Abstract
Population studies have consistently shown a highly inverse correlation between plasma concentration of high-density lipoprotein and the risk of atherosclerotic cardiovascular disease in humans. High-density lipoprotein (HDL) as a therapeutic target is an intense area of ongoing investigation. Aiming to solve the shortcomings of native HDL application, we prepared recombinant human HDL (rhHDL) that contains a similar composition and has similar functions with native HDL. Six kinds of recombinant human apolipoproteins (rhapo)—rhapoA-I, rhapoA-II, rhapoA-IV, rhapoC-I, rhapoC-II, and rhapoE—were expressed in Pichia pastoris and purified with chromatography. By the facilitation of cholate, six kinds of rhapo penetrated among the phosphatidylcholine acyl chains. After purification by density-gradient centrifugation, rhHDL was acquired. Based on morphological observation, we confirmed that the micellar complexes of rhapo with phosphatidylcholine and cholesterol were prepared. We carried on comparative studies in vitro and in vivo between native HDL and rhHDL. Cellular cholesterol efflux assays showed that rhHDL could promote the efflux of excess cholesterol from macrophages. Furthermore, rhHDL has similar effects with native HDL on the blood lipid metabolism in hyperlipidemic mice. In conclusion, rhHDL has similar effects on antiatherosclerosis with native HDL through reverse cholesterol transport, antioxidative, and antithrombotic properties. It could be used as a therapeutic HDL-replacement agent.
Introduction
Atherosclerosis is the pathological basis for ischemic cardiovascular disease, which is the leading cause of morbidity and mortality in the United States and other industrialized nations.1 Major risk factors for atherosclerosis include high plasma levels of low-density lipoprotein cholesterol (LDL-c) and low levels of plasma high-density lipoprotein cholesterol (HDL-c).2 In addition, population studies have consistently shown a highly inverse correlation between plasma concentration of HDL-c and the risk of atherosclerotic cardiovascular disease in humans.3 HDL may reduce atherosclerosis through several different mechanisms, including increasing, that is, the removal of free cholesterol from blood vessels to the liver,4,5 inhibiting physical and chemical modifications of LDL,6,7 and thus reducing foam cell formation, protecting against endothelial dysfunction,8,9 inhibiting chronic inflammation10 by suppressing adhesion molecules and macrophage chemotactic proteins, and reducing arterial lipoprotein retention.11
HDL as a therapeutic target is an intense area of ongoing investigation.12 Simply, there are two main strategies: increasing the quantity of HDL-c and/or the quality (function) of HDL. Studies in animals strongly suggest that HDL has direct antiatherogenic properties.13 ApoA-I is by far the most common protein component of HDL, and thus a native choice in developing a therapeutic HDL-replacement agent.14 Many studies are interested in the use of apoA-I Milano and apoA-I mimetic peptides as HDL-replacement agents,15,16 which showed their potential to prevent atherosclerosis. However, gene deletion of apoA-II in mice remarkably reduced HDL-c levels, suggesting that apoA-II is also required for normal HDL metabolism.17 The multifunction of HDL depends on its complicated composition. HDL is the most abundant lipoprotein particle in the plasma, which has equal composition of lipids and proteins. Identified proteins in HDL were the dominating apoA-I, apoA-II, apoA-IV, apoC-I, apoC-II, apoC-III, apoE, and recently discovered apoM, serum amyloid A and serum amyloid A-IV. Furthermore, α-antitrypsin and α-ptyalin were identified in HDL for the first time.18 Similar composition can lead to similar functions; thereby, we prepared recombinant human high-density lipoprotein (rhHDL) that contains a similar composition with native HDL as an HDL-replacement agent by biotechnology. As a result, it will increase HDL-c levels as well as the ability of HDL to participate in reverse cholesterol transport (RCT) or improve the anti-inflammatory and antioxidative properties in atherosclerosis.
In this study, we prepared rhHDL that contains egg phosphatidylcholine, cholesterol, and six kinds of recombinant human apolipoproteins (rhapo)—rhapoA-I, rhapoA-II, rhapoA-IV rhapoC-I, rhapoC-II, and rhapoE—in vitro by the facilitation of cholate. After that, we carried on comparative studies in vitro and in vivo about pharmacodynamic functions between native HDL and rhHDL to evaluate the effects of rhHDL on RCT, blood lipid metabolism, antioxidative, and antithrombotic properties, furthermore, to assess the effects of rhHDL on antiatherosclerosis.
Materials And Methods
Preparation of Apolipoproteins
Six kinds of rhapo—rhapoA-I, rhapoA-II, rhapoA-IV, rhapoC-I, rhapoC-II, and rhapoE—were prepared with same methods as mentioned previously.19,20 Briefly, the six kinds of rhapo were, respectively, expressed in Pichia pastoris and purified with cation-exchange chromatography (SP Sepharose XL) and reverse-phase chromatography (Source 30). They were identified by western blot and amino-terminal sequence analysis.
