Abstract
Duchenne muscular dystrophy (DMD) is a severe and the most prevalent form of muscular dystrophy, characterized by rapid progression of muscle degeneration. Antisense-mediated exon skipping is currently one of the most promising therapeutic options for DMD. However, unmodified antisense oligos such as morpholinos require frequent (weekly or bi-weekly) injections. Recently, new generation morpholinos such as vivo-morpholinos are reported to lead to extensive and prolonged dystrophin expression in the dystrophic mdx mouse, an animal model of DMD. The vivo-morpholino contains a cell-penetrating moiety, octa-guanidine dendrimer. Here, we sought to test the efficacy of multiple exon skipping of exons 6–8 with vivo-morpholinos in the canine X-linked muscular dystrophy, which harbors a splice site mutation at the boundary of intron 6 and exon 7. We designed and optimized novel antisense cocktail sequences and combinations for exon 8 skipping and demonstrated effective exon skipping in dystrophic dogs in vivo. Intramuscular injections with newly designed cocktail oligos led to high levels of dystrophin expression, with some samples similar to wild-type levels. This is the first report of successful rescue of dystrophin expression with morpholino conjugates in dystrophic dogs. Our results show the potential of phosphorodiamidate morpholino oligomer conjugates as therapeutic agents for DMD.
Introduction
Duchenne muscular dystrophy (DMD) is a lethal and the most common form of muscular dystrophy worldwide, which affects 1 in 3,500 boys (Duchenne, 1867; Zellweger and Antonik, 1975). There is currently no effective cure for DMD. Most patients die in their 20s–30s with respiratory or heart failure. DMD and its milder form, Becker muscular dystrophy, are caused by mutations in the dystrophin (DMD) gene (Hoffman et al., 1987; Koenig et al., 1987). Antisense oligonucleotide-mediated exon skipping therapy is a most promising approach to curing DMD (Pramono et al., 1996; Dunckley et al., 1998; Goyenvalle et al., 2011; Lu et al., 2011). Antisense oligos such as phosphorodiamidate morpholino oligomers (PMOs, or morpholinos) and 2'O- methyl antisense oligos with phosphorothioate bonds (2'OMePS) against dystrophin mRNA lead to the production of internally deleted in-frame transcripts both in vitro and in vivo (Pramono et al., 1996; Dunckley et al., 1998; Lu et al., 2005; Yokota et al., 2009a; Yokota et al., 2012). The truncated quasi-dystrophin retains some functions like mild Becker dystrophy or even leads to asymptomatic individuals in some cases (Beroud et al., 2006; Nakamura et al., 2008; Aoki et al., 2010; Goyenvalle et al., 2010). Exon skipping therapies with PMO or 2'OMePS antisense oligos targeting the exon 51 are currently under phase-2/3 clinical trials (Aartsma-Rus and van Ommen, 2007; van Deutekom et al., 2007; Kinali et al., 2009; Cirak et al., 2011; Goemans et al., 2011).
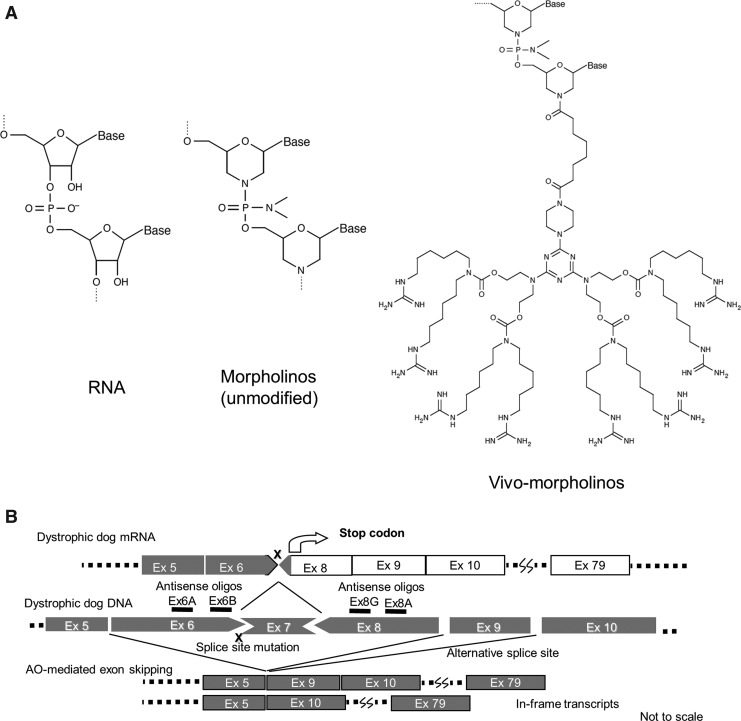
One of the biggest challenges of exon-skipping therapy is that the single exon skipping is applicable to only approximately 50% of DMD patients (total of each single individual target exon). In contrast, double or multiple exon skipping is potentially applicable to 90% of patients (Aartsma-Rus et al., 2006; Yokota et al., 2007a). The dystrophic dog requires more than one exon skipping (multiple exon skipping targeting exon 6 and exon 8 in the dystrophin mRNA). Previously we reported the first successful multiple (double) exon-skipping treatment in body-wide skeletal muscles in Canine X-linked muscular dystrophy (CXMD) with a cocktail of antisense phosphorodiamidate morpholino oligomers (PMOs, morpholinos) (Yokota et al., 2009a). The dog trial targeting exon 6 and exon 8 of dystrophin mRNA led to 27% normal levels of dystrophin expression in body-wide skeletal muscles detected by western blotting analysis on average. However, unmodified morpholinos exhibit inefficient long-term delivery. The half-life of dystrophin expression was approximately 1–2 months (Wu et al., 2010).
