogousslavsky J, Fornari E, Thiran JP, Clarke S. Sound recognition and localization in man: specialized cortical networks and effects of acute circumscribed lesions. Exp Brain Res. 2003;153:591–604. doi: 10.1007/s00221-003-1616-0. [DOI] [PubMed] [Google Scholar]
- Alain C, Arnott SR, Hevenor S, Graham S, Grady CL. ‘What’ and ‘where’ in the human auditory system. Proc Natl Acad Sci U S A. 2001;98:12301–12306. doi: 10.1073/pnas.211209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, King AJ. The non-lemniscal auditory cortex in ferrets: convergence of corticotectal inputs in the superior colliculus. Front Neuroanat. 2010b;4:18. doi: 10.3389/fnana.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 2007;17:475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci. 2010a;13:253–260. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, King AJ. Visual-auditory spatial processing in auditory cortical neurons. Brain Res. 2008;1242:24–36. doi: 10.1016/j.brainres.2008.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Bajo VM, Nelken I, King AJ. Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb Cortex. 2007a;17:2172–2189. doi: 10.1093/cercor/bhl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Parsons CH, King AJ. Role of auditory cortex in sound localization in the midsagittal plane. J Neurophysiol. 2007b;98:1763–1774. doi: 10.1152/jn.00444.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Walker KMM, Silverman BW, King AJ, Schnupp JWH. Interdependent encoding of pitch, timbre, and spatial location in auditory cortex. J Neurosci. 2009;29:2064–2075. doi: 10.1523/JNEUROSCI.4755-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SA, DeAngelis GC. Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron. 2008;60:367–377. doi: 10.1016/j.neuron.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber B, Edwards D, Jones SJ, Shackleton TM, Goldschmidt J, Wallace MN, Palmer AR. Cortical inactivation by cooling in small animals. Front Syst Neurosci. 2011;5:53. doi: 10.3389/fnsys.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol. 2007;17:456–464. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Ghose GM. Learning in mammalian sensory cortex. Curr Opin Neurobiol. 2004;14:513–518. doi: 10.1016/j.conb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Li W, Piech V. Perceptual learning and adult cortical plasticity. J Physiol. 2009;587:2743–2751. doi: 10.1113/jphysiol.2009.171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington IA, Heffner RS, Heffner HE. An investigation of sensory deficits underlaying the aphasia-like behaviour of macaques with auditory cortex lesions. Neuroreport. 2001;12:1271–1221. doi: 10.1097/00001756-200105080-00032. [DOI] [PubMed] [Google Scholar]
- Harrington IA, Stecker GC, Macpherson EA, Middlebrooks JC. Spatial sensitivity of neurons in the anterior, posterior, and primary fields of cat auditory cortex. Hear Res. 2008;240:22–41. doi: 10.1016/j.heares.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart HC, Palmer AR, Hall DA. Different areas of human non-primary auditory cortex are activated by sounds with spatial and nonspatial properties. Hum Brain Mapp. 2004;21:178–190. doi: 10.1002/hbm.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. J Neurophysiol. 1986;56:683–701. doi: 10.1152/jn.1986.56.3.683. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Hofman PM, Van Riswick JGA, Van Opstal JA. Relearning sound localization with new ears. Nat Neurosci. 1998;1:417–421. doi: 10.1038/1633. [DOI] [PubMed] [Google Scholar]
- Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu ZL. Perceptual learning improves contrast sensitivity of V1 neurons in cats. Curr Biol. 2010;20:887–894. doi: 10.1016/j.cub.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving S, Moore DR, Liberman MC, Sumner CJ. Olivocochlear efferent control in sound localization and experience-dependent learning. J Neurosci. 2011;31:2493–2501. doi: 10.1523/JNEUROSCI.2679-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Masterton RB. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2:1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Contribution of auditory cortex to sound localization by the ferret (Mustela putorius. J Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4:627–638. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Whitfield IC. Effects of auditory cortical lesions on discriminations of rising and falling frequency-modulated tones. J Neurophysiol. 1971;24:802–816. doi: 10.1152/jn.1971.34.5.802. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Wang G, Tanaka K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J Neurophysiol. 1998;80:324–330. doi: 10.1152/jn.1998.80.1.324. [DOI] [PubMed] [Google Scholar]