Preparation of rhHDL
In accordance with Kato et al.,21 we prepared rhHDL that contains six kinds of rhapo. An ethanol solution (0.5 mL), containing 7.2 mg egg phosphatidylcholine and 1.6 mg cholesterol, was rapidly injected into 12 mL of the phosphate buffer (pH=7.4) through a glass syringe with a 25-gauge needle. After mixing for 15 min under a stream of N2, 10.8 mg cholate and 3.2 mg rhapoA-I, 0.84 mg rhapoA-II, 0.05 mg rhapoA-IV, 0.64 mg rhapoC-I, 0.05 mg rhapoC-II, and 0.15 mg rhapoE in phosphate buffer (1 mL) were added to the lipid mixture with stirring. The mixture was incubated for 30 min at room temperature, and then it was incubated at 4°C for 12 h. The solution was dialyzed totally at 4°C (about 2 days) against the phosphate buffer to remove ethanol and cholate (Table 1).
Table 1.
Protocol Table for Recombinant Human High-Density Lipoprotein Preparation and Purification
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Phosphate buffer | 12 mL | pH 7.4 |
| 2 | Egg phosphatidylcholine and cholesterol | 0.5 mL | Dissolved in ethanol |
| 3 | Mixing step | 15 min | Under a stream of N2 |
| 4 | Six kinds of rhapo | 1 mL | Dissolved in phosphate buffer |
| 5 | Incubation time | 30 min | Room temperature |
| 6 | Incubation time | 12 h | 4°C |
| 7 | Dialysis | 2 days | 4°C, against phosphate buffer |
| 8 | Density-gradient centrifugation | 20 h | As desired |
Step Notes
1. 50-mL centrifuge tube, 12 mL phosphate buffer, pH 7.4.
2. An ethanol solution containing 7.2 mg egg phosphatidylcholine and 1.6 mg cholesterol, rapidly injected into phosphate buffer.
3. Mixing for 15 min under a stream of N2.
4. Six kinds of rhapo were dissolved in phosphate buffer (1 mL) as their proportion in native. HDL and added to the lipid mixture with stirring.
5. Mixture was incubated for 30 min at room temperature.
6. Mixture was incubated at 4°C for 12 h.
7. Solution was dialyzed totally at 4°C (about 2 days) against phosphate buffer to remove ethanol and cholate.
8. A discontinuous NaCl/KBr density gradient was used to purify rhHDL.
rhHDL, recombinant human high-density lipoprotein; rhapo, recombinant human apolipoprotein.
Purification of rhHDL by Density Gradient Centrifugation
A discontinuous NaCl/KBr density gradient was formed by adjusting the density (d) of the rhHDL solution to 1.30 g/mL with KBr and layering normal saline (d, 1.006 g/mL) over the adjusted rhHDL solution. The tubes were housed in a 45-Ti rotor and centrifuged at 41,500 rpm (200,000 g) for 20 h in the Beckman Optima bench (LE-80K); then, rhHDL (d 1.063–1.21 g/mL) was isolated and dialyzed.
A multiplex enzyme-linked immunosorbent assay (ELISA) (Bioss) was used to quantify apolipoproteins and calculate percentages of composition of each apolipoprotein. If the percentage of composition of apolipoproteins was rhapoA-I 65%–75%, rhapoA-II 10%–23%, rhapoA-IV 1%–3%, rhapoC-I 10%–15%, rhapoC-II 1%–3%, and rhapoE 1%–3%, the rhHDL can be used for further research. After filtration, sterilization, and quantitative determination by a commercial kit (Beihuakangtai), the solution of rhHDL was reserved at 4°C for further studies.
Morphologic Observation of rhHDL
We analyzed and photographed the morphological feature and grain diameters with a transmission electron microscope.
Separation of Native HDL from Human Plasma
Native human HDL (d, 1.063–1.21 g/mL) was separated by density-gradient centrifugation as reported.22 After filtration and sterilization, the solution of HDL was reserved at 4°C for further studies.
Animals
Pathogen-free male C57BL/6J mice (5-week old) were obtained from the Center of Experimental Animals of the Jilin University and were caged in animal rooms with alternating 12-h periods of light (from 7.00 a.m. to 7.00 p.m.) and dark (from 7.00 p.m. to 7.00 a.m.), with ad libitum access to water and mouse chow diet. All animal protocols followed the National Guidelines for the Care and Use of Animals.
Cellular Cholesterol Efflux Assays In Vitro
After 1 week of acclimatation, 10 mice received a high-fat diet, which includes 87.7% basal feed, 2% cholesterol, 0.3% sodium cholate, and 10% lard,23 for 15 days to establish an experimental hyperlipidemic model. Peritoneal macrophages were isolated by peritoneal lavage with D-Hanks 3 days after intraperitoneal injection of 1.0 mL 4% brewer thioglycollate medium as described.24 Macrophages were plated in an RPMI-1640 medium supplemented with 10% fetal bovine serum and were allowed to adhere for 4 h at 37°C under 5% CO2-humidified air. Then, nonadherent cells were removed by washing twice with phosphate-buffered saline (PBS), followed by loading of the macrophages with 50 μg/mL acetylated LDL and 3 μCi/mL [3H]cholesterol for 24 h and equilibrated for 18 h as previously published.25
To measure the relationship of dose–effect between the percentage of cellular cholesterol efflux and the dosage of rhHDL, macrophages were incubated with bovine serum albumin (BSA; 100 μg/mL, n=6) or native HDL (12.5, 25, and 50 μg/mL, respectively, n=6) or rhHDL (12.5, 25, and 50 μg/mL, respectively, n=6) for 24 h. After 24 h, radioactivity within the medium was determined by liquid scintillation counting. The cell layer was washed twice with PBS, and thereafter 0.1 M NaOH was added. Plates were incubated for 30 min at room temperature, and the radioactivity remaining within the cells was assessed by liquid scintillation counting. Wells incubated with BSA without added HDL or rhHDL were used as blanks to determine the HDL-independent efflux, and these values were subtracted from the respective experimental values. Efflux is given as the percentage of counts recovered from the medium in relation to the total counts present on the plate (sum of medium and cells). To measure the relationship of time–effect of cellular cholesterol efflux, macrophages were incubated with BSA (50 μg/mL) or native HDL (50 μg/mL) or rhHDL (50 μg/mL) for different time points: 12, 24, and 36 h, respectively. At different points of time, the efflux was measured as described above.