Recently, new generation morpholinos such as cell-penetrating peptide conjugated phosphorodiamidate morpholino oligomers (PPMOs) and vivo-morpholinos (vPMOs) were reported to induce prolonged and extensive rescue of dystrophin expression and ameliorate the function in cardiac muscles in dystrophic mdx mice (Wu et al., 2009; Goyenvalle et al., 2010; Jearawiriyapaisarn et al., 2010; Crisp et al., 2011; Widrick et al., 2011; Wu et al., 2011a). vPMOs are morpholino oligomers conjugated with delivery moiety containing eight terminal guanidinium groups on a dendrimer scaffold that enable entry into cells (Fig. 1A) (Morcos et al., 2008). New generation morpholinos are efficiently delivered into various tissues including muscle fibers in vivo (Wu et al., 2009). Vivo-morpholino-mediated splice modulation efficiently also rescued Fukuyama congenital muscular dystrophy model mice and primary myotubes from human patients (Taniguchi-Ikeda et al., 2011). Their delivery efficacy is reported to be more than 50 times higher than unmodified morpholinos (Wu et al., 2009).
FIG. 1.
Antisense chemistry and design of multiple exon skipping for dystrophic dogs. (A) Comparison of antisense oligos. (B) Schematic design of multi (double) exon skipping therapy for dystrophic dogs. At least two exons (exons 6 and 8) need to be skipped (removed) with antisense oligos to correct their reading frame. Gua, Guanidine.
In this study, we focused on 2 aims. First, we employed a novel backbone (vivo-morpholino) for the antisense therapy in the dog model. Second, we tested novel antisense oligo cocktails designed for multiple exon skipping (exons 6 and 8) in the canine DMD gene. We hypothesized that (1) vivo-morpholinos induce extensive and prolonged dystrophin expression in dystrophic dogs, and (2) our novel antisense oligo cocktail can improve the efficacy of exon 6–8 multiple skipping. We tested these hypotheses and the efficacy of multiple (double) exon skipping in dystrophic dogs in vivo. Vivo-morpholinos with newly optimized sequences induced near normal level of dystrophin protein and prolonged expression recovery.
Materials and Methods
Ethics Statement
All animal works have been conducted according to relevant national and international guidelines. The Experimental Animal Care and Use Committee of the National Institute of Neuroscience, National Center of Neurology and Psychiatry (NCNP) Japan approved all experimental protocols in this study. We obtained consent from all of the owners of the dogs involved in this study (All dogs are owned by NCNP).
Animals
The CXMD dog is the beagle dog model of DMD (Shimatsu et al., 2003). They were allowed ad libitum access to food and drinking water. Dogs carrying mutations were identified by reverse-transcription polymerase chain reaction (RT-PCR) analysis as previously described (Sharp et al., 1992). Three- to five-month-old dogs were used. Five dystrophic dogs were used for injections. Four dystrophic dogs and three wild-type dogs were used as non-treated controls. Animals were euthanized by exsanguination under general anesthesia.
Antisense oligos
Antisense oligos for targeted skipping of exons 6 and exon 8 in the canine DMD gene were used as previously described (Tables 1, 2) (Yokota et al., 2009a; Saito et al., 2010). All PMOs and vPMOs were obtained from Gene-tools, Inc (Morcos et al., 2008). As control oligos, we employed Ex6A only (GTTGATTGTCGGACCCAGCTCAGG) or 3-oligo cocktail containing Ex6A (GTTGATTGTCGGACCCAGCTCAGG), Ex6B (ACCTATGACTGTGGATGAGAGCGTT), and Ex8A (CTTCCTGGATGGCTTCAATGCTCAC) for intramuscular injections as indicated. The dose selection is based on previous mouse studies with PMOs and vPMOs, showing that vPMOs induce more than 10×higher efficacy, and dog studies with PMOs (Wu et al., 2009). The Ex8G dose and ratio were determined based on previous cell experiments (Saito et al., 2010).
Table 1.
Antisense Oligo Sequences
| Oligo name | Sequence (5′–3′) |
|---|---|
| Ex6A | GTTGATTGTCGGACCCAGCTCAGG |
| Ex6B | ACCTATGACTGTGGATGAGAGCGTT |
| Ex8A | CTTCCTGGATGGCTTCAATGCTCAC |
Table 2.
Additional Antisense Oligo Sequences for Exon 8
| Oligo name | Sequence (5′–3′) |
|---|---|
| Ex8G | GGCAAAACTTGGAAGAGTGATGTGA |
| Ex8I | CCTTGGCAACATTTCCACTTCCTGG |
| Ex8K | TTTACCTGTTGAGAATAGTGCATTT |
Injections
Animals were anesthetized with thiopental sodium induction and maintained by isoflurane (Nacalai Tesque, Inc.) for all intramuscular injections and muscle biopsies. General anesthesia was maintained with isoflurane administered through an endotracheal tube. Skin was excised over the site of injection, muscle exposed, and the injection site marked with a suture in the muscle. Antisense oligonucleotides were delivered by intramuscular injection using 1 mL saline bolus into indicated skeletal muscles using a 27-gauge needle. Antisense oligonucleotides were delivered as a singular or in mixtures as previously described (Yokota et al., 2011). Tibialis anterior, extensor digitorum longus, extensor carpi ulnaris, flexor digitorum superficialis (FDS), flexor carpi ulnaris (FCU), and flexor carpi radialis (FCR) muscles were used for injections. Muscles samples were obtained 2 or 8 weeks after the intramuscular injections. Muscles were obtained immediately, snap-frozen in liquid nitrogen–cooled isopentane, and stored at −80°C for immunohistochemistry and western blotting. Skeletal muscle tissues were cut and collected in microtubes and snap-frozen in liquid nitrogen for RT-PCR analysis.
Immunohistochemical analysis
Antibodies
The following monoclonal antibodies were used for immunofluorescence: anti-dystrophin DYS-1 (Novocastra, Newcastle upon Tyne, UK). Alexa 488, or Alexa 594 conjugated goat anti mouse secondary antibodies (Invitrogen).