- Kumpik DP, Kacelnik O, King AJ. Adaptive reweighting of auditory localization cues in response to chronic unilateral earplugging in humans. J Neurosci. 2010;30:4883–4894. doi: 10.1523/JNEUROSCI.5488-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Lomber SG. Sound localization during homotopic and heterotopic bilateral cooling deactivation of primary and nonprimary auditory cortical areas in the cat. J Neurophysiol. 2007;97:26–43. doi: 10.1152/jn.00720.2006. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Stecker GC, Middlebrooks JC, Lomber SG. Sound localization deficits during reversible deactivation of primary auditory cortex and/or the dorsal zone. J Neurophysiol. 2008;99:1628–1642. doi: 10.1152/jn.01228.2007. [DOI] [PubMed] [Google Scholar]
- Miller LM, Recanzone GH. Populations of auditory cortical neurons can accurately encode acoustic space across stimulus intensity. Proc Natl Acad Sci U S A. 2009;106:5931–5935. doi: 10.1073/pnas.0901023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff WD, Fisher JF, Diamond IT, Yela M. Role of auditory cortex in discrimination requiring localization of sound in space. J Neurophysiol. 1956;19:500–512. doi: 10.1152/jn.1956.19.6.500. [DOI] [PubMed] [Google Scholar]
- Nelken I, Bizley JK, Nodal FR, Ahmed B, Schnupp JW, King AJ. Large-scale organization of ferret auditory cortex revealed using continuous acquisition of intrinsic optical signals. J Neurophysiol. 2004;92:2574–2588. doi: 10.1152/jn.00276.2004. [DOI] [PubMed] [Google Scholar]
- Nelken I, Bizley JK, Nodal FR, Ahmed B, King AJ, Schnupp JW. Responses of auditory cortex to complex stimuli: functional organization revealed using intrinsic optical signals. J Neurophysiol. 2008;99:1928–1941. doi: 10.1152/jn.00469.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Bajo VM, Parsons CH, Schnupp JW, King AJ. Sound localization behavior in ferrets: comparison of acoustic orientation and approach-to-target responses. Neuroscience. 2008;154:397–408. doi: 10.1016/j.neuroscience.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Kacelnik O, Bajo VM, Bizley JK, Moore DR, King AJ. Lesions of the auditory cortex impair azimuthal sound localization and its recalibration in ferrets. J Neurophysiol. 2010;103:1209–1225. doi: 10.1152/jn.00991.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl FW, Wetzel W, Wagner T, Rech A, Scheich H. Bilateral ablation of auditory cortex in Mongolian gerbil affects discrimination of frequency modulated but not of pure tones. Learn Mem. 1999;6:347–362. [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci. 2006;26:6589–6602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH. Representation of con-specific vocalizations in the core and belt areas of the auditory cortex in the alert macaque monkey. J Neurosci. 2008;28:13184–13193. doi: 10.1523/JNEUROSCI.3619-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol. 2000;83:2723–2739. doi: 10.1152/jn.2000.83.5.2723. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadwinkel S, Gutschalk A. Activity associated with stream segregation in human auditory cortex is similar for spatial and pitch cues. Cereb Cortex. 2010;20:2863–2873. doi: 10.1093/cercor/bhq037. [DOI] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Smith AL, Cordery PM, Thompson ID. Manufacture and release characteristics of Elvax polymers containing glutamate receptor antagonists. J Neurosci Methods. 1995;60:211–217. doi: 10.1016/0165-0270(95)00014-l. [DOI] [PubMed] [Google Scholar]
- Smith AL, Parsons CH, Lanyon RG, Bizley JK, Akerman CJ, Baker GE, Dempster AC, Thompson ID, King AJ. An investigation of the role of auditory cortex in sound localization using muscimol-releasing Elvax. Eur J Neurosci. 2004;19:3059–3072. doi: 10.1111/j.0953-816X.2004.03379.x. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Relearning sound localization with a new ear. J Neurosci. 2005;25:5413–5424. doi: 10.1523/JNEUROSCI.0850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KMM, Bizley JK, King AJ, Schnupp JWH. Multiplexed and robust representations of sound features in auditory cortex. J Neurosci. 2011;31:14565–14576. doi: 10.1523/JNEUROSCI.2074-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C, Bourgeon S, Coq JO. Perceptual context-dependent remodeling of the forepaw map in the SI cortex of rats trained on tactile discrimination. Behav Brain Res. 2005;162:207–221. doi: 10.1016/j.bbr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Yang T, Maunsell JHR. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Celebrini S, Britten KH, Newsome WT. Neuronal plasticity that underlies improvement in perceptual performance. Science. 1994;263:1289–1292. doi: 10.1126/science.8122114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