Lipid Profile of Hyperlipidemic Mice
After 1 week of acclimatation, 40 mice were randomly divided into five groups: the normal control group (NC; n=8, normal diet and vehicle-treated group), the model control group (MC; n=8, high-fat diet and vehicle-treated group), the rhHDL-treated groups (n=8, high-fat diet and 2 and 4 mg/kg/day rhHDL, respectively), and the native HDL-treated group (n=8, high-fat diet and 4 mg/kg/day native HDL). All groups were supplemented with vehicle or rhHDL for 3 weeks. Vehicle or rhHDL was administered by intravenous injection once daily.
At the end of the third week, after a 12-h fasting, the mice were anaesthetized with sodium pentobarbital. Blood samples were obtained by heart puncture and used to determine triglyceride (TG), total cholesterol (TC), HDL-c, LDL-c, malondialdehyde (MDA), superoxide dismutase (SOD), prostaglandin (PG) I2, and thromboxane (TX) A2. The atherogenic index (AI) was calculated by using the formula, AI=(TC−HDL-c)/HDL-c. At the same time, the liver samples were dissected out immediately, chilled and washed with ice-cold saline, weighed, and prepared a 10% (w/v) homogenate to determine MDA and SOD.
Biochemical Analysis
Serum and liver homogenates were obtained to determine TG, TC, HDL-c, LDL-c, SOD, and MDA using the commercial kits (Beihuakangtai). Specifically, TC was measured using an enzymatic photometric method, and TG using an enzymatic colorimetric method; additionally, HDL-c and LDL-c were determined by a precipitation procedure, and SOD activity was assayed by the nitroblue tetrazolium reduction method. One unit of enzyme activity was defined as the amount of enzyme necessity to inhibit the reaction to 50%. The amount of MDA formed was quantified by a reaction with thiobarbituric (TBA) and used as an index of lipid peroxidation. Mouse blood was also collected in tubes containing 0.1% ethylenediaminetetraacetic acid, and plasma was separated to measure PGI2 and TXA2 using the commercial radioimmunoassay kits (Institute of Radioimmunoassay).
Histopathological Evaluation
For histopathological examination, tissue samples from the liver were acquired from all animals. The liver samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin.
Statistical Analysis
Data are presented as means±standard error of the means. All statistical analyses were performed using Student's t-test. Probability values <0.05 were considered statistically significant.
Results
Preparation of rhHDL
The results of western blot and amino-terminal sequence analysis showed that six kinds of rhapo, namely rhapoA-I, rhapoA-II, rhapoA-IV, rhapoC-I, rhapoC-II, and rhapoE, were correctly expressed in P. pastoris, respectively. After being purified with cation-exchange chromatography and reverse-phase chromatography, the purity of every six kinds of rhapo was over 95%.
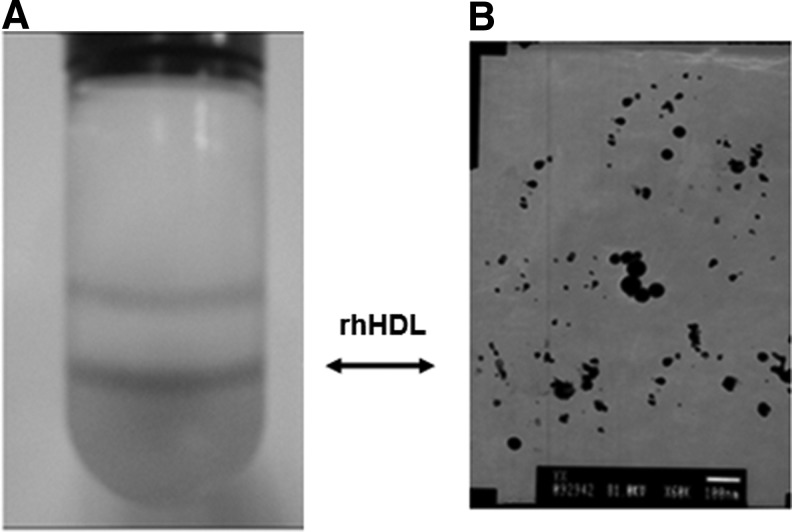
We synthesized large-particle, mature rhHDL in vitro with rhapoA-I, rhapoA-II, rhapoA-IV, rhapoC-I, rhapoC-II, rhapoE, egg phosphatidylcholine, and cholesterol. After purified by density-gradient centrifugation, the contents of each rhapo were quantified by ELISA. Based on morphological observation with a transmission electron microscope, we confirmed that the micellar complexes of rhapo with phosphatidylcholine and cholesterol were prepared. After being dialyzed, the solution of rhHDL was proven to consist of particles of different diameters (10–30 nm) (Fig. 1).