Immunofluorescence
Cryosections (7.5 μm) were blocked with 20% goat serum in phosphate buffered saline, and then incubated with a primary antibody at 4°C overnight. Alexa 488, or 594-conjugated anti-mouse goat antibody (Invitrogen, Camarillo, CA) was used as the secondary antibody. The sections were viewed and photographed by a laser scanning microscope, FluoView™ (Olympus, Tokyo, Japan) The number of positive fibers for DYS-1 was counted and compared in sections containing the largest number of positive fibers as described previously (Yokota et al., 2006). At least 200 muscle fibers were counted in each section for the analysis.
Western blotting analysis
Muscle proteins from cryosections were extracted with lysis buffer containing 75 mM Tris-HCl (pH 6.8), 10% sodium dodecyl sulfate, 10 mM EDTA, and 5% 2-mercaptoethanol. Four to 40 μg proteins were loaded onto precase 3%–8% resolving sodium dodecyl sulfate polyacrylamide gel electrophoresis gels following manufacturer's instructions (Bio-Rad, Hercules, CA). The gels were transferred by semidry blotting at 400 mA for 1.5 hours. DYS-1 (Novocastra) antibody against dystrophin and rabbit polyclonal antibody against desmin (Abcam) were used as primary antibodies. Horseradish peroxidase–conjugated anti-mouse or anti-rabbit goat immunoglobulin (Cedarlane Laboratories, Hornby, Ontario, Canada) was used as a secondary antibody. Enzyme chemiluminescence kit (GE, Fairfield, CT) was used for the detection. Blots were analyzed by ImageJ software (COLLINS, 2007).
Reverse transcriptase polymerase chain reaction
Total RNA was extracted from frozen tissue sections using TRIzol (Invitrogen). Then RT-PCR was performed on 200 ng of total RNA for 35 cycles of amplification using One-Step RT-PCR kit (Qiagen, Chatsworth, CA) following manufacturer's instructions with 0.6 μM of an exon 5 (CTGACTCTTGGTTTGATTTGGA) forward primer. Reverse primers were exon 10 (TGCTTCGGTCTCTGTCAATG).
Statistical analysis
The data between samples were compared using F-test and Student's or Welch's t-test. P<0.05 was considered statistically significant.
Results
Design of antisense vivo-morpholinos
In this study, we employed a cocktail of antisense vivo-morpholino oligos (Gene-tools) to induce exon skipping of exon 6 and exon 8 in the canine dystrophin (DMD) gene (Fig. 1A). A vivo-morpholino is comprised of a morpholino oligo with a covalently linked delivery moiety, an octa-guanidine dendrimer. As previously demonstrated, at least two exons (exon 6 and exon 8) need to be removed to restore the reading frame of the splice site mutation in the CXMD (Fig. 1B) (McClorey et al., 2006; Yokota et al., 2009a). Initially, we employed a cocktail oligo with the same sequences and combinations, Ex6A, Ex6B, and Ex8A, as previously used (Yokota et al., 2009a) (Table 1). We compared the efficacy of exon skipping by vPMOs and unmodified morpholinos.
Sustained dystrophin expression after cocktail vPMO injections
Since sustained recovery of dystrophin expression was previously reported after vivo-morpholino injections into dystrophic mdx mice, we tested the dystrophin expression levels 2 weeks and 8 weeks after vPMO injections in cranial tibialis (tibialis anterior in humans) muscles in dystrophic dogs (Fig. 2) (Jearawiriyapaisarn et al., 2008; Wu et al., 2009; Widrick et al., 2011; Wu et al., 2011a). In this study, we employed 3- to 5-month-old dystrophic dogs. At this stage the disease progression was relatively mild in these dogs. We employed a cocktail of three antisense oligos named Ex6A, Ex6B, and Ex8A (Table 1). We used anti-rod domain dystrophin antibody because anti-C-terminus dystrophin antibodies cross-react with other dystrophin isoforms (e.g., Dp71).
FIG. 2.
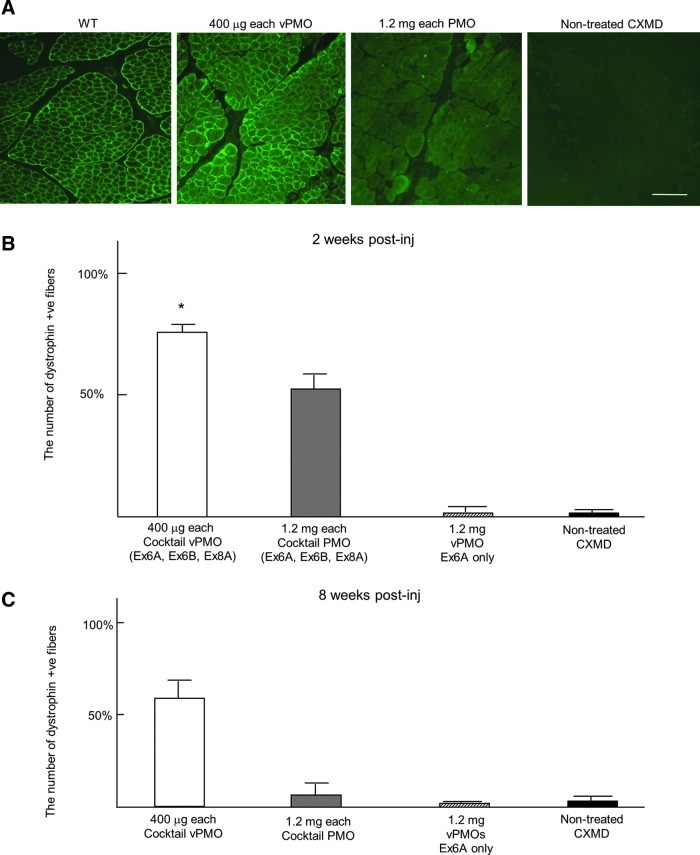
Vivo phosphorodiamidate morpholino oligomer (vPMO) local injections restore dystrophin expression in TA 8 weeks later. (A) Immunohistochemistry with dystrophin (DYS1) antibody 8 weeks after the cocktail vPMO treatment containing Ex6A, Ex6B, and Ex8A in canine X-linked muscular dystrophy (CXMD) (1.2 mg in total as a cocktail, 400 μg of each oligo), and unmodified morpholino treatment containing Ex6A, Ex6B, and Ex8A in CXMD (3.6 mg in total as a cocktail, 1.2 mg of each oligo). (B) The number of dystrophin positive fibers 2 weeks after injections. (C) The number of dystrophin positive fibers 8 weeks after injections. Scale bar=200 μm; n=2–4 in each group; *P<0.05 compared with non-treated control group.
While unmodified PMO (1.2 mg each, or 3.6 mg in total as a cocktail) injected muscle showed almost no detectable dystrophin expression 8 weeks after injections, extensive dystrophin expression was observed after 400 μg vPMO injected muscles (Fig. 2A). At 2 weeks after the vPMO (400 μg each) injection, approximately 75% of fibers were positive with dystrophin DYS-1 antibody, while 55% were positive after unmodified PMO injection (1.2 mg each) (Fig. 2B). A cocktail of antisense vPMOs was required to induce dystrophin expression (Fig.2B). Injections with single antisense vPMO targeting exon 6 only (Ex6A) did not induce detectable level of dystrophin expression (Fig. 2B). At 8 weeks after vPMO injection, approximately 60% of fibers were still positive with DYS-1 antibody, while only 10% were positive after unmodified PMO injection (Fig. 2C).
The expression level of dystrophin was then examined by western blotting analysis (Fig. 3). Approximately 20% of the level of dystrophin in wild-type was detected in vPMO-injected muscles 8 weeks after the injection with 400 μg each of Ex6A, Exx6B, and Ex8A (or 1.2 mg in total as a cocktail).
FIG. 3.
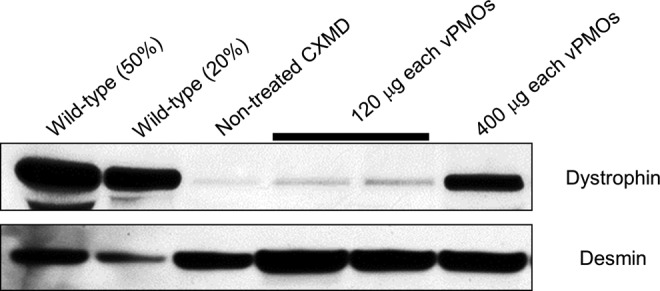
Prolonged dystrophin expression after vPMO injections. Western blotting analysis on dystrophin expression with DYS-1 antibody 8 weeks after vPMO cocktail injections (120 μg each or 400 μg each of Ex6A, Ex6B, and Ex8A) into TA muscles in dystrophic dogs as indicated.
Design of novel antisense sequences and combinations for dystrophin exon 8
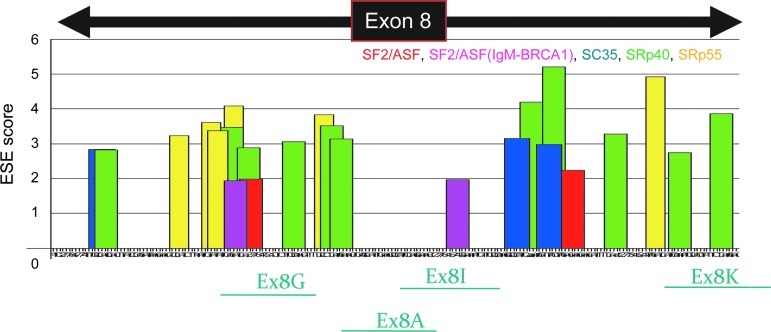
Next, to further optimize the antisense oligo sequences and combinations, we tested new oligos named Ex8G, Ex8I, and Ex8K (Fig. 4, Table 2). In previous study, these oligos efficiently induced exon 8 skipping in vitro in dog and human myotubes (Saito et al., 2010). These oligos were designed to target exon/intron borders or exonic splice enhancer (ESE) sites. ESE scores were obtained by using ESE finder software (Cartegni et al., 2003). These oligos target the same conserved sequences in both dog and human dystrophin mRNA.
FIG. 4.
Schematic outline of the antisense morpholinos targeting exon 8 of dog and human dystrophin mRNA. Antisense oligos against exon 8 of human/dog DMD gene used in this study. These 4 oligos were previously reported to be effective for exon 8 skipping in myotubes of dystrophic dogs and human patients in vitro (Saito et al., 2010).