Fig. 1.
Preparation of rhHDL. (A) Tube contains rhHDL after centrifugation. (B) Morphologic observation of rhHDL by a transmission electron microscope (×6,000). rhHDL, recombinant human high-density lipoprotein.
Cellular Cholesterol Efflux Assays
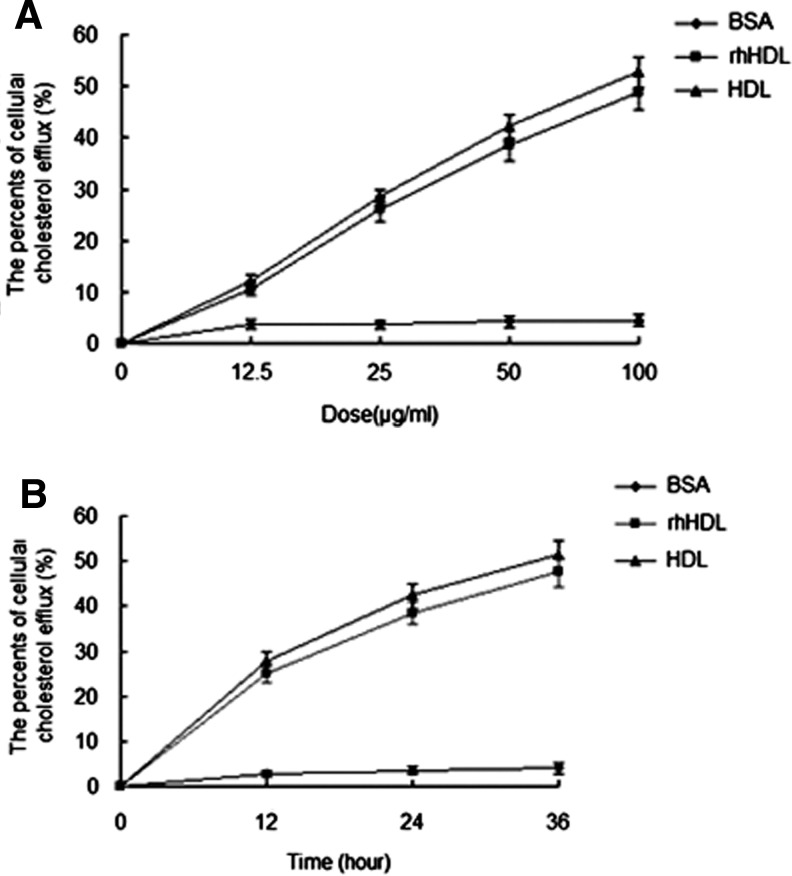
HDL is believed to play a prominent role in the process of RCT, in which it promotes the efflux of excess cholesterol from peripheral tissues (including macrophages) and returns it to the liver for biliary excretion. In our studies, after treated with rhHDL at different doses or time, the medium and macrophages were collected to detect the cellular cholesterol efflux. As a result, rhHDL treatment caused a dose-dependent increase of cellular cholesterol efflux in macrophages at 24 h (Fig. 2A) and a time-dependent increase of cellular cholesterol efflux in macrophages at a dose of 50 μg/mL (Fig. 2B) as well as native HDL.
Fig. 2.
Cellular cholesterol efflux assays. (A) Dose–effect curve of cellular cholesterol efflux facilitated by BSA (♦), rhHDL (■), and native HDL (▴). (B) Time–effect curve of cellular cholesterol efflux facilitated by BSA (♦), rhHDL (■), and native HDL (▴). BSA, bovine serum albumin.
Effects of rhHDL on Lipid Metabolism in Serum
As shown in Table 2, the mean concentrations of serum TC (P<0.001), TG (P<0.05), and LDL-c (P<0.001) were significantly higher, but the concentrations of HDL-c were quite lower (P<0.05) in the MC group than the NC group. Thus, the AI was significantly higher in the high-fat diet group than in the normal basal diet group (P<0.001). Moreover, these results indicated that the high-fat administration developed lipid metabolic dysfunction and promoted hyperlipidemia.
Table 2.
Serum Lipid Profile of Hyperlipidemic Mice
| Group | TC (mmol/L) | TG (mmol/L) | LDL-c (mmol/L) | HDL-c (mmol/L) | AI |
|---|---|---|---|---|---|
| Normal control | 2.11±0.15 | 0.69±0.23 | 0.23±0.04 | 1.46±0.22 | 0.47±0.19 |
| Model control | 21.6±6.37a | 1.25±0.37b | 19.35±5.29a | 1.16±0.19b | 17.9±6.86a |
| rhHDL (1.25 mg/kg) | 7.34±1.01c | 0.78±0.19d | 2.03±0.33c | 2.24±0.25c | 2.27±0.46c |
| rhHDL (2.5 mg/kg) | 5.91±0.53c | 0.72±0.21d | 1.77±0.29c | 2.42±0.30c | 1.44±0.34c |
| Native HDL (2.0 mg/kg) | 5.06±0.48c | 0.75±0.32d | 1.46±0.22c | 2.43±0.21c | 1.09±0.25c |
Normal control (NC) and model control (MC) groups were treated with vehicle; low-dose (LD) and high-dose (HD) groups were treated with rhHDL 2 mg/kg and 4 mg/kg, respectively; the positive control group (PC) was treated with native HDL 4 mg/kg once daily.