A novel antisense cocktail induces more efficient exon 8 skipping
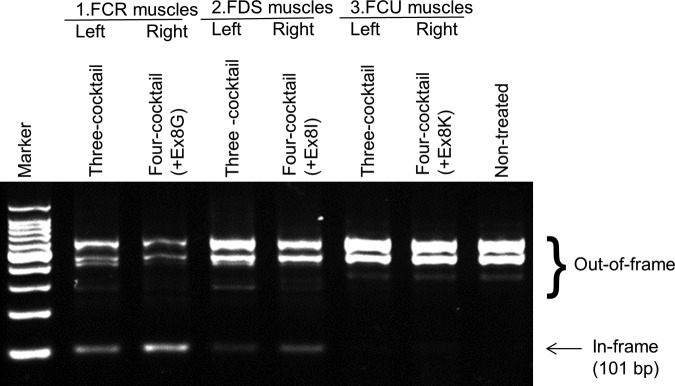
We tested the efficacy of newly designed oligos by intramuscular injections into skeletal muscles in dystrophic dogs (Fig. 5). We previously reported a cocktail oligo containing Ex6A, Ex6B, Ex8A, and Ex8G led to the most efficient double exon skipping of exon 6–8 (or triple skipping of exon 6–9) in both human and dog myotubes in vitro (Saito et al., 2010). Exon 9 was not targeted by antisense oligos but the exon is known as an alternative splice site which is spontaneously skipped with exon 6–8 skipping induced by antisense oligos in previous studies (Reiss and Rininsland 1994; McClorey et al., 2006). Here, we compared the efficacy of multiple exon skipping induced by the three oligos cocktail which we have previously reported effective for systemic trials in dystrophic dogs (Yokota et al., 2009a), and newly designed 4-oligo cocktails, which we found to be more effective in vitro (Saito et al., 2010). Here, 3-oligo cocktail oligos (Ex6A + Ex6B + Ex8A) were injected into right-side muscles of flexor carpi radialis (FCR), Flexor digitorum superficialis (FDS), or flexor carpi ulnaris (FCU) as controls. Different combinations of 4-oligo cocktails were injected into contralateral (left side) muscles (Fig. 5). The same total doses (120 μg) of 3-oligo cocktails or of 4-oligo cocktails were injected (i.e., 40 μg each in 3-oligo cocktails, and 30 μg each in 4-oligo cocktails). The efficacy of multiple exon skipping was initially examined by RT-PCR analysis. While all combinations led to substantial amount of exon 6–9 skipped in-frame mRNA products, the highest efficacy was achieved with the 4-oligo cocktail containing Ex8G (Ex6A + Ex6B + Ex8A + Ex8G), which is consistent with our previous report in myotubes in vitro (Saito et al., 2010).
FIG. 5.
A 4-oligo cocktail containing Ex8G induces efficient dystrophin expression. Detection of exon 6–9 skipped band with reverse-transcription polymerase chain reaction analysis. Equal amounts (120 μg) of oligos in total were injected into indicated muscles (i.e., 40 μg each in three-oligo cocktails, 30 μg each in four-oligo cocktails). FCR, flexor carpi radialis; FDS, flexor digitorum superficialis; FCU, flexor carpi ulnaris.
Efficient dystrophin recovery after injections with four-oligo cocktail vPMOs
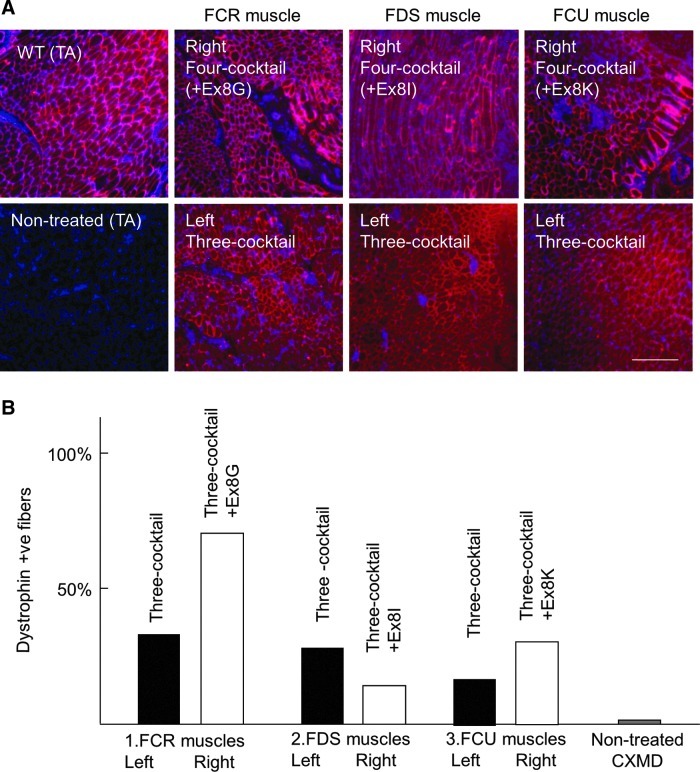
Next, we examined the recovery of dystrophin expression by immunohistochemistry with DYS-1 anti-dystrophin monoclonal antibody 2 weeks after intramuscular vPMO injections (Fig. 6). Although all tested cocktail oligos induced extensive expression of dystrophin, the highest recovery was obtained with the 4-oligo cocktail containing Ex6A, Ex6B, Ex8A, and Ex8G (Fig. 6). Approximately 70 percent of fibers was positively stained with the four-oligo cocktail (Fig. 6B).
FIG. 6.
Immunohistochemistry shows four-oligo cocktails induce efficient dystrophin expression in vivo. (A) Immunohistochemistry with anti-dystrophin antibody (DYS-1; red) and DAPI nuclei staining (blue). Equal amounts (120 μg) of oligos in total were injected into indicated muscles (i.e., 40 μg each in 3-oligo cocktails, 30 μg each in 4-oligo cocktails). Scale bars=200 μm. (B) The percentage of dystrophin positive fibers after cocktail oligo injections.
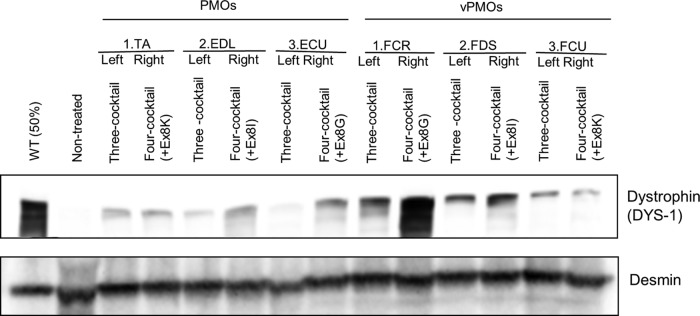
The expression levels of dystrophin after cocktail vPMO injections were also compared with western blotting analysis (Fig. 7). Desmin antibody was used as an internal control. Again, the 4-oligo cocktail injection with Ex6A, Ex6B, Ex8A, and Ex8G led to the highest levels of dystrophin expression.
FIG. 7.