Data are mean±standard error of the means (n=10).
aP<0.001 versus normal control.
bP<0.05 versus normal control.
cP<0.001 versus model control.
dP<0.05 versus model control.
TC, total cholesterol; TG, triglyceride; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; AI, atherogenic index.
After the treatment, the serum TC (P<0.001), TG (P<0.05), and LDL-c (P<0.001) were rather lower, whereas HDL-c was extremely higher (P<0.001) in the low-dose rhHDL group, high-dose rhHDL group, and native HDL group than the MC group. As a result, rhHDL treatment caused a dose-dependent decrease of TC, TG, and LDL-c, and at the same time, increase of HDL-c in 21-day treatment.
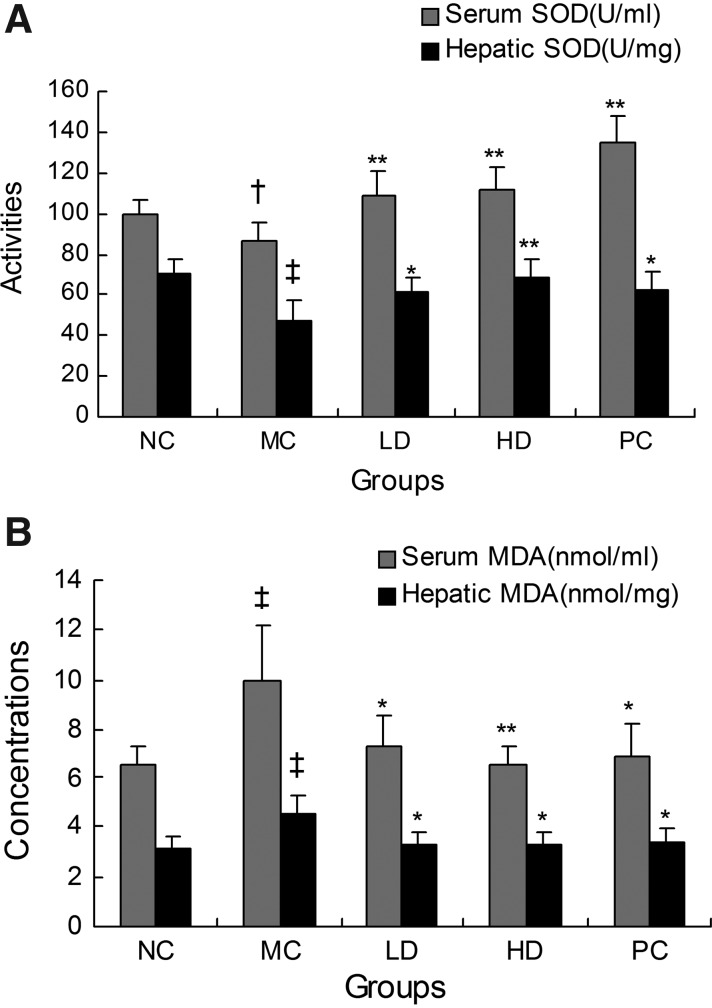
Since MDA is considered to be the major product of lipid peroxidation, an MDA assay is generally used to detect the existence of lipid peroxidation as a result of oxidative stress in any tissue.26 In our study, we found that 15 days of high-fat diet resulted in a prominent elevation of the concentration of MDA in serum and the liver compared with the NC group (P<0.01). Therefore, this indicated that oxidative damage in the MC group was more severe compared with the NC group. Moreover, the level of the antioxidant enzyme SOD, which is the scavenger of reactive oxygen species in oxidative stress, was found to be sharply reduced in the MC group (P<0.01 in the liver and P<0.05 in serum). To sum up, these results indicated that a high-fat diet lowered the activity of SOD and accelerated lipid oxidation, thereby resulting in an increased quantity of lipid peroxides. Both low dose and high dose of rhHDL decreased the concentration of MDA, and meanwhile increased the activities of SOD in the serum and liver homogenates remarkably (P<0.01 or P<0.05) compared with the MC group (Fig. 3).
Fig. 3.
Effects of rhHDL on SOD (A) and MDA (B) in experimental hyperlipidemic mice. Both SOD and MDA were measured in serum (■) and in the liver (■). NC and MC groups were treated with vehicle; LD and HD groups were treated with rhHDL 2 mg/kg and 4 mg/kg, respectively; the PC group was treated with native HDL 4 mg/kg once daily. †P<0.05 versus normal control; ‡P<0.01 versus normal control; *P<0.05 versus model control; **P<0.01 versus model control. SOD, superoxide dismutase; MDA, malondialdehyde; NC, normal control; MC, model control; PC, positive control; LD, low dose; HD, high dose.