Restoration of dystrophin expression with f4-oligo cocktail vPMOs. Western blotting analysis with anti-dystrophin (DYS-1) antibody 2 weeks after vPMO injections. Equal amounts (120 μg) of oligos in total were injected into indicated muscles (i.e., 40 μg each in 3-oligo cocktails, 30 μg each in 4-oligo cocktails). The 4-oligo cocktail (Ex6A + Ex6B + Ex8A + Ex8G) leads to the highest level of dystrophin expression. TA, tibialis anterior; EDL, extensor digitorum longus; ECU, extensor carpi ulnaris.
Discussion
Antisense mediated exon skipping is currently a most promising therapeutic approach to curing DMD (Yokota et al., 2007b; Hoffman et al., 2011; Partridge 2011; Pichavant et al., 2011). Although phase-2/3 clinical trials are currently underway, there are a couple of challenges. One of the most significant challenges is that the effect usually wears off after 3–4 weeks, thus repeated injections are required. Currently, weekly or bi-weekly injections are required for antisense systemic trials (Lu et al., 2005; Alter et al., 2006; Wu et al., 2011b). New generation morpholinos with cell-penetrating moiety, such as PPMOs and vPMOs, were developed to improve the efficacy in vivo (Moulton and Jiang 2009; Yokota et al., 2009b). Both PPMOs and vPMOs have the same backbones as conventional unmodified morpholinos (Fig. 1). In vPMOs, cell-penetrating octa-guanidine dendrimers are conjugated, while in PPMOs, arginine rich polypeptides are conjugated (Morcos et al., 2008). Peptide-morpholino conjugates (PPMOs) restored dystrophin to more than 80 percent of wild-type levels in skeletal muscles of mdx mice 9 weeks after injections, showing prolonged activity (Moulton et al., 2009). An injection with vPMOs in hDMD mice, a transgenic model carrying the full-length human dystrophin gene, led to more than 70% efficiency of targeted human dystrophin exon skipping in vivo systemically (Wu et al., 2011a). Therefore, use of morpholino conjugates such as PPMOs or vPMOs might be able to reduce the frequency of injections.
In this study, we demonstrated the first successful rescue of dystrophin expression with morpholino conjugates in dystrophic dogs. In previous in vitro experiments, we used a total of 30 μM for 3 or 4 sequences of PMOs (Saito et al., 2010). In this study, we employed 120μg–1.2 mg of vPMOs for intramuscular injections. The induction of exon 6–9 multiple skipping mediated by cocktail vPMOs was significantly more efficient than that mediated by unconjugated PMOs (Fig. 2). The expression levels were remained very high (60% dystrophin-positive fibers) 2 months after the injection, indicating prolonged persistence (Figs. 2–3). We employed dogs in early stages of the disease, because muscle fibers are replaced by fibrous connective tissue at later stages. This might be generalized to the antisense drug products intended for use in the first-in-human trial. Importantly, a vPMO cocktail efficiently rescued other genetic disorders including the mutation in Fukuyama congenital muscular dystrophy (Taniguchi-Ikeda et al., 2011). These studies clearly indicate that morpholino conjugates are not only useful tools for gene-knockdown study, but also have great potential for treating genetic disorders.
We next compared newly designed antisense oligos against exon 8 of dystrophin mRNA in vivo (Figs. 5–7). In accordance with the previous study in vitro by Saito et al., the most efficient vPMO cocktail was a 2 oligo cocktail containing Ex8A and Ex8G (Saito et al., 2010). Since exon 6, 7, and 8 are all among the most prevalent targets of exon skipping therapy outside the deletion mutation hotspot (exon 45–55), optimization of antisense oligos against these exons is very important. In fact, approximately 3.0% of DMD patients can be treated with double skipping of exon 6 and exon 7 (ranked No. 9), and 2.3% can be treated with skipping exon 8 (ranked No. 10) (Aartsma-Rus et al., 2009). Because the exon-skipping approach is fundamentally a mutation-specific personalized medicine, an effective path of drug approval process will be also a key to rescue mutations with a relatively small number of patients.
A major concern of new generation morpholino-mediated antisense therapy is their toxicity. No toxicity of vPMOs has been recorded up to 12 mg/kg of systemic injections in mice (Wu et al., 2009). However, with PPMOs, a high dose (150 mg/kg) of systemic injections led to adverse events such as lethargy, weight loss, elevated blood urea nitrogen, and serum creatinine levels (Amantana et al., 2007). In addition, a test in the cynomolgus monkey revealed mild tubular degeneration in the kidneys after weekly injections with 9 mg/kg PPMOs (Moulton and MOULTON, 2010). Although AVI-5038, a PPMO targeting exon 50 of dystrophin mRNA, is in preclinical development, the toxicity of PPMOs might pose a challenge for determination of an effective and safe regimen in man. An immune suppression regimen such as one used for robust adeno-associated virus expression might be effective for systemic trials (Shin et al., 2012; Wang et al., 2007). To test the systemic effect of vPMOs in dogs, precise pharmacokinetics, biodistribution, stability, and toxicity remain to be done. Nevertheless, our results indicate clear potential of the morpholino conjugate as a therapeutic agent to treat DMD and other genetic disorders.
Acknowledgments
We thank Michihiro Imamura, Jing Hong Shin, Takashi Okada, Michiko Wada, Sachiko Ohshima, Jun Tanihata, Satoru Masuda, Kazue Kinoshita, Hideki Kita, Shinichi Ichikawa, Yumiko Yahata, Yuko Kasahara, and Yuko Shimizu (NCNP); and Merryl Rodriguez and Dharminder Panesar (University of Alberta) for useful discussions and technical assistance. This work was supported by Grants-in-Aid for Research on Nervous and Mental Disorders (19A-7), Health and Labor Sciences Research Grants for Translation Research (H19-Translational Research-003 and H21-Clinical Research-015), and Health Sciences Research Grants for Research on Psychiatry and Neurological Disease and Mental Health (H18-kokoro-019) from the Ministry of Health, Labour, and Welfare of Japan, U.S. National Institutes of Health (1P50AR060836; 5T32AR056993), US Department of Defense (W81XWH-09-1-0599) The Friends of Garrett Cumming Research, HM Toupin Neurological Science Research, and Muscular Dystrophy Canada.
TY, TS, AN, and ST conceived and designed study. TY, AN, MK, and TS performed experiments. ST, EH, TN, YA, YO, YE and TP analyzed the data. TY, YA, and TS contributed reagents/materials/analysis tools. TY wrote the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- AARTSMA-RUS A. FOKKEMA I. VERSCHUUREN J. GINJAAR I., et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- AARTSMA-RUS A. KAMAN W.E. WEIJ R. DEN DUNNEN J.T., et al. Exploring the frontiers of therapeutic exon skipping for Duchenne muscular dystrophy by double targeting within one or multiple exons. Mol. Ther. 2006;14:401–407. doi: 10.1016/j.ymthe.2006.02.022. [DOI] [PubMed] [Google Scholar]
- AARTSMA-RUS A. VAN OMMEN G.J. Antisense-mediated exon skipping: A versatile tool with therapeutic and research applications. RNA. 2007;13:1609–1624. doi: 10.1261/rna.653607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTER J. LOU F. RABINOWITZ A. YIN H., et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat. Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- AMANTANA A. MOULTON H.M. CATE M.L. REDDY M.T, et al. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconjug. Chem. 2007;18:1325–1331. doi: 10.1021/bc070060v. [DOI] [PubMed] [Google Scholar]
- AOKI Y. NAKAMURA A. YOKOTA T. SAITO T., et al. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52-deficient mdx mouse. Mol. Ther. 2010;18:1995–2005. doi: 10.1038/mt.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEROUD C. TUFFERY-GIRAUD S. MATSUO M. HAMROUN D., et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum. Mutat. 2006;28:196–202. doi: 10.1002/humu.20428. [DOI] [PubMed] [Google Scholar]
- CARTEGNI L. WANG J. ZHU Z. ZHANG M.Q., et al. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRAK S. ARECHAVALA-GOMEZA V. GUGLIERI M. FENG L., et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS T.J. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- CRISP A. YIN H. GOYENVALLE A. BETTS C., et al. Diaphragm rescue alone prevents heart dysfunction in dystrophic mice. Hum. Mol. Genet. 2011;20:413–421. doi: 10.1093/hmg/ddq477. [DOI] [PubMed] [Google Scholar]
- DUCHENNE. The pathology of paralysis with muscular degeneration (paralysie myosclerotique), or paralysis with apparent hypertrophy. Br. Med. J. 1867;2:541–542. doi: 10.1136/bmj.2.363.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCKLEY M.G. MANOHARAN M. VILLIET P. EPERON I.C., et al. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum. Mol. Genet. 1998;7:1083–1090. doi: 10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- GOEMANS N.M. TULINIUS M. VAN DEN AKKER J.T. BURM B.E., et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N. Engl. J. Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- GOYENVALLE A. BABBS A. POWELL D. KOLE R., et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol. Ther. 2010;18:198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOYENVALLE A. SETO J.T. DAVIES K.E. CHAMBERLAIN J. Therapeutic approaches to muscular dystrophy. Hum. Mol. Genet. 2011;20:R69–78. doi: 10.1093/hmg/ddr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN E.P. BRONSON A. LEVIN A.A. TAKEDA S., et al. Restoring dystrophin expression in Duchenne muscular dystrophy muscle progress in exon skipping and stop codon read through. Am. J. Pathol. 2011;179:12–22. doi: 10.1016/j.ajpath.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN E.P. BROWN R.H., JR. KUNKEL L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- JEARAWIRIYAPAISARN N. MOULTON H.M. BUCKLEY B. ROBERTS J., et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol. Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEARAWIRIYAPAISARN N. MOULTON H.M. SAZANI P. KOLE R., et al. Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc. Res. 2010;85:444–453. doi: 10.1093/cvr/cvp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINALI M. ARECHAVALA-GOMEZA V. FENG L. CIRAK S., et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOENIG M. HOFFMAN E.P. BERTELSON C.J. MONACO A.P., et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- LU Q.L. RABINOWITZ A. CHEN Y.C. YOKOTA T., et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc. Natl. Acad. Sci. U. S. A. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU Q.L. YOKOTA T. TAKEDA S. GARCIA L., et al. The status of exon skipping as a therapeutic approach to duchenne muscular dystrophy. Mol. Ther. 2011;19:9–15. doi: 10.1038/mt.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLOREY G. MOULTON H.M. IVERSEN P.L. FLETCHER S., et al. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 2006;13:1373–1381. doi: 10.1038/sj.gt.3302800. 1373–1381. [DOI] [PubMed] [Google Scholar]
- MORCOS P.A. LI Y. JIANG S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 2008;45:613–614. doi: 10.2144/000113005. 616, 618 passim. [DOI] [PubMed] [Google Scholar]
- MOULTON H.M. MOULTON J.D. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta. 2010;1798:2296–2303. doi: 10.1016/j.bbamem.2010.02.012. [DOI] [PubMed] [Google Scholar]
- MOULTON H.M. WU B. JEARAWIRIYAPAISARN N. SAZANI P., et al. Peptide-morpholino conjugate: a promising therapeutic for Duchenne muscular dystrophy. Ann. N. Y. Acad. Sci. 2009;1175:55–60. doi: 10.1111/j.1749-6632.2009.04976.x. [DOI] [PubMed] [Google Scholar]