Effects of rhHDL on PGI2 and TXA2 in Plasma
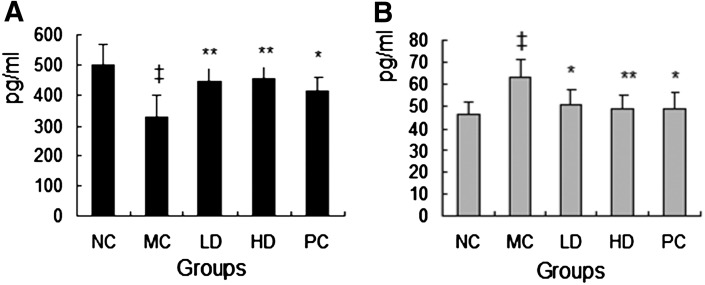
As known, PGI2 and TXA2 are major prostanoids in the cardiovascular system; specifically, PGI2 is synthesized in the endovascular system and acts as a vasodilator in addition to its inhibitory effect on platelet aggregation. In contrast, TXA2 is synthesized by platelets, acts as a vasoconstrictor, and induces platelet aggregation. According to those, their balance is critical in various vascular occlusive diseases, including coronary heart disease. In this study, the mean concentrations of plasma TXA2 were quite higher (P<0.01), but the concentrations of PGI2 were remarkably lower (P<0.01) in the MC group compared with the NC group. After the treatment of rhHDL and native HDL, the concentrations of PGI2 were increased (P<0.01), and the concentrations of TXA2 were fairly decreased (P<0.01 or P<0.05) compared with the MC group (Fig. 4).
Fig. 4.
Effects of rhHDL on PIG2 (A) and TXA2 (B) in experimental hyperlipidemic mice. NC and MC groups were treated with vehicle; LD and HD groups were treated with rhHDL 2 and 4 mg/kg, respectively; the PC group was treated with native HDL 4 mg/kg once daily. ‡P<0.01 versus normal control; *P<0.05 versus model control; **P<0.01 versus model control. PGI2, prostaglandin I2; TXA2, thromboxane A2.
Histopathological Examination of the Liver
Compared to the NC group, the MC group demonstrated marked swelling of hepatocytes and fatty degeneration due to accumulation of lipid droplets. In addition, the infiltration of inflammatory cells and single-cell necrosis were observed in the MC group. When compared to the MC group, the swelling of hepatocytes was suppressed in a dose-dependent manner in the rhHDL-treated groups, and the fatty degeneration of hepatocytes, infiltration of inflammatory cells, and single-cell necrosis were also improved (Fig. 5).
Fig. 5.
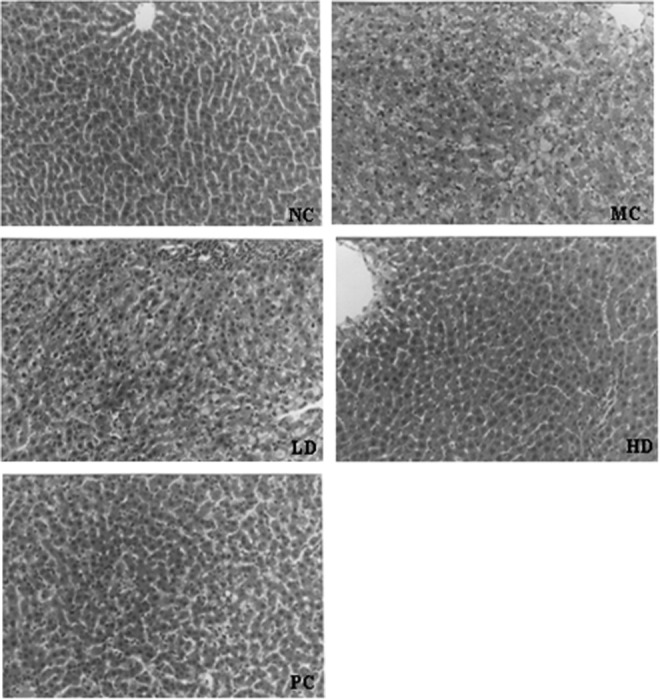
Histopathological examination of the liver, stained with hematoxylin and eosin (×200). NC and MC groups were treated with vehicle; LD and HD groups were treated with rhHDL 2 and 4 mg/kg, respectively; PC group was treated with native HDL 4.0 mg/kg once daily.
Discussion
It is well known that the HDL fraction in human plasma is heterogeneous, consisting of a number of discrete subpopulations that vary in size, density, composition of lipids, and apolipoproteins.26 The distribution profile of HDL subpopulations of patients with mixed hyperlipoproteinemia appears to be abnormal, that is, higher levels of small-sized particles (preβ-HDL and HDL3) and lower levels of large-sized particles (HDL2),27,28 which have been reported to be associated with cardiovascular disease severity in previous angiographic studies.29 Exchangeable apolipoproteins, including apoAs, apoE, and apoCs, are constituents of HDL.30 We prepared rhHDL that contained six kinds of rhapo, rhapoA-I, rhapoA-II, rhapoA-IV, rhapoE, rhapoC-I, and rhapoC-II, according to the apolipoprotein percentages of native mature HDL. Cholate can facilitate penetration of apolipoproteins between the phosphatidylcholine acyl chains. When phosphatidylcholine/cholate is from 1:2 to 2:l, the phosphatidylcholine–cholate mixtures exist in bilayer discs, and are stabilized by an annular arrangement.31 In our studies, as phosphatidylcholine/cholate was 3:2, which is between 1:2 and 2:l, we prepared stable rhHDL. Since cholate disrupts the phosphatidylcholine lattice by intercalation and/or solubilization, it must be removed by dialysis after incubation at 4°C. The particle diameter of rhHDL was 10–30 nm, which was much larger than native HDL (5–17 nm). We detected the activity of rhHDL by cellular cholesterol efflux assays and the effects on lipid metabolism.