- MOULTON J.D. JIANG S. Gene knockdowns in adult animals: PPMOs and vivo-morpholinos. Molecules. 2009;14:1304–1323. doi: 10.3390/molecules14031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA A. YOSHIDA K. FUKUSHIMA K. UEDA H., et al. Follow-up of three patients with a large in-frame deletion of exons 45–55 in the Duchenne muscular dystrophy (DMD) gene. J. Clin. Neurosci. 2008;15:757–763. doi: 10.1016/j.jocn.2006.12.012. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE T.A. Impending therapies for Duchenne muscular dystrophy. Curr. Opin. Neurol. 2011;24:415–422. doi: 10.1097/WCO.0b013e32834aa3f1. [DOI] [PubMed] [Google Scholar]
- PICHAVANT C. AARTSMA-RUS A. CLEMENS P.R. DAVIES K.E., et al. Current status of pharmaceutical and genetic therapeutic approaches to treat DMD. Mol. Ther. 2011;19:830–840. doi: 10.1038/mt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRAMONO Z.A. TAKESHIMA Y. ALIMSARDJONO H. ISHII A., et al. Induction of exon skipping of the dystrophin transcript in lymphoblastoid cells by transfecting an antisense oligodeoxynucleotide complementary to an exon recognition sequence. Biochem. Biophys. Res. Commun. 1996;226:445–449. doi: 10.1006/bbrc.1996.1375. [DOI] [PubMed] [Google Scholar]
- REISS J. RININSLAND F. An explanation for the constitutive exon 9 cassette splicing of the DMD gene. Hum. Mol. Genet. 1994;3:295–298. doi: 10.1093/hmg/3.2.295. [DOI] [PubMed] [Google Scholar]
- SAITO T. NAKAMURA A. AOKI Y. YOKOTA T., et al. Antisense PMO found in dystrophic dog model was effective in cells from exon 7-deleted DMD patient. PLoS One. 2010;5:e12239. doi: 10.1371/journal.pone.0012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP N.J. KORNEGAY J.N. VAN CAMP S.D. HERBSTREITH M.H., et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- SHIMATSU Y. KATAGIRI K. FURUTA T. NAKURA M., et al. Canine X-linked muscular dystrophy in Japan (CXMDJ) Exp. Anim. 2003;52:93–97. doi: 10.1538/expanim.52.93. [DOI] [PubMed] [Google Scholar]
- SHIN J.H. YUE Y. SRIVASTAVA A. SMITH B., et al. A Simplified Immune Suppression Scheme Leads to Persistent Micro-dystrophin Expression in Duchenne Muscular Dystrophy Dogs. Hum. Gene Ther. 2012;23:202–209. doi: 10.1089/hum.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI-IKEDA M. KOBAYASHI K. KANAGAWA M. YU C.C., et al. Pathogenic exon-trapping by SVA retrotransposon and rescue in Fukuyama muscular dystrophy. Nature. 2011;478:127–131. doi: 10.1038/nature10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEUTEKOM J.C. JANSON A.A. GINJAAR I.B. FRANKHUIZEN W.S., et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 2007;357:2677–286. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- WANG Z. KUHR C.S. ALLEN J.M. BLANKINSHIP M., et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol. Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- WIDRICK J.J. JIANG S. CHOI S.J. KNUTH S.T., et al. An octaguanidine-morpholino oligo conjugate improves muscle function of mdx mice. Muscle Nerve. 2011;44:563–570. doi: 10.1002/mus.22126. [DOI] [PubMed] [Google Scholar]
- WU B. BENRASHID E. LU P. CLOER C., et al. Targeted skipping of human dystrophin exons in transgenic mouse model systemically for antisense drug development. PLoS One. 2011a;6:e19906. doi: 10.1371/journal.pone.0019906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B. LI Y. MORCOS P.A. DORAN T.J., et al. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol. Ther. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B. LU P. BENRASHID E. MALILK S., et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 2010;17:132–140. doi: 10.1038/gt.2009.120. [DOI] [PubMed] [Google Scholar]
- WU B. XIAO B. CLOER C. SHABAN M., et al. One-year treatment of morpholino antisense oligomer improves skeletal and cardiac muscle functions in dystrophic mdx mice. Mol. Ther. 2011b;19:576–583. doi: 10.1038/mt.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOTA T. DUDDY W. ECHIGOYA Y. KOLSKI H. Exon skipping for nonsense mutations in Duchenne muscular dystrophy: too many mutations, too few patients? Expert Opin. Biol. Ther. 2012 doi: 10.1517/14712598.2012.693469. [DOI] [PubMed] [Google Scholar]
- YOKOTA T. DUDDY W. PARTRIDGE T. Optimizing exon skipping therapies for DMD. http://informahealthcare.com/doi/abs/10.1517/14712598.2012.693469. [Jun 1;2012 ];Acta Myol. 2007a 26:179–184. [PMC free article] [PubMed] [Google Scholar]
- YOKOTA T. HOFFMAN E.P. TAKEDA S. Antisense oligo-mediated multiple exon skipping in a dog model of Duchenne muscular dystrophy. Methods Mol. Biol. 2011;709:299–312. doi: 10.1007/978-1-61737-982-6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOTA T. LU Q.L. MORGAN J.E. DAVIES K.E., et al. Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J. Cell Sci. 2006;119:2679–2687. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- YOKOTA T. LU Q.L. PARTRIDGE T. KOBAYASHI M., et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann. Neurol. 2009a;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOTA T. PISTILLI E. DUDDY W. NAGARAJU K. Potential of oligonucleotide-mediated exon-skipping therapy for Duchenne muscular dystrophy. Expert Opin. Biol. Ther. 2007b;7:831–842. doi: 10.1517/14712598.7.6.831. [DOI] [PubMed] [Google Scholar]
- YOKOTA T. TAKEDA S. LU Q.L. PARTRIDGE T.A., et al. A renaissance for antisense oligonucleotide drugs in neurology: exon skipping breaks new ground. Arch. Neurol. 2009b;66:32–38. doi: 10.1001/archneurol.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELLWEGER H. ANTONIK A. Newborn screening for Duchenne muscular dystrophy. Pediatrics. 1975;55:30–34. [PubMed] [Google Scholar]