Apolipoprotein-mediated cholesterol efflux is a critical process of RCT that describes the transfer of cholesterol from nonhepatic cells to the liver. In this study, rhHDL can induce cellular cholesterol efflux in macrophages. Furthermore, rhHDL can regulate lipid metabolism in vivo. After the treatment by rhHDL, the serum TC, TG, and LDL-c of hyperlipidemic mice were really lower, whereas HDL-c was significantly higher compared with the MC group. It means that rhHDL can reverse hyperlipidemia that was caused by a high-fat diet.
Increased levels of lipid peroxidation, that is, MDA levels, in animals treated with high-fat diet reflect excessive formation of free radicals and a greater formation of lipid peroxides. SOD is a metalloenzyme that was known to be the first-line cellular defense against free-radical damage32,33 MDA levels, together with SOD activity levels, can be measured to monitor the degree of lipid peroxidation.34 In the present study, SOD activity decreased, whereas MDA increased rapidly in the high-fat diet group. Administration of rhHDL effectively prevented the depletion of SOD activities and significantly decreased the MDA levels. All in all, these results indicated that rhHDL can reduce lipid peroxidation and plays an important role in antioxidation as well as native HDL.
PGI2 and TXA2 are important regulators of vascular homeostasis, and their respective levels dictate the response to vascular injury.35 Separately speaking, PGI2 could provide cardioprotection; nevertheless, the actions of TXA2 on platelets and vasculature should contribute to the pathogenesis of atherosclerosis and/or vasospasm, which are prerequisites for coronary heart disease.36 rhHDL can reverse the disproportionality of TXA2 and PGI2 caused by a high-fat diet.
In conclusion, rhHDL has the similar effects on antiatherosclerosis with the native HDL via its effects on RCT, blood lipid metabolism, and antioxidative and antithrombotic properties of rhHDL. It could be used as a therapeutic HDL-replacement agent.
Abbreviations
- AI
atherogenic index
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- HD
high dose
- HDL
high-density lipoprotein
- HDL-c
high-density lipoprotein cholesterol
- LD
low dose
- LDL-c
low-density lipoprotein cholesterol
- MC
model control
- MDA
malondialdehyde
- NC
normal control
- PBS
phosphate-buffered saline
- PC
positive control
- PGI2
prostaglandin I2
- RCT
reverse cholesterol transport
- rhapo
recombinant human apolipoprotein
- rhHDL
recombinant human high-density lipoprotein
- SOD
superoxide dismutase
- TC
total cholesterol
- TG
triglyceride
- TXA2
thromboxane A2
Acknowledgments
This work was supported by grants from the Jilin University Seed Fund, the National Natural Science Foundation of China (Grants 30973187), the Jilin Province Development and Reform Commission, and the Jilin Science and Technology Bureau (Grants 20120957).
Disclosure Statement
No competing financial interests exist.
References
- 1.Wang X. Paigen B. Genetics of variation in HDL cholesterol in humans and mice. Circ Res. 2005;96:27–42. doi: 10.1161/01.RES.0000151332.39871.13. [DOI] [PubMed] [Google Scholar]
- 2.Gotto AM., Jr Brinton EA. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J Am Coll Cardiol. 2004;43:717–724. doi: 10.1016/j.jacc.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 3.Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans affairs high–density lipoprotein intervention trial. Am J Cardiol. 2000;86:19L–22L. doi: 10.1016/s0002-9149(00)01464-8. [DOI] [PubMed] [Google Scholar]
- 4.Pieters MN. Schouten D. Van Berkel TJ. In vitro and in vivo evidence for the role of HDL in reverse cholesterol transport. Biochim Biophys Acta. 1994;1225:125–134. doi: 10.1016/0925-4439(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 5.von Eckardstein A. Nofer JR. Assmann G. High density lipoproteins and arteriosclerosis role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Navab M. Imes SS. Hama SY. Hough GP. Ross LA. Bork RW, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parthasarathy S. Barnett J. Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044:275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 8.Cockerill GW. Rye KA. Gamble JR. Vadas MA. Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 9.Xia P. Vadas MA. Rye KA. Barter PJ. Gamble JR. High density lipoproteins (HDL) interrupt the sphingosine kinase signaling pathway. A possible mechanism for protection against atherosclerosis by HDL. J Biol Chem. 1999;274:33143–33147. doi: 10.1074/jbc.274.46.33143. [DOI] [PubMed] [Google Scholar]
- 10.Shah PK. Kaul S. Nilsson J. Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part I. Circulation. 2001;104:2376–2383. doi: 10.1161/hc4401.098467. [DOI] [PubMed] [Google Scholar]
- 11.Assmann G. Nofer JR. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 12.Duffy D. Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 13.Lewis GF. Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 14.Remaley AT. Amar M. Sviridov D. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther. 2008;6:1203–1215. doi: 10.1586/14779072.6.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls SJ. Tuzcu EM. Sipahi I. Schoenhagen P. Crowe T. Kapadia S, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006;47:992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Bloedon LT. Dunbar R. Duffy D. Pinell-Salles P. Norris R. DeGroot BJ, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikewaki K. Zech LA. Kindt M. Brewer HB., Jr Rader DJ. Apolipoprotein A-II production rate is a major factor regulating the distribution of apolipoprotein A-I among HDL subclasses LpA-I and LpA-I:A-II in normolipidemic humans. Arterioscler Thromb Vasc Biol. 1995;15:306–312. doi: 10.1161/01.atv.15.3.306. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson H. Leanderson P. Tagesson C. Lindahl M. Lipoproteomics II: mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1431–1445. doi: 10.1002/pmic.200401010. [DOI] [PubMed] [Google Scholar]
- 19.Su M. Xu T. Wang D. Zhou Y. Niu C. Yan W. High yield and purification of recombinant human apolipoprotein E3 in Pichia pastoris. Protein Expr Purif. 2009;68:7–11. doi: 10.1016/j.pep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Su M. Zhou Y. Wang D. Xu T. Chang W. Wang M, et al. Expression and purification of recombinant human apolipoprotein C-I in Pichia pastoris. Protein Expr Purif. 2011;78:22–26. doi: 10.1016/j.pep.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Kato H. Nakanishi T. Arai H. Nishida HI. Nishida T. Purification, microheterogeneity, and stability of human lipid transfer protein. J Biol Chem. 1989;264:4082–4087. [PubMed] [Google Scholar]
- 22.Chung BH. Wilkinson T. Geer JC. Segrest JP. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980;21:284–291. [PubMed] [Google Scholar]
- 23.Wang M. Liu JR. Gao JM. Parry JW. Wei YM. Antioxidant activity of Tartary buckwheat bran extract and its effect on the lipid profile of hyperlipidemic rats. J Agric Food Chem. 2009;57:5106–5112. doi: 10.1021/jf900194s. [DOI] [PubMed] [Google Scholar]
- 24.Tietge UJ. Pratico D. Ding T. Funk CD. Hildebrand RB. Van Berkel T. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. J Lipid Res. 2005;46:1604–1614. doi: 10.1194/jlr.M400469-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.de Boer JF. Annema W. Schreurs M. van der Veen JN. van der Giet M. Nijstad N, et al. Type I diabetes mellitus decreases in vivo macrophage-to-feces reverse cholesterol transport despite increased biliary sterol secretion in mice. J Lipid Res. 2012;53:348–357. doi: 10.1194/jlr.M018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med. 2002;32:303–308. doi: 10.1016/s0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- 27.Barter PJ. Nicholls S. Rye KA. Anantharamaiah GM. Navab Giet M. Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 28.Saïdi Y. Sich D. Camproux A. Egloff M. Federspiel MC. Gautier V, et al. Interrelationships between postprandial lipoprotein B: CIII particle changes and high-density lipoprotein subpopulation profiles in mixed hyperlipoproteinemia. Metabolism. 1999;48:60–67. doi: 10.1016/s0026-0495(99)90011-2. [DOI] [PubMed] [Google Scholar]
- 29.Miida T. Yamaguchi T. Tsuda T. Okada M. High preβ1-HDL levels in hypercholesterolemia are maintained by probucol but reduced by a low-cholesterol diet. Atherosclerosis. 1998;138:129–134. doi: 10.1016/s0021-9150(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 30.Johansson J. Carlson LA. Landou C. Hamsten A. High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb. 1991;11:174–182. doi: 10.1161/01.atv.11.1.174. [DOI] [PubMed] [Google Scholar]
- 31.Gursky O. Apolipoprotein structure and dynamics. Curr Opin Lipidol. 2005;16:287–294. doi: 10.1097/01.mol.0000169348.61191.ac. [DOI] [PubMed] [Google Scholar]
- 32.Matz CE. Jonas A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J Biol Chem. 1982;257:4535–4540. [PubMed] [Google Scholar]
- 33.Ji LL. Stratman FW. Lardy HA. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys. 1988;263:150–160. doi: 10.1016/0003-9861(88)90623-6. [DOI] [PubMed] [Google Scholar]
- 34.Ceyran H. Narin F. Narin N. Akgun H. Ceyran AB. Ozturk F, et al. The effect of high dose melatonin on cardiac ischemia-reperfusion injury. Yonsei Med J. 2008;49:735–741. doi: 10.3349/ymj.2008.49.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topsakal C. Erol FS. Ozveren MF. Yilmaz N. Ilhan N. Effects of methylprednisolone and dextromethorphan on lipid peroxidation in an experimental model of spinal cord injury. Neurosurg Rev. 2002;25:258–266. doi: 10.1007/s101430100183. [DOI] [PubMed] [Google Scholar]
- 36.Xiao CY. Hara A. Yuhki K. Fujino T. Ma H. Okada Y, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a studyusing mice lacking their respective receptors. Circulation. 2001;104:2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]